Antitumor Activity of USP7 Inhibitor GNE-6776 in Non-Small Cell Lung Cancer Involves Regulation of Epithelial-Mesenchymal Transition, Cell Cycle, Wnt/β-Catenin, and PI3K/AKT/mTOR Pathways
Abstract
1. Introduction
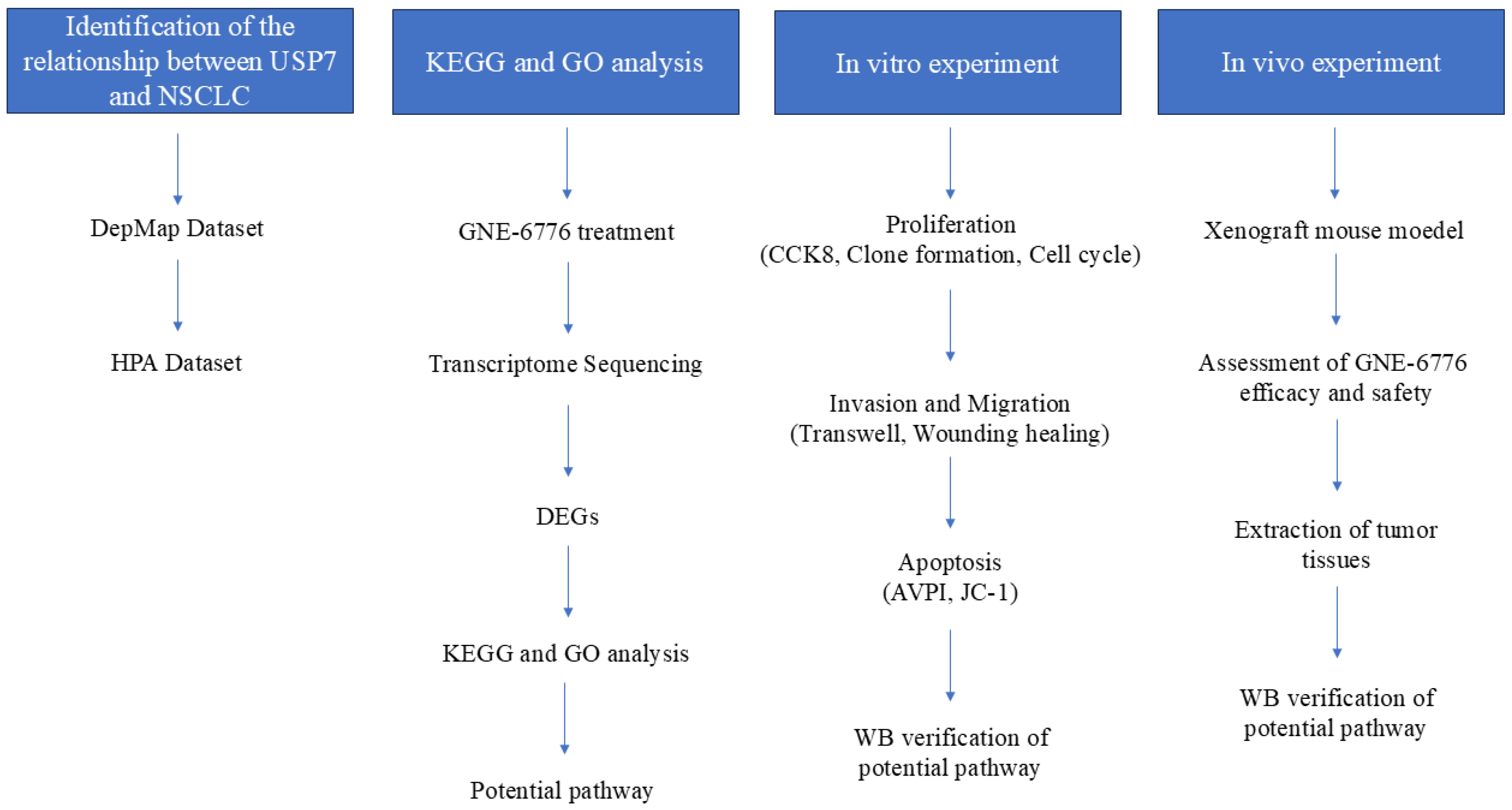
2. Results
2.1. GNE-6776 Inhibits the Proliferation and the Clonogenic Ability of A549 and H1299 Cells
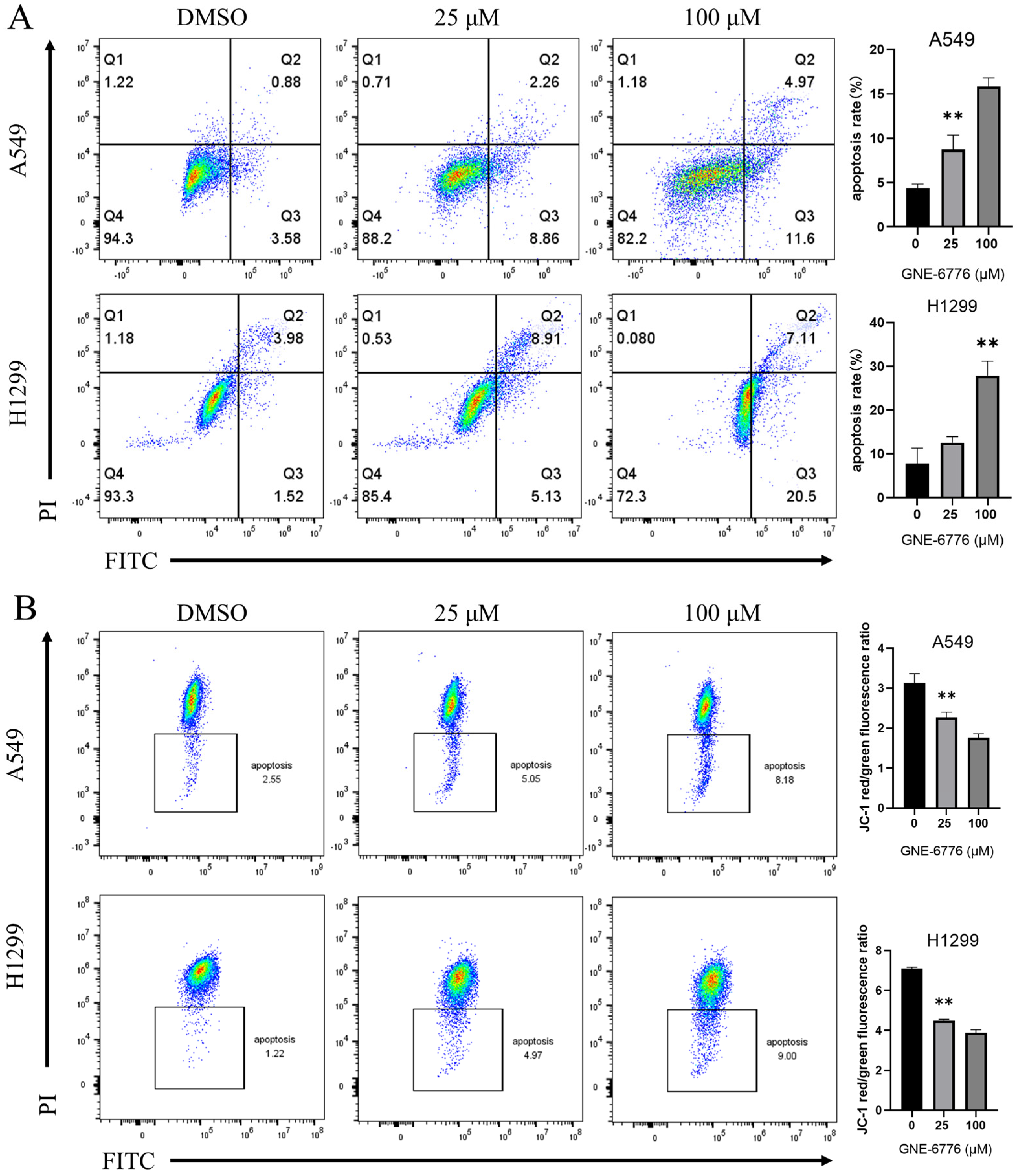
2.2. GNE-6776 Promotes Apoptosis and Reduces the Mitochondrial Membrane Potential in A549 and H1299 Cells
2.3. GNE-6776 Arrests A549 and H1299 Cells in the G1 Phase
2.4. GNE-6776 Inhibits Migration and Invasion of A549 and H1299 Cells
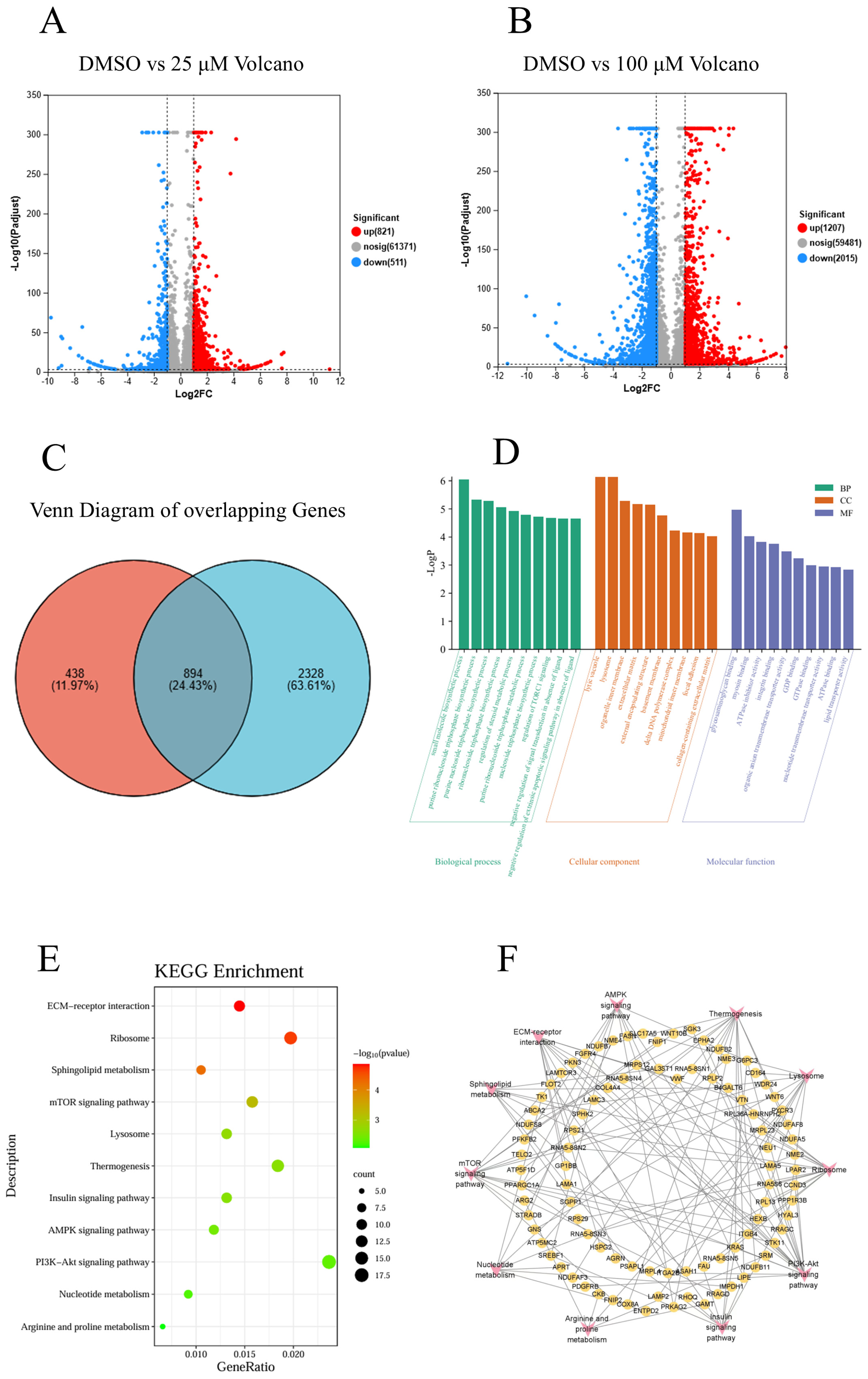
2.5. Transcriptome Sequencing of NSCLC Cells in the Control Group, Low-Concentration GNE-6776 Group and High-Concentration GNE-6776 Group
2.5.1. Differential Gene Expression Analysis Between Control and Experimental Groups
2.5.2. GO and KEGG Analysis of Common Differential Genes in Both Groups
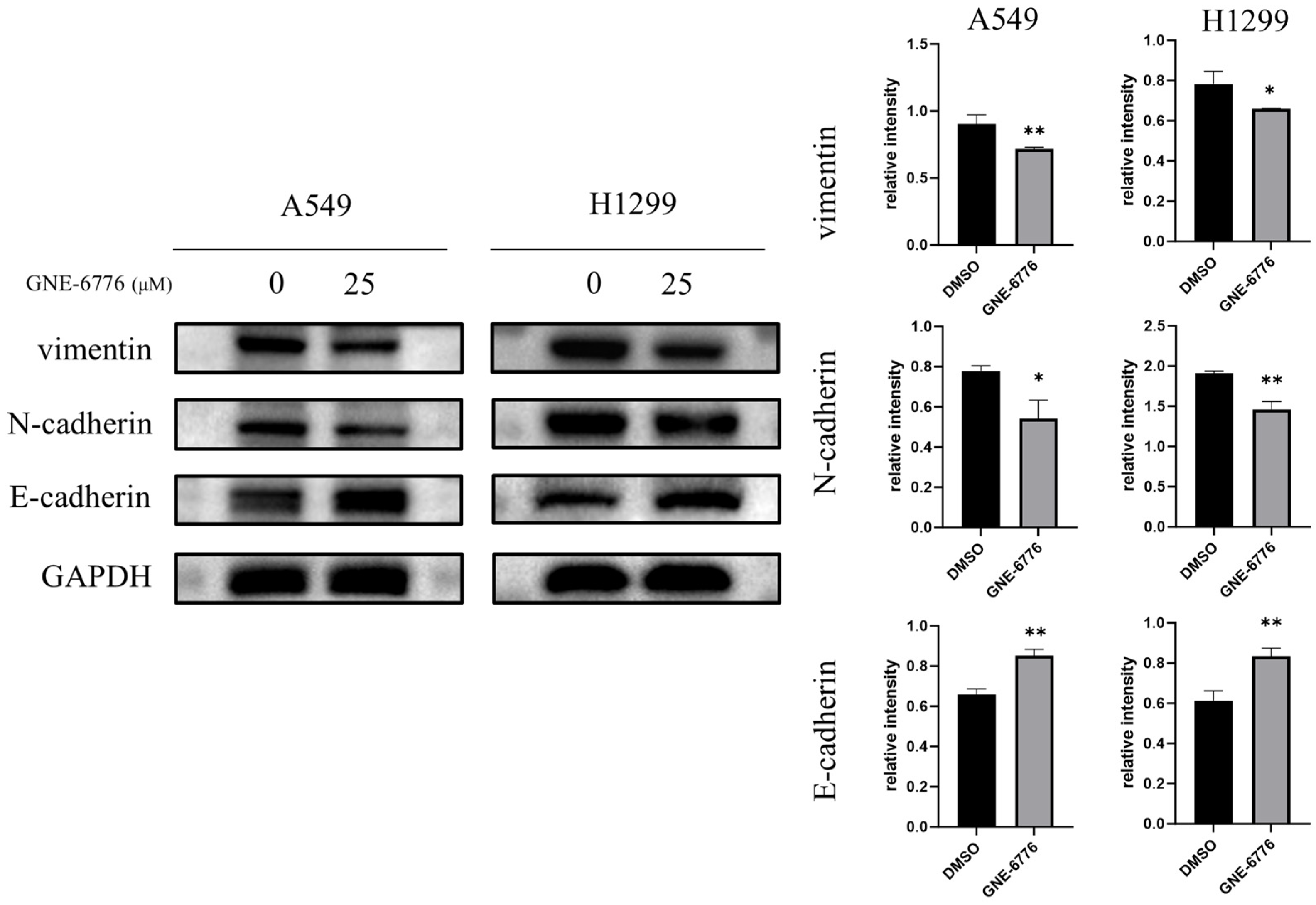
2.6. GNE-6776 Down-Regulates Vimentin and N-Cadherin, but Up-Regulates the Epithelial Marker E-Cadherin in NSCLC Cells
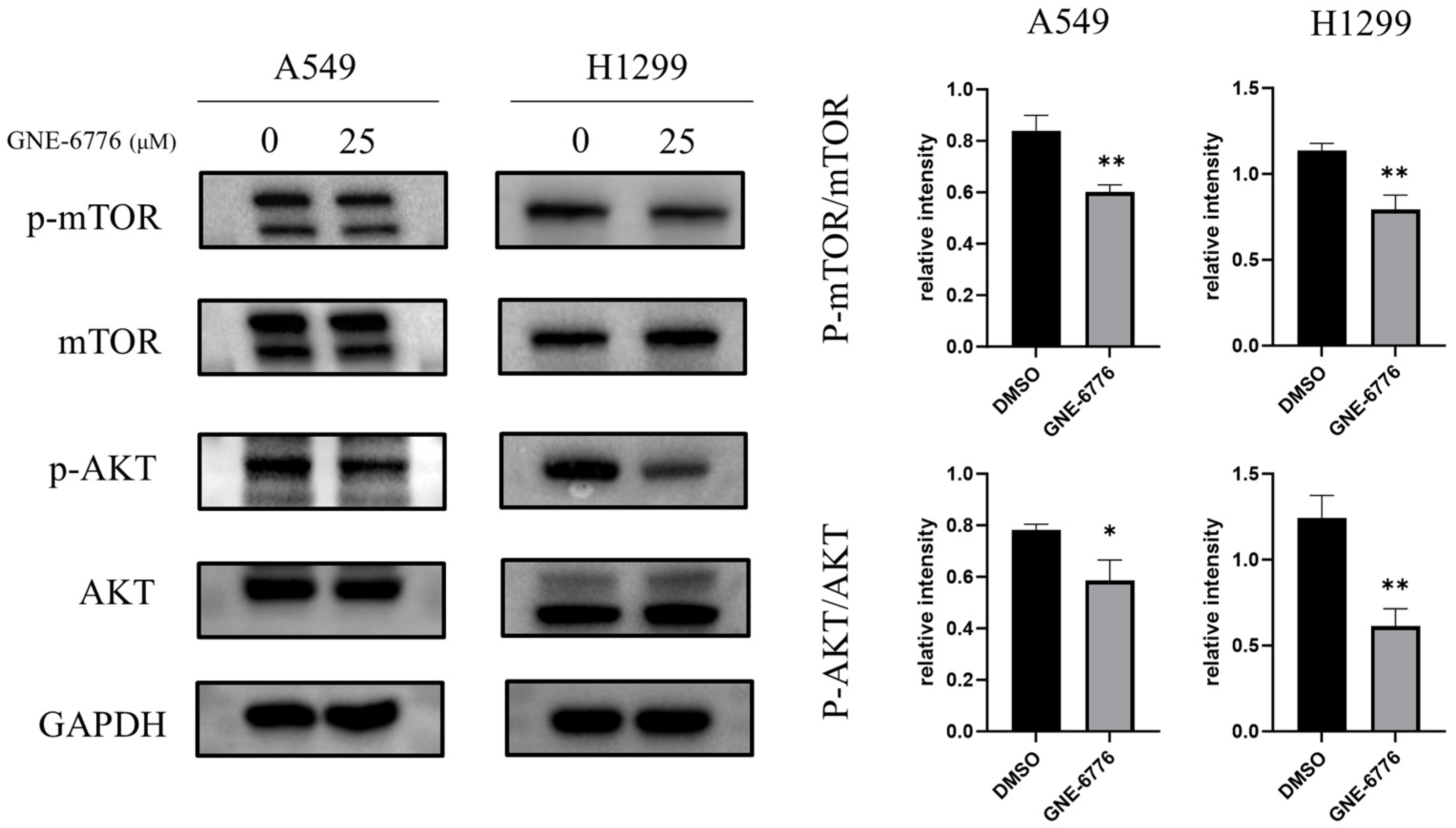
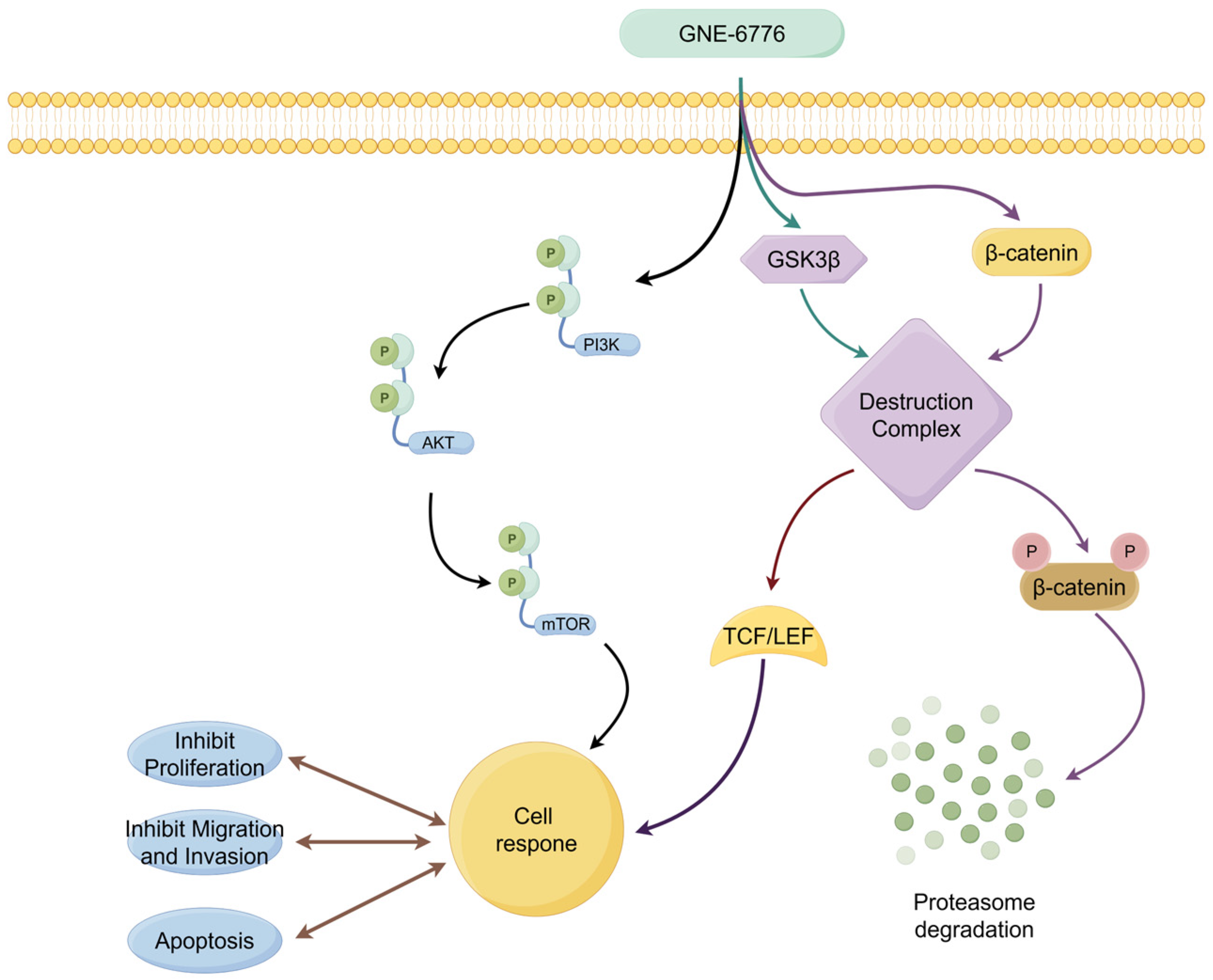
2.7. GNE-6776 Down-Regulates the PI3K/AKT/mTOR Pathway in NSCLC Cells
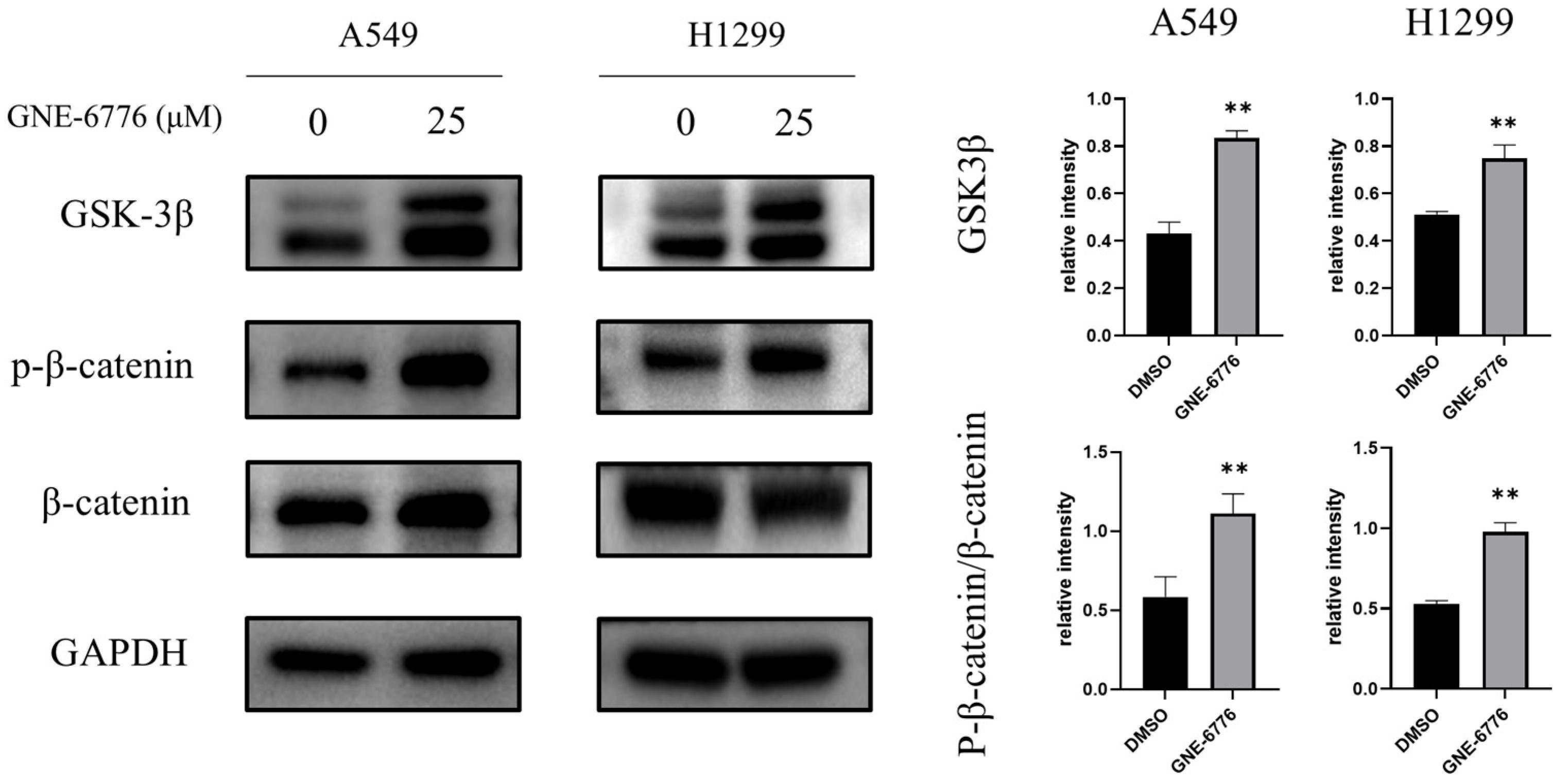
2.8. GNE-6776 Suppresses Wnt/β-Catenin Pathway in NSCLC Cells
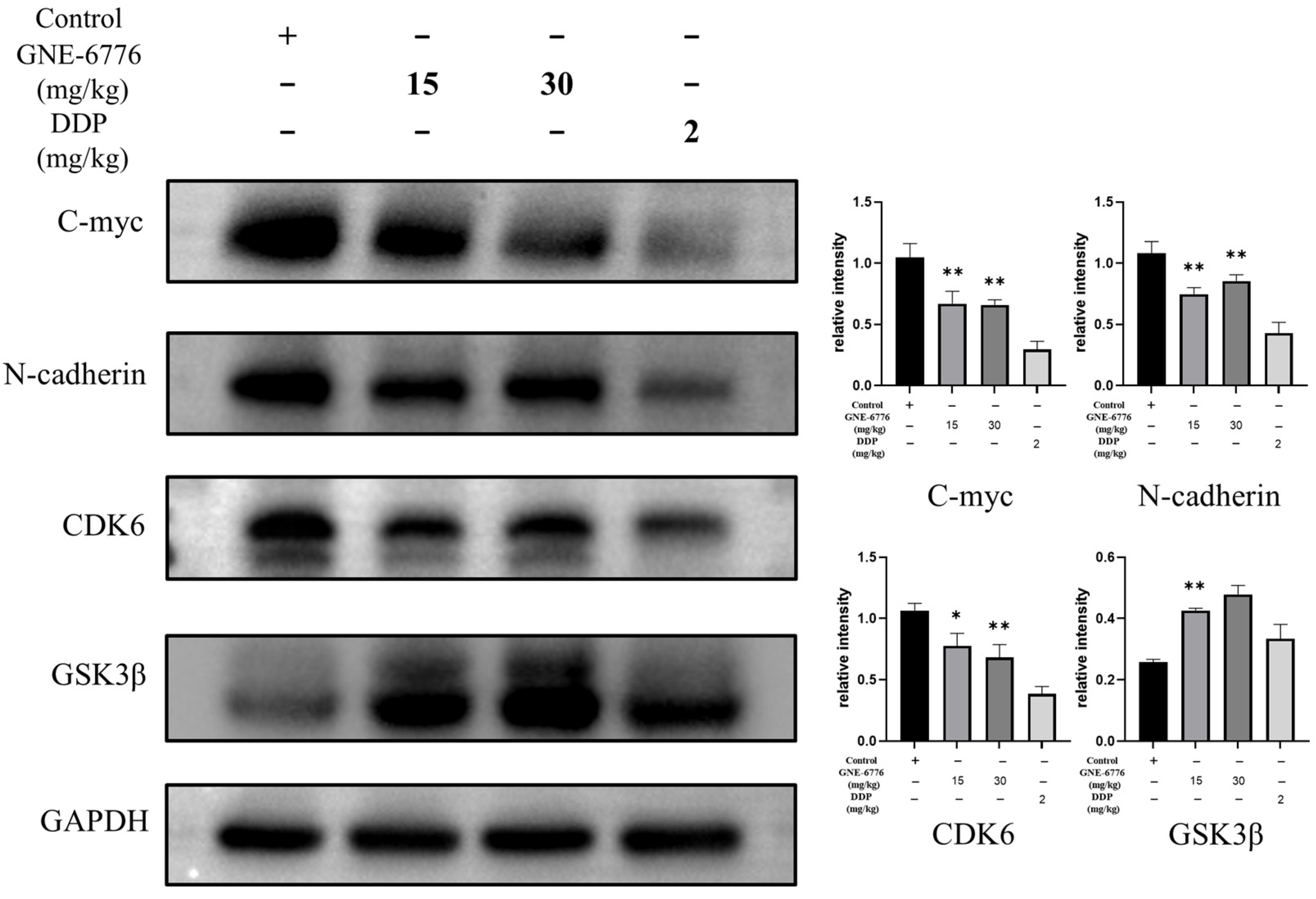
2.9. GNE-6776 Suppresses Tumor Growth in an A549 Xenograft Mouse Model Without Causing Significant Body Weight Loss
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Drug
4.2. RNA Sequencing, Differential Expression Analysis, and KEGG Analysis
4.3. CCK-8 Assay for Cell Viability
4.4. Clone Formation Assay
4.5. Annexin V-FITC Apoptosis Assay for Cell Apoptosis Detection
4.6. JC-1 Assay for Mitochondrial Membrane Potential Assessment
4.7. Flow Cytometry with PI Staining for Cell Cycle Analysis
4.8. Wound Healing Assay
4.9. Transwell Migration Assay
4.10. Transwell Invasion Assay
4.11. Western Blot
4.12. Xenograft Mouse Model
