Abstract
Background: Migraine, a complex neurological condition, poses significant challenges for both sufferers and healthcare providers. While prescription medications play a vital role in managing migraine attacks, the quest for natural, non-pharmacological alternatives has garnered increasing interest. This review explores the efficacy and safety of natural supplements as treatments for migraine relief, comparing them with conventional prescription medications. Methods: The review delves into herbal supplements, clinical studies on natural remedies, aromatherapy, dietary influences, and lifestyle modifications in the context of migraine management in several databases. Results: The findings shed light on the potential of natural supplements as complementary or alternative approaches to traditional migraine therapies, offering insights into a holistic and personalized treatment paradigm for migraine sufferers. Conclusions: Natural supplements have gained attention as potential treatments for migraine relief, often perceived as safer alternatives to conventional medications.
1. Introduction
Migraine is a common and debilitating neurological disorder that affects individuals across all age groups. It is a leading cause of disability worldwide, with significant societal and personal impacts, particularly during the most productive years of life [1,2,3,4]. Migraine is characterized by recurrent headaches that are typically unilateral or bilateral, pulsating in nature, and often accompanied by symptoms such as nausea, vomiting, photophobia, and phonophobia [5]. The International Classification of Headache Disorders (ICHD-3) identifies migraine as a primary headache disorder, distinct from more than 250 other types of headaches [6]. Despite its significant impact, the mechanisms underlying migraines remain incompletely understood. Research points to a combination of genetic predisposition, neurovascular processes, and inflammatory pathways, including the activation of the trigeminovascular system and the release of neuropeptides such as calcitonin gene-related peptide (CGRP) [7,8,9,10].
Conventional migraine treatment focuses on acute symptom relief and prevention, with options primarily including triptans and nonsteroidal anti-inflammatory drugs (NSAIDs) for acute management, as well as preventive medications like beta-blockers and CGRP inhibitors [11]. However, limitations such as medication overuse headaches, adverse effects, and contraindications in certain populations highlight the need for alternative and complementary therapies [12]. Several natural products such as ginger, butterbur, turmeric, feverfew, feverfew+ white willow, and ginkgo have promising potential for the management of migraine [13].
This comprehensive review addresses these gaps by synthesizing current knowledge on natural and complementary approaches, including herbal products (plant extracts, pure compounds, or their supplements), dietary supplements (non-herbal agents), aromatherapy, and lifestyle modifications.
2. Migraine Prevalence
Migraine is a highly prevalent neurological disorder, affecting approximately 1.1 billion people globally as of 2019, and ranks as the second leading cause of years lived with disability (YLDs), contributing to significant societal and individual burden [3]. Chronic migraine affects 1–2% of the population, and 2.5% of individuals with episodic migraines will develop chronic migraine over time [14]. Migraine has significant demographic variations, as its prevalence is notably higher in women, reaching nearly 18.9% compared to 9.8% in men (Figure 1), largely due to hormonal influences such as estrogen fluctuations [15]. It affects approximately 5% of youth by age 10 and increases in prevalence throughout adolescence, with nearly 28% of girls and 20% of boys experiencing migraines by their late teenage years (Figure 1) [1,16]. While regional disparities in prevalence exist, high-income countries like Belgium and Italy report the highest rates (22,400 and 20,337 per 100,000) and lower rates are reported in regions like Ethiopia (8277 per 100,000), highlighting differences in healthcare access and diagnostic capabilities (Figure 1) [15,16]. Additionally, despite a decline in active migraines among older populations, up to 25.7% of women aged 60 and older continue to live with inactive migraines, underscoring the long-term impact of the condition [16]. These statistics emphasize the urgent need for innovative, accessible, and holistic interventions that address the multifaceted burden of migraine and improve quality of life for diverse populations and age groups worldwide.
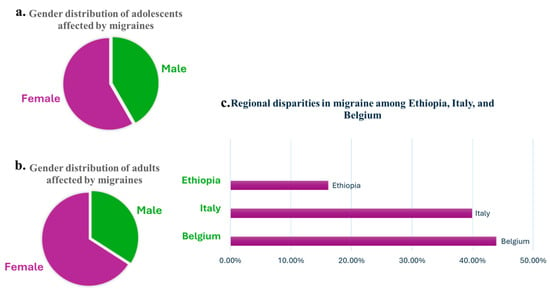
Figure 1.
Overview of migraine prevalence and distribution: (a) gender distribution of adolescents affected by migraines, showing a higher prevalence among girls compared to boys; (b) gender distribution of adults affected by migraines, emphasizing a greater proportion of women compared to men; (c) comparison of migraine prevalence in selected high-income (Belgium and Italy) and low-income (Ethiopia) countries, used as examples to highlight disparities in healthcare access and diagnostic capabilities.
3. Migraine Phases
A comprehensive understanding of this progression, which unfolds in four phases: prodromal, aura, headache, and postdromal (Figure 2), is crucial for elucidating both the variability of the migraine experience and the underlying pathophysiological mechanisms.
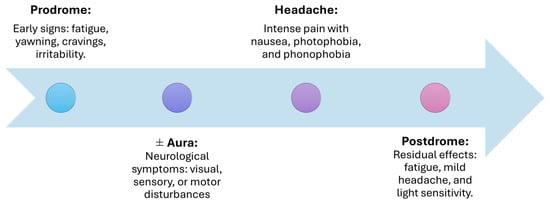
Figure 2.
Phases of migraine: from onset to resolution.
3.1. Prodromal Phase
The period of subclinical signs and symptoms that precedes the onset of a migraine headache is referred to as the prodrome phase. This phase occurs 24–48 h before headaches and is marked by early symptoms such as fatigue, yawning, irritability, muscle stiffness, food cravings, nausea, and sleeping difficulty. These symptoms suggest early dysfunction in the hypothalamus and brainstem, which prime the nervous system for the subsequent phases [17,18].
3.2. Aura Phase
Aura refers to a range of neurological symptoms—visual, sensory, speech, auditory, and motor disturbances—that can occur before or during a migraine attack. Experienced by about 25% of migraine sufferers, these symptoms include transient issues like visual flashes, changes in hearing, or motor disruptions. Caused by cortical spreading depression, these symptoms typically develop over 5 to 60 min and are fully reversible [18,19].
3.3. Headache Phase
The hallmark of migraine, the headache phase, can last from several hours to three days. Pain is typically unilateral but may become bilateral and is often accompanied by nausea, photophobia, and phonophobia. The activation of the trigeminovascular system and the release of neuropeptides like CGRP drive the pain and associated symptoms during this phase [17,18].
3.4. Postdromal Phase
The postdromal phase, also called the “migraine hangover”, occurs after the headache subsides. Lingering fatigue, mild headache, and sensitivity to light or smells are common. This phase reflects the residual activation of pain pathways and can be aggravated by environmental triggers [17,18].
4. Migraine Pathophysiology
The intricate biology of migraine involves multifactorial mechanisms that disrupt central and peripheral sensory processing, resulting in episodic or chronic pain and associated symptoms. Understanding these mechanisms is essential to appreciate the disorder’s complexity and guide effective treatment strategies.
4.1. Trigeminovascular System
Migraine pain primarily stems from the activation of the trigeminovascular system. This system involves nerve fibers from the trigeminal ganglion, which connect to the blood vessels in the cranial meninges. When triggered, these fibers release neuropeptides, such as CGRP. CGRP acts as a strong vasodilator, causing blood vessels to expand and trigger inflammation. This process heightens the sensitivity of pain pathways in the brain, which sustains the migraine [9,20,21,22]. By understanding how this system links both vascular and neural processes, it is clear why therapies targeting CGRP, such as CGRP antagonists, are an effective approach to migraine treatment.
4.2. Cortical Spreading Depression (CSD)
CSD is a self-propagating wave of neuronal depolarization and hyperpolarization strongly associated with aura symptoms. It activates the trigeminovascular system and releases inflammatory mediators, contributing to the initiation of headache. While pivotal in the aura phase, CSD is considered an epiphenomenon in migraine without aura [23,24].
4.3. Brainstem and Hypothalamic Dysfunction
The brainstem, particularly the periaqueductal gray (PAG) and locus coeruleus, modulates pain perception and plays a vital role during migraine episodes. Concurrently, the hypothalamus regulates premonitory symptoms such as yawning, thirst, and fatigue, reflecting its influence on cyclic migraine patterns. Functional imaging reveals altered connectivity between the hypothalamus, brainstem, and cortical areas in migraine patients, underscoring their role in initiating and perpetuating migraine attacks [25].
4.4. Neuroinflammation and Sensitization
Neurogenic inflammation amplifies migraine pain. During attacks, pro-inflammatory mediators like CGRP and substance P are released, leading to vasodilation, mast cell degranulation, and increased nitric oxide production. These processes sensitize trigeminal afferents, escalating pain and causing symptoms like photophobia and allodynia [22,24].
4.5. Thalamic and Cortical Involvement
The thalamus and cortex are central to migraine pathophysiology, contributing to sensory, cognitive, and emotional symptoms. The thalamus acts as a relay, amplifying nociceptive signals from the trigeminovascular system, which heightens pain perception and sensory sensitivities like photophobia and phonophobia. It also integrates signals from other brain regions, influencing the emotional and cognitive aspects of migraines. Structural and functional changes in cortical regions, particularly the occipital cortex, are linked to aura and visual symptoms, while dysregulated cortical networks further enhance pain perception [25].
5. Migraine-Contributing Risk Factors
Migraine episodes are deeply influenced by a variety of triggers, making their identification and management vital for reducing attack frequency and severity. Among the most significant contributors, stress stands out, affecting over 70% of patients and highlighting the profound connection between emotional strain and migraines [26,27]. Hormonal fluctuations, particularly during menstruation, are another powerful trigger, increasing migraine risk by up to 96%, underscoring the need for gender-specific approaches [28,29]. Dietary factors like fasting, alcohol, and chocolate frequently precipitate attacks, while environmental triggers such as noise, strong odors, and weather change further complicate its management [26,28,29]. Interestingly, some factors, like relaxation after stress, may unexpectedly reduce migraine risk, emphasizing the complexity of these triggers [28]. To provide a comprehensive understanding, these influences are categorized into intrinsic factors, such as hormonal changes and genetic predisposition, and extrinsic factors, including environmental and lifestyle elements, with each discussed in detail.
5.1. Intrinsic Risk Factors
Intrinsic factors originate within the individual and are closely linked to their biological and physiological makeup.
5.1.1. Genetic and Epigenetic Factors
Genetics plays a crucial role in determining susceptibility to migraines, with specific mutations strongly linked to certain migraine subtypes. Familial hemiplegic migraine (FHM), a rare and severe form, is inherited in an autosomal dominant pattern, meaning that a single copy of the mutated gene from either parent is enough to increase the risk of developing the condition. This pattern of inheritance means that if one parent carries the mutation, each child has a 50% chance of inheriting it [30]. FHM is associated with mutations in the CACNA1A, ATP1A2, and SCN1A genes, which affect ion channels responsible for regulating neuronal signaling. These mutations lead to an increased level of neuronal excitability, which can trigger migraine episodes, along with symptoms like temporary motor weakness and heightened sensitivity to light or sound [31]. Additionally, genetic factors influencing glutamate neurotransmission, synaptic development, and ion channel homeostasis contribute to migraine risk [18]. A large meta-analysis involving 375,000 individuals identified several migraine-related single nucleotide polymorphisms linked to arterial and smooth muscle function, suggesting a connection between vascular health and migraine pathogenesis. Furthermore, epigenetic changes, such as DNA methylation, can alter gene expression related to pain and inflammation, further elevating migraine susceptibility [32].
5.1.2. Hormonal Imbalances
Fluctuations in hormone levels, particularly estrogen, significantly affect migraines, explaining their higher prevalence in women [33]. Estrogen influences pain-processing pathways in the brain by modulating neurotransmitters like serotonin and dopamine. Drops in estrogen levels, such as those occurring before menstruation or during menopause, can destabilize these pathways, leading to heightened sensitivity to pain and triggering migraine episodes [34,35].
Similarly, elevated cortisol levels during stress disrupt circadian rhythms, which regulate essential functions like sleep–wake cycles. Chronic cortisol elevation increases brain excitability, heightening sensitivity to pain and triggering migraine attacks [36]. Additionally, circadian disruptions lead to poor sleep, a well-known migraine trigger. Together, these factors amplify the frequency and severity of migraines, highlighting the importance of stress management in prevention [37,38].
5.1.3. Metabolic Factors
Metabolic disorders, including obesity, diabetes, hypertension, and dyslipidemia, contribute to vascular changes and systemic inflammation, which are critical in migraine pathophysiology. For example, obesity is linked to increased inflammatory markers and altered adipokine levels (signaling proteins secreted by fat tissue that regulate inflammation and metabolism), both of which can amplify neuronal excitability [39]. Hypertension and dyslipidemia can compromise blood flow regulation in the brain, triggering migraines or prolonging their duration [40,41]. Interestingly, while type 1 diabetes may have a protective effect, type 2 diabetes shows no clear association, highlighting complex interplays between metabolic health and migraines [37,42].
5.1.4. Neurological Disorders
Neurological conditions, such as epilepsy and multiple sclerosis (MS), frequently co-occur with migraines due to shared pathways. Both epilepsy and migraines involve paroxysmal episodes caused by neuronal hyperexcitability, with evidence suggesting overlapping genetic and biochemical mechanisms [43]. Similarly, in MS, inflammation and demyelination in the central nervous system can disrupt neural communication, leading to migraines as a secondary manifestation [44]. Movement disorders like restless leg syndrome and Parkinson’s disease also share dopaminergic and iron metabolism dysfunctions, which may explain their link to migraines [37,45,46,47].
5.1.5. Psychological and Psychiatric Factors
Psychological stress and psychiatric conditions are among the most potent contributors to migraines. Depression and anxiety disorders are particularly common, with stress acting as both a trigger and a consequence of migraines. Chronic stress can sensitize pain pathways in the brain, lowering the threshold for migraine attacks [37,48,49]. Similarly, conditions like post-traumatic stress disorder and bipolar disorder involve disruptions in neurotransmitters like serotonin and dopamine, which also regulate pain perception [50]. This bidirectional relationship between psychiatric health and migraines underscores the importance of holistic management strategies.
5.2. Extrinsic Risk Factors
Extrinsic factors are external influences that interact with the individual’s internal environment to precipitate or exacerbate migraines.
5.2.1. Lifestyle and Behavioral Triggers
Disruptions in sleep patterns, such as insomnia or obstructive sleep apnea, are strongly linked to migraines. Sleep deprivation impairs the brain’s ability to regulate pain by increasing cortical excitability and inflammatory responses. Dietary habits, including fasting or irregular meal patterns, can destabilize glucose levels, triggering migraines. Additionally, excessive alcohol consumption is a well-known trigger, though the mechanism remains unclear; it likely involves the dilation of blood vessels and inflammatory responses [51,52].
5.2.2. Environmental Factors
Environmental stressors such as noise, strong odors, and bright or flickering lights can act as direct triggers for migraines by overstimulating sensory pathways. Changes in weather, including barometric pressure drops, have been shown to precipitate migraines in sensitive individuals, likely due to vascular responses in the brain. Prolonged exposure to stressful environments, whether social or occupational, not only increases the frequency of migraines but also contributes to their chronicity by maintaining heightened pain sensitivity [37,53,54].
5.2.3. Medication Overuse
The overuse of acute migraine medications, such as triptans, opioids, or analgesics, paradoxically increases migraine frequency and can lead to medication overuse headaches (MOHs) [55]. This occurs through mechanisms like central sensitization, where the brain becomes increasingly sensitive to pain signals, and the disruption of pain modulation pathways, which exacerbates dependency and diminishes the efficacy of treatments [56]. Over time, this misuse induces neuroinflammatory and neurotoxic changes in critical pain-processing regions, such as the trigeminal nucleus caudalis and the periaqueductal gray matter, while the withdrawal–rebound mechanism perpetuates a harmful cycle of escalating medication use [57]. Additionally, failure to effectively manage early migraine episodes can contribute to the progression from episodic to chronic migraines, with structural changes observed in pain-processing circuits [55,56,57]. Addressing medication overuse is vital to breaking this cycle and preventing long-term consequences, emphasizing the importance of preventive therapies, multidisciplinary care, and patient education tailored to reduce reliance on acute medications and promote sustainable headache management.
