Thermodynamic and Structural Characterization of a Mechanochemically Synthesized Pyrazinamide–Acetylsalicylic–Acid Eutectic Mixture
Abstract
1. Introduction
2. Results and Discussion
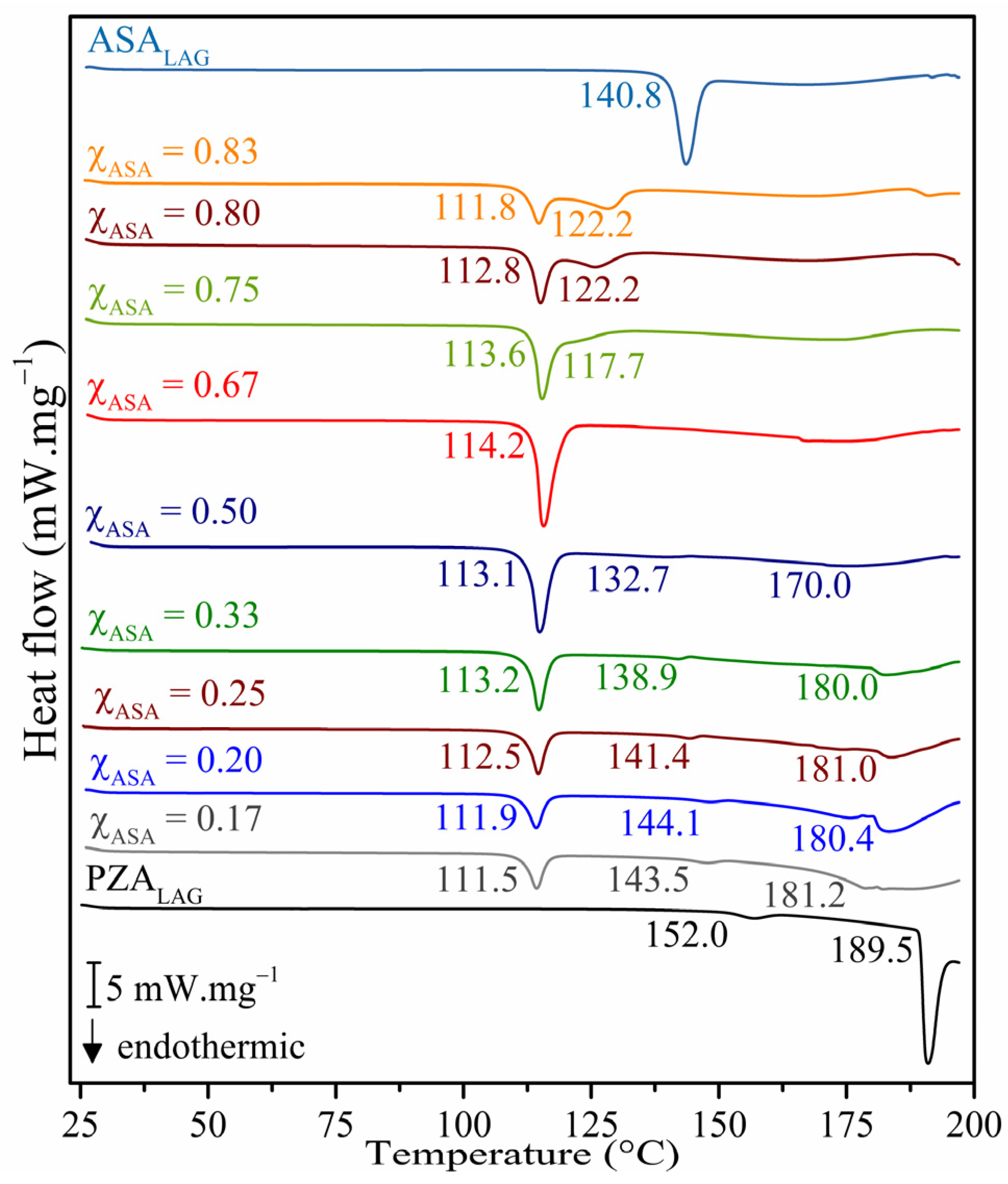
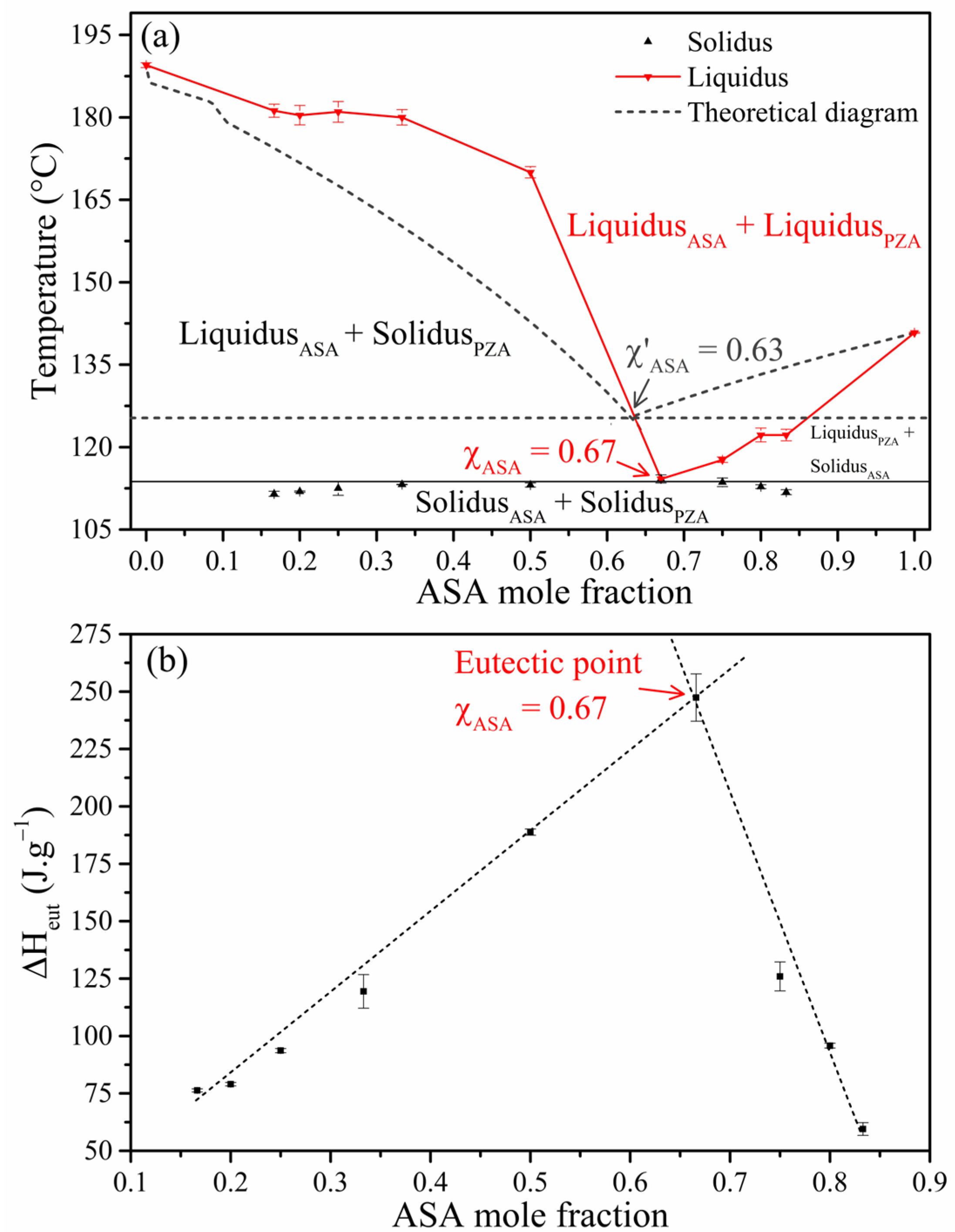
2.1. Thermal Analysis
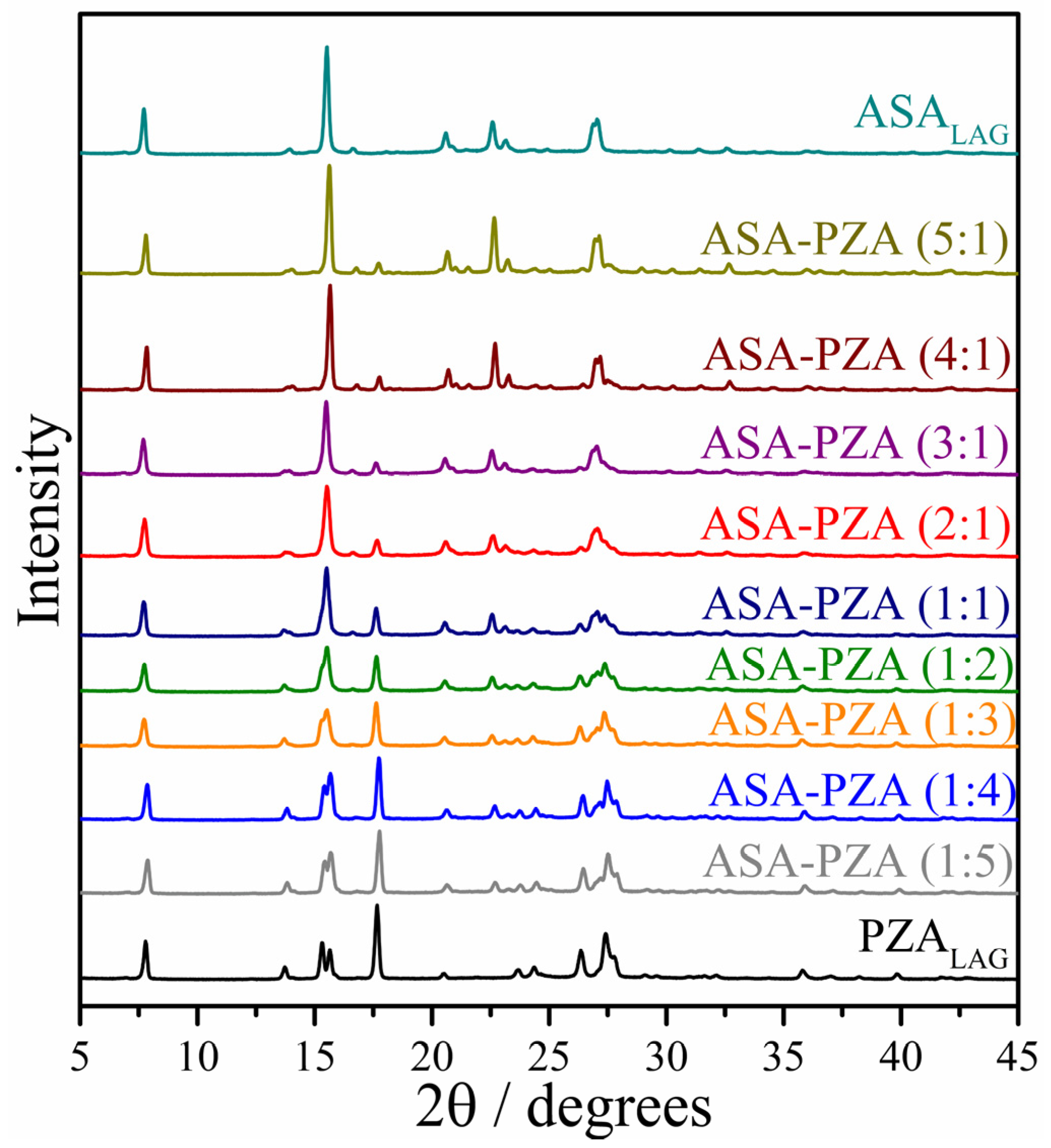
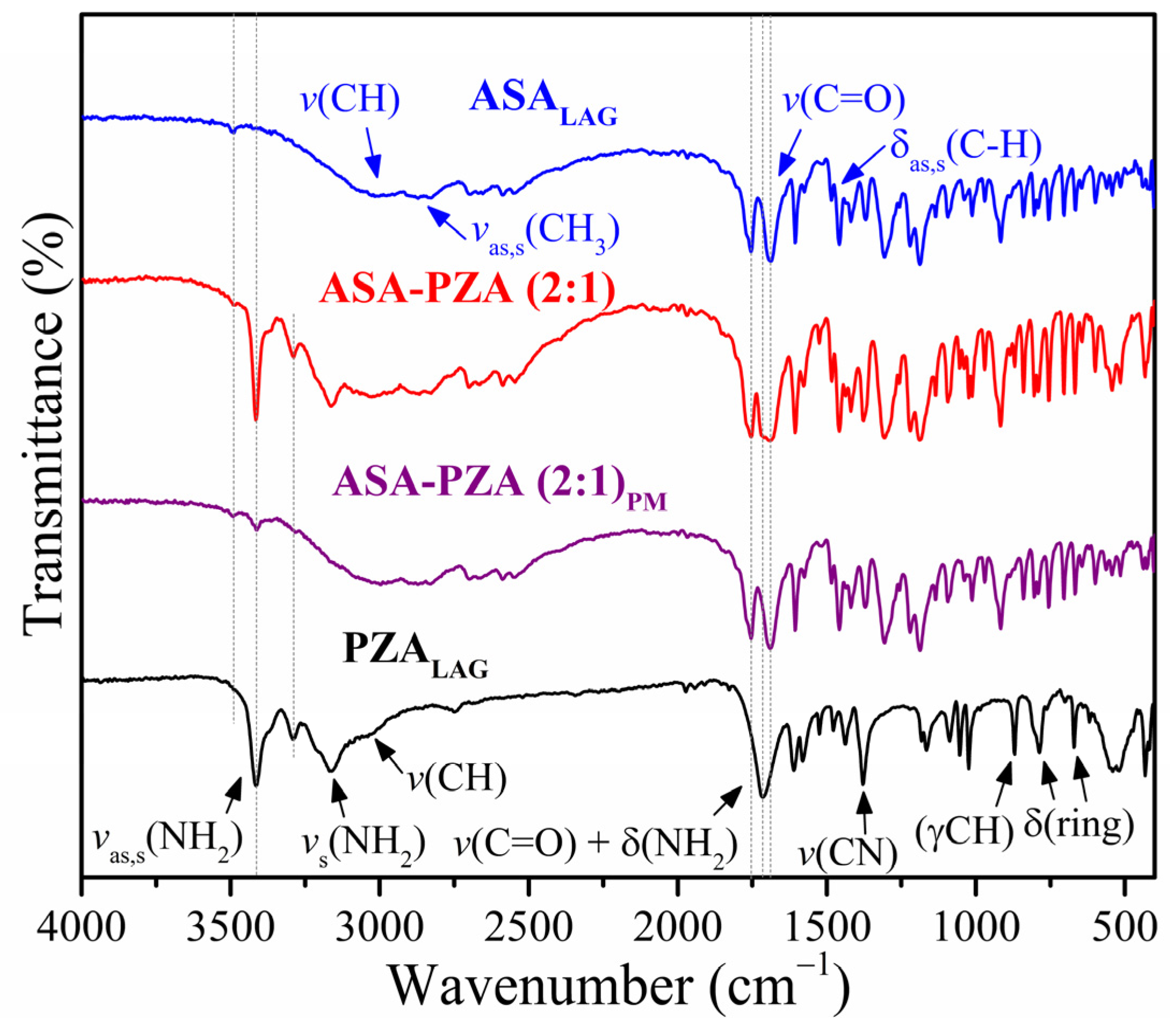
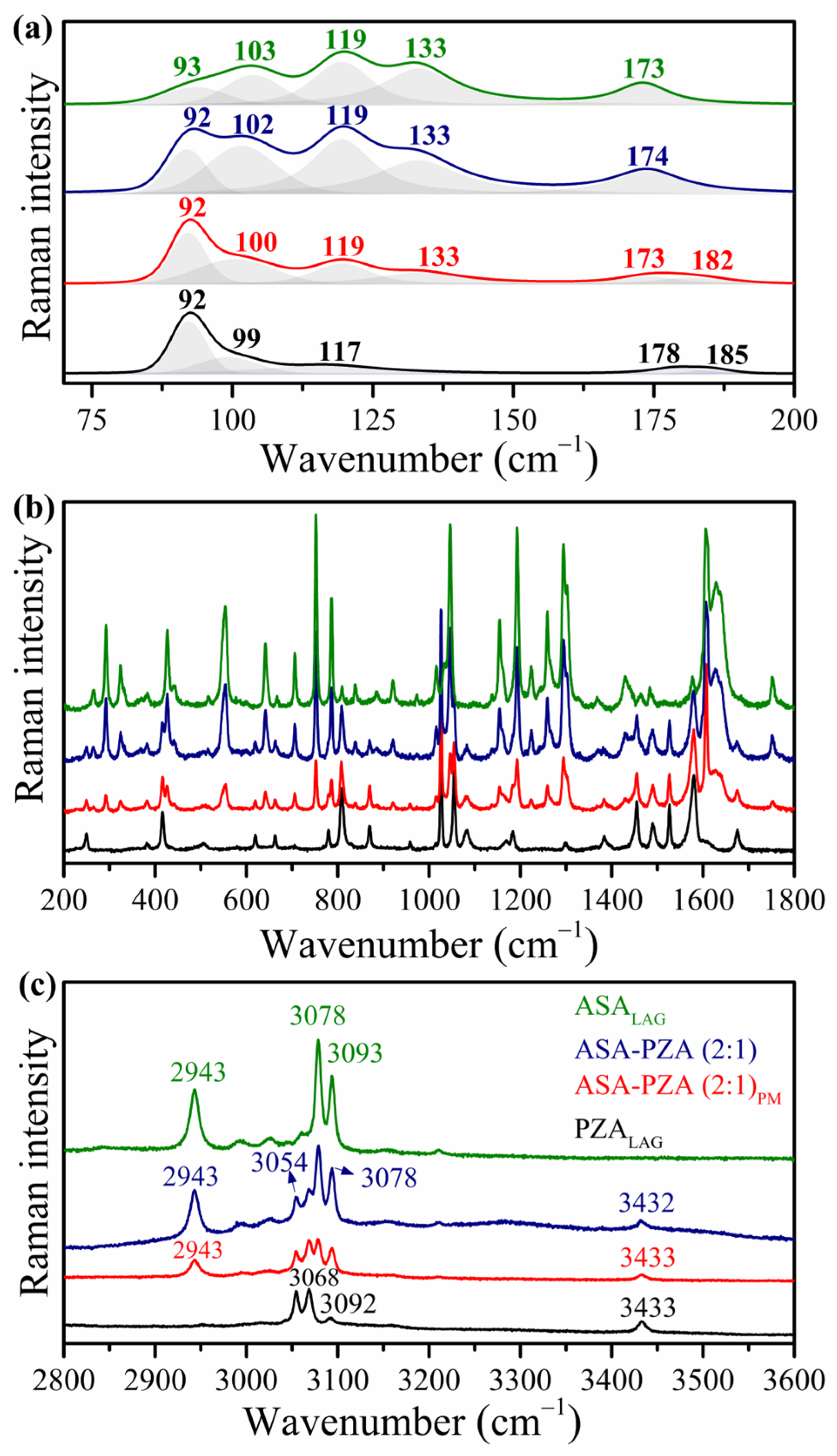
2.2. Structural Characterization and Molecular Interactions
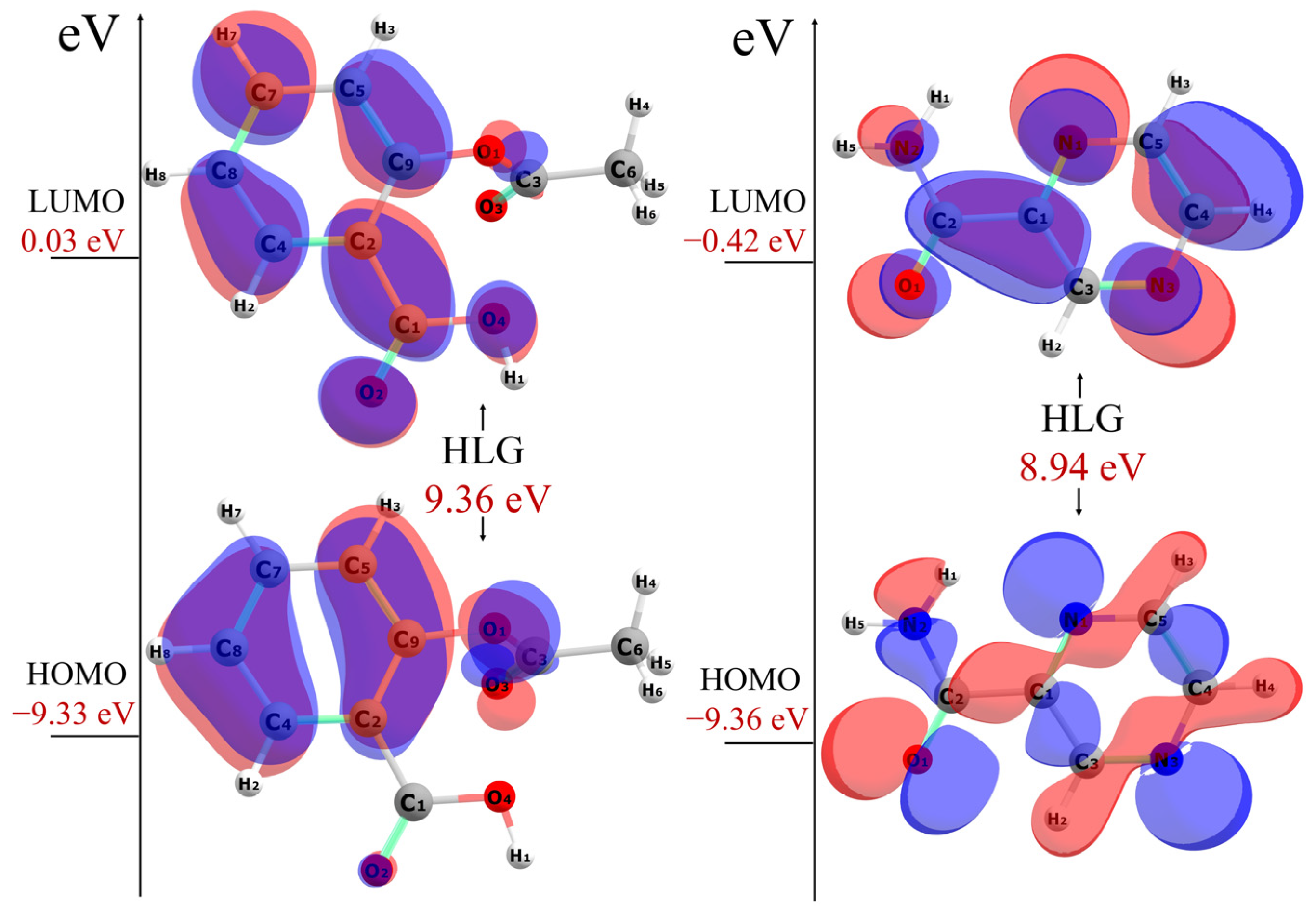
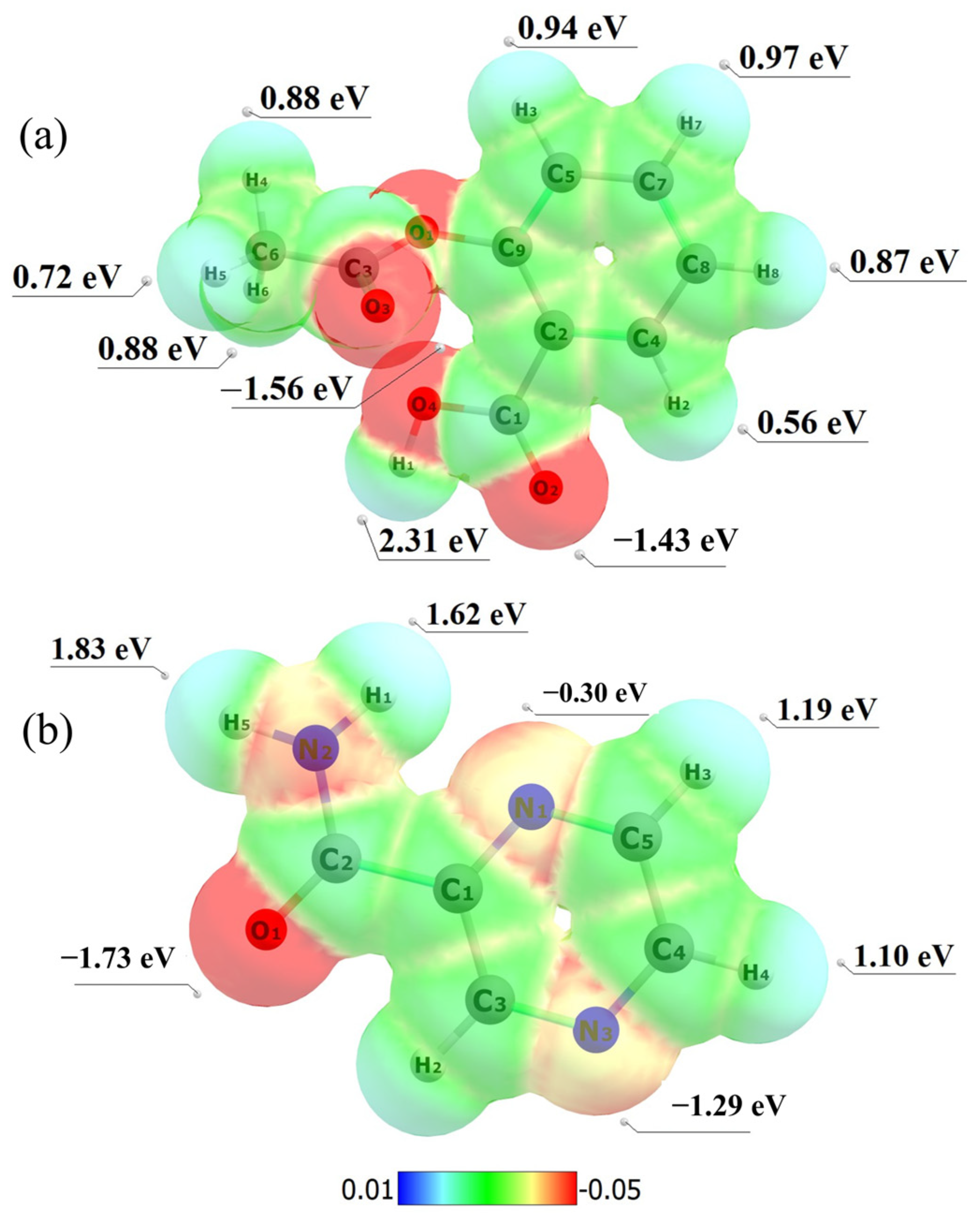
2.3. Computational Studies
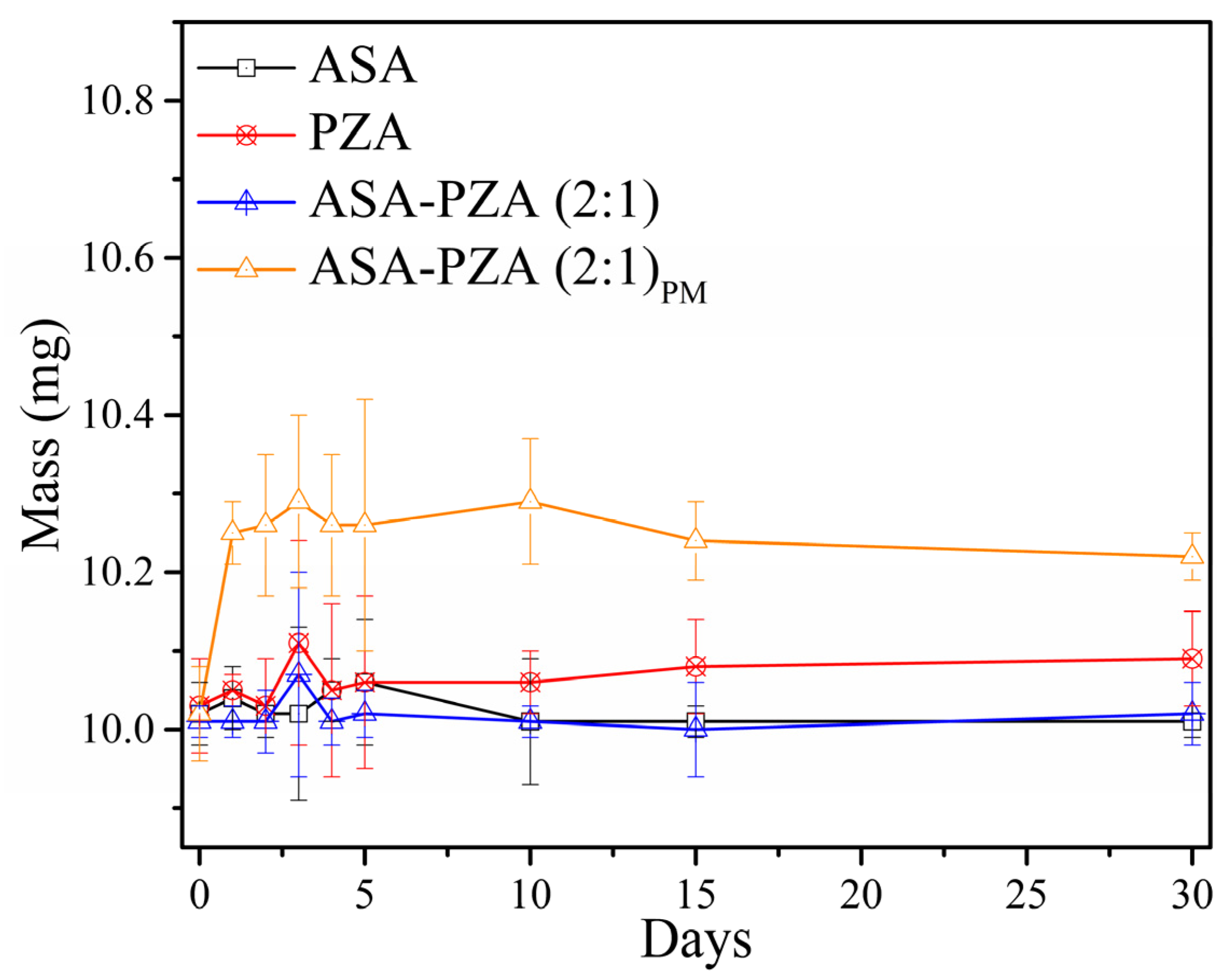
2.4. Hygroscopicity Measurements
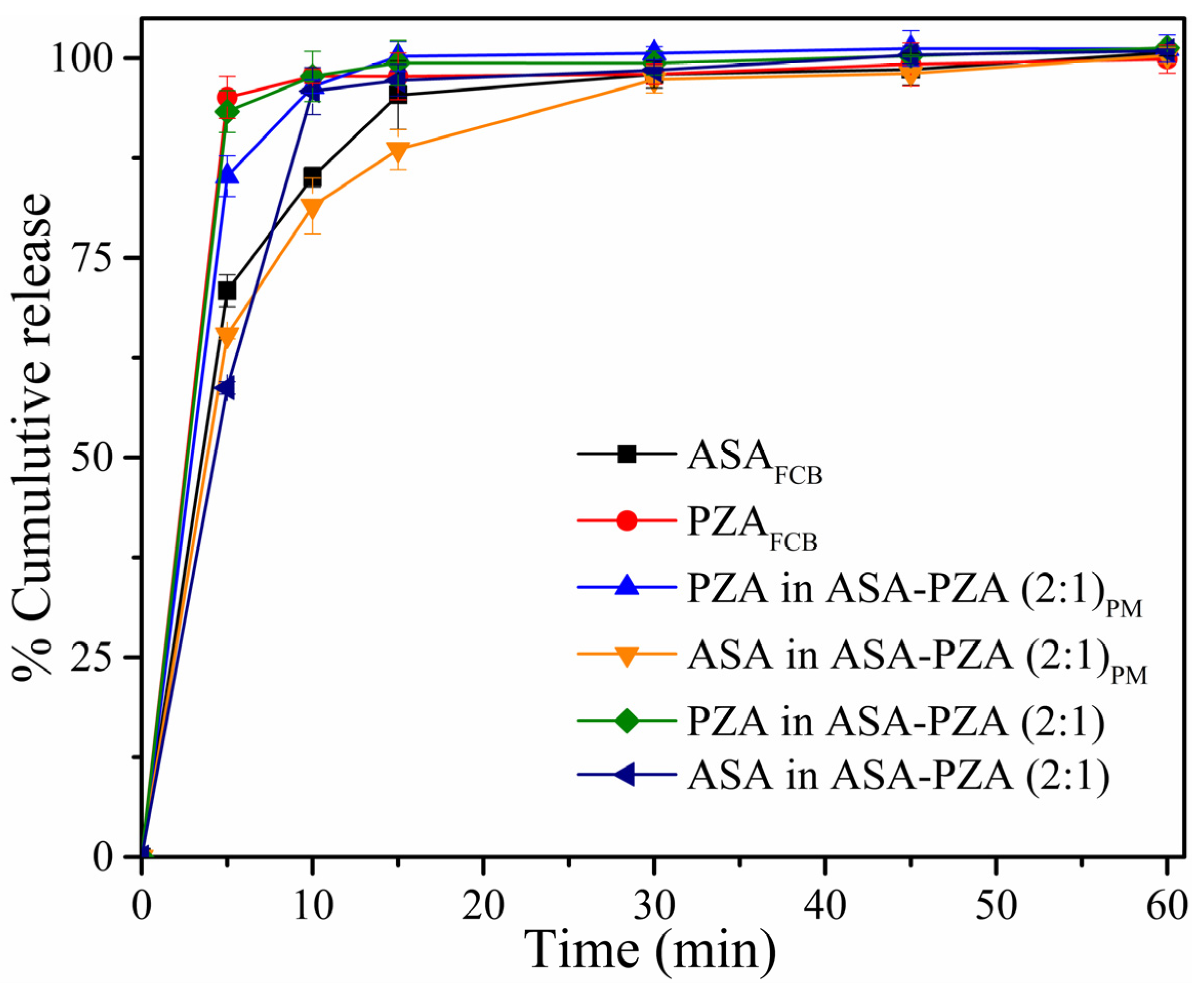
2.5. Solubility and Dissolution Behavior
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Solid State Characterization
3.3.1. Differential Scanning Calorimetry (DSC)
- Binary phase diagram and Tammann’s triangle
- Thermochemistry
3.3.2. Thermogravimetry and Differential Thermal Analysis (TG-DTA)
3.3.3. Powder X-Ray Diffraction (PXRD)
3.3.4. Fourier Transform Infrared Spectroscopy (FT-IR)
3.3.5. Raman Spectroscopy
3.4. Computational Details
3.5. Hygroscopicity Measurements
3.6. Saturation Solubility Study
3.7. Dissolution Profile
3.8. Quantification by UV–VIS
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DDEM | Drug–drug eutectic mixture |
| ASA | Acetylsalicylic acid |
| PZA | Pyrazinamide |
| EM | Eutectic mixture |
| LAG | Liquid-assisted grinding |
| TB | Tuberculosis |
| BSC | Biopharmaceutical classification system |
| DSC | Differential Scanning Calorimetry |
| TG-DTA | Thermogravimetry and differential thermal analysis |
| PXRD | Powder X-ray diffraction |
| FT-IR | Fourier transform infrared spectroscopy |
| POA | Pyrazinoic acid |
| FT-IR | Fourier transform infrared spectroscopy |
| DFT | Density functional theory |
References
- Yuri Gerônimo Kobata, P.; Ticona Chambi, J.; Maria do Espirito Santo, A.; Lucia Cuffini, S. Eutectic mixtures containing the active pharmaceutical ingredient ezetimibe: Phase diagrams, solid state characterization and dissolution profiles. J. Cryst. Growth 2024, 642, 127775. [Google Scholar] [CrossRef]
- Bazzo, G.C.; Pezzini, B.R.; Stulzer, H.K. Eutectic mixtures as an approach to enhance solubility, dissolution rate and oral bioavailability of poorly water-soluble drugs. Int. J. Pharm. 2020, 588, 119741. [Google Scholar] [CrossRef]
- Singh Raman, A.P.; Jain, P.; Kumar, A.; Singh, P.; Kumari, K.; Bahadur, I.; Mohammad, F.; Kaushik, N.K. Eutectic Mixtures to Enhance the Solubility of Active Pharmaceutical Ingredients: Density Functional Theory and Infrared Spectroscopy Approaches. ChemistrySelect 2023, 8, e202300824. [Google Scholar] [CrossRef]
- Solares-Briones, M.; Coyote-Dotor, G.; Páez-Franco, J.C.; Zermeño-Ortega, M.R.; de la O Contreras, C.M.; Canseco-González, D.; Avila-Sorrosa, A.; Morales-Morales, D.; Germán-Acacio, J.M. Mechanochemistry: A Green Approach in the Preparation of Pharmaceutical Cocrystals. Pharmaceutics 2021, 13, 790. [Google Scholar] [CrossRef]