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCK-8 | Cell Counting Kit -8 |
| CDKI | CDK inhibitor |
| DDP | Cisplatin |
| DEGs | Differentially expressed genes |
| DUBs | Deubiquitinases |
| EGFR-TKIs | Epidermal growth factor receptor tyrosine kinase inhibitors |
| EMT | Epithelial-mesenchymal transition |
| Foxo | Forkhead Proteins |
| GO | Gene Ontology |
| H&E | Hematoxylin and eosin |
| HIF | Hypoxia-inducible factor |
| IHC | Immunohistochemistry |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MMPs | Matrix metalloproteinases |
| NEK | Never in mitosis A-related kinase |
| NSCLC | Non-small cell lung cancer |
| PFS | Progression-free survival |
| USP7 | Ubiquitin-specific protease 7 |
References
- Frankell, A.M.; Dietzen, M.; Al Bakir, M.; Lim, E.L.; Karasaki, T.; Ward, S.; Veeriah, S.; Colliver, E.; Huebner, A.; Bunkum, A.; et al. The evolution of lung cancer and impact of subclonal selection in TRACERx. Nature 2023, 616, 525–533. [Google Scholar] [CrossRef]
- Liu, G.; Pei, F.; Yang, F.; Li, L.; Amin, A.D.; Liu, S.; Buchan, J.R.; Cho, W.C. Role of Autophagy and Apoptosis in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2017, 18, 367. [Google Scholar] [CrossRef] [PubMed]
- Bajbouj, K.; Al-Ali, A.; Ramakrishnan, R.K.; Saber-Ayad, M.; Hamid, Q. Histone Modification in NSCLC: Molecular Mechanisms and Therapeutic Targets. Int. J. Mol. Sci. 2021, 22, 11701. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, Y.; Zhu, Q.; Gu, X.; Xiang, B.; Gu, X.; Xie, T.; Sui, X. β-Elemene Reverses Gefitinib Resistance in NSCLC Cells by Inhibiting lncRNA H19-Mediated Autophagy. Pharmaceuticals 2024, 17, 626. [Google Scholar] [CrossRef] [PubMed]
- Raskova Kafkova, L.; Mierzwicka, J.M.; Chakraborty, P.; Jakubec, P.; Fischer, O.; Skarda, J.; Maly, P.; Raska, M. NSCLC: From tumorigenesis, immune checkpoint misuse to current and future targeted therapy. Front. Immunol. 2024, 15, 1342086. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Sanborn, R.E.; Negrao, M.V.; Vaishnavi, A.; Smit, E.F. BRAF(V600E)-mutant metastatic NSCLC: Disease overview and treatment landscape. npj Precis. Oncol. 2024, 8, 90. [Google Scholar] [CrossRef]