6. Search Strategy
To conduct a comprehensive literature review on the efficacy of natural therapies and complementary approaches for migraine management, we searched academic databases including PubMed, Google Scholar, EKB, and PsycINFO. We used MeSH terms and keywords such as “migraine”, “natural products”, “herbal remedies”, “aromatherapy”, “dietary supplements”, “lifestyle modifications”, and “alternative medicine”. We combined keywords with Boolean operators (AND and OR) for precision, e.g., “migraine AND natural products” or “herbal remedies OR clinical studies”. We focused on studies published since 2010 to prioritize recent advancements, emphasizing reviews and observational studies that assess the efficacy of natural supplements for managing migraines. We excluded non-English studies, case reports, editorials, and those with unclear methodologies or results to maintain relevance and rigor.
7. Results
7.1. Acute and Preventive Medications for Migraine Management
Migraine management involves both acute and preventive therapies tailored to individual patient needs. Acute treatments aim to relieve symptoms during attacks, while preventive therapies reduce the frequency, intensity, and duration of migraines over time. Medications for acute relief include triptans, NSAIDs, and gepants, and they are selected based on migraine severity, patient-specific factors, and prior treatment responses. Preventive options, such as beta-blockers, anticonvulsants, and CGRP monoclonal antibodies, are intended for patients with frequent or severe migraines that significantly impair quality of life [58,59]. The following tables (Table 1 and Table 2) summarize the mechanisms, efficacy, side effects, and safety considerations for these medications.

Table 1.
Acute migraine relief medications.

Table 2.
Preventive migraine medications.
The complexity and variability of migraines, combined with the limitations of current pharmacological treatments, highlight the need for alternative and complementary strategies. While medications such as triptans, beta-blockers, and CGRP inhibitors are effective, they often carry challenges such as side effects, including cardiovascular risks, medication overuse headaches (MOHs), and adherence issues. These limitations underscore the importance of developing holistic approaches that integrate natural therapies with evidence-based preventive treatments to address the diverse needs of migraine patients.
Natural and complementary therapies have gained attention as potential alternatives or adjuncts to conventional migraine treatments. Feverfew has shown promise in reducing migraine frequency, yet several studies have failed to replicate these outcomes, likely due to differences in trial design, dosing, and product standardization [86]. Butterbur, despite its effectiveness, is overshadowed by safety concerns, including hepatotoxicity, necessitating the use of pyrrolizidine alkaloid-free formulations to mitigate risks [87]. Ginger is primarily recognized for its acute effects, such as alleviating nausea and vomiting, but its role in prevention remains inconclusive due to mixed clinical evidence [88,89]. Other natural therapies, including dietary supplements like magnesium and riboflavin, show potential in both preventive and acute settings [90,91]. However, these approaches require rigorous clinical validation to confirm their efficacy and safety.
When compared to conventional treatments, natural therapies are generally more accessible and affordable, particularly in resource-constrained settings. For example, feverfew and magnesium supplements are widely available over the counter, reducing barriers to access. However, their inconsistent clinical outcomes and limited regulatory oversight raise concerns about their efficacy and safety [92,93]. In contrast, conventional treatments, such as CGRP inhibitors and beta-blockers, are backed by robust clinical evidence and regulatory approval, offering more predictable outcomes. CGRP inhibitors, including Erenumab and Fremanezumab, have demonstrated significant efficacy in reducing migraine frequency, particularly in patients unresponsive to older therapies, but their high costs and limited availability in low-income regions present substantial barriers to access [94]. Preventive therapies, such as beta-blockers and anticonvulsants, are effective, but they are often associated with side effects like fatigue, cognitive dysfunction, and mood disturbances which can lead to adherence challenges [8,71,72,73,74,75,76,77,78,79,80].
To address these gaps, combining evidence-based natural products with validated preventive treatments like CGRP inhibitors could optimize outcomes by reducing reliance on acute medications and enhancing cost-effectiveness. Rigorous clinical validation and the standardization of natural therapies are also needed to ensure safety and efficacy, paving the way for their integration into evidence-based migraine management.
7.2. Herbal Products (Plant Extracts, Pure Compounds, or Their Supplements) for Migraine Management
Tracing the literature highlights the pivotal role of natural products in migraine treatment. Today, several herbal supplements containing plant extracts or purified plant constituents are available for both prophylactic and abortive migraine management. Randomized clinical trials, along with in vivo, in vitro, and molecular docking studies, have demonstrated the effectiveness and safety of numerous natural products. These include plants like feverfew, butterbur, ginger, and ginkgo, as well as phytoconstituents such as parthenolide, petasin, isopetasin, curcumin, ergotamine, dihydroergotamine, Δ9-tetrahydrocannabinol, and cannabidiol.
Figure 3 summarizes our findings from the literature on natural products studied for their anti-migraine activity. Reported investigations into herbal supplements, Ayurvedic formulations, plant extracts, and isolated plant constituents with demonstrated efficacy are presented in Table 3. Additionally, the chemical structures of key plant-derived constituents with anti-migraine activity are depicted in Figure 4. The mechanisms of action of natural products or phytochemicals involved in migraine treatment were displayed in Figure 5.
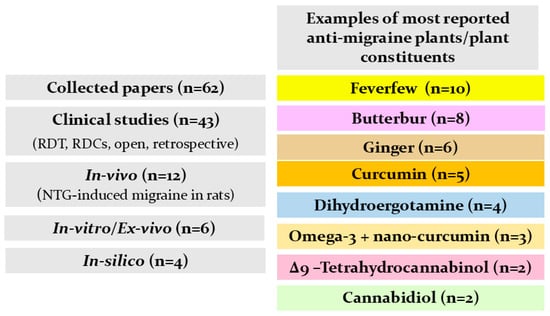
Figure 3.
Literature searching scheme for natural plants and plant constituents used for migraine management.
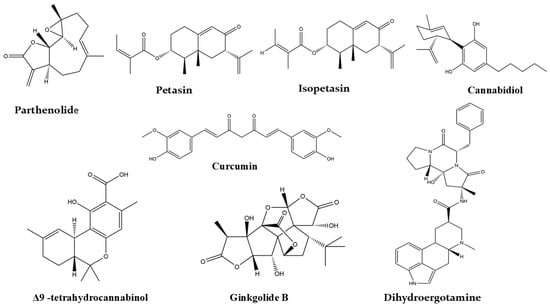
Figure 4.
Selected chemical structures of plant constituents for the management of migraine.
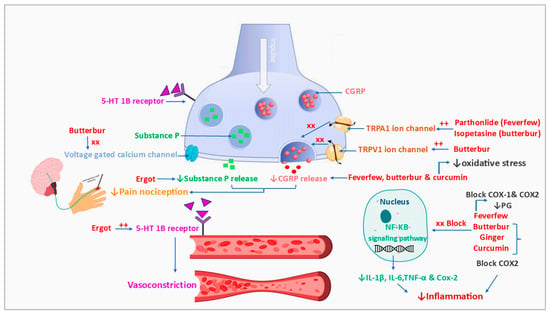
Figure 5.
Mechanisms of action of natural products or phytochemicals involved in migraine treatment. CGRP; calcitonin gene-related peptide, Cox-1; cyclooxiginase-1 enzyme, Cox-2; cyclooxiginase-2 enzyme, 5-HT-1B; 5-hydroxytryptamin 1B (serotonin receptor), IL; interleukin, NF-KB; nuclear factor kappa-light-chain-enhancer of activated B cells, PG; prostaglandin, TNF-α; tumor necrosis factor alpha, TRPA1; transient receptor potential ankyrin 1, TRPV1; transient receptor potential cation channel subfamily V member 1, also known as vanilloid receptor 1 and capsaicin receptor.
7.2.1. Feverfew
Feverfew (Tanacetum parthenium (L.) Sch. Bip.), a member of the Asteraceae family, has traditionally been used to treat fever, asthma, and inflammatory conditions such as psoriasis, insect bites, and rheumatism [95]. Modern research highlights its efficacy in migraine prophylaxis, and this is attributed primarily to parthenolide, a sesquiterpene lactone that inhibits serotonin release and prostaglandin biosynthesis, key mechanisms in migraine pathophysiology [86]. Furthermore, feverfew exhibits antiplatelet activity by suppressing thromboxane synthesis, which may address the vascular components of migraines [96].
Standardized supercritical CO2 extracts of feverfew, either alone or combined with other herbs such as Salix alba L. (white willow) or ginger, are commonly incorporated into migraine-specific herbal supplements. These formulations, designed for migraine management, include MIG-99 and Mig-RL (capsules), GelStat Migraine (sublingual), and LipiGesic and Antemig (tablets) [97,98,99,100]. While feverfew generally causes mild side effects, including nausea, stomach upset, and skin irritation, it is contraindicated in pregnancy due to its emmenagogue properties, in children under two years, and in individuals that are allergic to Asteraceae plants [96,101].
7.2.2. Butterbur
Butterbur (Petasites hybridus (L.) G. Gaertn., B. Mey. and Scherb.), another Asteraceae family member, has been traditionally used for central nervous system conditions, including migraine, hypertension, and dysmenorrhea [87]. Studies have demonstrated that its anti-migraine effects are primarily due to its active sesquiterpenes, petasin, and isopetasin. These compounds exert their effects through multiple mechanisms, including blocking voltage-dependent calcium channels, inhibiting CGRP secretion, and activating TRPA1 and TRPV1 channels. These actions help desensitize nociceptors and reduce inflammation, key factors in migraine pathophysiology [102,103,104,105]. Randomized clinical trials confirm the efficacy of standardized butterbur extracts (containing up to 15% petasin) in migraine prophylaxis for adults, adolescents, and children [106,107,108]. Products like Petadolex have shown promise but must be pyrrolizidine alkaloid-free to avoid hepatotoxicity [87].
7.2.3. Ginger
Ginger (Zingiber officinale Roscoe), a member of the Zingiberaceae family and a staple in Ayurvedic medicine, is traditionally used for digestive and respiratory ailments [109]. Randomized clinical trials have shown that ginger rhizome powder or extracts (400–500 mg/day, standardized to ≥5% gingerol) are effective for acute migraine relief, reducing pain intensity and duration within two hours [109,110,111,112]. Notably, combined formulations like GelStat and LipiGesic, which pair ginger with feverfew, show enhanced efficacy [98].
Ginger’s anti-migraine activity is primarily attributed to bioactive compounds like gingerols and shogaols, which inhibit cyclooxygenase-2, thereby reducing prostaglandin biosynthesis. Additionally, ginger’s antiemetic properties mitigate nausea and vomiting commonly associated with migraines [88]. Despite its benefits in acute management, studies investigating prophylactic effects have yielded mixed results, suggesting that ginger’s role may be limited to symptomatic relief [89].
7.2.4. Curcumin
Curcumin, a polyphenol from turmeric (Curcuma longa L.), exhibits antioxidant and anti-inflammatory properties, making it a potential migraine therapy. Its poor bioavailability necessitates advanced formulations, such as nanoparticles and liposomes, to enhance its absorption [113]. Clinical trials have highlighted curcumin’s benefits in reducing pain severity, oxidative stress, and inflammatory mediators like CGRP and IL-6. For instance, 100 mg/day of curcumin or 250 mg/day of phytosomal curcumin over two months has shown symptom improvement [114,115]. Synergistic effects have been observed when combined with omega-3 fatty acids or fish oil [116,117,118]. Preclinical studies in animal models have further demonstrated its antioxidant and analgesic effects, especially when paired with other drugs like sumatriptan or naproxen [119,120,121].

Table 3.
Reported herbal supplements or their constituents for migraine.
Table 3.
Reported herbal supplements or their constituents for migraine.