- Queiroz, L.H.S.; Barros, R.S.; de Sousa, F.F.; Lage, M.R.; Sarraguça, M.C.; Ribeiro, P.R.S. Preparation and Characterization of a Rifampicin Coamorphous Material with Tromethamine Coformer: An Experimental–Theoretical Study. Mol. Pharm. 2024, 21, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Adalder, T.K.; Sankolli, R.; Dastidar, P. Homo- or Heterosynthon? A Crystallographic Study on a Series of New Cocrystals Derived from Pyrazinecarboxamide and Various Carboxylic Acids Equipped with Additional Hydrogen Bonding Sites. Cryst. Growth. Des. 2012, 12, 2533–2542. [Google Scholar] [CrossRef]
- Bodart, L.; Derlet, A.; Buol, X.; Leyssens, T.; Tumanov, N.; Wouters, J. Combining two antitubercular drugs, clofazimine and 4-aminosalicylic acid, in order to improve clofazimine aqueous solubility and 4-aminosalicylic acid thermal stability. J. Pharm. Sci. 2020, 109, 3645–3652. [Google Scholar] [CrossRef]
- Yadav, J.P.A.; Bansal, A.K.; Jain, S. Molecular Understanding and Implication of Structural Integrity in the Deformation Behavior of Binary Drug–Drug Eutectic Systems. Mol. Pharm. 2018, 15, 1917–1927. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, R.; Chen, Y.; Zhang, Y.; Liu, X.; Liu, B.-F.; Zhang, G.; Hao, C. Drug-drug eutectic mixtures of celecoxib with tapentadol and milnacipran which could improve analgesic and antidepressant efficacy. J. Drug Deliv. Sci. Technol. 2022, 67, 102995. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L. On the physical processes of mechanochemically induced transformations in molecular solids. Chem. Commun. 2024, 60, 14750–14761. [Google Scholar] [CrossRef] [PubMed]
- Andre, V.; Duarte, M.T.; Gomes, C.S.B.; Sarraguca, M.C. Mechanochemistry in Portugal-A Step towards Sustainable Chemical Synthesis. Molecules 2022, 27, 241. [Google Scholar] [CrossRef]
- Ying, P.; Yu, J.; Su, W. Liquid-Assisted Grinding Mechanochemistry in the Synthesis of Pharmaceuticals. Adv. Synth. Catal. 2021, 363, 1246–1271. [Google Scholar] [CrossRef]
- Dartois, V.A.; Rubin, E.J. Anti-tuberculosis treatment strategies and drug development: Challenges and priorities. Nat. Rev. Microbiol. 2022, 20, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, T.; Godinez-Leon, J.A.; Gref, R. Nanosized Drug Delivery Systems to Fight Tuberculosis. Pharmaceutics 2023, 15, 393. [Google Scholar] [CrossRef]
- Ali, A.E.; Elasala, G.S.; Mohamed, E.A.; Kolkaila, S.A. Spectral, thermal studies and biological activity of pyrazinamide complexes. Heliyon 2019, 5, e02912. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, W.; Zhang, W.; Mitchison, D. Mechanisms of Pyrazinamide Action and Resistance. Microbiol. Spectr. 2014, 2, 10–1128. [Google Scholar] [CrossRef]
- Whitfield, M.G.; Soeters, H.M.; Warren, R.M.; York, T.; Sampson, S.L.; Streicher, E.M.; van Helden, P.D.; van Rie, A. A Global Perspective on Pyrazinamide Resistance: Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0133869. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, J.; Bo, Y.; Xue, J.; Liu, J.; Hong, Z.; Du, Y. Terahertz and Raman Spectroscopic Investigation of Anti-tuberculosis Drug-Drug Cocrystallization Involving 4-aminosalicylic Acid and Pyrazinamide. J. Mol. Struct. 2021, 1227, 129547. [Google Scholar] [CrossRef]
