Association of Tramadol-Induced Ovarian Damage and Reproductive Dysfunction with Adenosine Triphosphate and the Protective Role of Exogenous ATP Treatment
Abstract
1. Introduction
2. Results
2.1. Biochemical Results
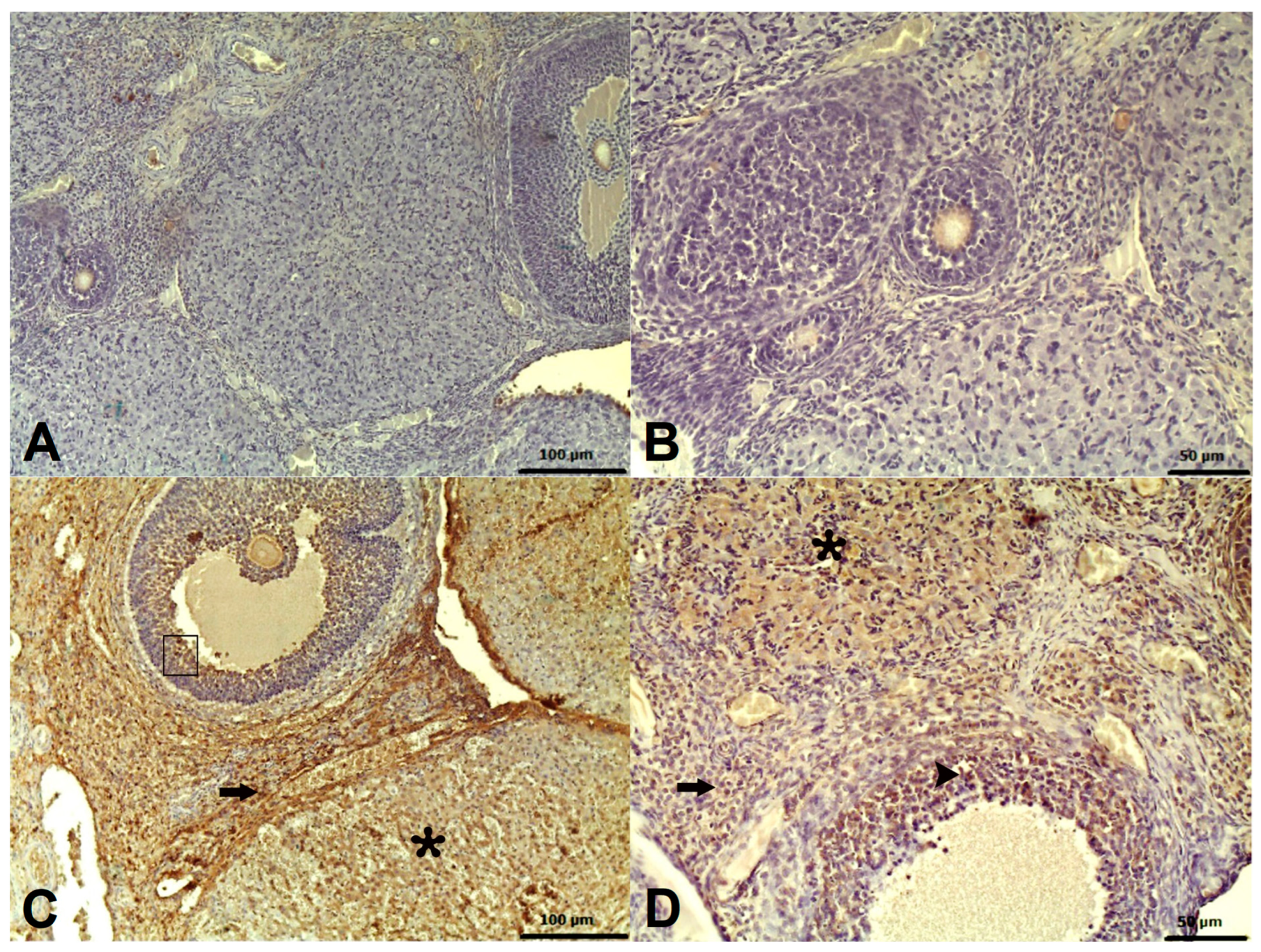
2.2. Histopathological Results
2.3. Immunohistochemical Results
2.4. Fertility Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Experimental Groups
4.4. Experimental Procedure
4.5. Biochemical Analyses
Preparation of Samples
4.6. MDA, tGSH, SOD, CAT, Protein, and IL-6 Levels Analysis in Ovarian Tissue
4.7. Histopathological Examination
4.8. Immunohistochemical Method
4.9. Statistical Analysis
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kader, G.A.; Ibrahim, M.A.; Khalifa, A.M.; Mirza, U.; Rashwan, E.K.; Abdel-Hady, Z. Evaluation of vitamin C protective effect on the cerebrocortical antioxidant defense, histopathological, pro-apoptotic p53 and anti-apoptotic Bcl2 expressions against tramadol neurotoxicity in rats. J. Chem. Neuroanat. 2021, 112, 101893. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Umehara, M.; Homan, T.; Okamoto, K.; Oka, M.; Oyama, T. The analgesic effect of tramadol in animal models of neuropathic pain and fibromyalgia. Neurosci. Lett. 2014, 562, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, F.A. Tramadol for the treatment of premature ejaculation. Eur. Urol. 2012, 61, 744–745. [Google Scholar] [CrossRef]
- Grond, S.; Sablotzki, A. Clinical pharmacology of tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, A.; El Khishin, I.; Megahed, O.; Mazen, F. Effect of Nigella sativa Linn oil on tramadol-induced hepato- and nephrotoxicity in adult male albino rats. Toxicol. Rep. 2015, 2, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; Barbosa, J.; Leal, S.; Afonso, L.P.; Lobo, J.; Moreira, R.; Queirós, O.; Carvalho, F.; Dinis-Oliveira, R.J. Effective analgesic doses of tramadol or tapentadol induce brain, lung and heart toxicity in Wistar rats. Toxicology 2017, 385, 38–47. [Google Scholar] [CrossRef]
- El-Ghawet, H.A. Effects of tramadol on the reproductive function of wistar albino rats. Eur. J. Exp. Biol. 2015, 5, 56–64. [Google Scholar]
- Koohsari, M.; Ahangar, N.; Mohammadi, E.; Shaki, F. Ameliorative Effect of Melatonin Against Reproductive Toxicity of Tramadol in Rats via the Regulation of Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis-related Gene Expression Signaling Pathway. Addict. Health 2020, 12, 118–129. [Google Scholar]