| Plant/Herbal Supplement | Plant Part | Extract Type/ Composition | Study Design | Method | Result | References |
|---|---|---|---|---|---|---|
| Preclinical Data | ||||||
| Butterbur | Root | Isopetasin (isolated from the acetone extract) | In vitro In vivo | The ability of isopetasin to target TRPA1 channel was tested in vitro in rodent and human TRPA1-expressing cells using patch-clamp recording and single-cell calcium imaging. CGRP release was tested in mouse spinal cord. Meningeal blood flow and facial rubbing were tested in vivo in rats and mice. | Isopetasin could activate TRPA1 channels, causing the excitation of neuropeptide containing nociceptors and the desensitization of peptidergic trigeminal nerve terminals, attenuating their ability to release CGRP. | [105] |
| Butterbur | - | Petasin Isopetasin (isolated from the ethanolic extract) | In vitro | The mechanism of anti-migraine activity of petasin and isopetasin was tested in vitro by evaluating CGRP release in the hemisected skull of a mouse and rats and dissected trigeminal ganglia. | Petasin and isopetasin are the active constituents of butterbur root extract; they reduced CGRP levels by affecting both TRPA1 and TRPV1 receptor channels. | [104] |
| - | - | Curcumin | In vivo | NTG-induced rats treated with either curcumin (10 mg/100 g), propranolol (100 µg/100 g), or Indomethacin (0.5 mg/100 g). The control group was treated with NTG only, and the non-migraine group was treated with normal saline. Formalin test and oxidative stress parameters were determined. | Curcumin showed an analgesic effect, reduced nociception (↓ number of shakes and flinches) when received before pain stimuli, and enhanced antioxidant activity (↓ NO, TOS, and MDA) | [119] |
| - | - | Curcumin Liposomal curcumin | In vivo | NTG-treated rats were divided into 6 groups and received either NTG only (control group), sumatriptan only (1 mg/100 g), 2 different doses of curcumin (1 mg/100 g and 2 mg/100 g) along with sumatriptan, or 2 different doses of liposomal curcumin along with sumatriptan. In addition, the non-migraine control group received normal saline. Formalin test and oxidative parameters were measured | Liposomal curcumin (2 mg/100 g) adjuvant with sumatriptan showed the most powerful potential for reducing nociception (↓ number of shakes and flinches), and it improved antioxidant potential (↑ TAC levels and serum thiol). ↓ NO, TOS, and MDA in all curcumin groups, especially liposomal curcumin (2 mg/100 g) | [120] |
| - | - | Curcumin Liposomal curcumin | In vivo | NTG-treated rats were divided into 6 groups and received either NTG only (control group), naproxen only (2.8 mg/kg), 2 different doses of curcumin (1 mg/100 g and 2 mg/100 g) along with naproxen, or 2 different doses of liposomal curcumin along with naproxen. In addition, the non-migraine control group received normal saline. Formalin test and oxidative parameters were measured | Combination of liposomal curcumin with naproxen showed enhanced anti-nociception and antioxidant potential. (↑ TAC levels and ↓ NO, TOS, and MDA in all curcumin-treated groups) | [121] |
| - | - | Cannabidiol | In vivo | Cannabidiol anti-migraine activity was tested in a CGRP-induced migraine mice model. Cephalic allodynia, anxiety-like behavior, spontaneous pain, and photophobia were determined | Cannabidiol could be used as an abortive treatment for migraine attacks. It reversed CGRP-evoked allodynia, decreased pain traits in female mice, and relieved anxiety in male mice. | [122] |
| - | - | Δ9-tetrahydrocannabinol (THC) | In vivo | The anti-migraine potential of THC was tested in rats using AITC-induced depression of wheel running. Rats were injected with 10 µL of 10% AITC or mineral oil in the dura mater, then immediately injected with either THC or vehicle at different doses: 0.1, 0.32, and 1 mg/kg | THC was effective in treating migraine-like pain when taken immediately after AITC at a dose of 0.32 mg/kg. The anti-migraine potential is related to CB1. | [123] |
| Feverfew | Flowering arial parts | Parthenolide isolated from the ethanolic extract | In vitro In vivo | Electrophysiologic recording, calcium imaging, and CGRP-like immunoreactivity assays were performed in vitro using human- and mouse-cultured neurons, cells, and tissues isolated from rats. Changes in meningeal blood flow and the eye wiping assay were used to detect nociceptive response; behavior testing and dural cannulation were evaluated in in vivo models using mice and rats. | Parthenolide may produce the anti-migraine activity by acting as a partial agonist to TRPA1, desensitizing the TRPA1 channel to any stimulus, which resulted in the inhibition of CGRP release. | [124] |
| Feverfew | - | Methanol combined with hexane, dichloromethane, and ether (standardized to (0.5% parthenolide w/w) | In vivo | Rats in treatment groups received either 100 mg/kg of FE or 100 mg/kg of FD and 15 mg/kg of purified parthenolide for 6 days followed by NTG injection, and they were compared with 3 groups that received the same medication without NTG. Control groups received either vehicle or NTG. The effect of extracts and purified parthenolide on NTG-induced Fos expression was measured as a marker for neural activation | Feverfew extracts enriched with parthenolide showed reduced Fos expression in the nucleus trigeminalis caudalis. Purified parthenolide inhibited neuronal activation in another brain nuclei and nuclear factor-kB. | [125] |
| Feverfew Salix alba | - | Water extract (standardized to (0.5% parthenolide w/w) and 15% salicin w/w) | Ex vivo In silico | The effect of combined feverfew and Salix alba extracts on serotonin, nitrites, and lactate dehydrogenase levels were determined in cortex tissue homogenates of mice via HPLC. A docking study of major detected metabolites; the caffeic acid and epicatechin to MAO-A enzyme assay was performed. | The combination of feverfew and Salix alba extracts showed a protective effect against migraines by reducing lactate, nitrites, and serotonin degradation. The prevention of serotonin depletion may be due to the inhibition of MAO-A enzyme that is responsible for serotonin catabolism using caffeic acid and epicatechin | [126] |
| Ficus deltoidea | Leaves | A standardized aqueous extract was obtained from Zach Biotech Sdn. Bhd, Malaysia. | In vivo | The anti-hyperalgesic behavior of F. deltoidea leaves’ aqueous extract (50, 100, and 200 mg/kg daily) and C-fos expression level in TNC were evaluated in NTG-induced mice. | F. deltoidea at 100 and 200 mg/kg showed a marked reduction in the nociceptive behaviors induced by NTG and inhibited C-Fos production. | [127] |
| Formula of Ayurvedic herbs (consisted of 8 plants, 10 g each) | Fruits of Terminalia bellerica, Terminalia chebula, Piper nigrum, and Emblica officinalis. Roots of Valerina villaichi and Premna Integrifolia. Tinospora cordifolia stem and Azadiracta indica bark | Hydro–alcoholic extract (50:50); yield is standardized to 6–8 gm. | In vivo | The migraine activity of the extract was assessed in mice using 3 models; morphine withdrawal caused hyperalgesia in the hot plate test; acetic acid-induced abdominal constrictions; and apomorphine induced climbing. | The Ayurvedic herb extract showed promising efficacy for anti-migraine activity by acting on dopamine. | [128] |
| Ginkgo biloba EGb 761® | Leaves | Standardized extract | In vivo | Rats were divided into 6 groups. The control, model, and sham operation groups received distilled water, and 3 groups received Gingko biloba extract at different doses of 25, 50, and 100 mg/kg for 1 week. Then, NTGs were injected subcutaneously into the gingko biloba and sham operation groups. | Gingo biloba could regulate nitric oxide, platelets aggregation rate, and 5-hydroxytryptamine levels during migraine attacks. | [129] |
| Ligusticum chuanxiong Hort. (LCH) | Edible herb | Aqueous extract | In vivo | The migraine activity of LCH aqueous extract was tested in NTG-induced rats. Cerebral blood flow was detected using laser speckle contrast imaging. Molecular changes were determined via immunofluorescence, RT-qPCR, and immunohistochemistry. | LCH could effectively reduce cerebral injury and migraine pain attack threshold by regulating glutamate, glucose metabolism, and Nrf-2 signals | [130] |
| Sapindus trifoliatus L. | Pericarp | Aqueous extract | In vitro | The aqueous extract of S. trifoliatus was tested in vitro to illustrate its action to the following receptors; HT1B/1D, 5-HT2B, GABA, Ca2+ channel, vanilloid receptor, and platelet serotonin release assay. | Sapindus trifoliatus showed a prophylactic effect against migraine via the mediation of the 5-HT2B receptor and the inhibition of serotonin release from platelets | [131] |
| Yufeng Ningxin tablet (YNT) | Pueraria lobata root | - | In vivo In vitro In silico | An in silico study using network pharmacology analysis. An in vivo model of NTG-induced rats was used to evaluate 3 doses of YNT (378, 576, and 1512 mg/kg, orally) for 10 days. Cortex and serum CGRP, 5-HT, IL-1β, NF-κB, and c-fos were determined. YNT extract was tested in vitro on LPS-induced NF-κB p65 expression in BV2 cells. | A network pharmacology analysis showed that the major anti-migraine targets for YNT constituents were 5-HT, NF-κB, CGRP, nociceptive factors, and inflammation. The in vivo study revealed that YNT could elevate 5-HT expression and decrease NF-κB, CGRP, IL-1β, and c-fos. YNT inhibited LPS-induced inflammation through NF-κB in BV2 cells. | [132] |
| Clinical data | ||||||
| Antemig®, PiLeJe tablets | - | Feverfew Coenzyme Q10 Magnesium | Clinical study | Patients received 1 tablet/day consisting of 100 mg of feverfew, 100 mg of coenzyme Q10, and 112.5 mg of magnesium, as well as 1.4 mg of vitamin B6 for 3 months; after 1 month, baseline period without treatment was established. | The consumption of Antemig® supplement at 1 tablet/day for 3 months exerted a prophylactic effect against migraine by reducing the number of attacks over the period of use. | [133] |
| Butterbur | Root | Extract standardized to at least 15% petasins | RCT | Patients (n = 33) received butterbur root extract at 50 mg/twice daily or placebo (n = 27) for 3 months. | Butterbur extract showed an effective role in migraine prophylaxis. The frequency of attacks reduced by 52.9% after 3 months of use. | [134] |
| Butterbur | Root | Extract standardized to at least 15% petasins | RCT | Patients (n = 245) were divided into 3 groups and received either butterbur extract 50 mg/twice daily, 75 mg/twice daily, or placebo for 4 months. | Butterbur extract at 75 mg/twice daily could reduce the frequency of migraine attacks in 68% of patients after 4 months of treatment. | [107] |
| Butterbur | Root | Extract standardized to at least 15% petasins | RCT | Patients (n = 108), children and adolescents (age 6–17 years), received 50 to 150 mg of butterbur root extract according to their age for 4 months. | Butterbur root extract showed an effective prophylactic effect against migraine in children and adolescents. A total of 77% of the patients showed a reduced number of migraine attacks by 50%. | [108] |
| Butterbur | Root | Extract standardized to at least 15% petasins | RCT | Patients (n = 58) were divided into 3 groups; each group received either butterbur root extract, music therapy, or placebo for 3 months. The frequency of attacks was assessed at 2- and 6-month post treatment. | The music group showed a reduction in the number of migraine attacks post treatment, while after 6 months, both the music therapy and butterbur root extract groups showed a prophylactic effect. | [135] |
| Curcuma | - | Curcumin supplements contained a standardized 95% turmeric extract in pellet form (475 mg curcuminoids, covering 70–80% curcumin, 15–20% dimethoxy curcumin and 2.5–6.5% bis-demethoxy curcumin) | RCT | Women patients (n = 44) with migraine received either 500 mg of curcumin twice/day or placebo for 2 months. Serum CGRP and IL-6, as well as pain severity, duration, and frequency were measured. | Curcumin showed a significant decrease in CGRP and IL-6, as well as in pain severity and duration. | [136] |
| - | - | Nano-curcumin | RCT | Patients diagnosed with episodic migraine (n = 40) were randomly assigned to receive 80 mg/day of nano-curcumin or placebo for 2 months. Gene expression and serum levels of TGF-b and IL-4 in PBMCs isolated from the migraine patients were determined via real-time PCR and ELISA, respectively. | The nano-curcumin intake showed group a significant increase in serum levels of IL-4, exerting anti-inflammatory effects, while no remarkable action was seen for serum levels and gene expression of TGF-b. | [137] |
| - | - | Phytosomal curcumin | RCT | Patients (n = 60) with migraine received either 250 mg/day phytosomal curcumin or maltodextrin as placebo for 2 months. NO, hs-CRP, and oxidative stress were measured | The intake of phytosomal curcumin at 250 mg/day for 2 months could relief symptoms of migraine and inhibit oxidative stress and inflammation. | [115] |
| - | - | Omega-3 fatty acids Nano-curcumin | RCT | Patients (n = 72) with episodic migraine were randomized into 4 groups: group 1 received 2 capsules/day of omega-3 (600 mg DHA, 1200 mg EPA and 200 mg other omega-3); group 2 received 1 capsule containing 80 mg of nanocurcumin/day; group 3 took 2 capsules of omega-3 and 1 capsule of nanocurcumin/day; group 4 received placebo. The gene expression and serum level of ICAM-1 were measured after 2 months of consumption. | The combination of omega-3 and nanocurcumin for 2 months showed a significant decrease in frequency of migraine attacks and serum level of ICAM-1, while no significant difference was seen in the gene expression of ICAM-1. | [116] |
| - | - | Omega-3 fatty acids Nano-curcumin | RCT | Patients (n = 80) with episodic migraine were randomized into 4 groups: group 1 received 2 capsules/day of omega-3 (600 mg DHA,1200 mg EPA and 200 mg other omega-3); group 2 received 1 capsule containing 80 mg of nanocurcumin/day; group 3 took 2 capsules of omega-3 and 1 capsule of nanocurcumin/day; group 4; received placebo. The gene expression and serum level of VCAM were measured after 2 months of consumption. | The combination of omega-3 and nanocurcumin for 2 months showed a significant decrease in gene expression and serum level of VCAM compared to the intake of omega-3 alone. No significant change was seen in patients who received nanocurcumin alone. | [118] |
| - | - | Omega-3 fatty acids Nano-curcumin | RCT | Patients with episodic migraine (n = 80) were randomized into 4 groups: group 1: 2500 mg/day of fish oil capsule and 80 mg/day of nano-curcumin; group 2: 2500 mg/day of fish oil capsule; group 3: 80 mg/day of nano-curcumin; group 4: paraffin oil placebo capsule. The serum level and gene expression of IL-1β were determined after 2 months of treatment. | The combination of omega-3 fatty acids and nano-curcumin showed a significant decrease in the frequency of migraine attacks and serum level of IL-1β, while a non-significant differences was seen in the gene expression of IL-1β. | [117] |
| - | - | Δ9-tetrahydrocannabinol (THC) Cannabidiol (CBD) | RCT | Patients (n = 92) were divided into 4 groups and received, for 4 separate acute migraine attacks, either vaporized 6% THC, 11% CBD; a combination of 6% THC + 11% CBD; and cannabis flower as placebo. | The combination of 6% THC and 11% CBD showed an effective relief of acute migraine pain after 2 h of intake and a sustained effect for 24 and 48 h. | [138] |
| Feverfew | Arial parts | 80% Ethanolic extract (standardized to 0.4% parthenolide) | RCT | Patients (n = 60) were divided into 2 groups. Both groups received 40 mg/day of propranolol in the form of a tablet and 10 drops of parthenolide (0.4%) or placebo for 3 months. The MIDAS questionnaire was used to evaluate the results. | Feverfew extract exerted a prophylactic effect against migraine. The frequency and severity of migraine attacks decreased by 61.62% and 56.2%, respectively, for 3 months. | [139] |
| - | - | Magnesium at 300 mg (1:1 ratio of magnesium citrate and oxide), riboflavin at 400 mg, and feverfew at 100 mg (standardized to 0.7% parthenolide) | RCT | Patients received a combination of feverfew at 100 mg, riboflavin at 400 mg, and magnesium at 300 mg for 3 months, while placebo group took 25 mg riboflavin daily. | A combination of feverfew, riboflavin, and magnesium showed a similar effect to the placebo. | [140] |
| GelStat Migraine® | - | Feverfew Ginger | Clinical study | Patients (n = 30) received 2 mL of feverfew/ginger sublingually when they felt mild pain | GelStat Migraine® could relieve mild pain after 2 h in 48% of the patients. | [99] |
| Ginger | - | A total of 500 mg/d of ginger (two capsules of 250 mg Zintoma (Gol Darou Co.) along with 500 mg/d of Depakene. | RDBCT | Patients (n = 80) were randomized into 2 groups. The treatment group received 2 ginger capsules (500 mg/day) orally and Depakene (500 mg/day of sodium valproate) for 4 months, while the control group received only Depakene at 500 mg/day. | Ginger intake at 500 mg/day adjunct to Depakene could relief the pain intensity of migraine compared to using Depakene alone. | [112] |
| Ginger | Rhizome | Standardized to 5% gingerols. | RCT | Patients (n = 103) were randomization into 2 groups. The treatment group received 500 mg/day ginger dry extract, and the control group received placebo starch for 3 months. | The ginger dry extract group showed reduced duration and severity of migraine attacks compared to placebo. No significant effect on the number of migraine attacks. | [111] |
| Ginger | - | Extract standardized to at least 5% gingerols | RCT | Participants with acute migraine were randomized into 2 groups that received 400 mg of ginger extract or placebo, and both received 100 mg of ketoprofen. | Ginger could be used as an adjuvant to non-steroidal anti-inflammatory drugs to relief acute migraine pain. | [110] |
| Ginger | - | Extract standardized to at least 5% gingerols | RCT | Patients (n = 107) with chronic migraine were randomized into treatment and placebo groups that received capsules (200 mg, 3 times/day) of ginger dry extract or placebo (cellulose) for 3 months. | Ginger showed no significant effect on migraine prophylaxis compared to the placebo. | [89] |
| Ginger | Rhizome | Extract standardized to at least 5% gingerols. | RDBCT | Patients (n = 100) with acute migraine without aura were randomly divided into 2 groups and received either 1 capsule containing 250 mg/day of ginger powder or 50 mg sumatriptan for 1 month. | The activity of ginger powder was similar to sumatriptan in relieving the pain of acute migraine without aura within 2 h of use. | [109] |
| Iaraj Fiqra tablet | Rose flower, cinnamon bark, ginger rhizome, Aloe vera, saffron flower, balsam incense, asaroon root, Pistacia lentiscus fruit, Commiphora opobalsamum balsam | - | RCT | Patients received a 500 mg/day Iaraj Fiqra tablet for 2 months. Headache was tested using the MIDAS HIT-6 test. | Iaraj Fiqra herbal supplements showed a significant decrease in duration, frequency, and severity of migraine attacks compared to placebo | [141] |