- Zhang, Y.; Mitchison, D. The curious characteristics of pyrazinamide: A review. Int. J. Tuberc. Lung Dis. 2003, 7, 6–21. [Google Scholar]
- Becker, C.; Dressman, J.; Amidon, G.; Junginger, H.; Kopp, S.; Midha, K.; Shah, V.; Stavchansky, S.; Barends, D. Biowaiver monographs for immediate release solid oral dosage forms: Pyrazinamide. J. Pharm. Sci. 2008, 97, 3709–3720. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Cherukuvada, S.; Thakuria, R.; Nangia, A. Pyrazinamide polymorphs: Relative stability and vibrational spectroscopy. Cryst. Growth. Des. 2010, 10, 3931–3941. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Z.; Ji, S.; Wang, X.; Lan, P. The thermal behavior of pyrazinamide in 12 solvents from 288.15 to 328.15 k. J. Mol. Liq. 2021, 329, 115572. [Google Scholar] [CrossRef]
- McMahon, J.A.; Bis, J.A.; Vishweshwar, P.; Shattock, T.R.; McLaughlin, O.L.; Zaworotko, M.J. Crystal engineering of the composition of pharmaceutical phases. 3. Primary amide supramolecular heterosynthons and their role in the design of pharmaceutical co-crystals. Z. Für Krist.-Cryst. Mater. 2005, 220, 340–350. [Google Scholar] [CrossRef]
- Thorat, S.H.; Sahu, S.K.; Gonnade, R.G. Crystal structures of the pyrazinamide–p-aminobenzoic acid (1/1) cocrystal and the transamidation reaction product 4-(pyrazine-2-carboxamido) benzoic acid in the molten state. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 1010–1016. [Google Scholar] [CrossRef]
- Al-Otaibi, J.S.; Mary, Y.S.; Mary, Y.S.; Panicker, C.Y.; Thomas, R. Cocrystals of pyrazinamide with p-toluenesulfonic and ferulic acids: DFT investigations and molecular docking studies. J. Mol. Struct. 2019, 1175, 916–926. [Google Scholar] [CrossRef]
- Kulla, H.; Greiser, S.; Benemann, S.; Rademann, K.; Emmerling, F. In situ investigation of a self-accelerated cocrystal formation by grinding pyrazinamide with oxalic acid. Molecules 2016, 21, 917. [Google Scholar] [CrossRef]
- Rajbongshi, T.; Sarmah, K.K.; Sarkar, A.; Ganduri, R.; Cherukuvada, S.; Thakur, T.S.; Thakuria, R. Preparation of Pyrazinamide Eutectics versus Cocrystals Based on Supramolecular Synthon Variations. Cryst. Growth. Des. 2018, 18, 6640–6651. [Google Scholar] [CrossRef]
- Bommaka, M.K.; Mannava, M.C.; Suresh, K.; Gunnam, A.; Nangia, A. Entacapone: Improving aqueous solubility, diffusion permeability, and cocrystal stability with theophylline. Cryst. Growth. Des. 2018, 18, 6061–6069. [Google Scholar] [CrossRef]
- Évora, A.O.; Castro, R.A.; Maria, T.M.; Rosado, M.T.; Ramos Silva, M.; Matos Beja, A.; Canotilho, J.; Eusébio, M.E.S. Pyrazinamide-diflunisal: A new dual-drug co-crystal. Cryst. Growth. Des. 2011, 11, 4780–4788. [Google Scholar] [CrossRef]
