In Vitro Bactericidal Activity of a Neomycin-Polymyxin B-Nystatin Combination Compared to Metronidazole and Clindamycin Against the Main Bacteria Involved in Bacterial Vaginosis and Aerobic Vaginitis
Abstract
1. Introduction
2. Results
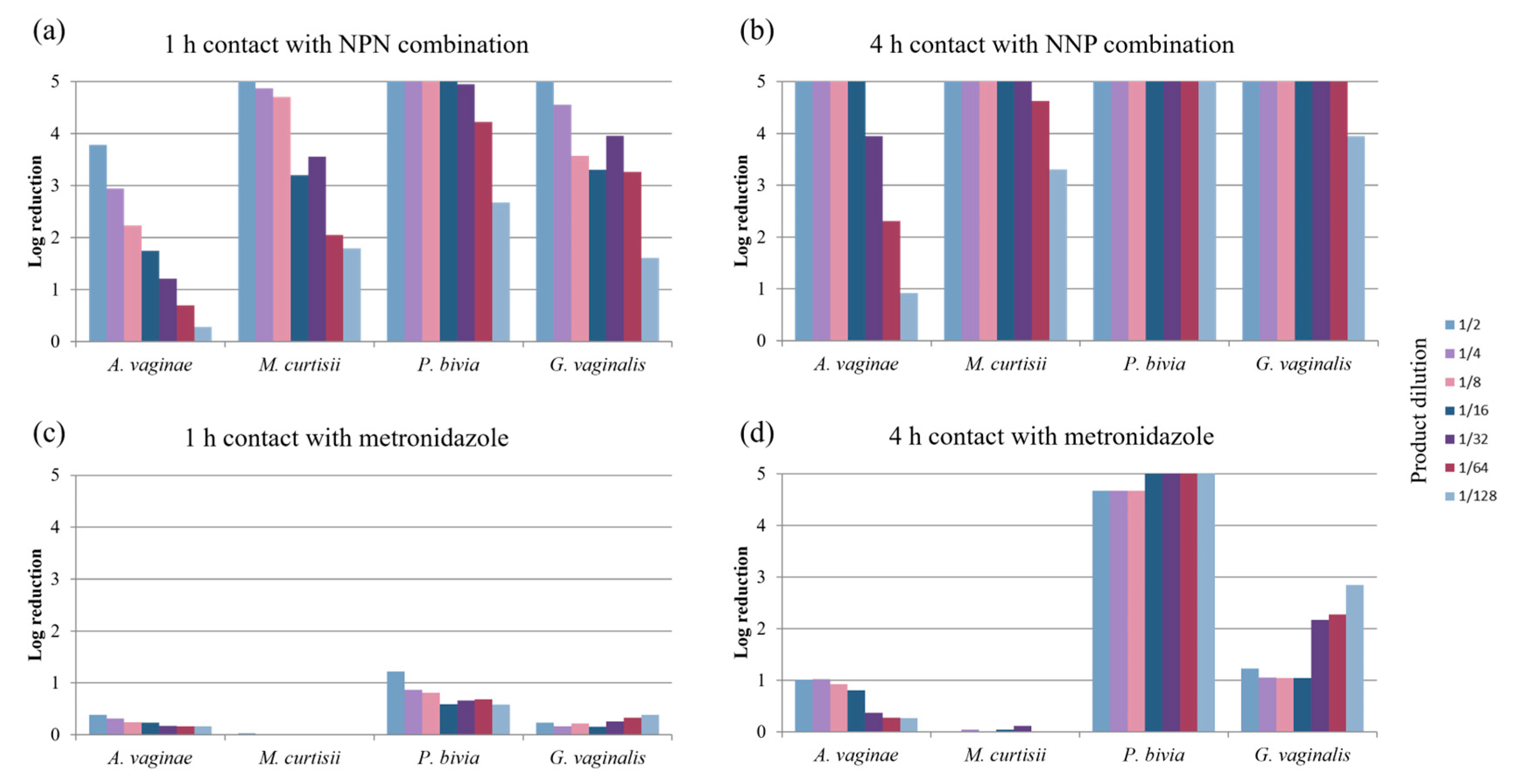
2.1. Bactericidal Activity Against Bacteria Involved in Bacterial Vaginosis
2.2. Bactericidal Activity Against Bacteria Involved in Aerobic Vaginitis
2.2.1. Gram-Positive Bacteria
2.2.2. Gram-Negative Bacteria
3. Discussion
4. Materials and Methods
4.1. Antimicrobial Products
4.2. Bacterial Strains
4.3. Assays
- -
- c is the sum of the CFU values taken into account;
- -
- n1 is the number of CFU values taken into account for the lower dilution, i.e., 10−x;
- -
- n2 is the number of CFU values taken into account for the higher dilution, i.e., 10−(x+1);
- -
- 10−x is the dilution factor corresponding to the lower dilution.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Egan, M.E.; Lipsky, M.S. Diagnosis of vaginitis. Am. Fam. Physician 2000, 62, 1095–1104. [Google Scholar] [PubMed]
- Nyirjesy, P.; Sobel, J.D. Advances in Diagnosing Vaginitis: Development of a New Algorithm. Curr. Infect. Dis. Rep. 2005, 7, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Sherrard, J.; Wilson, J.; Donders, G.; Mendling, W.; Jensen, J.S. 2018 European (IUSTI/WHO) International Union against Sexually Transmitted Infections (IUSTI) World Health Organisation (WHO) Guideline on the Management of Vaginal Discharge. Int. J. STD AIDS 2018, 29, 1258–1272. [Google Scholar] [CrossRef]
- Bautista, C.T.; Wurapa, E.; Sateren, W.B.; Morris, S.; Hollingsworth, B.; Sanchez, J.L. Bacterial Vaginosis: A Synthesis of the Literature on Etiology, Prevalence, Risk Factors, and Relationship with Chlamydia and Gonorrhea Infections. Mil. Med. Res. 2016, 3, 4. [Google Scholar] [CrossRef]
- Coudray, M.S.; Madhivanan, P. Bacterial Vaginosis—A Brief Synopsis of the Literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 143–148. [Google Scholar] [CrossRef]
- Donders, G.G.G.; Vereecken, A.; Bosmans, E.; Dekeersmaecker, A.; Salembier, G.; Spitz, B. Definition of a Type of Abnormal Vaginal Flora That Is Distinct from Bacterial Vaginosis: Aerobic Vaginitis. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 34–43. [Google Scholar] [CrossRef]
- Peebles, K.; Kiweewa, F.M.; Palanee-Phillips, T.; Chappell, C.; Singh, D.; Bunge, K.E.; Naidoo, L.; Makanani, B.; Jeenarain, N.; Reynolds, D.; et al. Elevated Risk of Bacterial Vaginosis Among Users of the Copper Intrauterine Device: A Prospective Longitudinal Cohort Study. Clin. Infect. Dis. 2021, 73, 513–520. [Google Scholar] [CrossRef]
- Jung, H.-S.; Ehlers, M.M.; Lombaard, H.; Redelinghuys, M.J.; Kock, M.M. Etiology of Bacterial Vaginosis and Polymicrobial Biofilm Formation. Crit. Rev. Microbiol. 2017, 43, 651–667. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Muzny, C.A.; Schwebke, J.R. Pathogenesis of Bacterial Vaginosis: Discussion of Current Hypotheses. J. Infect. Dis. 2016, 214, S1–S5. [Google Scholar] [CrossRef]