- El-Mottaleb, A.; Ahmed, H.A.; Mahmoud, S.F.; Hassan, A.I.; Tealeb, A.-S.M.I.; Almorsy, G.Z. Effects of Chronic Use of Tramadol on Uterus and Ovary of Albino Rats. Egypt. J. Hosp. Med. 2019, 76, 3184–3190. [Google Scholar] [CrossRef]
- El-Twab, A.; Sanaa, M. Biochemical and molecular studies on tramadol-mediated pituitary-gonadal axis and ovarian dysfunctions in adult female albino rats. Egypt. J. Zool. 2016, 66, 27–50. [Google Scholar] [CrossRef]
- Adelakun, S.A.; Ukwenya, V.O.; Akintunde, O.W. Vitamin B(12) ameliorate Tramadol-induced oxidative stress, endocrine imbalance, apoptosis and NO/iNOS/NF-κB expression in Sprague Dawley rats through regulatory mechanism in the pituitary-gonadal axis. Tissue Cell 2022, 74, 101697. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, K.; Manthari, R.K.; Najibi, A.; Jia, Z.; Ommati, M.M.; Heidari, R. Mitochondrial dysfunction and oxidative stress are involved in the mechanism of tramadol-induced renal injury. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100049. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Ghelichkhani, Z.; Aghakhani, R.; Klionsky, D.J.; Motaghinejad, O.; Motaghinejad, M.; Koohi, M.K.; Hassan, J. Minocycline Acts as a Neuroprotective Agent Against Tramadol-Induced Neurodegeneration: Behavioral and Molecular Evidence. Int. J. Prev. Med. 2024, 28, 47. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; Barbosa, J.; Queirós, O.; Moreira, R.; Carvalho, F.; Dinis-Oliveira, R.J. Comparative study of the neurotoxicological effects of tramadol and tapentadol in SH-SY5Y cells. Toxicology 2016, 359–360, 1–10. [Google Scholar] [CrossRef]
- Saquet, A.; Streif, J.; Bangerth, F. Changes in ATP, ADP and pyridine nucleotide levels related to the incidence of physiological disorders in ‘Conference’pears and ‘Jonagold’apples during controlled atmosphere storage. J. Hortic. Sci. Biotechnol. 2000, 75, 243–249. [Google Scholar] [CrossRef]
- Yi, C.; Jiang, Y.; Shi, J.; Qu, H.; Xue, S.; Duan, X.; Shi, J.; Prasad, N.K. ATP-regulation of antioxidant properties and phenolics in litchi fruit during browning and pathogen infection process. Food Chem. 2010, 118, 42–47. [Google Scholar] [CrossRef]