- Thakuria, R.; Cherukuvada, S.; Nangia, A. Crystal structures of pyrogallol, its hydrate, and stable multiple Z′ cocrystals with N-heterocycles containing metastable conformers of pyrogallol. Cryst. Growth. Des. 2012, 12, 3944–3953. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Arhangelskis, M.; Fabian, L.; Tizzard, G.J.; Coles, S.J.; Jones, W. Investigation of an amide-pseudo amide hydrogen bonding motif within a series of theophylline: Amide cocrystals. Cryst. Growth. Des. 2016, 16, 51–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Xue, J.; Du, Y.; Liu, J.; Hong, Z. Vibrational spectroscopic investigation into novel ternary eutectic formed between pyrazinamide, fumaric acid, and isoniazid. ACS Omega 2020, 5, 17266–17274. [Google Scholar] [CrossRef]
- Górniak, A.; Wojakowska, A.; Karolewicz, B.; Pluta, J. Phase diagram and dissolution studies of the fenofibrate–acetylsalicylic acid system. J. Therm. Anal. Calorim. 2011, 104, 1195–1200. [Google Scholar] [CrossRef]
- Dressman, J.B.; Nair, A.; Abrahamsson, B.; Barends, D.M.; Groot, D.; Kopp, S.; Langguth, P.; Polli, J.E.; Shah, V.P.; Zimmer, M. Biowaiver monograph for immediate-release solid oral dosage forms: Acetylsalicylic acid. J. Pharm. Sci. 2012, 101, 2653–2667. [Google Scholar] [CrossRef] [PubMed]
- Crowell, E.L.; Dreger, Z.A.; Gupta, Y.M. High-pressure polymorphism of acetylsalicylic acid (aspirin): Raman spectroscopy. J. Mol. Struct. 2015, 1082, 29–37. [Google Scholar] [CrossRef]
- Kousková, L.; Leitner, J. DSC study of the system o-acetylsalicylic acid–caffeine and thermodynamic modelling of the system o-acetylsalicylic acid–caffeine–paracetamol. Thermochim. Acta 2019, 674, 63–67. [Google Scholar] [CrossRef]
- Osmanović Omerdić, E.; Alagić-Džambić, L.; Krstić, M.; Pašić-Kulenović, M.; Odović, J.; Vasiljević, D. In vitro dissolution study of acetylsalicylic acid and clopidogrel bisulfate solid dispersions: Validation of the RP-HPLC method for simultaneous analysis. Appl. Sci. 2020, 10, 4792. [Google Scholar] [CrossRef]
- Policianova, O.; Brus, J.; Hruby, M.; Urbanova, M. In vitro dissolution study of acetylsalicylic acid solid dispersions. Tunable drug release allowed by the choice of polymer matrix. Pharm. Dev. Technol. 2015, 20, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Tita, D.; Jurca, T.; Fulias, A.; Marian, E.; Tita, B. Compatibility study of the acetylsalicylic acid with different solid dosage forms excipients. J. Therm. Anal. Calorim. 2013, 112, 407–419. [Google Scholar] [CrossRef]
- Wang, F.; Huang, P.; Huang, D.; Hu, Y.; Ma, K.; Cai, X.; Jiang, T. Preparation and functionalization of acetylsalicylic acid loaded chitosan/gelatin membranes from ethanol-based suspensions via electrophoretic deposition. J. Mater. Chem. B 2018, 6, 2304–2314. [Google Scholar] [CrossRef]
- Byrne, S.T.; Denkin, S.M.; Zhang, Y. Aspirin and ibuprofen enhance pyrazinamide treatment of murine tuberculosis. J. Antimicrob. Chemother. 2007, 59, 313–316. [Google Scholar] [CrossRef]
- Alea, G.V.; Lagua, F.M.G.; Ajero, M.D.M. Synthesis and characterization of pyrazinamide analogs of acetylsalicylic acid and salicylic acid. Philipp. J. Sci. 2017, 146, 457–468. [Google Scholar]