- Muzny, C.A.; Taylor, C.M.; Swords, W.E.; Tamhane, A.; Chattopadhyay, D.; Cerca, N.; Schwebke, J.R. An Updated Conceptual Model on the Pathogenesis of Bacterial Vaginosis. J. Infect. Dis. 2019, 220, 1399–1405. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Muzny, C.A.; Josey, W.E. Role of Gardnerella vaginalis in the Pathogenesis of Bacterial Vaginosis: A Conceptual Model. J. Infect. Dis. 2014, 210, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.S.; Brotman, R.M. Making Inroads into Improving Treatment of Bacterial Vaginosis—Striving for Long-Term Cure. BMC Infect. Dis. 2015, 15, 292. [Google Scholar] [CrossRef] [PubMed]
- Fettweis, J.M.; Brooks, J.P.; Serrano, M.G.; Sheth, N.U.; Girerd, P.H.; Edwards, D.J.; Strauss, J.F.; The Vaginal Microbiome Consortium; Jefferson, K.K.; Buck, G.A. Differences in Vaginal Microbiome in African American Women versus Women of European Ancestry. Microbiology 2014, 160, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N.M.; Lewis, W.G.; Li, G.; Sojka, D.K.; Lubin, J.B.; Lewis, A.L. Gardnerella vaginalis and Prevotella bivia Trigger Distinct and Overlapping Phenotypes in a Mouse Model of Bacterial Vaginosis. J. Infect. Dis. 2019, 220, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal Microbiota, Women’s Health, and Reproductive Outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef]
- Leyva-Gómez, G.; Del Prado-Audelo, M.L.; Ortega-Peña, S.; Mendoza-Muñoz, N.; Urbán-Morlán, Z.; González-Torres, M.; González-Del Carmen, M.; Figueroa-González, G.; Reyes-Hernández, O.D.; Cortés, H. Modifications in Vaginal Microbiota and Their Influence on Drug Release: Challenges and Opportunities. Pharmaceutics 2019, 11, 217. [Google Scholar] [CrossRef]
- Mendling, W.; Palmeira-de-Oliveira, A.; Biber, S.; Prasauskas, V. An Update on the Role of Atopobium Vaginae in Bacterial Vaginosis: What to Consider When Choosing a Treatment? A Mini Review. Arch. Gynecol. Obstet. 2019, 300, 1–6. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, R.; Duarte, P.; Palmeira-de-Oliveira, A.; Das Neves, J.; Amaral, M.H.; Breitenfeld, L.; Martinez-de-Oliveira, J. Women’s Experiences, Preferences and Perceptions Regarding Vaginal Products: Results from a Cross-Sectional Web-Based Survey in Portugal. Eur. J. Contracept. Reprod. Health Care 2015, 20, 259–271. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New Strategies for Local Treatment of Vaginal Infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef]
- Srinivasan, S.; Hoffman, N.G.; Morgan, M.T.; Matsen, F.A.; Fiedler, T.L.; Hall, R.W.; Ross, F.J.; McCoy, C.O.; Bumgarner, R.; Marrazzo, J.M.; et al. Bacterial Communities in Women with Bacterial Vaginosis: High Resolution Phylogenetic Analyses Reveal Relationships of Microbiota to Clinical Criteria. PLoS ONE 2012, 7, e37818. [Google Scholar] [CrossRef] [PubMed]