- Yue, B.; Xiong, D.; Chen, J.; Yang, X.; Zhao, J.; Shao, J.; Wei, D.; Gao, F.; Huang, M.; Chen, J. SPP1 induces idiopathic pulmonary fibrosis and NSCLC progression via the PI3K/Akt/mTOR pathway. Respir. Res. 2024, 25, 362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Westover, D.; Tang, Z.; Liu, Y.; Sun, J.; Sun, Y.; Zhang, R.; Wang, X.; Zhou, S.; Hesilaiti, N.; et al. Wnt/β-catenin signaling in the development and therapeutic resistance of non-small cell lung cancer. J. Transl. Med. 2024, 22, 565. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, C.; He, B.; Du, X.; Liu, J.; Xia, H.; Wang, P.; Wu, M.; Wu, H.; Liu, Q. Cigarette smoking, by accelerating the cell cycle, promotes the progression of non-small cell lung cancer through an HIF-1α-METTL3-m(6)A/CDK2AP2 axis. J. Hazard. Mater. 2023, 455, 131556. [Google Scholar] [CrossRef]
- Cui, B.; Song, L.; Wang, Q.; Li, K.; He, Q.; Wu, X.; Gao, F.; Liu, M.; An, C.; Gao, Q.; et al. Non-small cell lung cancers (NSCLCs) oncolysis using coxsackievirus B5 and synergistic DNA-damage response inhibitors. Signal Transduct. Target. Ther. 2023, 8, 366. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ye, R.; Li, Z.; Zhang, B.; Huang, Y.; Du, J.; Wang, B.; Meng, H.; Xian, H.; Yang, X.; et al. KIAA1429 promotes tumorigenesis and gefitinib resistance in lung adenocarcinoma by activating the JNK/ MAPK pathway in an m(6)A-dependent manner. Drug Resist. Updat. 2023, 66, 100908. [Google Scholar] [CrossRef] [PubMed]
- Priest, K.; Le, A.; Gebregzabheir, A.; Nijmeh, H.; Reis, G.B.; Mandell, M.; Davies, K.D.; Lawrence, C.; O’Donnell, E.; Doebele, R.C.; et al. Evolution of acquired resistance in a ROS1(+) KRAS G12C(+) NSCLC through the MAPK pathway. npj Precis. Oncol. 