- Villagracia, A.; Ong, H.L.; Lagua, F.M.; Alea, G. Chemical reactivity and bioactivity properties of pyrazinamide analogs of acetylsalicylic acid and salicylic acid using conceptual density functional theory. Heliyon 2020, 6, e04239. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, Z.-F.; Li, Y.-Y.; Zhang, Y.; Ren, Y.; Fu, Z.; Xu, S.-Q. Nutrient-starved incubation conditions enhance pyrazinamide activity against Mycobacterium tuberculosis. Chemotherapy 2007, 53, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Hall II, R.G.; Leff, R.D.; Gumbo, T. Treatment of Active Pulmonary Tuberculosis in Adults: Current Standards and Recent Advances. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2009, 29, 1468–1481. [Google Scholar] [CrossRef]
- Alshaikh, R.A.; Essa, E.A.; El Maghraby, G.M. Eutexia for enhanced dissolution rate and anti-inflammatory activity of nonsteroidal anti-inflammatory agents: Caffeine as a melting point modulator. Int. J. Pharm. 2019, 563, 395–405. [Google Scholar] [CrossRef]
- Fernandes, R.P.; de Carvalho, A.C.S.; Ekawa, B.; do Nascimento, A.L.S.C.; Pironi, A.M.; Chorilli, M.; Caires, F.J. Synthesis and characterization of meloxicam eutectics with mandelic acid and saccharin for enhanced solubility. Drug Dev. Ind. Pharm. 2020, 46, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Seo, H.J.; Ha, E.-S.; Hong, S.-h.; Kim, J.-S.; Kim, M.-S.; Hwang, S.-J. Preparation and characterization of glimepiride eutectic mixture with l-arginine for improvement of dissolution rate. Int. J. Pharm. 2020, 581, 119288. [Google Scholar] [CrossRef]
- Rai, U.S.; Rai, R.N. Physical chemistry of the organic analog of metal–metal eutectic and monotectic alloys. J. Cryst. Growth 1998, 191, 234–242. [Google Scholar] [CrossRef]
- Law, D.; Wang, W.; Schmitt, E.A.; Qiu, Y.; Krill, S.L.; Fort, J.J. Properties of Rapidly Dissolving Eutectic Mixtures of Poly(ethylene glycol) and Fenofibrate:The Eutectic Microstructure. J. Pharm. Sci. 2003, 92, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.; Shah, H.S.; Nahar, K.; Dave, R.; Morris, K.R. Contribution of Crystal Lattice Energy on the Dissolution Behavior of Eutectic Solid Dispersions. ACS Omega 2020, 5, 9690–9701. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Rai, U.S.; Rai, R.N. Thermal and physico-chemical studies on binary organic eutectic systems: 4-Aminoacetophenone with benzoin and 4-nitrophenol. J. Therm. Anal. Calorim. 2012, 110, 551–557. [Google Scholar] [CrossRef]
- Reddi, R.S.B.; Satuluri, V.S.A.K.; Rai, U.S.; Rai, R.N. Thermal, physicochemical and microstructural studies of binary organic eutectic systems. J. Therm. Anal. Calorim. 2012, 107, 377–385. [Google Scholar] [CrossRef]
- Goud, N.R.; Suresh, K.; Sanphui, P.; Nangia, A. Fast dissolving eutectic compositions of curcumin. Int. J. Pharm. 2012, 439, 63–72. [Google Scholar] [CrossRef]
- Matsumoto, T.; Yamano, A.; Sato, T.; Ferrara, J.D.; White, F.J.; Meyer, M. “What is This?” A Structure Analysis Tool for Rapid and Automated Solution of Small Molecule Structures. J. Chem. Crystallogr. 2021, 51, 438–450. [Google Scholar] [CrossRef]
- Takaki, Y.; Sasada, Y.; Watanabe, T. The crystal structure of [alpha]-pyrazinamide. Acta Crystallogr. 1960, 13, 693–702. [Google Scholar] [CrossRef]