- Marincsák, R.; Tóth, B.I.; Czifra, G.; Szabó, T.; Kovács, L.; Bíró, T. The analgesic drug, tramadol, acts as an agonist of the transient receptor potential vanilloid-1. Anesth. Analg. 2008, 106, 1890–1896. [Google Scholar] [CrossRef]
- Arve, K. “You get stuck in it”: Young people’s accounts of attempting to quit non-medical tramadol use. Nordisk Alkohol. Nark. 2023, 40, 355–370. [Google Scholar] [CrossRef]
- Sabi Boun, S.; Omonaiye, O.; Yaya, S. Protocol for a scoping review study on the prevalence and public health consequences of non-medical use (NMU) of tramadol in Africa. PLoS ONE 2023, 18, e0285809. [Google Scholar] [CrossRef] [PubMed]
- Paulis, M.G.; Abbas, M.F. Tramadol subchronic toxicity on pituitarygonadal axis and ovarian functions in adult female rats. Egypt. J. Forensic Sci. Appl. Toxicol. 2015, 220. Available online: https://www.researchgate.net/publication/299612495_Tramadol_toxicity_onpituitarygonadal_axis_and_ovarian_functions_in_female_rats (accessed on 2 February 2025).
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Guan, Z.H.; Yang, D.; Wang, Y.; Ma, J.B.; Wang, G.N. EDA2R knockdown alleviates myocardial ischemia/reperfusion injury through inhibiting the activation of the NF-κB signaling pathway. Exp. Anim. 2024, 73, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, H.; Ozcicek, A. Molecular mechanism of ischemia reperfusion injury. Arch. Basic Clin. Res. 2019, 2, 25–27. [Google Scholar] [CrossRef]
- Hindawy, R.F.; Hendawy, F.F.; Ali, N.E. Ameliorative effect of aloe vera gel on tramadol reproductive toxicity in adult albino rats. Zagazig J. Forensic Med. 2019, 17, 71–83. [Google Scholar] [CrossRef]
- Kawakami, K.; Matsuo, H.; Kajitani, N.; Matsumoto, K.I. Treatment of spontaneously hypertensive rats during pregnancy and lactation with the antioxidant tempol lowers blood pressure and reduces oxidative stress. Exp. Anim. 2024, 73, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids. 2012, 2012, 736837. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D. Glutathione and glutathione-dependent enzymes: From biochemistry to gerontology and successful aging. Ageing Res. Rev. 2023, 92, 102066. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef]