2023, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Huang, Y.; Gu, W.; Gan, J.; Wang, W.; Zhang, S.; Wang, K.; Zhan, J.; Yang, Y.; Huang, Y.; et al. PI3K-AKT-mTOR pathway alterations in advanced NSCLC patients after progression on EGFR-TKI and clinical response to EGFR-TKI plus everolimus combination therapy. Transl. Lung Cancer Res. 2020, 9, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Fan, Q.; Liang, X.; Han, S.; He, J.; Wang, Q.Q.; Gao, H.; Feng, Y.; Yang, S. Cucurbitacin B inhibits TGF-β1-induced epithelial-mesenchymal transition (EMT) in NSCLC through regulating ROS and PI3K/Akt/mTOR pathways. Chin. Med. 2022, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sanchez, A.; Torres, G.; Estrada, S.; Perez, D.; Garcia, C.; Milian, M.; Velazquez, E.; Molina, V.; Delgado, Y. Unraveling the Impact of Six Pentacyclic Triterpenes Regulating Metabolic Pathways on Lung Carcinoma Cells. Pharmaceuticals 2024, 17, 694. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, K.; He, B.; You, L.; Xu, Z.; McCormick, F.; Jablons, D.M. Activation of the Wnt pathway in non small cell lung cancer: Evidence of dishevelled overexpression. Oncogene 2003, 22, 7218–7221. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Cai, L.; Li, S.; Hou, Y.; Wang, Y.D.; Yang, D.; Xia, Y.; Nie, X. WNT ligands in non-small cell lung cancer: From pathogenesis to clinical practice. Discov. Oncol. 2023, 14, 136. [Google Scholar] [CrossRef]
- da Silva-Oliveira, R.J.; Gomes, I.N.F.; da Silva, L.S.; Lengert, A.V.H.; Laus, A.C.; Melendez, M.E.; Munari, C.C.; Cury, F.P.; Longato, G.B.; Reis, R.M. Efficacy of Combined Use of Everolimus and Second-Generation Pan-EGRF Inhibitors in KRAS Mutant Non-Small Cell Lung Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 7774. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Liu, Y.P. MERTK Inhibition: Potential as a Treatment Strategy in EGFR Tyrosine Kinase Inhibitor-Resistant Non-Small Cell Lung Cancer. Pharmaceuticals 2021, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Wang, L.; Zhou, F.M.; Liu, S.W.; Wang, W.; Zhao, E.J.; Yao, Q.J.; Li, W.; Zhao, Y.Q.; Shi, Z.; et al. Elevated NOX4 promotes tumorigenesis and acquired EGFR-TKIs resistance via enhancing IL-8/PD-L1 signaling in NSCLC. Drug Resist. Updat. 2023, 70, 100987. [Google Scholar] [CrossRef]