- Rampersaud, R.; Randis, T.M.; Ratner, A.J. Microbiota of the Upper and Lower Genital Tract. Semin. Fetal Neonatal Med. 2012, 17, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Turovskiy, Y.; Sutyak Noll, K.; Chikindas, M.L. The Aetiology of Bacterial Vaginosis: Aetiology of Bacterial Vaginosis. J. Appl. Microbiol. 2011, 110, 1105–1128. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.G.; Bellen, G.; Grinceviciene, S.; Ruban, K.; Vieira-Baptista, P. Aerobic Vaginitis: No Longer a Stranger. Res. Microbiol. 2017, 168, 845–858. [Google Scholar] [CrossRef]
- Sangeetha, T.; Saroj, G.; Vasudha, L. A Study of Aerobic Bacterial Pathogens Associated with Vaginitis in Reproductive Age Group Women (15–45 Years) and Their Sensitivity Pattern. Int. J. Res. Med. Sci. 2015, 2268–2273. [Google Scholar] [CrossRef]
- Tansarli, G.S.; Kostaras, E.K.; Athanasiou, S.; Falagas, M.E. Prevalence and Treatment of Aerobic Vaginitis among Non-Pregnant Women: Evaluation of the Evidence for an Underestimated Clinical Entity. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 977–984. [Google Scholar] [CrossRef]
- Tomusiak, A.; Heczko, P.B.; Janeczko, J.; Adamski, P.; Pilarczyk-Zurek, M.; Strus, M. Bacterial Infections of the Lower Genital Tract in Fertile and Infertile Women from the Southeastern Poland. Ginekol. Pol. 2013, 84, 352–358. [Google Scholar] [CrossRef]
- Vieira-Baptista, P.; Stockdale, C.K.; Sobel, J. (Eds.) International Society for the Study of Vulvovaginal Disease Recommendations for the Diagnosis and Treatment of Vaginitis; Admedic: Lisbon, Portugal, 2023; ISBN 978-989-53-4893-0. [Google Scholar]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Management of Symptomatic Sexually Transmitted Infections; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-002416-8. [Google Scholar]
- Bagnall, P.; Rizzolo, D. Bacterial Vaginosis: A Practical Review. J. Am. Acad. Physician Assist. 2017, 30, 15–21. [Google Scholar] [CrossRef]
- Donders, G.G.G.; Zodzika, J.; Rezeberga, D. Treatment of Bacterial Vaginosis: What We Have and What We Miss. Expert. Opin. Pharmacother. 2014, 15, 645–657. [Google Scholar] [CrossRef]
- Laghi, L.; Picone, G.; Cruciani, F.; Brigidi, P.; Calanni, F.; Donders, G.; Capozzi, F.; Vitali, B. Rifaximin Modulates the Vaginal Microbiome and Metabolome in Women Affected by Bacterial Vaginosis. Antimicrob. Agents Chemother. 2014, 58, 3411–3420. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Desmond, R.A. A Randomized Trial of the Duration of Therapy with Metronidazole plus or Minus Azithromycin for Treatment of Symptomatic Bacterial Vaginosis. Clin. Infect. Dis. 2007, 44, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.S.; Sobel, J.D. Current Treatment of Bacterial Vaginosis—Limitations and Need for Innovation. J. Infect. Dis. 2016, 214, S14–S20. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Castro, J.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Cerca, N. Bacterial Vaginosis Biofilms: Challenges to Current Therapies and Emerging Solutions. Front. Microbiol. 2016, 6, 1528. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Cerca, N. Influence of Biofilm Formation by Gardnerella vaginalis and Other Anaerobes on Bacterial Vaginosis. J. Infect. Dis. 2015, 212, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, H.; Swidsinski, A. The Biofilm in Bacterial Vaginosis: Implications for Epidemiology, Diagnosis and Treatment: 2018 Update. Curr. Opin. Infect. Dis. 2019, 32, 38–42. [Google Scholar] [CrossRef]