- Buettner, G.R.; Ng, C.F.; Wang, M.; Rodgers, V.G.; Schafer, F.Q. A new paradigm: Manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic. Biol. Med. 2006, 41, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Cronstein, B.N. Interleukin-6—A key mediator of systemic and local symptoms in rheumatoid arthritis. Bull. NYU Hosp. Jt. Dis. 2007, 65 (Suppl. S1), S11–S15. Available online: https://pubmed.ncbi.nlm.nih.gov/17708739/#:~:text=The%20biological%20activities%20of%20IL,%2Dreactive%20protein%20(CRP) (accessed on 2 February 2025). [PubMed]
- Tanaka, T.; Narazaki, M.; Masuda, K.; Kishimoto, T. Regulation of IL-6 in Immunity and Diseases. Adv. Exp. Med. Biol. 2016, 941, 79–88. [Google Scholar] [PubMed]
- Uciechowski, P.; Dempke, W.C.M. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Chaudry, I.H. Does ATP cross the cell plasma membrane. Yale J. Biol. Med. 1982, 55, 1–10. [Google Scholar]
- Mandel, L.J.; Takano, T.; Soltoff, S.P.; Murdaugh, S. Mechanisms whereby exogenous adenine nucleotides improve rabbit renal proximal function during and after anoxia. J. Clin. Investig. 1988, 81, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.J.; Zhu, D.; Opdebeeck, B.; D’Haese, P.; Millán, J.L.; Bourne, L.E.; Wheeler-Jones, C.P.D.; Arnett, T.R.; MacRae, V.E.; Orriss, I.R. Inhibition of arterial medial calcification and bone mineralization by extracellular nucleotides: The same functional effect mediated by different cellular mechanisms. J. Cel. Physiol. 2018, 233, 3230–3243. [Google Scholar] [CrossRef]
- Ozer, M.; Ince, S.; Altuner, D.; Suleyman, Z.; Cicek, B.; Gulaboglu, M.; Mokhtare, B.; Gursul, C.; Suleyman, H. Protective Effect of Adenosine Triphosphate against 5-Fluorouracil-Induced Oxidative Ovarian Damage in vivo. Asian Pac. J. Cancer Prev. 2023, 24, 1007–1013. [Google Scholar] [CrossRef]
- Chen, T.H.; Wang, H.C.; Chang, C.J.; Lee, S.Y. Mitochondrial Glutathione in Cellular Redox Homeostasis and Disease Manifestation. Int. J. Mol. Sci. 2024, 25, 1314. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.Q.; Huang, L.; Huang, H.Q.; Cai, Z. Effects of chronic tramadol exposure on the zebrafish brain: A proteomic study. J. Proteom. 2012, 75, 3351–3364. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Pourahmad, J.; Taghizadeh, G.; Vousooghi, N.; Yoonessi, A.; Naserzadeh, P.; Behzadfar, L.; Rouini, M.R.; Sharifzadeh, M. Mitochondrial impairments contribute to spatial learning and memory dysfunction induced by chronic tramadol administration in rat: Protective effect of physical exercise. