The Potential of Polyphenols in Modulating the Cellular Senescence Process: Implications and Mechanism of Action
Abstract
1. Introduction
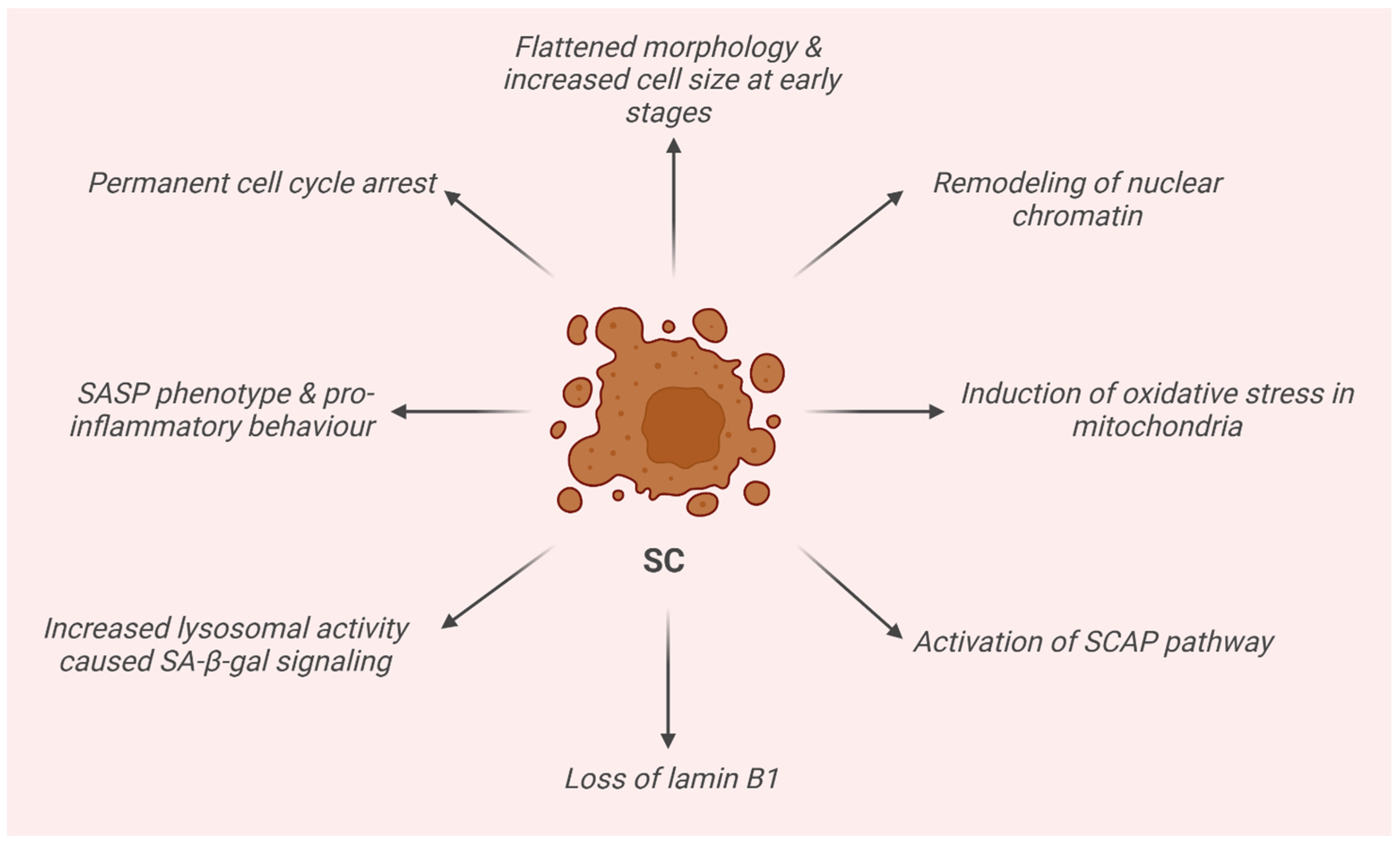
2. Senescence Cells (SCs)
3. Therapeutic Approaches
- The SCs and phenotype must be strictly associated, and if an individual does not present SCs, then he or she will not present the phenotype;
- The phenotype is caused by an induced accumulation of SCs, and as a consequence, removing the induced SCs ameliorates the phenotype;
- Senolytics have minimal effects on the phenotype in young individuals without SCs;
- An intermittent administration of senolytics has been proven to be effective;
- Senolytic drugs are able to alleviate various age-related diseases.