| Jodeungsan (JDS) (Granular product of herbal supplement consisted of 11 plants) | Uncariae Ramulus et Uncus Liriopis seu Ophiopogonis tuber, Pinelliae tuber, Poria Sclerotium, Ginseng radix, Saposhnikoviae radix, Citri unshius pericarp, Chrysanthemi Indici flower, Zingiberis rhizome, Glycyrrhizae Radix et rhizome, Gypsum Fibrosum | - | RCT | Patients (n = 32 at each group) were given 22.5 g/day of JDS or placebo for 28 days. | JDS showed no significant difference on chronic migraine in HAD compared to the control group. However, the MSQoL and HIT results exerted significant enhancements in both groups. | [142] |
| - | - | Flaxseed oil L-carnitine | RCT | Women patients with migraine were assigned into 2 groups (n = 40, each); placebo group and treatment group that received flaxseed oil (1000 mg/day) and L-carnitine (500 mg/day) for 3 months | Flaxseed oil and L-carnitine intake showed a marked reduction in migraine symptoms, oxidative stress, inflammation, and enhanced quality of life. | [143] |
| LipiGesicTMM | - | Feverfew Ginger | RCT | Patients (n = 45) took sublingual feverfew/ginger for 163 migraine attacks for 1 month. | The feverfew/ginger combination showed a relief of mild pain before the onset of severe or moderate pain after 2 h in 63% of the patients. | [98] |
| Migrasoll® | - | Ginkgo biloba terpenes phytosome (Ginkgolide B) Coenzyme Q 10 Vitamin B2 | Clinical study | Patients (n = 50) with migraine with aura received 120 mg of ginkgo biloba terpenes phytosome, 17.4 mg of vitamin B2, and 22 mg of coenzyme Q 10 daily for 4 months. | Ginkgolide B terpenes was effective in decreasing the duration and frequency of migraine attacks with aura after two months of use. | [144] |
| Migrasoll® | - | Ginkgo biloba terpenes phytosome (Ginkgolide B) Coenzyme Q 10 Vitamin B2 | Clinical study | Patients (n = 25) diagnosed with migraine with aura received 120 mg of ginkgo biloba terpenes phytosome, 17.4 mg of vitamin B2, and 22 mg of coenzyme Q 10 at the onset of second attack. | Ginkgolide B, the terpene fraction of ginkgo, showed a significant decrease in duration of neurological symptoms in 60% of the patients of acute migraine with aura, and the pain disappeared completely in 18% of the patients. | [145] |
| - | - | Ginkgolide B Riboflavin Coenzyme Q10 Magnesium | Clinical study | Children patients (n = 119; mean age 9.7 ± 1.42) suffering from migraine without aura received a combination of ginkgolide B, riboflavin, coenzyme Q10, and magnesium for 3 months | The combination of ginkgolide B, riboflavin, coenzyme Q10, and magnesium was effective as a prophylaxis treatment of childhood migraine without aura by reducing the frequency of attacks. | [146] |
| - | - | Ginkgolide B Coenzyme Q 10 Vitamin B2 Magnesium | Clinical study | Young patients (n = 30, age: 8–18) suffering from migraine without aura received 160 mg of ginkgolide B, 3.2 mg of vitamin B2, 40 mg of coenzyme Q, and 400 mg of magnesium daily for 3 months. | Ginkgolide B showed a prophylactic effect against migraine without aura in young patients. It could decrease the number of attacks and the regular use of analgesics. | [147] |
| MIG-99 (Soft gelatin capsule) | Feverfew (Flowering arial parts) | Supercritical CO2 extraction | RDBCT | The patients (n = 147) were divided into 3 groups, and each took a different dose of feverfew extract: 2.08 mg, 6.25 mg, and 18.75 mg 3 times/daily for 12 weeks. The efficacy of the treatment during the last 4 weeks were compared to the 4-week baseline period before starting the treatment. | MIG-99 did not show a significant prophylactic effect. Feverfew extract (6.25 mg 3 times/daily) could reduce the number of migraine attacks in a small group of patients that received at least 4 attacks/month. | [97] |
| MIG-99 | Feverfew (Flowering arial parts) | Supercritical CO2 extraction | RCT | Patients (n = 170) were divided into 2 groups and received either 18.75 mg of feverfew extract or placebo for 4 months after 1 month at baseline. | Feverfew was effective in the prophylaxis of migraine using 18.75 mg/day for 4 months. It could reduce the number of attacks by 1.9/month compared to baseline. | [148] |
| MYRLIQ® | - | Ginkgo biloba L. Vitamin B2 Coenzyme Q10 | Clinical pilot study | Patients suffering from migraine headache without aura or tension-type headache received a combination of Ginkgo biloba, vitamin B2, and coenzyme Q10 at the start of headache attack for 6 months. | The natural combination of Ginkgo biloba, vitamin B2, and coenzyme Q10 showed analgesic and anti-inflammatory activity. It could relief the pain in 75% of the patients and reduce inflammatory markers such as TNF-α, IL6, and IL-8. | [149] |
| Neurasites® (A standardized extract of butterbur) | - | Butterbur (Petasites hybridus) 150 mg contains at least 7.5% petasin | Clinical Study | Migraine activity was assessed through the Neurasites® Questionnaire Survey (NQS) in 85 patients assigned in 2 groups; placebo and Neurasites®-based treatment, using imigran as positive controls for migraine with aura and topiramate for chronic migraine. | Neurasites® could effectively reduce the intensity of pain, the duration of headache attacks, and the numbers of migraine episodes. | [150] |
| Petadolex® | Rhizome | CO2 extract, standardized to at least 15% petasins. | RDBCT | Patients (n = 60) received 2 Petadolex capsules (each containing 25 mg of butterbur extract) twice daily or placebo for 3 months. | Butterbur showed a prophylactic effect against migraine by reducing the number of migraine attacks by 60% | [106] |
| Computational data | ||||||
| - | - | Asarinin | In silico | Molecular docking of asarinin with CGRP receptor, molecular dynamic assessment, MMGBSA analysis, and network analysis were used to compared the anti-migraine drug Atogepant | Asarinin was considered a promising anti migraine candidate. It showed good binding affinity (−10.3 kcal/mol) with CGRP receptor (PDB: 6ZHO). Network analysis highlighted the major proteins that interacted with the targets of migraine with aura such as OPRM1, GNB1, and GNAS | [151] |
| Ginger | - | 98% Ethanol | In silico | The anti-migraine activity of ginger constituents detected via GC-MS was evaluated using molecular docking and compared to the anti-migraine drug Telcagepant. | Among 28 tested compounds; Estra-1,3,5(10)-trien-17-beta-ol showed the best binding scores (6.8 and −8.8 1 Kcalmol−1) in CGRP (PDB:3n7r) and human NK1 tachykinin receptors (PDB:6e59), respectively, which are very close to the control Telcagepant | [152] |
AITC; allyl isothiocyanate, CB1; cannabinoid receptor 1, CGRP; calcitonin gene-related peptide, FD; feverfew-depleted extract of parthenolide, feverfew-enriched parthenolide, GNAS; gene protein subunit alpha S, GNB1; gene protein subunit beta 1, HAD; headache attack days, HIT; headache impact test, HPLC; high-performance liquid chromatography, hs-CRP; high-sensitivity C-reactive protein, 5-HT; 5-hydroxytryptamine, ICAM-1; intercellular adhesion molecule-1, IL; interleukin, LPS; lipopolysaccharides, MAO-A; monoamineoxxidase-A, MDA; malondialdehyde, MIDAS; migraine disability assessment, MSQoL; migraine-specific quality of life, NF-κB; nuclear factor kappa, Nrf-2; nuclear factor erythroid 2-related factor 2, NO; nitric oxide, NTG; nitroglycerin, OPRM1; opioid receptor Mu 1, PBMCs; peripheral blood mononuclear cells, RDBCT; randomized double-blind clinical trial, RCT; randomized double-blind, placebo-controlled clinical study, TGF-b; Transforming growth factor beta, TNF-α; tumor necrosis factor alpha, TOS; total oxidative capacity, TRPA1; transient receptor potential ankyrin 1, TRPV1; transient receptor potential cation channel subfamily V member 1, known as vanilloid receptor 1 and capsaicin receptor, TNC; trigeminal nucleus caudalis, VCAM; vascular cell adhesion molecule.
7.3. Complementary (Non-Pharmacological) Approaches
Aromatherapy, dietary supplements, and lifestyle modifications are increasingly recognized as effective complementary treatments for migraines, as illustrated in Figure 6.
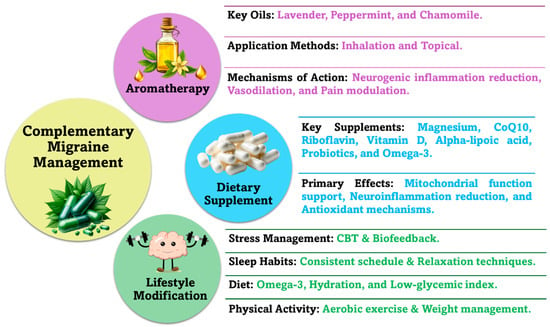
Figure 6.
Complementary (non-pharmacological) approaches in migraine management.
7.3.1. Migraine and Aromatherapy
Aromatherapy, the use of high-quality plant-based essential oils through inhalation or topical application, is increasingly recognized as a complementary approach for managing migraines [153,154]. This practice offers an alternative to conventional pharmacological treatments, which are often accompanied by side effects and contraindications [155]. Essential oils contain bioactive compounds such as terpenoids, esters, and aldehydes, which contribute to their therapeutic effects [156]. Specifically, research has demonstrated that essential oils can alleviate migraine symptoms such as nausea, photophobia, and phonophobia by modulating neurotransmitter levels, reducing neurogenic inflammation, and promoting vascular relaxation. For instance, lavender oil’s topical application to the temples has been associated with reflex actions that relax blood vessels and reduce pain perception, highlighting its unique mechanism of action [155].
The method of essential oil application plays a crucial role in optimizing therapeutic effects. Topical application, such as to the temples or forehead, utilizes the oils’ skin penetration properties, while inhalation leverages their volatility to activate sensory pathways. The choice of application method depends on the oil’s properties and the patient’s tolerance to optimize therapeutic effects [154]. For example, gentle oils like lavender are often applied directly to the skin, while oils such as peppermint are usually diluted in a carrier oil to appropriate concentrations to prevent skin irritation. Additionally, some essential oils are formulated into suitable pharmaceutical forms, like gels, for targeted application. Inhalation methods can accommodate flexible concentrations, ranging from 1% to 100%, depending on the enclosed space, making them adaptable to individual needs [155].
Several essential oils were evaluated for their effectiveness in managing migraines. Essential oils such as lavender, peppermint, chamomile, anise, basil, rose, and a mixed blend of lavender and grape seed oil demonstrated promising outcomes as essential oils aromatherapy in reducing migraine intensity, frequency, and symptoms like nausea, photophobia, and phonophobia. These oils primarily work through mechanisms targeting vasodilation, the inhibition of neurogenic inflammation, and a reduction in central pain sensitization, offering multifaceted relief in both experimental and clinical settings [157].
Lavender Oil
Lavender oil, one of the most extensively studied essential oils in aromatherapy, has shown significant effectiveness in anti-migraine clinical and animal studies. It mainly contains linalool and linalyl acetate as its main active constituents. Clinical trials involved patients applying 2–3 drops of lavender oil to their upper lips for inhalation at the onset of migraine symptoms, while animal models used inhalation at concentrations of 1%, 10%, and 100% in controlled environments. Both human and animal studies found significant reductions in headache severity, nausea, and sensitivity to light and sound within two hours of inhalation. Lavender’s active components, such as linalool and linalyl acetate, contribute to its effects by relaxing blood vessels, inhibiting neurogenic inflammation, and potentially modulating neurotransmitter activity. This combination of actions makes lavender oil a valuable option for managing acute migraine symptoms [155,158].
Peppermint Oil
Peppermint oil has also been widely researched for its anti-migraine properties. In clinical settings, peppermint oil diluted to 1.5% was applied to the nostrils during the onset of migraines. Patients experienced relief within five minutes, with notable reductions in both headache intensity and frequency like the lidocaine group [159]. The rapid action of peppermint oil is largely attributed to menthol, a key compound that activates TRPM8 receptors, producing a cooling sensation that helps modulate pain and inflammation. Additionally, peppermint oil promotes vasodilation, making it highly effective in managing acute migraine symptoms [160].
Basil Oil
Basil oil has been studied in clinical trials where it was applied topically on the forehead and temples in concentrations of 2%, 4%, and 6%. Over the study period, patients experienced gradual declines in migraine intensity and attack frequency. The oil’s active components, such as estragole and eugenol, are thought to contribute to basil’s therapeutic effects by modulating blood flow and reducing vascular tension. Through a combination of vasodilation and anti-inflammatory actions, basil oil offers both immediate and preventive migraine relief [161].
Chamomile Oil
Chamomile oil has been examined for its potential to relieve migraine symptoms when applied topically. In clinical trials, a 5.5% chamomile essential oil gel was applied to the temples, forehead, and areas behind the ears. Within 30 min, patients reported a decrease in pain intensity and relief from associated symptoms such as nausea, photophobia, and phonophobia. The oil’s active components, including chamazulene and bisabolol, are known for their anti-inflammatory properties, probably helping to inhibit neurogenic inflammation and reduce pain sensitization. Chamomile oil’s capacity to alleviate a broad range of migraine symptoms makes it especially useful for holistic migraine management [162].
Anise Oil
Anise oil, used in aromatherapy, has also shown promise in clinical trials, where a 7% anise essential oil cream was applied to the temples and forehead during migraine episodes. Routine application led to a reduction in migraine frequency from an average of 3.45 episodes per week to 1.89, and it decreased the average duration of migraines by approximately twelve hours [163]. The active compound anethole has notable anti-inflammatory properties, targeting the neurogenic inflammation associated with migraine pain. Anethole is structural similarity to dopamine, potentially allowing it to act as a dopamine receptor antagonist, thereby interrupting the dopamine-related cascade that induces migraines [164]. Also, anise oil’s preventive effects make it valuable for those experiencing frequent migraines.
Rose Oil
Rose oil, extracted from Rosa damascena, has shown particular effectiveness in managing “hot” migraines, which are characterized by symptoms like facial heat and red eyes. Clinical studies involved applying rose oil to the forehead and temples with a formulation derived from soaking rose petals in 20% sesame oil. This treatment significantly reduced pain intensity in patients with hot migraine syndrome. Rose oil’s active compounds, citronellol and geraniol, are believed to calm vascular responses and reduce inflammation, making it well suited for migraines with pronounced vasodilation-related symptoms [165].
Lavender Oil and Grape Seed Oil
A mixed essential oil blend of lavender and grape seed oil has shown promising results for migraine relief in aromatherapy. In clinical settings, this blend, formulated with a 3:2:10 ratio of lavender to grape seed to base oil, was applied to the forehead, face, and neck. Over a 20-day period, most patients reported complete symptom relief, with some experiencing longer intervals between migraine attacks. This combination is effective due to the synergy of lavender’s anti-inflammatory and vasodilatory properties with grape seed oil’s antioxidant effects, providing comprehensive relief from both migraine pain and its associated symptoms [155,157].
7.3.2. Migraine and Dietary Supplements
Dietary supplements are increasingly recognized as an effective complementary approach to managing migraines, much like aromatherapy. Oxidative stress and low antioxidant intake are significant risk factors for migraines, as they can trigger inflammation and neuroinflammatory responses. Research shows that supplements targeting mitochondrial function, neuroinflammation, brain energy metabolism, and vascular health can help reduce migraine frequency and severity. Nutrients such as Coenzyme Q10, riboflavin, omega-3 fatty acids, magnesium, alpha-lipoic acid, and probiotics have shown promising effects by addressing deficiencies or physiological imbalances linked to migraine pathogenesis, helping to stabilize cellular energy, reduce neuroinflammation, and regulate pathways involved in migraine attacks [166].
Magnesium
Magnesium, the fourth most abundant cation in the body, is crucial for nerve function, enzyme activity, DNA and protein synthesis, and maintaining the electrical potential of neurons. It is found mainly in leafy green vegetables as well as in nuts and seeds. Deficiency in magnesium, due to inadequate intake, excessive loss, or cellular redistribution, is a risk factor for migraines, potentially due to its impact on neurotransmission and vascular health [166]. Magnesium deficiency has been linked to migraine pathogenesis through mechanisms such as platelet aggregation, glutamate release, and decreased mitochondrial function, which promote the formation of vasoconstrictive agents like 5-hydroxytryptamine (5-HT), and they are often associated with migraine onset [167,168]. A U.S. National Health and Nutrition Study (including data from 3626 participants) and research by Assarzadegan et al. both reported significantly lower serum magnesium levels in individuals with migraines compared to healthy controls [169,170]. Clinical studies also highlight the effectiveness of magnesium supplementation in migraine management. Chiu et al. found that intravenous magnesium quickly alleviated acute migraine symptoms, while oral magnesium reduced attack frequency and intensity [90]. Moreover, Khani et al. reported that combining magnesium with sodium valproate was more effective than either treatment alone, lowering attack frequency, intensity, and medication requirements [171]. Given the prevalence of magnesium deficiency in migraine sufferers, magnesium supplementation, especially as adjunct therapy, holds potential for enhancing migraine treatment outcomes.
Coenzyme Q10
Coenzyme Q10 (CoQ10) is a natural antioxidant crucial for mitochondrial energy production and cellular respiration functioning within the electron transport chain. Known for its benefits in conditions like cardiovascular disease, fibromyalgia, and neurodegenerative disorders, CoQ10 may also help prevent migraines [166]. Migraine has been associated with mitochondrial dysfunction, specifically energy deficits affecting oxidative phosphorylation, which can disrupt vascular tone and promote oxidative stress. CoQ10 supports mitochondrial function, reduces inflammation, and has shown negative associations with inflammatory markers, which may influence migraine pathogenesis [172]. Several studies highlight CoQ10’s potential for migraine management. A clinical trial demonstrated that 400 mg/day of CoQ10 for three months reduced headache severity, frequency, and duration, as well as inflammatory markers like tumor necrosis factor-α (TNF-α) and calcitonin gene-related peptide (CGRP) [173]. Shoeibi et al. found that 100 mg/day of CoQ10 reduced migraine attack severity and frequency [174], while other trials noted improvements in oxidative stress markers and HDL cholesterol in migraine patients receiving 400 mg/day of CoQ10 [175]. A meta-analysis of six randomized trials confirmed CoQ10’s efficacy in lowering attack frequency, with enhanced effects when used as adjunct therapy [176]. Although evidence supports CoQ10’s safety and efficacy in reducing migraine symptoms, further studies are recommended to confirm its optimal dosing and effects in combination therapies.