- Bilardi, J.; Walker, S.; McNair, R.; Mooney-Somers, J.; Temple-Smith, M.; Bellhouse, C.; Fairley, C.; Chen, M.; Bradshaw, C. Women’s Management of Recurrent Bacterial Vaginosis and Experiences of Clinical Care: A Qualitative Study. PLoS ONE 2016, 11, e0151794. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Morton, A.N.; Hocking, J.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. High Recurrence Rates of Bacterial Vaginosis over the Course of 12 Months after Oral Metronidazole Therapy and Factors Associated with Recurrence. J. Infect. Dis. 2006, 193, 1478–1486. [Google Scholar] [CrossRef]
- Vodstrcil, L.A.; Muzny, C.A.; Plummer, E.L.; Sobel, J.D.; Bradshaw, C.S. Bacterial Vaginosis: Drivers of Recurrence and Challenges and Opportunities in Partner Treatment. BMC Med. 2021, 19, 194. [Google Scholar] [CrossRef]
- Hay, P. Recurrent Bacterial Vaginosis. Curr. Opin. Infect. Dis. 2009, 22, 82–86. [Google Scholar] [CrossRef]
- Fan, A.; Yue, Y.; Geng, N.; Zhang, H.; Wang, Y.; Xue, F. Aerobic Vaginitis and Mixed Infections: Comparison of Clinical and Laboratory Findings. Arch. Gynecol. Obstet. 2013, 287, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Abou Chacra, L.; Fenollar, F.; Diop, K. Bacterial Vaginosis: What Do We Currently Know? Front. Cell. Infect. Microbiol. 2022, 11, 672429. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, J.-M.; Sednaoui, P.; Verriere, F.; Achhammer, I. Diversité étiologique des vaginites. Gynécol. Obs. Fertil. 2012, 40, 578–581. [Google Scholar] [CrossRef]
- Qian, Z.; Zhu, H.; Zhao, D.; Yang, P.; Gao, F.; Lu, C.; Yin, Y.; Kan, S.; Chen, D. Probiotic Lactobacillus sp. Strains Inhibit Growth, Adhesion, Biofilm Formation, and Gene Expression of Bacterial Vaginosis-Inducing Gardnerella vaginalis. Microorganisms 2021, 9, 728. [Google Scholar] [CrossRef]
- Bohbot, J.M.; Goubard, A.; Aubin, F.; Mas, Y.; Coatantiec, E.; Lucas, N.; Verrière, F. PRISM Study: Comparison of a Nystatin-Neomycin-Polymyxin B Combination with Miconazole for the Empirical Treatment of Infectious Vaginitis. Méd. Mal. Infect. 2019, 49, 194–201. [Google Scholar] [CrossRef]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Thompson, P.E.; Li, J. Pharmacology of Polymyxins: New Insights into an ‘Old’ Class of Antibiotics. Future Microbiol. 2013, 8, 711–724. [Google Scholar] [CrossRef]
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E.W. Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef]
- Lima, M.R.; Ferreira, G.F.; Nunes Neto, W.R.; Monteiro, J.d.M.; Santos, Á.R.C.; Tavares, P.B.; Denadai, Â.M.L.; Bomfim, M.R.Q.; dos Santos, V.L.; Marques, S.G.; et al. Evaluation of the Interaction between Polymyxin B and Pseudomonas aeruginosa Biofilm and Planktonic Cells: Reactive Oxygen Species Induction and Zeta Potential. BMC Microbiol. 2019, 19, 115. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef]