4. Anti-Cellular Senescence Activity by Isolated Dietary Polyphenols
4.1. Senomorphic Activity
4.1.1. Resveratrol
4.1.2. Kaempferol
4.1.3. Apigenin
4.1.4. Genistein
4.1.5. Pterostilbene
4.1.6. Oleuropein Aglycone and Hydroxytyrosol
4.1.7. Rutin
4.1.8. Luteolin
4.1.9. Hesperidin and Hesperetin
4.1.10. Naringenin
| Senomorphic Polyphenol | |
|---|---|
| Resveratrol | Activates SIRT1, an NAD+-dependent deacetylase, leading to reduced cellular senescence [49]. At low concentrations, it acts as an antioxidant and prevents senescence, suppressing SASPs, while at higher concentrations, it triggers senescence or apoptosis [50]. |
| Kaempferol | Inhibits SASP production by downregulating NF-κB signaling through the IRAK1/IκBα pathway [60]. Also enhances mitochondrial function and reduces oxidative stress [61]. |
| Apigenin | Suppresses SASP by modulating IL-1α signaling and inhibits NF-κB and p38-MAPK pathways [66]. It activates Nrf2, reducing oxidative damage and promoting anti-senescent effects [64]. |
| Genistein | Reduces senescence via the SIRT1/LKB1/AMPK pathway and modulates autophagy in vascular cells, reducing oxidative stress and senescence markers [69]. |
| Pterostilbene | Modulates oxidative stress and inflammation through Nrf2 activation [76,77]. Reduces SASP factors [81] and inhibits cellular senescence in various models by increasing SIRT1 activity and reducing p21 and p53 expression [79]. |
| Oleuropein aglycone and hydroxytyrosol | Acts as an SASP inhibitor, reducing pro-inflammatory cytokines, maintaining lamin B1 expression in irradiated cells, and protecting against radiation-induced senescence [84]. |
| Rutin | A potent senomorphic agent targeting ATM kinase, HIF1α, and TRAF6 to inhibit SASP development. Shows potential for age-related pathologies by modulating early SASP signaling pathways [89]. |
| Luteolin | Activates SIRT1, modulating the GLO1/AGE/RAGE pathway to reduce oxidative stress and inflammation. It also has protective effects against cognitive decline and senescence in neural cells [91]. |
| Hesperidin and hesperetin | Inhibits SASP by modulating NF-κB, Nrf2, and FOXO pathways. Shows antioxidant and anti-inflammatory effects, protecting against cellular senescence in human chondrocytes and improving bone density in aging models [94]. |
| Naringenin | Upregulates SIRT1, downregulates NF-κB, and modulates oxidative stress and inflammation to suppress SASP formation. Promotes regenerative and anti-aging effects in skin and myocardial cells [60]. |
4.2. Senolytic Activity
4.2.1. Fisetin
4.2.2. Epigallocatechin Gallate
4.2.3. Quercetin and Dasatinib
4.2.4. Procyanidin C1 (PCC1)
4.2.5. Wogonin and GL-V9
4.2.6. Curcumin and Analogs
4.2.7. Piperlongumine and Analogs
| Senolytic Polyphenol | |
|---|---|
| Fisetin | Induces apoptosis in senescent cells by inhibiting survival pathways and reducing SASP markers. Shown to improve tissue homeostasis and extend lifespans in animal models [132]. In clinical trials on humans, fisetin exhibited senomorphic activity by modulating SASP markers and reducing senescence-associated β-galactosidase activity [106]. |
| Epigallocatechin gallate (EGCG) | Modulates PI3K/Akt/mTOR signaling and promotes senescent cell apoptosis by regulating pro- and anti-apoptotic factors like Bax and Bcl-2. Shows as both senomorphic and senolytic behavior, depending on concentration and cell type [107,108,109]. |