Riboflavin
Riboflavin (vitamin B2) is a water-soluble vitamin that converts vitamins into the cofactors flavin mononucleotide (FMN) and flavin–adenosine dinucleotide (FAD), both essential for flavoenzymes in the electron transport chain, which is vital for cellular energy production [91]. Riboflavin also has anti-inflammatory, antioxidant, anti-nociceptive, and anti-aging properties [166]. It is primarily found in dairy products and green vegetables, although its structure is not susceptible to cooking heat, and it can be degraded by light. Riboflavin deficiency, affecting about 10–15% of the population due to genetic issues, is associated with neuroinflammation and conditions like migraines [177,178]. Several studies have demonstrated the effectiveness of riboflavin supplementation in preventing migraines. High doses (400 mg/day) have shown reductions in attack frequency, duration, severity, and the use of anti-migraine medications [91]. Even low doses (100 mg/day) were found to be as effective as propranolol, a common migraine treatment [179]. Additionally, riboflavin’s effects were comparable to sodium valproate (500 mg/day) for migraine prophylaxis [180]. Based on these findings, riboflavin is considered a promising option for migraine prevention, with supplementation for at least 12 weeks being beneficial [179]. Further research is needed to determine the optimal dosage and duration.
Vitamin D
Vitamin D, a fat-soluble vitamin primarily synthesized in the skin through sunlight exposure and obtained from dietary sources like fatty fish, fortified foods, and supplements, is crucial for calcium absorption, bone health, and various cellular processes. Its active form, calcitriol, binds to nuclear vitamin D receptors (nVDRs) in the central nervous system, modulating neuroplasticity, oxidative stress, calcium homeostasis, immune responses, and neurotrophic factor production [166]. Vitamin D deficiency, affecting 30–80% of the global population, has been linked to neurodegenerative and neurological conditions, including migraines [166,181]. Studies demonstrate a connection between vitamin D deficiency and increased migraine risk. Rapisarda et al. found that migraine patients often exhibit severe vitamin D deficiency, with an inverse relationship between serum vitamin D levels and headache frequency [182]. Clinical trials, including those by Mottaghi et al. and Ghorbani et al., reveal that vitamin D supplementation (e.g., 50,000 IU weekly or 2000 IU daily) significantly reduces migraine frequency, severity, and associated inflammatory markers like IL-6 and iNOS [183,184]. Hu et al.’s meta-analysis confirms vitamin D’s efficacy in lowering headache days and disability scores, though its effects on attack duration and intensity remain inconsistent [185]. While promising, further studies are needed to solidify vitamin D’s role in migraine management and establish optimal dosing regimens.
Alpha-Lipoic Acid
Alpha-lipoic acid (α-LA) is an organosulfur antioxidant synthesized in the mitochondria, and it is essential in energy metabolism and antioxidant defense. Acting as a cofactor in enzyme complexes, α-LA supports mitochondrial function and has high potential in neutralizing ROS and RNS, which are elevated in migraines due to oxidative stress and mitochondrial dysfunction. This imbalance, involving damaged cellular components such as DNA, proteins, and lipids, is a key factor in migraine pathogenesis [166]. Several studies highlight α-LA’s potential in migraine management. Eren et al. found a significant correlation between low thiol levels and migraine severity, suggesting that thiol-containing compounds like α-LA may offer therapeutic benefits [186]. Gross et al. reported abnormally low serum α-LA levels in migraine patients, indicating it as a potential biomarker for prevention and treatment [187]. Clinical trials support these findings. Rezaei et al. observed that 300 mg/day of α-LA for three months reduced markers of oxidative stress and inflammation, including MDA and CRP in women [188]. Cavestro et al. found that 400 mg/day of α-LA over six months reduced headache days in migraine patients with insulin resistance [189], while Magis et al. showed that 600 mg/day over three months reduced migraine frequency and severity [190]. Despite the positive results, further studies are needed to confirm optimal dosing and its mechanisms, though current evidence suggests α-LA’s safety and efficacy in reducing oxidative stress and improving symptoms associated with migraine.
Probiotics
Probiotics, often derived from fermented foods or supplements, are live microorganisms that support health by enhancing gut microbiota balance. Psychobiotics, a class of probiotics, specifically benefit brain function by affecting neuronal pathways through the gut–brain axis. Migraine, a neurovascular condition often linked to gut dysbiosis, appears to be influenced by interactions between the gut and the brain via neural, immune, and endocrine pathways. Research highlights that gut microbiota imbalance can contribute to inflammation and increase intestinal permeability, allowing pro-inflammatory substances to enter the bloodstream and activate trigeminal pathways, potentially triggering migraine attacks. Stress-induced changes in the hypothalamic–pituitary–adrenal axis, which elevate cortisol and weaken gut barrier integrity, are additional factors [166]. Martami et al. found that daily probiotic supplements reduced migraine frequency, severity, and medication use in both episodic and chronic migraine patients, although attack duration decreased only in chronic cases [191]. Studies by Xie et al. and Ghavami et al. also demonstrated that probiotics, especially when paired with an elimination diet or combined with prebiotics, decreased migraine attacks, medication use, and inflammation markers like zonulin and Hs-CRP [192,193]. However, inconsistent results across studies, likely due to varying probiotic formulations and microbial strains, highlight the need for further large-scale trials to confirm probiotics’ role in migraine management.
Omega-3
Omega-3 polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid and docosahexaenoic acid, play an important role in managing migraine. Using rich sources of these omega-3s like fish oil increases the PUFA content, which exhibit anti-inflammatory and neuroprotective mechanisms. These fatty acids reduce neuroinflammation by suppressing cytokine production, inhibiting NF-κB activity via lipopolysaccharide (LPS) receptors, and decreasing the production of NO and ROS in active microglia. They also influence transcription factors, intracellular signaling pathways, and gene expression, contributing to their antinociceptive effects [166]. Clinical studies support these mechanisms, and a recent randomized controlled trial (RCT) demonstrated that supplementing a diet with 1.8 g/day of eicosapentaenoic acid over 12 weeks reduced attack severity, frequency, and associated disability while improving quality of life [194]. Another study found that combining 180 mg/day of fish oil with sodium valproate was more effective than the medication alone in reducing attack frequency [195]. Diets rich in omega-3 and low in omega-6 further enhanced pain relief and antinociceptive lipid mediator levels [196]. Although omega-3 supplementation has shown benefits in reducing migraine attack frequency and improving quality of life, further research is needed to determine optimal dosages and treatment protocols.
7.3.3. Migraine and Lifestyle
Migraine imposes a significant burden on patients, affecting daily functioning, productivity, and overall well-being. While pharmacological treatment is essential, lifestyle modifications have emerged as critical, non-pharmacological strategies to reduce migraine frequency and severity. These changes target modifiable risk factors such as the overuse of acute migraine medications, metabolic syndrome, obesity, depression, and stress. [51]. Figure 7 summarizes evidence-based lifestyle strategies, including dietary habits, physical activity, sleep optimization, and stress management, which have been shown to alleviate migraine symptoms and enhance quality of life.
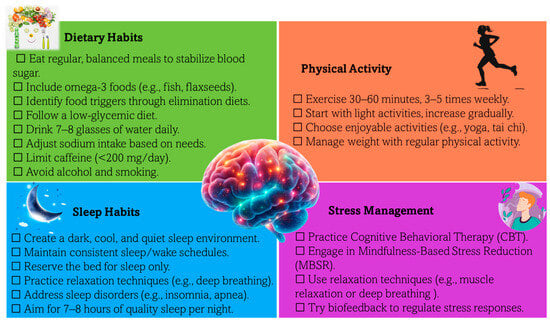
Figure 7.
Checklist of lifestyle modifications for effective migraine management. This visual summary highlights evidence-based lifestyle changes, including dietary, sleep, physical activity, and stress management strategies, aimed at reducing migraine frequency, severity, and overall burden while enhancing quality of life.
Dietary Habits
Balanced meals, omega-3 fatty acids, hydration, and caffeine management play a pivotal role in stabilizing blood sugar and preventing triggers. Elimination diets and low-glycemic food choices offer personalized approaches to migraine prevention [51,52]. Specific details on dietary recommendations are illustrated in Figure 7.
Sleep Optimization
Maintaining a consistent sleep schedule, optimizing the sleep environment, and addressing disorders like insomnia or apnea are essential for migraine management. Aim for 7–8 h of quality sleep per night to reduce attack frequency and severity [51,52].
Physical Activity
Regular exercise, such as aerobic activity or yoga, not only helps reduce migraine frequency and intensity but also supports weight management, a key factor in mitigating risk.
Stress Management
Techniques such as cognitive behavioral therapy (CBT), mindfulness-based stress reduction (MBSR), relaxation techniques, and biofeedback are proven to decrease migraine-related anxiety and stress [51,52,197,198,199,200].
Together, these modifications represent sustainable, low-cost approaches to migraine management. For a detailed, actionable checklist of recommendations, refer to Figure 7, which provides an easy-to-follow guide for patients and practitioners alike.
8. Future Prospectives
Embracing a personalized approach by tailoring natural supplement regimens to individual patient profiles and incorporating genetic predispositions, lifestyle factors, and comorbidities, holds the key to optimizing treatment efficacy and outcomes in migraine management. To substantiate the effectiveness and safety of natural supplements, further research through well-designed clinical trials and longitudinal studies is imperative. This robust evidence will not only guide clinical decision-making but also elevate the standard of patient care. By exploring synergies among natural supplements, prescription medications, behavioral therapies, and other non-pharmacological interventions, a novel and comprehensive treatment landscape can be unveiled for migraine sufferers. Empowering patients with comprehensive knowledge about natural supplements, their mechanisms of action, and potential benefits is crucial for informed decision-making and active involvement in their treatment plans. Collaborative efforts among diverse healthcare providers, including neurologists, primary care physicians, and complementary medicine practitioners, are essential to establish a holistic and integrated approach to migraine care, ensuring comprehensive support for patients on their healing journey. Establishing clear guidelines and standards for the quality, purity, and labeling of natural supplements will further bolster consumer safety and confidence in their utilization as adjunct therapies for migraine relief. By embracing these forward-looking perspectives and nurturing interdisciplinary collaboration, the field of migraine management is poised for a transformative evolution, offering innovative and patient-centered strategies that effectively address the intricate nature of migraines while enhancing overall care quality and patient outcomes.
9. Conclusions
By adopting a broader, more integrative perspective than systematic reviews, this review bridges traditional pharmacological insights with emerging evidence on natural therapies, aiming to deepen the understanding of migraine management, guide clinical practice and future research, and empower individuals to manage their condition more effectively. While prescription medications remain cornerstone interventions, the integration of natural supplements offers a personalized and comprehensive strategy that may address gaps in current treatment paradigms. Non-pharmacological approaches, such as lifestyle modifications and dietary habits, are increasingly recognized as critical components of migraine management.
Author Contributions
F.A.-E., H.E., D.K.H., R.A.E.-S. and A.M.E.-D.: Conceptualization, methodology, software, formal analysis, investigation, writing—original draft preparation, and writing—review and editing. A.A., E.M.Y. and H.M.R.M.S.: writing—review and editing and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available within the manuscript.
Acknowledgments
The authors would like to express sincere gratitude to Alfaisal University, Riyadh, Saudi Arabia, for its scientific support of this research. The authors would like to express sincere gratitude to Almaarefa University, Riyadh, Saudi Arabia, for its scientific support of this research. The authors would like to express sincere gratitude to King Saud bin Abdulaziz University for Health Sciences, for its scientific support. The authors would like to express sincere gratitude to King Abdullah International Medical Research Center, for its scientific support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, Y.; Cao, Y. Rising trends in the burden of migraine and tension-type headache among adolescents and young adults globally, 1990 to 2019. J. Headache Pain 2023, 24, 94. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Katsarava, Z.; Do, T.P.; Buse, D.C.; Pozo-Rosich, P.; Özge, A.; Krymchantowski, A.V.; Lebedeva, E.R.; Ravishankar, K.; Yu, S. Migraine: Epidemiology and systems of care. Lancet 2021, 397, 1485–1495. [Google Scholar] [CrossRef]
- Peres, M.F.P.; Sacco, S.; Pozo-Rosich, P.; Tassorelli, C.; Ahmed, F.; Burstein, R.; Ashina, S.; Uluduz, D.; Husøy, A.K.; Steiner, T.J. Migraine is the most disabling neurological disease among children and adolescents, and second after stroke among adults: A call to action. Cephalalgia 2024, 44, 03331024241267309. [Google Scholar] [CrossRef]
- Sahithi, A.S.; Muthu, T.; Saraswathy, R. Migraine: Update and future perspectives. Int. J. Nutr. Pharmacol. Neurol. Dis. 2020, 10, 179–187. [Google Scholar]
- Loder, E.; Renthal, W. Calcitonin gene-related peptide monoclonal antibody treatments for migraine. JAMA Intern. Med. 2019, 179, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J. Headache classification committee of the international headache society (IHS) the international classification of headache disorders. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Song, X.; Zhu, Q.; Su, L.; Shi, L.; Chi, H.; Yan, Y.; Luo, M.; Xu, X.; Liu, B.; Liu, Z. New perspectives on migraine treatment: A review of the mechanisms and effects of complementary and alternative therapies. Front. Neurol. 2024, 15, 1372509. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, M.; Fu, F.; Nie, L.; Miao, Q.; Zhao, L.; Liu, L.; Li, B. Mechanism of Traditional Chinese Medicine in Treating Migraine: A Comprehensive Review. J. Pain Res. 2024, 17, 3031–3046. [Google Scholar] [CrossRef]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the trigeminal system in migraine. Headache J. Head Face Pain 2019, 59, 659–681. [Google Scholar] [CrossRef]
- Zagami, A.S.; Edvinsson, L.; Goadsby, P.J. Pituitary adenylate cyclase activating polypeptide and migraine. Ann. Clin. Transl. Neurol. 2014, 1, 1036–1040. [Google Scholar] [CrossRef]
- Dodick, D.W.; Goadsby, P.J.; Schwedt, T.J.; Lipton, R.B.; Liu, C.; Lu, K.; Yu, S.Y.; Severt, L.; Finnegan, M.; Trugman, J.M. Ubrogepant for the treatment of migraine attacks during the prodrome: A phase 3, multicentre, randomised, double-blind, placebo-controlled, crossover trial in the USA. Lancet 2023, 402, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Buse, D.C.; Ashina, H.; Pozo-Rosich, P.; Peres, M.F.; Lee, M.J.; Terwindt, G.M.; Singh, R.H.; Tassorelli, C.; Do, T.P. Migraine: Integrated approaches to clinical management and emerging treatments. Lancet 2021, 397, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, S.; Wang, Y. Role of herbal medicine for prevention and treatment of migraine. Phytother. Res. 2022, 36, 730–760. [Google Scholar] [CrossRef] [PubMed]
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine: Epidemiology, Burden, and Comorbidity. Neurol. Clin. 2019, 37, 631–649. [Google Scholar] [CrossRef]
- Woldeamanuel, Y.W.; Cowan, R.P. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants. J. Neurol. Sci. 2017, 372, 307–315. [Google Scholar] [CrossRef]
- Piccininni, M.; Brinks, R.; Rohmann, J.L.; Kurth, T. Estimation of migraine prevalence considering active and inactive states across different age groups. J. Headache Pain 2023, 24, 83. [Google Scholar] [CrossRef]
- Understanding Migraine Phases. Available online: https://americanmigrainefoundation.org (accessed on 15 October 2024).