- Castro, R.A.; Maria, T.M.; Évora, A.O.; Feiteira, J.C.; Silva, M.R.; Beja, A.M.; Canotilho, J.; Eusébio, M.E.S. A new insight into pyrazinamide polymorphic forms and their thermodynamic relationships. Cryst. Growth. Des. 2010, 10, 274–282. [Google Scholar] [CrossRef]
- Sharma, P.; Nandi, R.; Gangopadhyay, D.; Singh, A.; Singh, R.K. Temperature dependent polymorphism of pyrazinamide: An in situ Raman and DFT study. Spectroc. Acta Pt. A-Mol. Biomol. 2018, 190, 177–180. [Google Scholar] [CrossRef]
- Chiş, V.; Pîrnău, A.; Jurcă, T.; Vasilescu, M.; Simon, S.; Cozar, O.; David, L. Experimental and DFT study of pyrazinamide. Chem. Phys. 2005, 316, 153–163. [Google Scholar] [CrossRef]
- Raman, A.P.S.; Singh, M.B.; Chaudhary, M.; Bahdur, I.; Jain, P.; Kaushik, N.; Choi, E.H.; Kaushik, N.K.; Lal, A.A.; Singh, P. DFT calculations, molecular docking and SAR investigation for the formation of eutectic mixture using thiourea and salicylic acid. J. Mol. Liq. 2022, 362, 119650. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, K.; Singh, S.; Bahadur, I.; Singh, P. Noscapine anticancer drug designed with ionic liquids to enhance solubility: DFT and ADME approach. J. Mol. Liq. 2021, 325, 115159. [Google Scholar] [CrossRef]
- Araya-Sibaja, A.M.; Vega-Baudrit, J.R.; Guillén-Girón, T.; Navarro-Hoyos, M.; Cuffini, S.L. Drug Solubility Enhancement through the Preparation of Multicomponent Organic Materials: Eutectics of Lovastatin with Carboxylic Acids. Pharmaceutics 2019, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, A.; Balachandran, V.; Karthick, T. Molecular orbital studies (hardness, chemical potential, electrophilicity, and first electron excitation), vibrational investigation and theoretical NBO analysis of 2-hydroxy-5-bromobenzaldehyde by density functional method. J. Mol. Struct. 2013, 1031, 221–233. [Google Scholar] [CrossRef]
- Miar, M.; Shiroudi, A.; Pourshamsian, K.; Oliaey, A.R.; Hatamjafari, F. Theoretical investigations on the HOMO–LUMO gap and global reactivity descriptor studies, natural bond orbital, and nucleus-independent chemical shifts analyses of 3-phenylbenzo[d]thiazole-2(3H)-imine and its para-substituted derivatives: Solvent and substituent effects. J. Chem. Res. 2020, 45, 147–158. [Google Scholar] [CrossRef]
- De Proft, F.; Sablon, N.; Tozer, D.J.; Geerlings, P. Calculation of negative electron affinity and aqueous anion hardness using Kohn–Sham HOMO and LUMO energies. Faraday Discuss. 2007, 135, 151–159. [Google Scholar] [CrossRef]
- Edim, M.M.; Louis, H.; Bisong, E.A.; Chioma, A.G.; Enudi, O.C.; Unimuke, T.O.; Bassey, A.B.; Prince, D.; Sam, Q.O.; Ubana, E.I.; et al. Electronic structure theory study of the reactivity and structural molecular properties of halo-substituted (F, Cl, Br) and heteroatom (N, O, S) doped cyclobutane. Phys. Sci. Rev. 2023, 8, 715–739. [Google Scholar] [CrossRef]