| Quercetin + dasatinib | This combination targets anti-apoptotic pathways (SCAPs) in senescent cells, promoting apoptosis through inhibition of PI3K/AKT and BCL2/BCL2L1 pathways. Effective at reducing senescent cells in various tissues [50,111]. |
| Procyanidin C1 (PCC1) | Shows dual behavior; at low concentrations, it is senomorphic, while at higher concentrations, it becomes senolytic by inducing mitochondrial dysfunction and ROS generation, leading to apoptosis in senescent cells [114]. |
| Wogonin and GL-V9 | Reduces the SASP by inhibiting the NF-κB pathway and enhances mitochondrial dysfunction and ROS production in senescent cells [60]. Its derivative GL-V9 also exhibits senolytic effects [115,116]. |
| Curcumin and analogs | Modulates SIRT1/AMPK/mTOR pathways, showing both senomorphic and senolytic effects, depending on the dose. It inhibits the SASP and enhances autophagy, reducing senescence and promoting apoptosis in higher concentrations [119]. |
| Piperlongumine | Promotes oxidative stress in senescent cells by targeting OXR1, leading to apoptosis [130]. |
5. The Effects of Combined Phytochemicals
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Cristofalo, V.J.; Lorenzini, A.; Allen, R.G.; Torres, C.; Tresini, M. Replicative senescence: A critical review. Mech. Ageing Dev. 2004, 125, 827–848. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; DELLA-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Alexander, P.B.; Wang, X.-F. Cellular senescence: From anti-cancer weapon to anti-aging target. Sci. China Life Sci. 2020, 63, 332–342. [Google Scholar] [CrossRef]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.-M.; Vijg, J.; Van Steeg, H.; Dollé, M.E.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Cañamero, M.; Maraver, A.; Gómez-López, G.; Contreras, J.; Murillo-Cuesta, S.; Rodríguez-Baeza, A.; Varela-Nieto, I.; Ruberte, J.; Collado, M.; et al. Programmed Cell Senescence during Mammalian Embryonic Development. Cell 2013, 155, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- Idda, M.L.; McClusky, W.G.; Lodde, V.; Munk, R.; Abdelmohsen, K.; Rossi, M.; Gorospe, M. Survey of senescent cell markers with age in human tissues. Aging 2020, 12, 4052–4066. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Zhao, J.; Bukata, C.; Wade, E.A.; McGowan, S.J.; Angelini, L.A.; Bank, M.P.; Gurkar, A.U.; McGuckian, C.A.; Calubag, M.F.; et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 2020, 19, e13094. [Google Scholar] [CrossRef] [PubMed]
- Shree, T.J.; Poompavai, S.; Begum SM, F.M.; Gowrisree, V.; Hemalatha, S.; Sieni, E.; Sundararajan, R. Cancer-Fighting Phyto-chemicals: Another Look. J. Nanomed. Biother. Discov. 2019, 9, 162. [Google Scholar]
- Ferrario, G.; Baron, G.; Gado, F.; Della Vedova, L.; Bombardelli, E.; Carini, M.; D’amato, A.; Aldini, G.; Altomare, A. Polyphenols from Thinned Young Apples: HPLC-HRMS Profile and Evaluation of Their Anti-Oxidant and Anti-Inflammatory Activities by Proteomic Studies. Antioxidants 2022, 11, 1577. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Queen, B.L.; Tollefsbol, T.O. Polyphenols and aging. Curr. Aging Sci. 2010, 3, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; O’Loghlen, A.; Banito, A.; Guijarro, M.V.; Augert, A.; Raguz, S.; Fumagalli, M.; Da Costa, M.; Brown, C.; Popov, N.; et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008, 133, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-Induced Senescence Relayed by an Interleukin-Dependent Inflammatory Network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Nuñez, S.; Heard, E.; Lin, A.W.; Hearn, S.A.; Spector, D.L.; Hannon, G.J.; Lowe, S.W. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003, 113, 703–716. [Google Scholar] [CrossRef]