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Mohammadnejad, L.; Soltaninejad, K.; Seyedabadi, M.; Ghasem Pouri, S.K.; Shokrzadeh, M.; Mohammadi, H. Evaluation of mitochondrial dysfunction due to oxidative stress in therapeutic, toxic and lethal concentrations of tramadol. Toxicol. Res. 2021, 10, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Klouda, C.B.; Stone, W.L. Oxidative Stress, Proton Fluxes, and Chloroquine/Hydroxychloroquine Treatment for COVID-19. Antioxidants 2020, 9, 894. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.Z.E.; Mohamed, H.K. Histological and immunohistochemical study of the effects of tramadol administration on the ovaries of adult albino rats and the possible recovery after its withdrawal. Egypt. J. Histol. 2021, 44, 256–270. [Google Scholar] [CrossRef]
- Elhomosany, N.M. Effect of tramadol on the ovaries of female mice. Egypt. J. Histol. 2021, 44, 805–813. [Google Scholar] [CrossRef]
- Amadi, N.M.; Achukwu, P.U.; Anoh, N.V.; Obeagu, E.I.; Achukwu, N.O.; Odo, O.F. Reproductive Organ Activities of Morinda lucida Ethanol Root Extract on Male and Female Albino Rats Tramadol HCL Induced Infertility. J. Pharm. Res. Int. 2021, 33, 287–295. [Google Scholar] [CrossRef]
- Kocaturk, H.; Bedir, F.; Turangezli, O.; Arslan, R.; Coban, T.A.; Altuner, D.; Suleyman, H. Effect of adenosine triphosphate, benidipine and their combinations on bevacizumab-induced kidney damage in rats. Adv. Clin. Exp. Med. 2021, 30, 1175–1183. [Google Scholar]
- Bayrakceken, K.; Ucgul, R.K.; Coban, T.; Yazici, G.; Suleyman, H. Effect of adenosine triphosphate on amiodarone-induced optic neuropathy in rats: Biochemical and histopathological evaluation. Cutan. Ocul. Toxicol. 2023, 42, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Balcı, S.; Çöllüoğlu, Ç.; Yavuzer, B.; Bulut, S.; Altındağ, F.; Akbaş, N.; Süleyman, H. Effect of low and high dose of favipiravir on ovarian and reproductive function in female rats: Biochemical and histopathological evaluation. Gen. Physiol. Biophys. 2022, 41, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Karateke, F.; Karateke, A.; Topdagi, B.; Atilgan, M.; Dokuyucu, R. The Role of Mannitol and Vitamin D in Ovarian Ischemia/Reperfusion Injury in Rats with Acute Abdominal. Curr. Issues Mol. Biol. 2024, 46, 8903–8913. [Google Scholar] [CrossRef]