- Al-Malah, K.I. Aqueous solubility of a simple (single-carbon) organic molecule as a function of its size & dipole moment. J. Mol. Model. 2011, 17, 1029–1034. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Willetts, S.; Foley, D.W. True or false? Challenges and recent highlights in the development of aspirin prodrugs. Eur. J. Med. Chem. 2020, 192, 112200. [Google Scholar] [CrossRef]
- Dolezal, M.; Kesetovic, D.; Zitko, J. Antimycobacterial Evaluation of Pyrazinoic Acid Reversible Derivatives. Curr. Pharm. Design 2011, 17, 3506–3514. [Google Scholar] [CrossRef]
- Callahan, J.C.; Cleary, G.W.; Elefant, M.; Kaplan, G.; Kensler, T.; Nash, R.A. Equilibrium Moisture Content of Pharmaceutical Excipients. Drug Dev. Ind. Pharm. 1982, 8, 355–369. [Google Scholar] [CrossRef]
- Thakur, T.S.; Thakuria, R. Crystalline Multicomponent Solids: An Alternative for Addressing the Hygroscopicity Issue in Pharmaceutical Materials. Cryst. Growth. Des. 2020, 20, 6245–6265. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Sun, B.-W. Pharmaceutical co-crystals of pyrazinecarboxamide (PZA) with various carboxylic acids: Crystallography, hirshfeld surfaces, and dissolution study. Cryst. Growth. Des. 2013, 13, 2098–2106. [Google Scholar] [CrossRef]
- Felder, E.; Pitre, D. Pyrazinamide. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: Cambridge, MA, USA, 1983; Volume 12, pp. 433–462. [Google Scholar]
- Rowland, M.; Riegelman, S.; Harris, P.A.; Sholkoff, S.D. Absorption Kinetics of Aspirin in Man follow Oral Administration of an Aqueous Solution. J. Pharm. Sci. 1972, 61, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Berthold-Pluta, A.; Pluta, A.; Garbowska, M. The effect of selected factors on the survival of Bacillus cereus in the human gastrointestinal tract. Microb. Pathog. 2015, 82, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Dressman, J.B.; Amidon, G.L.; Reppas, C.; Shah, V.P. Dissolution Testing as a Prognostic Tool for Oral Drug Absorption: Immediate Release Dosage Forms. Pharm. Res. 1998, 15, 11–22. [Google Scholar] [CrossRef]
- Goldberg, A.H.; Gibaldi, M.; Kanig, J.L. Increasing Dissolution Rates and Gastrointestinal Absorption of Drugs Via Solid Solutions and Eutectic Mixtures I: Theoretical Considerations and Discussion of the Literature. J. Pharm. Sci. 1965, 54, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Gunnam, A.; Thipparaboina, R.; Nangia, A.K.; Shastri, N.R. Hepatoprotective cocrystals of isoniazid: Synthesis, solid state characterization, and hepatotoxicity studies. Cryst. Growth. Des. 2019, 19, 5161–5172. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.C.H.; Johnson, J.F. Prediction of Eutectic Temperatures, Compositions and Phase Diagrams for Binary Mesophase Systemst. Mol. Cryst. Liquid Cryst. 1974, 27, 95–104. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The m PW and m PW1PW models. J. Chem. Phys. 1998, 108, 664–675. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16. In Revision A. 03; Gaussian. Inc.: Wallingford, CT, USA, 2016; Volume 3. [Google Scholar]
- Andrienko, G. Chemcraft-Graphical Software for Visualization of Quantum Chemistry Computations. 2010. Available online: https://www.chemcraftprog.com (accessed on 25 August 2024).