- Kim, E.-C.; Kim, J.-R. Senotherapeutics: Emerging strategy for healthy aging and age-related disease. BMB Rep. 2019, 52, 47–55. [Google Scholar] [CrossRef]
- Rajendran, P.; Alzahrani, A.M.; Hanieh, H.N.; Kumar, S.A.; Ben Ammar, R.; Rengarajan, T.; Alhoot, M.A. Autophagy and senescence: A new insight in selected human diseases. J. Cell. Physiol. 2019, 234, 21485–21492. [Google Scholar] [CrossRef]
- Fitzwalter, B.E.; Towers, C.G.; Sullivan, K.D.; Andrysik, Z.; Hoh, M.; Ludwig, M.; O’Prey, J.; Ryan, K.M.; Espinosa, J.M.; Morgan, M.J.; et al. Autophagy Inhibition Mediates Apoptosis Sensitization in Cancer Therapy by Relieving FOXO3a Turnover. Dev. Cell 2018, 44, 555–565.e3. [Google Scholar] [CrossRef]
- Al Bitar, S.; Gali-Muhtasib, H. The Role of the Cyclin Dependent Kinase Inhibitor p21cip1/waf1 in Targeting Cancer: Molecular Mechanisms and Novel Therapeutics. Cancers 2019, 11, 1475. [Google Scholar] [CrossRef]
- Sharpless, N.E.; Sherr, C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Indovina, P.; Marcelli, E.; Casini, N.; Rizzo, V.; Giordano, A. Emerging roles of RB family: New defense mechanisms against tumor progression. J. Cell. Physiol. 2013, 228, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Helmbold, H.; Deppert, W.; Bohn, W. Regulation of cellular senescence by Rb2/p130. Oncogene 2006, 25, 5257–5262. [Google Scholar] [CrossRef]
- Galanos, P.; Vougas, K.; Walter, D.; Polyzos, A.; Maya-Mendoza, A.; Haagensen, E.J.; Kokkalis, A.; Roumelioti, F.-M.; Gagos, S.; Tzetis, M.; et al. Chronic p53-independent p21 expression causes genomic instability by deregulating replication licensing. Nat. Cell Biol. 2016, 18, 777–789. [Google Scholar] [CrossRef]
- Lapasset, L.; Milhavet, O.; Prieur, A.; Besnard, E.; Babled, A.; Aït-Hamou, N.; Leschik, J.; Pellestor, F.; Ramirez, J.-M.; De Vos, J.; et al. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011, 25, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.I.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 1995, 55, 2284–2292. [Google Scholar]
- Kudlova, N.; De Sanctis, J.B.; Hajduch, M. Cellular Senescence: Molecular Targets, Biomarkers, and Senolytic Drugs. Int. J. Mol. Sci. 2022, 23, 4168. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Rovira, M.; Galiana, I.; Giménez, C.; Lozano-Torres, B.; Paez-Ribes, M.; Llanos, S.; Chaib, S.; Muñoz-Martín, M.; Ucero, A.C.; et al. A versatile drug delivery system targeting senescent cells. EMBO Mol. Med. 2018, 10, e9355. [Google Scholar] [CrossRef]
- Guerrero, A.; Guiho, R.; Herranz, N.; Uren, A.; Withers, D.J.; Martínez-Barbera, J.P.; Tietze, L.F.; Gil, J. Galactose-modified duocarmycin prodrugs as senolytics. Aging Cell 2020, 19, e13133. [Google Scholar] [CrossRef] [PubMed]
- Prata, L.G.L.; Ovsyannikova, I.G.; Tchkonia, T.; Kirkland, J.L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin. Immunol. 2018, 40, 101275. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 2015, 112, E6301–E6310. [Google Scholar] [CrossRef] [PubMed]