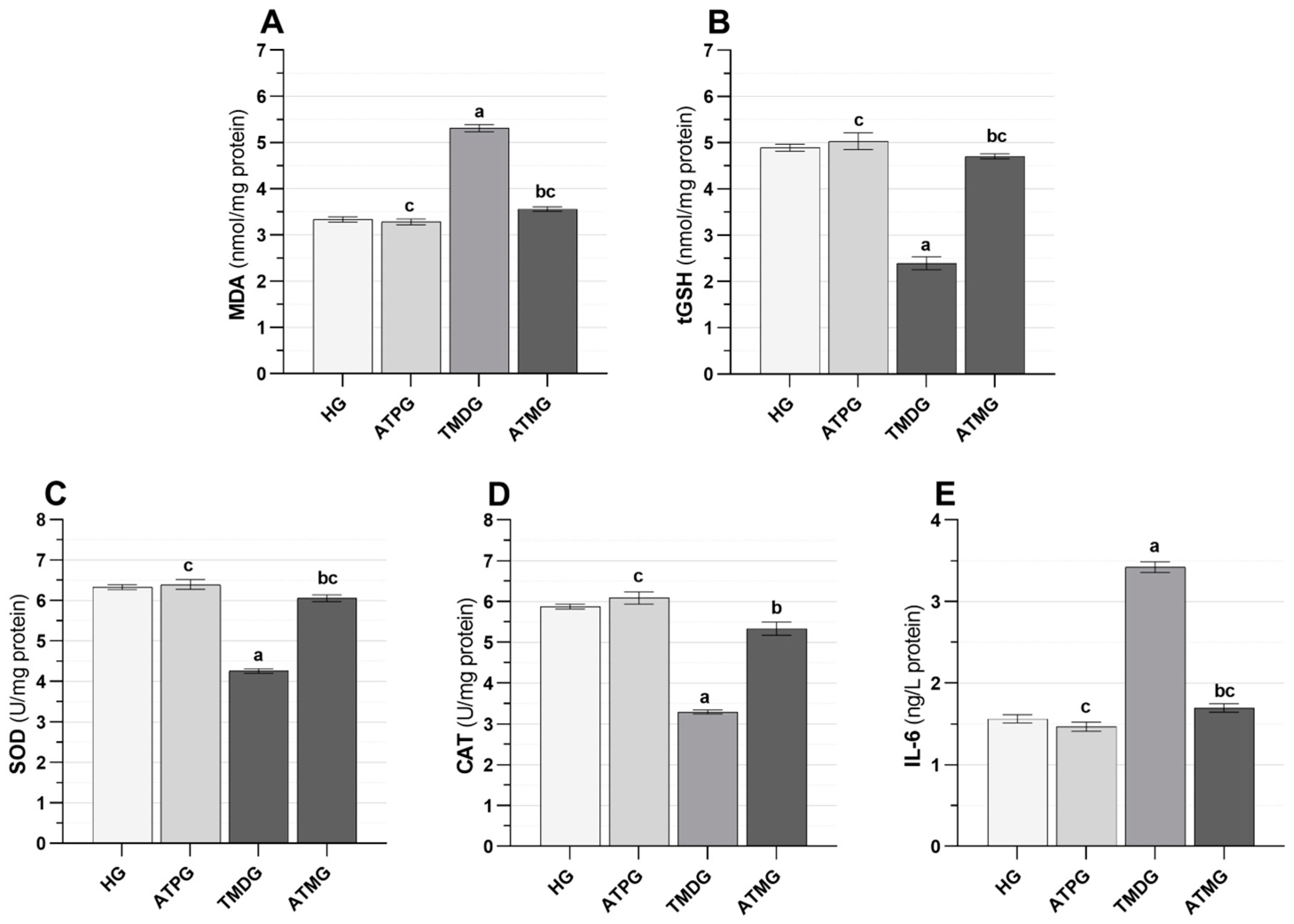

| Biochemical Variables | MDA * (nmol/mg Protein) | tGSH ** (nmol/mg Protein) | SOD * (U/mg Protein) | CAT * (U/mg Protein) | IL-6 (ng/L Protein) | |
|---|---|---|---|---|---|---|
| (Mean ± Standard Error of the Mean) | ||||||
| Groups (n = 6/each group) | HG | 3.34 ± 0.06 | 4.89 ± 0.08 | 6.33 ± 0.06 | 5.88 ± 0.06 | 1.56 ± 0.05 |
| ATPG | 3.28 ± 0.06 | 5.03 ± 0.18 | 6.40 ± 0.12 | 6.09 ± 0.15 | 1.47 ± 0.06 | |
| TMDG | 5.31 ± 0.08 | 2.40 ± 0.14 | 4.26 ± 0.05 | 3.29 ± 0.05 | 3.42 ± 0.06 | |
| ATMG | 3.56 ± 0.05 | 4.71 ± 0.06 | 6.06 ± 0.09 | 5.34 ± 0.16 | 1.70 ± 0.05 | |
| Group comparison p values | HG vs. ATPG | 0.936 | 0.890 | 0.950 | 0.583 | 0.633 |
| HG vs. TMDG | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| HG vs. ATMG | 0.080 | 0.272 | 0.127 | 0.019 | 0.357 | |
| ATPG vs. TMDG | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| ATPG vs. ATMG | 0.024 | 0.397 | 0.045 | 0.001 | 0.042 | |
| TMDG vs. ATMG | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| F (3, 20) | 238.701 | 102.659 | 144.115 | 120.014 | 275.024 | |
| p values | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Histopathological Variables | Groups | Stromal Hemorrhage | Follicular Hemorrhage | Follicular Degeneration | Edema |
|---|---|---|---|---|---|
| Mean ± Standard Error of the Mean | |||||
| Groups (n = 6/each group) | HG | 0 | 0 | 0 | 0 |
| ATPG | 0 | 0 | 0 | 0 | |
| TMDG | 64.67 ± 1.58 | 75.83 ± 1.54 | 65.83 ± 1.25 | 69.33 ± 1.15 | |
| ATMG | 13.17 ± 2.93 | 20.67 ± 0.67 | 16.50 ± 0.54 | 11.00 ± 0.73 | |
| Group comparison p values | HG vs. ATPG | - | - | - | - |
| HG vs. TMDG | <0.001 | <0.001 | <0.001 | <0.001 | |
| HG vs. ATMG | <0.001 | <0.001 | <0.001 | <0.001 | |
| ATPG vs. TMDG | <0.001 | <0.001 | <0.001 | <0.001 | |
| ATPG vs. ATMG | <0.001 | <0.001 | <0.001 | <0.001 | |
| TMDG vs. ATMG | <0.001 | <0.001 | <0.001 | <0.001 | |
| F (3, 20) | 961.568 | 1829.581 | 1160.764 | 2395.205 | |
| p values | <0.001 | <0.001 | <0.001 | <0.001 | |
| Caspase 3 (Immunohistochemical grading) | Groups (n = 6/each group) | H | p Value | |||
| HG | ATPG | TMDG | ATMG | |||
| Mean ± Standard Error of the Mean Median (minimum–maximum) | ||||||
| 0.00 ± 0.00 0 (0–0) | 0.00 ± 0.00 0 (0–0) | 2.83 ± 0.17 3 (2.75–3) | 1.00 ± 0.26 1 (1–1) | 220.913 | <0.001 | |
| Group comparison/p values | ||||||