- Dodick, D.W. A phase-by-phase review of migraine pathophysiology. Headache J. Head Face Pain 2018, 58, 4–16. [Google Scholar] [CrossRef]
- Viana, M.; Tronvik, E.A.; Do, T.P.; Zecca, C.; Hougaard, A. Clinical features of visual migraine aura: A systematic review. J. Headache Pain 2019, 20, 1–7. [Google Scholar] [CrossRef]
- Cipolla, M. The Cerebral Circulation, Morgan and Claypool Life Sciences; Morgan Claypool: San Rafael, CA, USA, 2009. [Google Scholar]
- Lv, X.; Wu, Z.; Li, Y. Innervation of the cerebral dura mater. Neuroradiol. J. 2014, 27, 293–298. [Google Scholar] [CrossRef]
- Edvinsson, L.; Goadsby, P. Neuropeptides in migraine and cluster headache. Cephalalgia 1994, 14, 320–327. [Google Scholar] [CrossRef]
- Fontaine, D.; Almairac, F.; Santucci, S.; Fernandez, C.; Dallel, R.; Pallud, J.; Lanteri-Minet, M. Dural and pial pain-sensitive structures in humans: New inputs from awake craniotomies. Brain 2018, 141, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.S.; Wolff, H.G. Experimental studies on headache: Pain-sensitive structures of the head and their significance in headache. Arch. Surg. 1940, 41, 813–856. [Google Scholar] [CrossRef]
- Puledda, F.; Silva, E.M.; Suwanlaong, K.; Goadsby, P.J. Migraine: From pathophysiology to treatment. J. Neurol. 2023, 270, 3654–3666. [Google Scholar] [CrossRef] [PubMed]
- Deniz, O.; Aygül, R.; Koçak, N.; Orhan, A.; Kaya, M.D. Precipitating factors of migraine attacks in patients with migraine with and without aura. Pain Clin. 2004, 16, 451–456. [Google Scholar] [CrossRef]
- Mateos, V.; Guerrero-Peral, Á.L.; García, M.; Armengol-Bertolin, S.; Plazas, M. Precipitating factors of migraine attacks in patients attended in neurology services. The FACTOR study. Rev. Neurol. 2012, 54, 641–648. [Google Scholar]
- Wöber, C.; Brannath, W.; Schmidt, K.; Kapitan, M.; Rudel, E.; Wessely, P.; Wöber-Bingöl, C. Prospective Analysis of Factors Related to Migraine Attacks: The PAMINA Study. Cephalalgia 2007, 27, 304–314. [Google Scholar] [CrossRef]
- Fukui, P.T.; Gonçalves, T.R.T.; Strabelli, C.G.; Lucchino, N.M.F.; Matos, F.C.; Santos, J.P.M.; Zukerman, E.; Zukerman-Guendler, V.; Mercante, J.P.P.; Masruha, M.R.; et al. Trigger factors in migraine patients. Arq. Neuro-Psiquiatr. 2008, 66, 494–499. [Google Scholar] [CrossRef]
- Ducros, A. Familial hemiplegic migraine: A model for the genetic studies of migraine. Cephalalgia 2014, 34, 1035–1037. [Google Scholar] [CrossRef]
- Pietrobon, D.; Moskowitz, M.A. Pathophysiology of migraine. Annu. Rev. Physiol. 2013, 75, 365–391. [Google Scholar] [CrossRef]
- Gormley, P.; Anttila, V.; Winsvold, B.S.; Palta, P.; Esko, T.; Pers, T.H.; Farh, K.-H.; Cuenca-Leon, E.; Muona, M.; Furlotte, N.A. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat. Genet. 2016, 48, 856–866. [Google Scholar] [CrossRef]
- Martin, V.T.; Behbehani, M.M. Ovarian Hormones and Migraine Headache: Understanding Mechanisms and Pathogenesis—Part I. Headache: J. Head Face Pain 2006, 46, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.C.; Peterlin, B.L.; Calhoun, A.H. Migraine and estrogen. Curr. Opin. Neurol. 2014, 27, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.A.H.; Maitrot-Mantelet, L.; Plu-Bureau, G.; Gompel, A. Migraine, hormones and the menopausal transition. Climacteric 2018, 21, 256–266. [Google Scholar] [CrossRef]
- Berry, J.K.M. How Does Stress Induce Headache? Ph.D. Thesis, Murdoch University, Murdoch, Australia, 2020. [Google Scholar]
- Amiri, P.; Kazeminasab, S.; Nejadghaderi, S.A.; Mohammadinasab, R.; Pourfathi, H.; Araj-Khodaei, M.; Sullman, M.J.M.; Kolahi, A.-A.; Safiri, S. Migraine: A review on its history, global epidemiology, risk factors, and comorbidities. Front. Neurol. 2022, 12, 800605. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.R. Stress and primary headache: Review of the research and clinical management. Curr. Pain Headache Rep. 2016, 20, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peterlin, B.L.; Sacco, S.; Bernecker, C.; Scher, A.I. Adipokines and migraine: A systematic review. Headache J. Head Face Pain 2016, 56, 622–644. [Google Scholar] [CrossRef]
- Scher, A.; Terwindt, G.; Picavet, H.; Verschuren, W.; Ferrari, M.; Launer, L. Cardiovascular risk factors and migraine: The GEM population-based study. Neurology 2005, 64, 614–620. [Google Scholar] [CrossRef]
- Onderwater, G.L.J.; Ligthart, L.; Bot, M.; Demirkan, A.; Fu, J.; van der Kallen, C.J.H.; Vijfhuizen, L.S.; Pool, R.; Liu, J.; Vanmolkot, F.H.M. Large-scale plasma metabolome analysis reveals alterations in HDL metabolism in migraine. Neurology 2019, 92, e1899–e1911. [Google Scholar] [CrossRef]
- Del Moro, L.; Pirovano, E.; Rota, E. Mind the Metabolic Gap: Bridging Migraine and Alzheimer’s disease through Brain Insulin Resistance. Aging Dis. 2024, 15, 2526. [Google Scholar]
- Mantegazza, M.; Cestèle, S. Pathophysiological mechanisms of migraine and epilepsy: Similarities and differences. Neurosci. Lett. 2018, 667, 92–102. [Google Scholar] [CrossRef]
- La Mantia, L. Headache and multiple sclerosis: Clinical and therapeutic correlations. Neurol. Sci. 2009, 30, 23–26. [Google Scholar] [CrossRef]
- Suzuki, S.; Suzuki, K.; Miyamoto, M.; Miyamoto, T.; Watanabe, Y.; Takashima, R.; Hirata, K. Evaluation of contributing factors to restless legs syndrome in migraine patients. J. Neurol. 2011, 258, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Marashly, E.T.; Bohlega, S.A. Riboflavin has neuroprotective potential: Focus on Parkinson’s disease and migraine. Front. Neurol. 2017, 8, 333. [Google Scholar] [CrossRef]
- Rotberg, O. An Overview of Headaches in Parkinson’s Disease Patients. Master’s Thesis, Lithuanian University of Health Sciences, Kaunas, Lithuania, 2024. [Google Scholar]
- Borkum, J.M. The migraine attack as a homeostatic, neuroprotective response to brain oxidative stress: Preliminary evidence for a theory. Headache J. Head Face Pain 2018, 58, 118–135. [Google Scholar] [CrossRef]
- Peng, K.-P.; May, A. Migraine understood as a sensory threshold disease. Pain 2019, 160, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Peterlin, B.L.; Rosso, A.L.; Sheftell, F.D.; Libon, D.J.; Mossey, J.M.; Merikangas, K.R. Post-traumatic stress disorder, drug abuse and migraine: New findings from the National Comorbidity Survey Replication (NCS-R). Cephalalgia 2011, 31, 235–244. [Google Scholar] [CrossRef]
- Agbetou, M.; Adoukonou, T. Lifestyle modifications for migraine management. Front. Neurol. 2022, 13, 719467. [Google Scholar] [CrossRef]
- Robblee, J.; Starling, A.J. SEEDS for success: Lifestyle management in migraine. Clevel. Clin. J. Med. 2019, 86, 741. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.I.; De Ver Dye, T. Migraine and the environment. Headache: J. Head Face Pain 2009, 49, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Beh, S.C.; Masrour, S.; Smith, S.V.; Friedman, D.I. The spectrum of vestibular migraine: Clinical features, triggers, and examination findings. Headache J. Head Face Pain 2019, 59, 727–740. [Google Scholar] [CrossRef]
- Ashina, S.; Terwindt, G.M.; Steiner, T.J.; Lee, M.J.; Porreca, F.; Tassorelli, C.; Schwedt, T.J.; Jensen, R.H.; Diener, H.-C.; Lipton, R.B. Medication overuse headache. Nat. Rev. Dis. Primers 2023, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Kebede, Y.T.; Mohammed, B.D.; Tamene, B.A.; Abebe, A.T.; Dhugasa, R.W. Medication overuse headache: A review of current evidence and management strategies. Front. Pain Res. 2023, 4, 1194134. [Google Scholar] [CrossRef] [PubMed]
- Ljubisavljevic, S.; Ljubisavljevic, M.; Damjanovic, R.; Kalinic, S.J. A descriptive review of medication-overuse headache: From pathophysiology to the comorbidities. Brain Sci. 2023, 13, 1408. [Google Scholar] [CrossRef] [PubMed]
- Kotowicz, Z.; Pabiś, J.; Podgórski, P.; Górecka, S.; Olszański, M.; Bogusz, A.; Kwiatkowski, O.; Kołodziej, A.; Król, A.J. Strategies for Treating Acute Migraines and Identifying Triggers. Literature review. J. Educ. Health Sport 2024, 69, 49179. [Google Scholar] [CrossRef]
- Al-Hassany, L.; Lyons, H.S.; Boucherie, D.M.; Farham, F.; Lange, K.S.; Marschollek, K.; Onan, D.; Pensato, U.; Storch, E.; Torrente, A.; et al. The sense of stopping migraine prophylaxis. J. Headache Pain 2023, 24, 9. [Google Scholar] [CrossRef]
- Lackovic, J.E. Mechanisms Underlying Migraine Headache Pathophysiology: Novel Insights From Preclinical Models. Ph.D. Thesis, The University of Texas at Dallas, Dallas, TX, USA, 2021. [Google Scholar]
- Wecker, L. Mechanism-Based Therapeutics. In Brody’s Human Pharmacology—E-Book; Dunaway, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Singh, R.K.; Pandey, S.K.; Singh, S.; Chittoda, A.; Chaudhari, S.A. Currently Available Interventions for the Management of Migraine Pain. In Management of Migraine Pain: Emerging Opportunities and Challenges; Springer: Berlin/Heidelberg, Germany, 2024; pp. 191–227. [Google Scholar]
- Noor, N.; LaChute, C.; Root, M.; Rogers, J.; Richard, M.; Varrassi, G.; Urits, I.; Viswanath, O.; Khater, N.; Kaye, A.D. A comprehensive review of celecoxib oral solution for the acute treatment of migraine. Heal. Psychol. Res. 2022, 10, 34265. [Google Scholar] [CrossRef]
- Mitsikostas, D.D.; Ward, T.N. Evidence-based symptomatic treatment of migraine. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2024; Volume 199, pp. 203–218. [Google Scholar]
- Domper Arnal, M.-J.; Hijos-Mallada, G.; Lanas, A. Gastrointestinal and cardiovascular adverse events associated with NSAIDs. Expert Opin. Drug Saf. 2022, 21, 373–384. [Google Scholar] [CrossRef]
- Hird, M.A.; Sandoe, C.H. Migraine Management in Medically Complex Patients: A Narrative Review. Curr. Neurol. Neurosci. Rep. 2024, 24, 423–438. [Google Scholar] [CrossRef]
- George, N.; Tepper, S.J. Novel migraine treatments: A review. J. Oral Facial Pain Headache 2023, 37, 3163. [Google Scholar] [CrossRef]
- Burgalassi, A.; Vigani, G.; Boccalini, A.; De Cesaris, F.; Mannaioni, G.; Chiarugi, A.; Geppetti, P.; Iannone, L.F. Rimegepant as an acute treatment in a refractory migraine patient non-responder to two anti-CGRP monoclonal antibodies and triptans: Case report and pharmacological considerations. Cephalalgia Rep. 2024, 7, 25158163241235130. [Google Scholar] [CrossRef]
- Begasse de Dhaem, O.; Takizawa, T.; Dodick, D.W. Long-term open-label and real-world studies of lasmiditan, ubrogepant, and rimegepant for the acute treatment of migraine attacks. Cephalalgia 2023, 43, 03331024221137092. [Google Scholar] [CrossRef] [PubMed]
- Orlova, Y.Y.; Mehla, S.; Chua, A.L. Drug safety in episodic migraine management in adults part 1: Acute treatments. Curr. Pain Headache Rep. 2022, 26, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, H.; Shanmuganathan, S.; Roy, J.S.; Gopal, G.; Ambi, S.V. Novel Treatment Approaches for the Management of Migraine Pain. In Management of Migraine Pain: Emerging Opportunities and Challenges; Springer: Berlin/Heidelberg, Germany, 2024; pp. 161–189. [Google Scholar]
- de Oliveira Souza, W.P.; Fortes, Y.M.L.; de Almeida Soares, A.; Silva-Néto, R.P. Propranolol: A migraine prophylactic since the 1960s. Headache Med. 2023, 14, 3–6. [Google Scholar] [CrossRef]
- Cojocariu, S.A.; Maștaleru, A.; Sascău, R.A.; Stătescu, C.; Mitu, F.; Leon-Constantin, M.M. Neuropsychiatric consequences of lipophilic beta-blockers. Medicina 2021, 57, 155. [Google Scholar] [CrossRef]
- Marti, H.-P.; Pavía López, A.A.; Schwartzmann, P. Safety and tolerability of β-blockers: Importance of cardioselectivity. Curr. Med Res. Opin. 2024, 40, 55–62. [Google Scholar] [CrossRef]
- Kitamura, E.; Imai, N. Molecular and Cellular Neurobiology of Spreading Depolarization/Depression and Migraine: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 11163. [Google Scholar] [CrossRef]
- Pearl, N.Z.; Babin, C.P.; Catalano, N.T.; Blake, J.C.; Ahmadzadeh, S.; Shekoohi, S.; Kaye, A.D. Narrative review of topiramate: Clinical uses and pharmacological considerations. Adv. Ther. 2023, 40, 3626–3638. [Google Scholar] [CrossRef]
- Walker, C.P.; Ray, S.D. Anti-epileptic medications. In Side Effects of Drugs Annual; Elsevier: Amsterdam, The Netherlands, 2020; Volume 42, pp. 91–114. [Google Scholar]
- Maroney, M.; Markovic, M. Anti-epileptic medications. In Side Effects of Drugs Annual; Elsevier: Amsterdam, The Netherlands, 2021; Volume 43, pp. 99–121. [Google Scholar]
- Błaszczyk, B.; Miziak, B.; Pluta, R.; Czuczwar, S.J. Epilepsy in pregnancy—Management principles and focus on valproate. Int. J. Mol. Sci. 2022, 23, 1369. [Google Scholar] [CrossRef]
- Young, M.R.; Bisaccia, E.K.; Romantseva, L.; Hovey, S.W. Valproic acid serum concentration and incidence of toxicity in pediatric patients. J. Child Neurol. 2022, 37, 461–470. [Google Scholar] [CrossRef]
- Iannone, L.F.; De Cesaris, F.; Ferrari, A.; Benemei, S.; Fattori, D.; Chiarugi, A. Effectiveness of anti-CGRP monoclonal antibodies on central symptoms of migraine. Cephalalgia 2022, 42, 1323–1330. [Google Scholar] [CrossRef]
- Janković, S.M.; Janković, S.V. Anti-calcitonin Gene-Related Peptide Monoclonal Antibodies in Migraine: Focus on Clinical Pharmacokinetics. Eur. J. Drug Metab. Pharmacokinet. 2024, 49, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, Y.; Xia, B.; Chen, J.; Liu, Y.; Pang, J.; Liu, F.; Cheng, H. Adverse event reporting of four anti-Calcitonin gene-related peptide monoclonal antibodies for migraine prevention: A real-world study based on the FDA adverse event reporting system. Front. Pharmacol. 2024, 14, 1257282. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, A.; Marichal-Cancino, B.A.; Villalón, C.M. The impact of CGRPergic monoclonal antibodies on prophylactic antimigraine therapy and potential adverse events. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassany, L.; Goadsby, P.J.; Danser, A.J.; MaassenVanDenBrink, A. Calcitonin gene-related peptide-targeting drugs for migraine: How pharmacology might inform treatment decisions. Lancet Neurol. 2022, 21, 284–294. [Google Scholar] [CrossRef]