- Nation, R.L.; Velkov, T.; Li, J. Colistin and Polymyxin B: Peas in a Pod, or Chalk and Cheese? Clin. Infect. Dis. 2014, 59, 88–94. [Google Scholar] [CrossRef]
- Davies, J.; Davis, B.D. Misreading of Ribonucleic Acid Code Words Induced by Aminoglycoside Antibiotics: The Effect of Drug Concentration. J. Biol. Chem. 1968, 243, 3312–3316. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.B.; Terry, D.S.; Altman, R.B.; Blanchard, S.C. Aminoglycoside Activity Observed on Single Pre-Translocation Ribosome Complexes. Nat. Chem. Biol. 2010, 6, 54–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belardinelli, R.; Sharma, H.; Peske, F.; Rodnina, M.V. Perturbation of Ribosomal Subunit Dynamics by Inhibitors of tRNA Translocation. RNA 2021, 27, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Misumi, M.; Nishimura, T.; Komai, T.; Tanaka, N. Interaction of Kanamycin and Related Antibiotics with the Large Subunit of Ribosomes and the Inhibition of Translocation. Biochem. Biophys. Res. Commun. 1978, 84, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Cabañas, M.J.; Vázquez, D.; Modolell, J. Inhibition of Ribosomal Translocation by Aminoglycoside Antibiotics. Biochem. Biophys. Res. Commun. 1978, 83, 991–997. [Google Scholar] [CrossRef]
- Deng, Z.-L.; Gottschick, C.; Bhuju, S.; Masur, C.; Abels, C.; Wagner-Döbler, I. Metatranscriptome Analysis of the Vaginal Microbiota Reveals Potential Mechanisms for Protection against Metronidazole in Bacterial Vaginosis. Msphere 2018, 3, e00262-18. [Google Scholar] [CrossRef]
- Ferris, M.J.; Masztal, A.; Martin, D.H. Use of Species-Directed 16S rRNA Gene PCR Primers for Detection of Atopobium Vaginae in Patients with Bacterial Vaginosis. J. Clin. Microbiol. 2004, 42, 5892–5894. [Google Scholar] [CrossRef]
- Gal, M.; Brazier, J.S. Metronidazole Resistance in Bacteroides spp. Carrying Nim Genes and the Selection of Slow-Growing Metronidazole-Resistant Mutants. J. Antimicrob. Chemother. 2004, 54, 109–116. [Google Scholar] [CrossRef]
- Ravel, J.; Brotman, R.M.; Gajer, P.; Ma, B.; Nandy, M.; Fadrosh, D.W.; Sakamoto, J.; Koenig, S.S.; Fu, L.; Zhou, X.; et al. Daily Temporal Dynamics of Vaginal Microbiota before, during and after Episodes of Bacterial Vaginosis. Microbiome 2013, 1, 29. [Google Scholar] [CrossRef]
- Schuyler, J.A.; Mordechai, E.; Adelson, M.E.; Sobel, J.D.; Gygax, S.E.; Hilbert, D.W. Identification of Intrinsically Metronidazole-Resistant Clades of Gardnerella vaginalis. Diagn. Microbiol. Infect. Dis. 2016, 84, 1–3. [Google Scholar] [CrossRef]
- Sobel, J.D. Recurrent Bacterial Vaginosis, Relapse or Reinfection: The Role of Sexual Transmission. BJOG 2021, 128, 768. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Cheu, R.K.; Lemke, M.M.; Gustin, A.T.; France, M.T.; Hampel, B.; Thurman, A.R.; Doncel, G.F.; Ravel, J.; Klatt, N.R.; et al. Quantitative Modeling Predicts Mechanistic Links between Pre-Treatment Microbiome Composition and Metronidazole Efficacy in Bacterial Vaginosis. Nat. Commun. 2020, 11, 6147. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.; Castro, J.; Sousa, C.; Cereija, T.B.; Cerca, N. Gardnerella vaginalis Outcompetes 29 Other Bacterial Species Isolated from Patients with Bacterial Vaginosis, Using in an in Vitro Biofilm Formation Model. J. Infect. Dis. 2014, 210, 593–596. [Google Scholar] [CrossRef]