- Chen, Q.; Jia, G.; Zhang, X.; Ma, W. Targeting HER3 to overcome EGFR TKI resistance in NSCLC. Front. Immunol. 2023, 14, 1332057. [Google Scholar] [CrossRef]
- Bonnet, J.; Romier, C.; Tora, L.; Devys, D. Zinc-finger UBPs: Regulators of deubiquitylation. Trends Biochem. Sci. 2008, 33, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, P.; Mandal, D.; Velayutham, R. The emerging role of Deubiquitinases (DUBs) in parasites: A foresight review. Front. Cell Infect. Microbiol. 2022, 12, 985178. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, J.A.; Jacq, X.; Martin, N.M.; Jackson, S.P. Deubiquitylating enzymes and drug discovery: Emerging opportunities. Nat. Rev. Drug Discov. 2018, 17, 57–78. [Google Scholar] [CrossRef]
- Huang, B.; Cao, D.; Yuan, X.; Xiong, Y.; Chen, B.; Wang, Y.; Niu, X.; Tian, R.; Huang, H. USP7 deubiquitinates KRAS and promotes non-small cell lung cancer. Cell Rep. 2024, 43, 114917. [Google Scholar] [CrossRef]
- Feng, T.; Ling, S.; Xu, C.; Ying, L.; Su, D.; Xu, X. Ubiquitin-specific peptidase 22 in cancer. Cancer Lett. 2021, 514, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Gu, L.; Li, M.; Jeffrey, P.D.; Gu, W.; Shi, Y. Structural basis of competitive recognition of p53 and MDM2 by HAUSP/USP7: Implications for the regulation of the p53-MDM2 pathway. PLoS Biol. 2006, 4, e27. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.L.; Li, M.; Hu, M.; Shi, Y.; Gu, W. The p53—Mdm2—HAUSP complex is involved in p53 stabilization by HAUSP. Oncogene 2007, 26, 7262–7266. [Google Scholar] [CrossRef] [PubMed]
- Agathanggelou, A.; Smith, E.; Davies, N.J.; Kwok, M.; Stankovic, T. USP7 inhibition alters homologous recombination repair and targets CLL cells independently of ATM/p53 functional status. Blood 2017, 130, 156–166. [Google Scholar] [CrossRef]
- Ma, M.; Yu, N. Ubiquitin-specific protease 7 expression is a prognostic factor in epithelial ovarian cancer and correlates with lymph node metastasis. Onco Targets Ther. 2016, 9, 1559–1569. [Google Scholar]