- Ernst, E.; Pittler, M. The efficacy and safety of feverfew (Tanacetum parthenium L.): An update of a systematic review. Public Health Nutr. 2000, 3, 509–514. [Google Scholar] [CrossRef]
- Kulinowski, Ł.; Luca, S.V.; Minceva, M.; Skalicka-Woźniak, K. A review on the ethnobotany, phytochemistry, pharmacology and toxicology of butterbur species (Petasites L.). J. Ethnopharmacol. 2022, 293, 115263. [Google Scholar] [CrossRef]
- Chen, L.; Cai, Z. The efficacy of ginger for the treatment of migraine: A meta-analysis of randomized controlled studies. Am. J. Emerg. Med. 2021, 46, 567–571. [Google Scholar] [CrossRef]
- Martins, L.B.; Rodrigues, A.M.d.S.; Monteze, N.M.; Tibaes, J.R.B.; Amaral, M.H.A.; Gomez, R.S.; Teixeira, A.L.; Ferreira, A.V.M. Double-blind placebo-controlled randomized clinical trial of ginger (Zingiber officinale Rosc.) in the prophylactic treatment of migraine. Cephalalgia 2020, 40, 88–95. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Yeh, T.-H.; Yin-Cheng, H.; Pin-Yuan, C. Effects of intravenous and oral magnesium on reducing migraine: A meta-analysis of randomized controlled trials. Pain Physician 2016, 19, E97. [Google Scholar]
- Schoenen, J.; Jacquy, J.; Lenaerts, M. Effectiveness of high-dose riboflavin in migraine prophylaxis A randomized controlled trial. Neurology 1998, 50, 466–470. [Google Scholar] [CrossRef]
- Rajapakse, T.; Gantenbein, A.R. Nutraceuticals in migraine. Handb. Clin. Neurol. 2024, 199, 125–144. [Google Scholar] [PubMed]
- Yamanaka, G.; Kanou, K.; Takamatsu, T.; Takeshita, M.; Morichi, S.; Suzuki, S.; Ishida, Y.; Watanabe, Y.; Go, S.; Oana, S. Complementary and integrative medicines as prophylactic agents for pediatric migraine: A narrative literature review. J. Clin. Med. 2021, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Underwood, M.; Naghdi, S.; Brown, A.; Duncan, C.; Matharu, M.; Mistry, H. A systematic review of economic evaluations of pharmacological treatments for adults with chronic migraine. J. Headache Pain 2022, 23, 122. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn. Rev. 2011, 5, 103. [Google Scholar] [CrossRef]
- Rajapakse, T.; Davenport, W.J. Phytomedicines in the Treatment of Migraine. CNS Drugs 2019, 33, 399–415. [Google Scholar] [CrossRef]
- Pfaffenrath, V.; Diener, H.; Fischer, M.; Friede, M.; Henneicke-von Zepelin, H. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis—A double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia 2002, 22, 523–532. [Google Scholar] [CrossRef]
- Cady, R.K.; Goldstein, J.; Nett, R.; Mitchell, R.; Beach, M.; Browning, R. A double-blind placebo-controlled pilot study of sublingual feverfew and ginger (LipigesicTMM) in the treatment of migraine. Headache J. Head Face Pain 2011, 51, 1078–1086. [Google Scholar] [CrossRef]
- Cady, R.K.; Schreiber, C.P.; Beach, M.E.; Hart, C.C. Gelstat Migraine® (sublingually administered feverfew and ginger compound) for acute treatment of migraine when administered during the mild pain phase. Med. Sci. Monit. 2005, 11, PI65. [Google Scholar]
- Shrivastava, R.; Pechadre, J.; John, G. Tanacetum parthenium and Salix alba (mig-RL®) combination in migraine prophylaxis: A prospective, open-label study. Clin. Drug Investig. 2006, 26, 287–296. [Google Scholar] [CrossRef]
- Kumar, N.; Ashaq, M. Safety and Toxicity of Botanical Medicines: A critical Appraisal. Int. J. All Res. Educ. Sci. Methods 2021, 9, 2455–6211. [Google Scholar]
- Horak, S.; Koschak, A.; Stuppner, H.; Striessnig, J. Use-dependent block of voltage-gated Cav2. 1 Ca2+ channels by petasins and eudesmol isomers. J. Pharmacol. Exp. Ther. 2009, 330, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Slavin, M.; Bourguignon, J.; Jackson, K.; Orciga, M.-A. Impact of food components on in vitro calcitonin gene-related peptide secretion—A potential mechanism for dietary influence on migraine. Nutrients 2016, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Kleeberg-Hartmann, J.; Vogler, B.; Messlinger, K. Petasin and isopetasin reduce CGRP release from trigeminal afferents indicating an inhibitory effect on TRPA1 and TRPV1 receptor channels. J. Headache Pain 2021, 22, 1–14. [Google Scholar] [CrossRef]
- Benemei, S.; De Logu, F.; Li Puma, S.; Marone, I.M.; Coppi, E.; Ugolini, F.; Liedtke, W.; Pollastro, F.; Appendino, G.; Geppetti, P. The anti-migraine component of butterbur extracts, isopetasin, desensitizes peptidergic nociceptors by acting on TRPA1 cation channel. Br. J. Pharmacol. 2017, 174, 2897–2911. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, W.; Schmidramsl, H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Altern. Med. Rev. 2001, 6, 303–310. [Google Scholar] [CrossRef]
- Lipton, R.; Gobel, H.; Einhaupl, K.; Wilks, K.; Mauskop, A. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology 2004, 63, 2240–2244. [Google Scholar] [CrossRef]
- Pothmann, R.; Danesch, U. Migraine prevention in children and adolescents: Results of an open study with a special butterbur root extract. Headache J. Head Face Pain 2005, 45, 196–203. [Google Scholar] [CrossRef]
- Maghbooli, M.; Golipour, F.; Moghimi Esfandabadi, A.; Yousefi, M. Comparison between the efficacy of ginger and sumatriptan in the ablative treatment of the common migraine. Phytother. Res. 2014, 28, 412–415. [Google Scholar] [CrossRef]
- Martins, L.B.; Rodrigues, A.M.d.S.; Rodrigues, D.F.; Dos Santos, L.C.; Teixeira, A.L.; Ferreira, A.V.M. Double-blind placebo-controlled randomized clinical trial of ginger (Zingiber officinale Rosc.) addition in migraine acute treatment. Cephalalgia 2019, 39, 68–76. [Google Scholar] [CrossRef]
- Helli, B.; Anjirizadeh, F.; Mehramiri, A.; Shalilahmadi, D.; Latifi, S.M. The effect of ginger (Zingiber officinale Rosc.) consumption in headache prophylaxis in patients with migraine: A randomized placebo-controlled clinical trial. Jundishapur J. Nat. Pharm. Prod. 2022, 17, e120449. [Google Scholar] [CrossRef]
- Jivad, N.; Shahrakei, Z.F.; Mardani, M. The effect of combined administration of Ginger (Zingiber officinale Roscoe) and Depakene on pain reduction in patients with migraine headaches compared to Depakene alone. Future Nat. Prod. 2023, 9, 10–15. [Google Scholar]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Taype-Rondan, A.; Tanaka, J.H.Z.; Merino-Garcia, N. Peruvian scientific production on abortion in scopus. Int. J. Prev. Med. 2017, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, M.; Sahebkar, A.; Khorvash, F.; Fallahpour, S.; Askari, G.; Bagherniya, M. The effects of phytosomal curcumin supplementation on clinical symptoms, and inflammatory and oxidative stress biomarkers in patients with migraine: A protocol for a randomized double-blind placebo-controlled trial. Avicenna J. Phytomedicine 2023, 13, 45. [Google Scholar]
- Soveyd, N.; Abdolahi, M.; Djalali, M.; Hatami, M.; Tafakhori, A.; Sarraf, P.; Honarvar, N.M. The combined effects of ω-3 fatty acids and nano-curcumin supplementation on intercellular adhesion molecule-1 (ICAM-1) gene expression and serum levels in migraine patients. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2017, 16, 1120–1126. [Google Scholar] [CrossRef]
- Honarvar, N.M.; Soveid, N.; Abdolahi, M.; Djalali, M.; Hatami, M.; Karzar, N.H. Anti-neuroinflammatory properties of n-3 fatty acids and nano-curcumin on migraine patients from cellular to clinical insight: A randomized, double-blind and placebo-controlled trial. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2021, 21, 365–373. [Google Scholar]
- Abdolahi, M.; Karimi, E.; Sarraf, P.; Tafakhori, A.; Siri, G.; Salehinia, F.; Sedighiyan, M.; Asanjarani, B.; Badeli, M.; Abdollahi, H. The omega-3 and Nano-curcumin effects on vascular cell adhesion molecule (VCAM) in episodic migraine patients: A randomized clinical trial. BMC Res. Notes 2021, 14, 1–7. [Google Scholar] [CrossRef]
- Bulboacă, A.E.; Bolboacă, S.D.; Stănescu, I.C.; Sfrângeu, C.A.; Bulboacă, A.C. Preemptive analgesic and antioxidative effect of curcumin for experimental migraine. BioMed Res. Int. 2017, 2017, 4754701. [Google Scholar] [CrossRef]
- Bulboacă, A.E.; Bolboacă, S.D.; Stănescu, I.C.; Sfrângeu, C.A.; Porfire, A.; Tefas, L.; Bulboacă, A.C. The effect of intravenous administration of liposomal curcumin in addition to sumatriptan treatment in an experimental migraine model in rats. Int. J. Nanomed. 2018, 13, 3093–3103. [Google Scholar] [CrossRef]
- Bulboacă, A.E.; Bolboacă, S.D.; Bulboacă, A.C.; Porfire, A.S.; Tefas, L.R.; Suciu, Ş.M.; Dogaru, G.; Stănescu, I.C. Liposomal curcumin enhances the effect of naproxen in a rat model of migraine. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 5087. [Google Scholar] [CrossRef]
- Sturaro, C.; Fakhoury, B.; Targowska-Duda, K.M.; Zribi, G.; Schoch, J.; Ruzza, C.; Calò, G.; Toll, L.; Cippitelli, A. Preclinical effects of cannabidiol in an experimental model of migraine. Pain 2023, 164, 2540–2552. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, R.; Dawson, C.T.; Craft, R.M.; Morgan, M.M. Anti-migraine effect of∆ 9-tetrahydrocannabinol in the female rat. Eur. J. Pharmacol. 2018, 818, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Materazzi, S.; Benemei, S.; Fusi, C.; Gualdani, R.; De Siena, G.; Vastani, N.; Andersson, D.A.; Trevisan, G.; Moncelli, M.R.; Wei, X. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. PAIN 2013, 154, 2750–2758. [Google Scholar] [CrossRef] [PubMed]
- Tassorelli, C.; Greco, R.; Morazzoni, P.; Riva, A.; Sandrini, G.; Nappi, G. Parthenolide is the component of tanacetum parthenium that inhibits nitroglycerin-induced Fos activation: Studies in an animal model of migraine. Cephalalgia 2005, 25, 612–621. [Google Scholar] [CrossRef]
- Di Simone, S.C.; Acquaviva, A.; Libero, M.L.; Chiavaroli, A.; Recinella, L.; Leone, S.; Brunetti, L.; Politi, M.; Giannone, C.; Campana, C. The association of Tanacetum parthenium and Salix alba extracts reduces cortex serotonin turnover, in an ex vivo experimental model of migraine. Processes 2022, 10, 280. [Google Scholar] [CrossRef]
- Salihan, S.; Moklas, M.A.M.; Khalid, M.H.b.; Chiromaa, S.M.; Sulaiman, M.R.; Baharuldin, M.T.H. Anti-hyperalgesic activity of Ficus deltoidea in an animal model of migraine. Indian J. Nat. Prod. Resour. 2024, 15, 426–435. [Google Scholar]
- Mony, R.S.; Vidyarthi, P. Anti-migraine activity: In vivo evaluation of polyherbal hydro alcoholic extract. Pharma Innov. 2024, 13, 214–219. [Google Scholar]
- Xue, L.; Lian, F.; Wu, X.; Pan, J. Effects of Ginkgo Biloba extract EGb761 on rats with nitroglycerin migraine and the mechanisms. Chin. J. Hosp. Pharm. 2015, 35, 1729–1732. (In Chinese) [Google Scholar]
- Xing, Z.; Peng, F.; Chen, Y.; Wan, F.; Peng, C.; Li, D. Metabolomic profiling integrated with molecular exploring delineates the action of Ligusticum chuanxiong hort. on migraine. Phytomedicine 2024, 134, 155977. [Google Scholar] [CrossRef]
- Arulmozhi, D.; Sridhar, N.; Bodhankar, S.; Veeranjaneyulu, A.; Arora, S. In vitro pharmacological investigations of Sapindus trifoliatus in various migraine targets. J. Ethnopharmacol. 2004, 95, 239–245. [Google Scholar] [CrossRef]
- Yu, S.; Fan, C.; Li, Y.; Pei, H.; Tian, Y.; Zuo, Z.; Wang, Z.; Liu, C.; Zhao, X.; Wang, Z. Network pharmacology and experimental verification to explore the anti-migraine mechanism of Yufeng Ningxin Tablet. J. Ethnopharmacol. 2023, 310, 116384. [Google Scholar] [CrossRef] [PubMed]
- Guilbot, A.; Bangratz, M.; Ait Abdellah, S.; Lucas, C. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: A prospective observational study. BMC Complement. Altern. Med. 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.; Rahlfs, V.; Danesch, U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: Reanalysis of efficacy criteria. Eur. Neurol. 2004, 51, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Oelkers-Ax, R.; Leins, A.; Parzer, P.; Hillecke, T.; Bolay, H.V.; Fischer, J.; Bender, S.; Hermanns, U.; Resch, F. Butterbur root extract and music therapy in the prevention of childhood migraine: An explorative study. Eur. J. Pain 2008, 12, 301–313. [Google Scholar] [CrossRef]
- Rezaie, S.; Askari, G.; Khorvash, F.; Tarrahi, M.J.; Amani, R. Effects of curcumin supplementation on clinical features and inflammation, in migraine patients: A double-blind controlled, placebo randomized clinical trial. Int. J. Prev. Med. 2021, 12, 161. [Google Scholar]
- Djalali, M.; Abdolahi, M.; Hosseini, R.; Miraghajani, M.; Mohammadi, H.; Djalali, M. The effects of nano-curcumin supplementation on Th2/tregulatory axis in migraine patients: A randomized, double-blind, placebo-controlled trial. Int. J. Neurosci. 2023, 133, 169–175. [Google Scholar] [CrossRef]
- Schuster, N.; Wallace, M.; Buse, D.; Marcotte, T.; Lee, E.; Liu, L.; Sexton, M. Vaporized Cannabis versus Placebo for Acute Migraine: A Randomized Controlled Trial (S22.010). Neurology 2024, 102, 3105. [Google Scholar] [CrossRef]
- Rafie, S.; Namjoyan, F.; Hassanzadeh, A.; Yousefbeyk, F.; Hamedi, S.; Golfakhrabadi, F. The Effect of Feverfew Cultivated in Iran for the Prophylactic Treatment of Migraine: A Randomized Placebo-controlled Clinical Trial. Jundishapur J. Nat. Pharm. Prod. 2016, 11, 29275. [Google Scholar] [CrossRef]
- Maizels, M.; Blumenfeld, A.; Burchette, R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: A randomized trial. Headache J. Head Face Pain 2004, 44, 885–890. [Google Scholar] [CrossRef]
- Mousavi, S.; Bidaki, M.Z.; Yazdi, N.; Tafazoli, V.; Dinani, M.S. The Effects of Combination Herbal Medicine Tablet on Migraine Headache: A Randomized, Double-blind, Placebo, Controlled Study. J. Pharm. Care 2024, 12, 32–39. [Google Scholar] [CrossRef]
- Kim, S.; Seo, J.; Kim, C.-h.; Sung, H.-K.; Go, H.-Y.; Jung, W.S.; Kwon, S.; Kwak, M.; Lee, S. Effect of herbal medicine (Jodeungsan) on migraine: A double-blind randomized clinical trial. Integr. Med. Res. 2022, 11, 100885. [Google Scholar] [CrossRef] [PubMed]
- Golpour-Hamedani, S.; Bagherniy, M.; Khorvash, F.; Feizi, A.; Sharma, M.; Askari, G. Investigating the effects of co-supplementation with alpha-linolenic acid and L-carnitine on inflammatory status, oxidative stress, clinical symptoms, mental health and quality of life in women with migraine: A protocol for a randomized, triple-blind, placebo-controlled trial. Trials 2024, 25, 666. [Google Scholar] [PubMed]