- Castro, J.; Alves, P.; Sousa, C.; Cereija, T.; França, Â.; Jefferson, K.K.; Cerca, N. Using an In-Vitro Biofilm Model to Assess the Virulence Potential of Bacterial Vaginosis or Non-Bacterial Vaginosis Gardnerella vaginalis Isolates. Sci. Rep. 2015, 5, 11640. [Google Scholar] [CrossRef]
- Janulaitiene, M.; Gegzna, V.; Baranauskiene, L.; Bulavaitė, A.; Simanavicius, M.; Pleckaityte, M. Phenotypic Characterization of Gardnerella vaginalis Subgroups Suggests Differences in Their Virulence Potential. PLoS ONE 2018, 13, e0200625. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Z.; Wang, F.; He, Y.; Zong, X.; Bai, H.; Liu, Z. Antimicrobial Susceptibility Testing of Metronidazole and Clindamycin against Gardnerella vaginalis in Planktonic and Biofilm Formation. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 1361825. [Google Scholar] [CrossRef]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Swidsinski, S.; Dörffel, Y.; Scholze, J.; Lochs, H.; Verstraelen, H. An Adherent Gardnerella vaginalis Biofilm Persists on the Vaginal Epithelium after Standard Therapy with Oral Metronidazole. Am. J. Obstet. Gynecol. 2008, 198, 97.e1–97.e6. [Google Scholar] [CrossRef] [PubMed]
- Mollin, A.; Katta, M.; Sobel, J.D.; Akins, R.A. Association of Key Species of Vaginal Bacteria of Recurrent Bacterial Vaginosis Patients before and after Oral Metronidazole Therapy with Short- and Long-Term Clinical Outcomes. PLoS ONE 2022, 17, e0272012. [Google Scholar] [CrossRef]
- Bohbot, J.-M.; Sednaoui, P.; Verriere, F. Nystatin-Neomycin-Polymyxin B Combination: Efficacy and Tolerance as 1st-Line Local Treatment of Infectious Vaginitis. Open J. Obstet. Gynecol. 2014, 4, 445–454. [Google Scholar] [CrossRef][Green Version]
- Choukri, F.; Benderdouche, M.; Sednaoui, P. In Vitro Susceptibility Profile of 200 Recent Clinical Isolates of Candida spp. to Topical Antifungal Treatments of Vulvovaginal Candidiasis, the Imidazoles and Nystatin Agents. J. Mycol. Médicale 2014, 24, 303–307. [Google Scholar] [CrossRef]
- Wang, F.-J.; Zhang, D.; Liu, Z.-H.; Wu, W.-X.; Bai, H.-H.; Dong, H.-Y. Species Distribution and In Vitro Antifungal Susceptibility of Vulvovaginal Candida Isolates in China. Chin. Med. J. 2016, 129, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Neut, C.; Verrière, F.; Nelis, H.J.; Coenye, T. Topical Treatment of Infectious Vaginitis: Effects of Antibiotic, Antifungal and Antiseptic Drugs on the Growth of Normal Vaginal Lactobacillus Strains. Open J. Obstet. Gynecol. 2015, 5, 173–180. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Namitha, B.N.; Natarajan, A. Vulvovaginitis Due to Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus among Women in Reproductive Age-Group. J. Clin. Sci. Res. 2023, 12, S11–S13. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamada, K.; Nagao, M.; Aoki, E.; Matsumoto, M.; Hirayama, T.; Yamamoto, H.; Hiramatsu, R.; Ichiyama, S.; Iinuma, Y. Antimicrobial Ointments and Methicillin-Resistant Staphylococcus aureus USA300. Emerg. Infect. Dis. 2011, 17, 1917–1920. [Google Scholar] [CrossRef]