- He, Y.; Jiang, S.; Zhong, Y.; Wang, X.; Cui, Y.; Liang, J.; Sun, Y.; Zhu, Z.; Huang, Z.; Mao, X. USP7 promotes non-small-cell lung cancer cell glycolysis and survival by stabilizing and activating c-Abl. Clin. Transl. Med. 2023, 13, e1509. [Google Scholar] [CrossRef]
- Qi, S.M.; Cheng, G.; Cheng, X.D.; Xu, Z.; Qin, J.J. Targeting USP7-Mediated Deubiquitination of MDM2/MDMX-p53 Pathway for Cancer Therapy: Are We There Yet? Front. Cell Dev. Biol. 2020, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Colland, F.; Formstecher, E.; Jacq, X.; Reverdy, C.; Planquette, C.; Conrath, S.; Trouplin, V.; Bianchi, J.; Aushev, V.N.; Camonis, J.; et al. Small-molecule inhibitor of USP7/HAUSP ubiquitin protease stabilizes and activates p53 in cells. Mol. Cancer Ther. 2009, 8, 2286–2295. [Google Scholar] [CrossRef]
- Dar, A.; Shibata, E.; Dutta, A. Deubiquitination of Tip60 by USP7 determines the activity of the p53-dependent apoptotic pathway. Mol. Cell Biol. 2013, 33, 3309–3320. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.A.; Moradi, A.; Hosseini, S.Y.; Razavi Nikoo, H.; Bamdad, T.; Faghih, Z.; Sarvari, J.; Tabarraei, A. A New Insight Into p53-Inhibiting Genes in Epstein–Barr Virus-Associated Gastric Adenocarcinoma. Iran. Biomed. J. 2023, 27, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Sun, J.; Hu, J.R.; Liu, C.F.; Li, Y.; Wang, W.; Fu, R.; Guo, M.; Wang, H.L.; Pang, M. ANKRD49 promotes the metastasis of NSCLC via activating JNK-ATF2/c-Jun-MMP-2/9 axis. BMC Cancer 2023, 23, 1108. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Jain, V.K.; Rizwanullah, M.; Ahmad, J.; Jain, K. PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: A review on drug discovery and future challenges. Drug Discov. Today 2019, 24, 2181–2191. [Google Scholar] [CrossRef]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef]
- Wang, J.; Zou, K.; Feng, X.; Chen, M.; Deng, W. Downregulation of NMI promotes tumor growth and predicts poor prognosis in human lung adenocarcinomas. Mol. Cancer 2017, 16, 158. [Google Scholar] [CrossRef]