- Kobayashi, Y.; Ito, S.; Itai, S.; Yamamoto, K. Physicochemical properties and bioavailability of carbamazepine polymorphs and dihydrate. Int. J. Pharm. 2000, 193, 137–146. [Google Scholar] [CrossRef]
- Murdande, S.B.; Pikal, M.J.; Shanker, R.M.; Bogner, R.H. Aqueous solubility of crystalline and amorphous drugs: Challenges in measurement. Pharm. Dev. Technol. 2011, 16, 187–200. [Google Scholar] [CrossRef]
- Melo, K.J.C.; Henostroza, M.A.B.; Löbenberg, R.; Bou-Chacra, N.A. Rifampicin nanocrystals: Towards an innovative approach to treat tuberculosis. Mater. Sci. Eng. C 2020, 112, 110895. [Google Scholar] [CrossRef]
- Patil, P.H.; Belgamwar, V.S.; Patil, P.R.; Surana, S.J. Enhancement of solubility and dissolution rate of poorly water soluble raloxifene using microwave induced fusion method. Braz. J. Pharm. Sci. 2013, 49, 571–578. [Google Scholar] [CrossRef]
- Commision, B.P. British Pharmacopoeia; The Stationery Office: London, UK, 2009. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Bharate, S.S.; Bharate, S.B. Spectrophotometric and Chromatographic determination of acetylsalicylic acid and Caffeine in pure and in tablet dosage form. J. Adv. Sci. Res. 2012, 3, 73–81. [Google Scholar]
- Khawas, S.; Parui, S.; Dey, S.; Mandal, S.; Sarkar, S. Simultaneous Spectrophotometric Estimation of Rifampicin, Isoniazid and Pyrazinamide in their Pharmaceutical Dosage Form. Asian J. Res. Chem. 2020, 13, 117. [Google Scholar] [CrossRef]
- Pinho, L.A.G.; Sá-Barreto, L.C.L.; Infante, C.M.C.; Cunha-Filho, M.S.S. Simultaneous determination of benznidazole and itraconazole using spectrophotometry applied to the analysis of mixture: A tool for quality control in the development of formulations. Spectroc. Acta Pt. A-Mol. Biomol. 2016, 159, 48–52. [Google Scholar] [CrossRef]
- Andrews, G.P.; Qian, K.; Jacobs, E.; Jones, D.S.; Tian, Y. High drug loading nanosized amorphous solid dispersion (NASD) with enhanced in vitro solubility and permeability: Benchmarking conventional ASD. Int. J. Pharm. 2023, 632, 122551. [Google Scholar] [CrossRef] [PubMed]
| Sample | Tfus (°C) | Tfus (K) | ΔHfus (kJ.mol−1) | ΔSfus (J/(mol.K)−1) | ΔSfus0 (ΔfusS/R) |
|---|---|---|---|---|---|
| ASALAG | 140.8 | 413.9 | 41.33 | 99.85 | 12.01 |
| PZALAG | 189.5 | 462.6 | 26.69 | 57.69 | 6.94 |
| DDEM | 114.2 | 387.3 | 119.31 | 308.05 | 37.05 |
| Compound | IE a | EA a | HLG | η a | σ b | χ a | μ a | ω a |
|---|---|---|---|---|---|---|---|---|
| ASAvac | 9.33 | −0.03 | 9.36 | 4.68 | 0.11 | 4.65 | −4.65 | 2.31 |
| PZAvac | 9.36 | 0.42 | 8.94 | 4.47 | 0.11 | 4.89 | −4.89 | 2.67 |
| Sample | Solubility of ASA (mg.mL−1) a | RSD (%) b | Solubility of PZA (mg.mL−1) a | RSD (%) b |
|---|---|---|---|---|
| ASA | 16.1 ± 0.4 | 2.4 | - | - |
| PZA | - | - | 22.6 ± 0.2 | 1.1 |
| ASA-PZA (2:1)PM | 19.1 ± 0.9 | 4.5 | 31.2 ± 1.4 | 4.4 |
| ASA-PZA (2:1) | 26.0 ± 1.3 | 5.0 | 42.0 ± 1.8 | 4.3 |
| Sample | ASA | ASA in PM | ASA in DDEM | PZA | PZA in PM | PZA in DDEM |
|---|---|---|---|---|---|---|
| Q | 85.1 | 81.5 | 95.9 | 97.7 | 96.4 | 97.7 |
| DE | 0.104 | 0.100 | 0.097 | 0.127 | 0.117 | 0.125 |
| f2 | - | 71.36 | 60.04 | - | 68.68 | 89.69 |
| Property (Abbreviation) | Math Expression | |
|---|---|---|
| Ionization energy (IE) | IE = −EHOMO | (4) |
| Electronic affinity (EA) | EA −ELUMO | (5) |
| HOMO-LUMO gap (HLG) | HLG = ELUMO − EHOMO | (6) |
| Hardness (η) | (7) | |
| Softness (S) | (8) | |
| Electronegativity (χ) | (9) | |
| Chemical potential (µ) | −µ = χ | (10) |
| Electrophilicity (ω) | (11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz, L.H.S.; Lage, M.R.; dos Santos, C.C.; Sarraguça, M.C.; Ribeiro, P.R.S. Thermodynamic and Structural Characterization of a Mechanochemically Synthesized Pyrazinamide–Acetylsalicylic–Acid Eutectic Mixture. Pharmaceuticals 2025, 18, 211. https://doi.org/10.3390/ph18020211
Queiroz LHS, Lage MR, dos Santos CC, Sarraguça MC, Ribeiro PRS. Thermodynamic and Structural Characterization of a Mechanochemically Synthesized Pyrazinamide–Acetylsalicylic–Acid Eutectic Mixture. Pharmaceuticals. 2025; 18(2):211. https://doi.org/10.3390/ph18020211
Chicago/Turabian StyleQueiroz, Luís H. S., Mateus R. Lage, Clenilton C. dos Santos, Mafalda C. Sarraguça, and Paulo R. S. Ribeiro. 2025. "Thermodynamic and Structural Characterization of a Mechanochemically Synthesized Pyrazinamide–Acetylsalicylic–Acid Eutectic Mixture" Pharmaceuticals 18, no. 2: 211. https://doi.org/10.3390/ph18020211
APA StyleQueiroz, L. H. S., Lage, M. R., dos Santos, C. C., Sarraguça, M. C., & Ribeiro, P. R. S. (2025). Thermodynamic and Structural Characterization of a Mechanochemically Synthesized Pyrazinamide–Acetylsalicylic–Acid Eutectic Mixture. Pharmaceuticals, 18(2), 211. https://doi.org/10.3390/ph18020211