- Coates, A.R.M.; Hu, Y.; Holt, J.; Yeh, P. Antibiotic Combination Therapy against Resistant Bacterial Infections: Synergy, Rejuvenation and Resistance Reduction. Expert Rev. Anti-Infect. Ther. 2020, 18, 5–15. [Google Scholar] [CrossRef]
- Tempera, G.; Mangiafico, A.; Genovese, C.; Giudice, E.; Mastrojeni, S.; Nicolosi, D.; Furneri, P.M. In Vitro Evaluation of the Synergistic Activity of Neomycin-Polymyxin B Association against Pathogens Responsible for Otitis Externa. Int. J. Immunopathol. Pharmacol. 2009, 22, 299–302. [Google Scholar] [CrossRef]
- Muzny, C.A.; Sobel, J.D. The Role of Antimicrobial Resistance in Refractory and Recurrent Bacterial Vaginosis and Current Recommendations for Treatment. Antibiotics 2022, 11, 500. [Google Scholar] [CrossRef]
- Giske, C.G.; Turnidge, J.; Cantón, R.; Kahlmeter, G. Update from the European Committee on Antimicrobial Susceptibility Testing (EUCAST). J. Clin. Microbiol. 2022, 60, e00276-21. [Google Scholar] [CrossRef]
- NF EN 13727+A2; Antiseptiques et Désinfectants Chimiques—Essai Quantitatif de Suspension Pour L’évaluation de L’activité Bactéricide en Médecine—Méthode D’essai et Prescriptions (Phase 2, Étape 1). AFNOR: Saint-Denis, France, 2015.
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef]


| Bacterial Strain | Subcultures | Main Characteristics | |
|---|---|---|---|
| Bacterial vaginosis | |||
| Gram-positive bacteria | Atopobium (Fannyhessea) vaginae DSM 15829 (T) * | Columbia agar + 5% sheep red cells, anaerobiosis ## | Type strain |
| Mobiluncus curtisii DSM 23059 (T) * | Type strain | ||
| Gram-negativebacteria | Prevotella bivia CIP 105105T ** | Columbia agar + 5% sheep red cells, anaerobiosis ## | Type strain |
| Gardnerella vaginalis CIP 70.74T * | Type strain | ||
| Aerobic vaginitis | |||
| Gram-positive bacteria | Staphylococcus aureus CIP 4.83 *** | Trypticase soy agar, aerobiosis # | Recommended strain for microbiological assays of antibiotics Quality control strain for European and American Pharmacopeia |
| Enterococcus hirae CIP 58.55 *** | Trypticase soy agar, aerobiosis # | Recommended strain for microbiological assays of antibiotics, assay of gramicidine, assays of tyrothricine, and assays of thiostreptone Quality control strain for European and American Pharmacopeia | |
| Enterococcus faecalis CIP 103015T | Trypticase soy agar, aerobiosis # | Type strain | |
| Streptococcus agalactiae CIP 103227T | Trypticase soy agar, 5% CO2 # | Type strain Lancefield’s Group B Non-hemolytic | |
| Streptococcus pyogenes CIP 106884 | Trypticase soy agar, aerobiosis # | Resistant to erythromycin, resistant to ciprofloxacin | |
| Corynebacterium amycolatum CIP 103452T | Columbia agar, aerobiosis # | Type strain | |
| Gram-negative bacteria | Pseudomonas aeruginosa CIP 103467 *** | Trypticase soy agar, aerobiosis # | Assay of slimicides Commercial germicides resistance |