- Fang, L.; Ford-Roshon, D.; Russo, M.; O’Brien, C.; Xiong, X.; Gurjao, C.; Grandclaudon, M.; Raghavan, S.; Corsello, S.M.; Carr, S.A.; et al. RNF43 G659fs is an oncogenic colorectal cancer mutation and sensitizes tumor cells to PI3K/mTOR inhibition. Nat. Commun. 2022, 13, 3181. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [PubMed]
- Lie, D.C.; Colamarino, S.A.; Song, H.J.; Désiré, L.; Mira, H.; Consiglio, A.; Lein, E.S.; Jessberger, S.; Lansford, H.; Dearie, A.R.; et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 2005, 437, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Berndt, J.D.; Moon, R.T.; Cell biology. Making a point with Wnt signals. Science 2013, 339, 1388–1389. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Nishizuka, M. Repression of RhoJ expression promotes TGF-β-mediated EMT in human non-small-cell lung cancer A549cells. Biochem. Biophys. Res. Commun. 2021, 566, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Debaugnies, M.; Rodríguez-Acebes, S.; Blondeau, J.; Parent, M.A.; Zocco, M.; Song, Y.; de Maertelaer, V.; Moers, V.; Latil, M.; Dubois, C.; et al. RHOJ controls EMT-associated resistance to chemotherapy. Nature 2023, 616, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Ma, X.; Hu, H. The Influence of Cell Cycle Regulation on Chemotherapy. Int. J. Mol. Sci. 2021, 22, 6923. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Thornburg, Z.R.; Bianchi, D.M.; Brier, T.A.; Gilbert, B.R.; Earnest, T.M.; Melo, M.C.R.; Safronova, N.; Sáenz, J.P.; Cook, A.T.; Wise, K.S.; et al. Fundamental behaviors emerge from simulations of a living minimal cell. Cell 2022, 185, 345–360.e328. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Schade, A.E.; Branigan, T.B.; Müller, G.A.; DeCaprio, J.A. Coordinating gene expression during the cell cycle. Trends Biochem. Sci. 2022, 47, 1009–1022. [Google Scholar] [CrossRef]
- D’Costa, M.N.; Bothe, A.; Das, S.; Udhaya Kumar, S.; Gnanasambandan, R.; George Priya Doss, C. CDK regulators-Cell cycle progression or apoptosis-Scenarios in normal cells and cancerous cells. Adv. Protein Chem. Struct. Biol. 2023, 135, 125–177. [Google Scholar]