| HG vs. | ATPG vs. | TMDG vs. | ||||
| ATPG | TMDG | ATMG | TMDG | ATMG | ATMG | |
| 1.000 | <0.001 | 0.247 | <0.001 | 0.247 | 0.395 | |
| Groups | n | Fertile Animals | Infertile Animals | Reproductive Process (A) (days) | Delay in Maternity (A-22) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | Mean ± Standard Error of the Mean | ||||||||
| HG | 6 | 6 | 100 | - | - | 24.00 ± 0.37 | 2.00 ± 0.37 | |||||
| ATPG | 6 | 6 | 100 | - | - | 23.83 ± 0.48 | 1.83 ± 0.48 | |||||
| TMDG | 6 | 2 | 33.3 | 4 | 66.7 | 34.50 ± 1.50 | 13.00 ± 1.00 | |||||
| ATMG | 6 | 5 | 83.3 | 1 | 16.7 | 24.20 ± 0.37 | 2.20 ± 0.37 | |||||
| F (3, 20) | 54.501 | |||||||||||
| p value | <0.001 | |||||||||||
| Group comparison p values | ||||||||||||
| HG vs. ATPG | HG vs. TMDG | HG vs.ATMG | ATPG vs. TMDG | ATPG vs. ATMG | TMDG vs. ATMG | |||||||
| 0.993 | <0.001 | 0.990 | <0.001 | 0.945 | <0.001 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumusburun, N.; Delibasi, I.B.; Bulut, S.; Suleyman, H.; Kalkan Yilmaz, B.; Coban, T.A.; Mendil, A.S.; Suleyman, Z. Association of Tramadol-Induced Ovarian Damage and Reproductive Dysfunction with Adenosine Triphosphate and the Protective Role of Exogenous ATP Treatment. Pharmaceuticals 2025, 18, 216. https://doi.org/10.3390/ph18020216
Gumusburun N, Delibasi IB, Bulut S, Suleyman H, Kalkan Yilmaz B, Coban TA, Mendil AS, Suleyman Z. Association of Tramadol-Induced Ovarian Damage and Reproductive Dysfunction with Adenosine Triphosphate and the Protective Role of Exogenous ATP Treatment. Pharmaceuticals. 2025; 18(2):216. https://doi.org/10.3390/ph18020216
Chicago/Turabian StyleGumusburun, Neset, Ilhan Bahri Delibasi, Seval Bulut, Halis Suleyman, Betul Kalkan Yilmaz, Taha Abdulkadir Coban, Ali Sefa Mendil, and Zeynep Suleyman. 2025. "Association of Tramadol-Induced Ovarian Damage and Reproductive Dysfunction with Adenosine Triphosphate and the Protective Role of Exogenous ATP Treatment" Pharmaceuticals 18, no. 2: 216. https://doi.org/10.3390/ph18020216
APA StyleGumusburun, N., Delibasi, I. B., Bulut, S., Suleyman, H., Kalkan Yilmaz, B., Coban, T. A., Mendil, A. S., & Suleyman, Z. (2025). Association of Tramadol-Induced Ovarian Damage and Reproductive Dysfunction with Adenosine Triphosphate and the Protective Role of Exogenous ATP Treatment. Pharmaceuticals, 18(2), 216. https://doi.org/10.3390/ph18020216