| Escherichia coli CIP 54127 *** | Trypticase soy agar, aerobiosis # | H21 Recommended strain for microbiological assays of antibiotics Quality control strain for European and American Pharmacopeia | |
| Proteus mirabilis CIP 103181T | Trypticase soy agar, aerobiosis # | Type strain Recommended strain for evaluation of antimicrobial preservatives | |
| Proteus hauseri CIP 58.60 | Trypticase soy agar, aerobiosis # | Recommended strain for testing bactericides | |
| Klebsiella pneumoniae CIP 82.91 | Trypticase soy agar, aerobiosis # | Capsular type 3 Recommended strain for assays of antimicrobial preservatives—bioresistance testing | |
| Klebsiella aerogenes CIP 60.86T | Trypticase soy agar, aerobiosis # | Recommended strain for assays of antimicrobial preservatives and slimicides | |
| Shigella flexneri CIP 82.48T | Trypticase soy agar, aerobiosis # | Type strain Serotype 2a | |
| Salmonella enterica enterica enteritidis CIP 105150 | Trypticase soy agar, aerobiosis # | Resistant to third-generation cephalosporins Resistant to cefoxitin | |
| Yersinia enterocolitica CIP 80.27T | Trypticase soy agar, aerobiosis # | Type strain Lysotype Xz Biotype 1B Serotype 7,8, Serotype 8, Serotype 19 | |
| Branhamella (Moraxella) catarrhalis CIP 73.21T | Trypticase soy agar, aerobiosis # | Type strain | |
| Haemophilus influenzae CIP 102514T | Chocolate agar, 5% CO2 # | Type strain Presence of fimbriae but absence of the fimbrial genes ghfA, ghfD, and ghfE | |
| Neisseria meningitidis A CIP 73.10T | Chocolate agar, 5% CO2 # | Type strain Reference strain for serogroup A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feuillolay, C.; Salvatico, S.; Escola, J.; Quioc-Salomon, B.; Carrois, F.; Roques, C. In Vitro Bactericidal Activity of a Neomycin-Polymyxin B-Nystatin Combination Compared to Metronidazole and Clindamycin Against the Main Bacteria Involved in Bacterial Vaginosis and Aerobic Vaginitis. Pharmaceuticals 2025, 18, 340. https://doi.org/10.3390/ph18030340
Feuillolay C, Salvatico S, Escola J, Quioc-Salomon B, Carrois F, Roques C. In Vitro Bactericidal Activity of a Neomycin-Polymyxin B-Nystatin Combination Compared to Metronidazole and Clindamycin Against the Main Bacteria Involved in Bacterial Vaginosis and Aerobic Vaginitis. Pharmaceuticals. 2025; 18(3):340. https://doi.org/10.3390/ph18030340
Chicago/Turabian StyleFeuillolay, Catherine, Sylvie Salvatico, Julie Escola, Barbara Quioc-Salomon, Frédéric Carrois, and Christine Roques. 2025. "In Vitro Bactericidal Activity of a Neomycin-Polymyxin B-Nystatin Combination Compared to Metronidazole and Clindamycin Against the Main Bacteria Involved in Bacterial Vaginosis and Aerobic Vaginitis" Pharmaceuticals 18, no. 3: 340. https://doi.org/10.3390/ph18030340
APA StyleFeuillolay, C., Salvatico, S., Escola, J., Quioc-Salomon, B., Carrois, F., & Roques, C. (2025). In Vitro Bactericidal Activity of a Neomycin-Polymyxin B-Nystatin Combination Compared to Metronidazole and Clindamycin Against the Main Bacteria Involved in Bacterial Vaginosis and Aerobic Vaginitis. Pharmaceuticals, 18(3), 340. https://doi.org/10.3390/ph18030340