- Zheng, Q.; Dong, H.; Mo, J.; Zhang, Y.; Huang, J.; Ouyang, S.; Shi, S.; Zhu, K.; Qu, X.; Hu, W.; et al. A novel STAT3 inhibitor W2014-S regresses human non-small cell lung cancer xenografts and sensitizes EGFR-TKI acquired resistance. Theranostics 2021, 11, 824–840. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Nilsson, M.B.; Yang, Y.; Le, X.; Tran, H.T.; Elamin, Y.Y.; Yu, X.; Zhang, F.; Poteete, A.; Ren, X.; et al. IL6 Mediates Suppression of T- and NK-cell Function in EMT-associated TKI-resistant EGFR-mutant NSCLC. Clin. Cancer Res. 2023, 29, 1292–1304. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Lin, L.; Yu, M.; Li, H.; Dang, Y.; Liang, H.; Chen, G.; Muhetaer, H.; Zheng, G.; Li, J.; et al. Antitumor Activity of USP7 Inhibitor GNE-6776 in Non-Small Cell Lung Cancer Involves Regulation of Epithelial-Mesenchymal Transition, Cell Cycle, Wnt/β-Catenin, and PI3K/AKT/mTOR Pathways. Pharmaceuticals 2025, 18, 245. https://doi.org/10.3390/ph18020245
Wu L, Lin L, Yu M, Li H, Dang Y, Liang H, Chen G, Muhetaer H, Zheng G, Li J, et al. Antitumor Activity of USP7 Inhibitor GNE-6776 in Non-Small Cell Lung Cancer Involves Regulation of Epithelial-Mesenchymal Transition, Cell Cycle, Wnt/β-Catenin, and PI3K/AKT/mTOR Pathways. Pharmaceuticals. 2025; 18(2):245. https://doi.org/10.3390/ph18020245
Chicago/Turabian StyleWu, Lipeng, Long Lin, Meng Yu, Huajian Li, Yuanye Dang, Huosheng Liang, Guangyang Chen, Halimulati Muhetaer, Guodong Zheng, Jingjing Li, and et al. 2025. "Antitumor Activity of USP7 Inhibitor GNE-6776 in Non-Small Cell Lung Cancer Involves Regulation of Epithelial-Mesenchymal Transition, Cell Cycle, Wnt/β-Catenin, and PI3K/AKT/mTOR Pathways" Pharmaceuticals 18, no. 2: 245. https://doi.org/10.3390/ph18020245
APA StyleWu, L., Lin, L., Yu, M., Li, H., Dang, Y., Liang, H., Chen, G., Muhetaer, H., Zheng, G., Li, J., Jia, X., Wu, B., & Li, C. (2025). Antitumor Activity of USP7 Inhibitor GNE-6776 in Non-Small Cell Lung Cancer Involves Regulation of Epithelial-Mesenchymal Transition, Cell Cycle, Wnt/β-Catenin, and PI3K/AKT/mTOR Pathways. Pharmaceuticals, 18(2), 245. https://doi.org/10.3390/ph18020245