- D’Andrea, G.; Bussone, G.; Allais, G.; Aguggia, M.; D’Onofrio, F.; Maggio, M.; Moschiano, F.; Saracco, M.G.; Terzi, M.G.; Petretta, V. Efficacy of Ginkgolide B in the prophylaxis of migraine with aura. Neurol. Sci. 2009, 30, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Allais, G.; D’Andrea, G.; Maggio, M.; Benedetto, C. The efficacy of ginkgolide B in the acute treatment of migraine aura: An open preliminary trial. Neurol. Sci. 2013, 34, 161–163. [Google Scholar] [CrossRef]
- Esposito, M.; Carotenuto, M. Ginkgolide B complex efficacy for brief prophylaxis of migraine in school-aged children: An open-label study. Neurol. Sci. 2011, 32, 79–81. [Google Scholar] [CrossRef]
- Usai, S.; Grazzi, L.; Bussone, G. Gingkolide B as migraine preventive treatment in young age: Results at 1-year follow-up. Neurol. Sci. 2011, 32, 197–199. [Google Scholar] [CrossRef]
- Diener, H.; Pfaffenrath, V.; Schnitker, J.; Friede, M.; Zepelin, H.H.-V.; Investigators. Efficacy and safety of 6.25 mg tid feverfew CO2-extract (MIG-99) in migraine prevention—A randomized, double-blind, multicentre, placebo-controlled study. Cephalalgia 2005, 25, 1031–1041. [Google Scholar] [CrossRef]
- Scarzella, L. Effects of a novel MYRLIQ®, Ginkgo Biloba, Q10 and vitamin B2 (riboflavin) based nutra-ceutical in patients suffering from migraine headache without aura or sporadic episodes of tension-type headache. A six-month pilot study. Gazz. Medica Ital. Arch. Per Le Sci. Mediche 2017, 4, 149–153. [Google Scholar]
- Lunguț, E.F.; Antal, C.; Turcu, S.; Varlas, V.-N.; Filipescu, A.G.; Balescu, I.; Bacalbașa, N.; Gorecki, G.-P. Neurasites®—A Standardized Plant Extract in the Treatment and Prevention of Migraine Attacks. J. Clin. Med. 2024, 13, 3364. [Google Scholar] [CrossRef]
- Rushendran, R.; Chitra, V. Antimigraine activity of Asarinin by OPRM1 pathway with multifaceted impacts through network analysis. Sci. Rep. 2024, 14, 20207. [Google Scholar] [CrossRef]
- Blessing, A.C.; Ijeoma, K.G. Elucidation of potential inhibitor compounds from zingiber officinale against migraine headache. World News Nat. Sci. 2024, 55, 184–205. [Google Scholar]
- Cooke, B.; Ernst, E. Aromatherapy: A systematic review. Br. J. Gen. Pract. 2000, 50, 493–496. [Google Scholar] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Yuan, R.; Zhang, D.; Yang, J.; Wu, Z.; Luo, C.; Han, L.; Yang, F.; Lin, J.; Yang, M. Review of aromatherapy essential oils and their mechanism of action against migraines. J. Ethnopharmacol. 2021, 265, 113326. [Google Scholar] [CrossRef]
- Khatib, C. Aromatherapy: Historical, Phytochemical Insights and Therapeutic Applications. In Alternative Medicine—New Insights; IntechOpen: London, UK, 2024. [Google Scholar]
- Penhollow, T.M.; Mielke, A.C. Aromatherapy: An alternative treatment for migraine headaches. Int. Phys. Med. Rehabil. J. 2024, 9, 1–4. [Google Scholar] [CrossRef]
- Sasannejad, P.; Saeedi, M.; Shoeibi, A.; Gorji, A.; Abbasi, M.; Foroughipour, M. Lavender essential oil in the treatment of migraine headache: A placebo-controlled clinical trial. Eur. Neurol. 2012, 67, 288–291. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Hasanpour-Dehkordi, A.; Lorigooini, Z.; Deris, F.; Solati, K.; Mahdiyeh, F. Comparing the effect of intranasal lidocaine 4% with peppermint essential oil drop 1.5% on migraine attacks: A double-blind clinical trial. Int. J. Prev. Med. 2019, 10, 121. [Google Scholar]
- Andersen, H.H.; Gazerani, P.; Arendt-Nielsen, L. High-concentration L-menthol exhibits counter-irritancy to neurogenic inflammation, thermal and mechanical hyperalgesia caused by trans-cinnamaldehyde. J. Pain 2016, 17, 919–929. [Google Scholar] [CrossRef]
- Ahmadifard, M.; Yarahmadi, S.; Ardalan, A.; Ebrahimzadeh, F.; Bahrami, P.; Sheikhi, E. The efficacy of topical basil essential oil on relieving migraine headaches: A randomized triple-blind study. Complement. Med. Res. 2020, 27, 310–318. [Google Scholar] [CrossRef]
- Zargaran, A.; Borhani-Haghighi, A.; Salehi-Marzijarani, M.; Faridi, P.; Daneshamouz, S.; Azadi, A.; Sadeghpour, H.; Sakhteman, A.; Mohagheghzadeh, A. Evaluation of the effect of topical chamomile (Matricaria chamomilla L.) oleogel as pain relief in migraine without aura: A randomized, double-blind, placebo-controlled, crossover study. Neurol. Sci. 2018, 39, 1345–1353. [Google Scholar] [CrossRef]
- Mosavat, S.H.; Jaberi, A.R.; Sobhani, Z.; Mosaffa-Jahromi, M.; Iraji, A.; Moayedfard, A. Efficacy of Anise (Pimpinella anisum L.) oil for migraine headache: A pilot randomized placebo-controlled clinical trial. J. Ethnopharmacol. 2019, 236, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Pourgholami, M.H.; Majzoob, S.; Javadi, M.; Kamalinejad, M.; Fanaee, G.H.R.; Sayyah, M. The fruit essential oil of Pimpinella anisum exerts anticonvulsant effects in mice. J. Ethnopharmacol. 1999, 66, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Niazi, M.; Hashempur, M.H.; Taghizadeh, M.; Heydari, M.; Shariat, A. Efficacy of topical Rose (Rosa damascena Mill.) oil for migraine headache: A randomized double-blinded placebo-controlled cross-over trial. Complement. Ther. Med. 2017, 34, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemy, Z.; Golpour-Hamedani, S.; Eshaghian, N.; Sadeghi, O.; Khorvash, F.; Askari, G. Practical supplements for prevention and management of migraine attacks: A narrative review. Front. Nutr. 2024, 11, 1433390. [Google Scholar] [CrossRef] [PubMed]
- Giniatullin, R. 5-hydroxytryptamine in migraine: The puzzling role of ionotropic 5-HT3 receptor in the context of established therapeutic effect of metabotropic 5-HT1 subtypes. Br. J. Pharmacol. 2022, 179, 400–415. [Google Scholar] [CrossRef]
- de Vries, T.; Villalón, C.M.; MaassenVanDenBrink, A. Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. Pharmacol. Ther. 2020, 211, 107528. [Google Scholar] [CrossRef]
- Slavin, M.; Li, H.; Khatri, M.; Frankenfeld, C. Dietary magnesium and migraine in adults: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2001–2004. Headache: J. Head Face Pain 2021, 61, 276–286. [Google Scholar] [CrossRef]
- Assarzadegan, F.; Asgarzadeh, S.; Hatamabadi, H.R.; Shahrami, A.; Tabatabaey, A.; Asgarzadeh, M. Serum concentration of magnesium as an independent risk factor in migraine attacks: A matched case–control study and review of the literature. Int. Clin. Psychopharmacol. 2016, 31, 287–292. [Google Scholar] [CrossRef]
- Khani, S.; Hejazi, S.A.; Yaghoubi, M.; Sharifipour, E. Comparative study of magnesium, sodium valproate, and concurrent magnesium-sodium valproate therapy in the prevention of migraine headaches: A randomized controlled double-blind trial. J. Headache Pain 2021, 22, 1–10. [Google Scholar] [CrossRef]
- Borkum, J.M. Brain energy deficit as a source of oxidative stress in migraine: A molecular basis for migraine susceptibility. Neurochem. Res. 2021, 46, 1913–1932. [Google Scholar] [CrossRef]
- Dahri, M.; Tarighat-Esfanjani, A.; Asghari-Jafarabadi, M.; Hashemilar, M. Oral coenzyme Q10 supplementation in patients with migraine: Effects on clinical features and inflammatory markers. Nutr. Neurosci. 2019, 22, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Shoeibi, A.; Olfati, N.; Soltani Sabi, M.; Salehi, M.; Mali, S.; Akbari Oryani, M. Effectiveness of coenzyme Q10 in prophylactic treatment of migraine headache: An open-label, add-on, controlled trial. Acta Neurol. Belg. 2017, 117, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Dahri, M.; Sadeghi, A.S.; Pahlavani, N.; Nattagh-Eshtivani, E.; Hashemilar, M.; Asghari-Jafarabadi, M.; Barghchi, H.; Tarighat-Esfanjani, A. The effects of coenzyme Q10 supplementation on oxidative status and lipid profile in migraine patients: A randomized double-blinded controlled clinical trial. Clin. Nutr. Res. 2023, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Sazali, S.; Badrin, S.; Norhayati, M.N.; Idris, N.S. Coenzyme Q10 supplementation for prophylaxis in adult patients with migraine—A meta-analysis. BMJ Open 2021, 11, e039358. [Google Scholar] [CrossRef]
- Kennedy, D.O. B vitamins and the brain: Mechanisms, dose and efficacy—A review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Sani, M.A.; Dahri, M.; Ghalichi, F.; Ghavami, A.; Arjang, P.; Tarighat-Esfanjani, A. The role of nutrients in the pathogenesis and treatment of migraine headaches. Biomed. Pharmacother. 2018, 102, 317–325. [Google Scholar] [CrossRef]
- Nambiar, N.; Aiyappa, C.; Srinivasa, R. Oral riboflavin versus oral propranolol in migraine prophylaxis: An open label randomized controlled trial. Neurol. Asia 2011, 16, 223. [Google Scholar]
- Rahimdel, A.; Zeinali, A.; Yazdian-Anari, P.; Hajizadeh, R.; Arefnia, E. Effectiveness of vitamin B2 versus sodium valproate in migraine prophylaxis: A randomized clinical trial. Electron. Physician 2015, 7, 1344. [Google Scholar]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Rapisarda, L.; Mazza, M.R.; Tosto, F.; Gambardella, A.; Bono, F.; Sarica, A. Relationship between severity of migraine and vitamin D deficiency: A case-control study. Neurol. Sci. 2018, 39, 167–168. [Google Scholar] [CrossRef]
- Mottaghi, T.; Askari, G.; Khorvash, F.; Maracy, M.R. Effect of Vitamin D supplementation on symptoms and C-reactive protein in migraine patients. J. Res. Med. Sci. 2015, 20, 477–482. [Google Scholar] [PubMed]
- Ghorbani, Z.; Togha, M.; Rafiee, P.; Ahmadi, Z.S.; Rasekh Magham, R.; Djalali, M.; Shahemi, S.; Martami, F.; Zareei, M.; Razeghi Jahromi, S. Vitamin D3 might improve headache characteristics and protect against inflammation in migraine: A randomized clinical trial. Neurol. Sci. 2020, 41, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Fan, Y.; Wu, S.; Zou, Y.; Qu, X. Vitamin D supplementation for the treatment of migraine: A meta-analysis of randomized controlled studies. Am. J. Emerg. Med. 2021, 50, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Eren, Y.; Dirik, E.; Neşelioğlu, S.; Erel, Ö. Oxidative stress and decreased thiol level in patients with migraine: Cross-sectional study. Acta Neurol. Belg. 2015, 115, 643–649. [Google Scholar] [CrossRef]
- Gross, E.C.; Putananickal, N.; Orsini, A.-L.; Vogt, D.R.; Sandor, P.S.; Schoenen, J.; Fischer, D. Mitochondrial function and oxidative stress markers in higher-frequency episodic migraine. Sci. Rep. 2021, 11, 4543. [Google Scholar] [CrossRef]
- Rezaei Kelishadi, M.; Alavi Naeini, A.; Askari, G.; Khorvash, F.; Heidari, Z. The efficacy of alpha-lipoic acid in improving oxidative, inflammatory, and mood status in women with episodic migraine in a randomised, double-blind, placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14455. [Google Scholar] [CrossRef]
- Cavestro, C.; Bedogni, G.; Molinari, F.; Mandrino, S.; Rota, E.; Frigeri, M.C. Alpha-lipoic acid shows promise to improve migraine in patients with insulin resistance: A 6-month exploratory study. J. Med. Food 2018, 21, 269–273. [Google Scholar] [CrossRef]
- Magis, D.; Ambrosini, A.; Sándor, P.; Jacquy, J.; Laloux, P.; Schoenen, J. A randomized double-blind placebo-controlled trial of thioctic acid in migraine prophylaxis. Headache: J. Head Face Pain 2007, 47, 52–57. [Google Scholar] [CrossRef]
- Martami, F.; Togha, M.; Seifishahpar, M.; Ghorbani, Z.; Ansari, H.; Karimi, T.; Jahromi, S.R. The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: A randomized double-blind controlled trial. Cephalalgia 2019, 39, 841–853. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, G.; Xu, Y.; He, B.; Wang, Y.; Ma, R.; Chang, Y.; He, D.; Xu, C.; Xiao, Z. Effects of diet based on IgG elimination combined with probiotics on migraine plus irritable bowel syndrome. Pain Res. Manag. 2019, 2019, 7890461. [Google Scholar] [CrossRef]
- Ghavami, A.; Khorvash, F.; Heidari, Z.; Khalesi, S.; Askari, G. Effect of synbiotic supplementation on migraine characteristics and inflammatory biomarkers in women with migraine: Results of a randomized controlled trial. Pharmacol. Res. 2021, 169, 105668. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Liu, W.-C.; Zailani, H.; Yang, C.-C.; Chen, T.-B.; Chang, C.-M.; Tsai, I.J.; Yang, C.-P.; Su, K.-P. A 12-week randomized double-blind clinical trial of eicosapentaenoic acid intervention in episodic migraine. Brain Behav. Immun. 2024, 118, 459–467. [Google Scholar] [CrossRef]
- Tajmirriahi, M.; Sohelipour, M.; Basiri, K.; Shaygannejad, V.; Ghorbani, A.; Saadatnia, M. The effects of sodium valproate with fish oil supplementation or alone in migraine prevention: A randomized single-blind clinical trial. Iran. J. Neurol. 2012, 11, 21. [Google Scholar] [PubMed]
- Ramsden, C.E.; Faurot, K.R.; Zamora, D.; Suchindran, C.M.; MacIntosh, B.A.; Gaylord, S.; Ringel, A.; Hibbeln, J.R.; Feldstein, A.E.; Mori, T.A. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: A randomized trial. PAIN 2013, 154, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Sorbi, M.J.; Balk, Y.; Kleiboer, A.M.; Couturier, E.G.M. Follow-up over 20 months confirms gains of online behavioural training in frequent episodic migraine. Cephalalgia 2017, 37, 236–250. [Google Scholar] [CrossRef]
- Gu, Q.; Hou, J.-C.; Fang, X.-M. Mindfulness meditation for primary headache pain: A meta-analysis. Chin. Med. J. 2018, 131, 829–838. [Google Scholar] [CrossRef]
- Williamson, D.A.; Monguillot, J.E.; Jarrell, M.P.; Cohen, R.A.; Pratt, J.M.; Blouin, D.C. Relaxation for the treatment of headache: Controlled evaluation of two group programs. Behav. Modif. 1984, 8, 407–424. [Google Scholar] [CrossRef]
- Blanchard, E.B.; Appelbaum, K.A.; Nicholson, N.L.; Radnitz, C.L.; Morrill, B.; Michultka, D.; Kirsch, C.; Hillhouse, J.; Dentinger, M.R. A controlled evaluation of the addition of cognitive therapy to a home-based biofeedback and relaxation treatment of vascular headache. Headache J. Head Face Pain 1990, 30, 371–376. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).