- Tilstra, J.S.; Robinson, A.R.; Wang, J.; Gregg, S.Q.; Clauson, C.L.; Reay, D.P.; Nasto, L.A.; Croix, C.M.S.; Usas, A.; Vo, N.; et al. NF-κB inhibition delays DNA damage–induced senescence and aging in mice. J. Clin. Investig. 2012, 122, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Lamming, D.W.; Ye, L.; Sabatini, D.M.; Baur, J.A. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J. Clin. Investig. 2013, 123, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, O.; Deschênes-Simard, X.; St-Germain, E.; Igelmann, S.; Huot, G.; Cadar, A.E.; Bourdeau, V.; Pollak, M.N.; Ferbeyre, G. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 2013, 12, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, K.; Jabbour, E.; Skinner, J.; Anderson, K.; Dellasala, S.; Yilmaz, M.; Ferrajoli, A.; Bose, P.; Thompson, P.; Alvarado, Y.; et al. Long-term follow-up of lower dose dasatinib (50 mg daily) as frontline therapy in newly diagnosed chronic-phase chronic myeloid leukemia. Cancer 2020, 126, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ottmann, O.; Saglio, G.; Apperley, J.F.; Arthur, C.; Bullorsky, E.; Charbonnier, A.; Dipersio, J.F.; Kantarjian, H.; Khoury, H.J.; Kim, D.-W.; et al. Long-term efficacy and safety of dasatinib in patients with chronic myeloid leukemia in accelerated phase who are resistant to or intolerant of imatinib. Blood Cancer J. 2018, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Neff, F.; Flores-Dominguez, D.; Ryan, D.P.; Horsch, M.; Schröder, S.; Adler, T.; Afonso, L.C.; Aguilar-Pimentel, J.A.; Becker, L.; Garrett, L.; et al. Rapamycin extends murine lifespan but has limited effects on aging. J. Clin. Investig. 2013, 123, 3272–3291. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.H.M.; D’rozario, J.; Mendonca, S.; Bhuvan, T.; Payne, N.L.; Zheng, D.; Hisana, A.; Wallis, G.; Barugahare, A.; Powell, D.; et al. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nat. Commun. 2021, 12, 6495. [Google Scholar] [CrossRef]
- Tripathi, U.; Misra, A.; Tchkonia, T.; Kirkland, J.L. Impact of Senescent Cell Subtypes on Tissue Dysfunction and Repair: Importance and Research Questions. Mech. Ageing Dev. 2021, 198, 111548. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef]
- Xia, L.; Wang, X.X.; Hu, X.S.; Guo, X.G.; Shang, Y.P.; Chen, H.J.; Zeng, C.L.; Zhang, F.R.; Chen, J.Z. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br. J. Pharmacol. 2008, 155, 387–394. [Google Scholar] [CrossRef]
- Csiszar, A.; Sosnowska, D.; Wang, M.; Lakatta, E.G.; Sonntag, W.E.; Ungvari, Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate macaca mulatta: Reversal by resveratrol treatment. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 811–820. [Google Scholar] [CrossRef]
- Giovannelli, L.; Pitozzi, V.; Jacomelli, M.; Mulinacci, N.; Laurenzana, A.; Dolara, P.; Mocali, A. Protective Effects of Resveratrol Against Senescence-Associated Changes in Cultured Human Fibroblasts. J. Gerontol. Ser. A 2011, 66A, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Baur, J.A.; Boyd, A.R.; de Cabo, R.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nelson, J.F.; et al. Rapamycin, But Not Resveratrol or Simvastatin, Extends Life Span of Genetically Heterogeneous Mice. J. Gerontol. Biol. Sci. Med. Sci. 2011, 66A, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.; Miller, R.A.; Astle, C.M.; Baur, J.A.; de Cabo, R.; Fernandez, E.; Guo, W.; Javors, M.; Kirkland, J.L.; Nelson, J.F.; et al. Evaluation of Resveratrol, Green Tea Extract, Curcumin, Oxaloacetic Acid, and Medium-Chain Triglyceride Oil on Life Span of Genetically Heterogeneous Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiao, K.; Zhou, Q.; Yang, J.; Yang, K.; Hu, C.; Zhou, M.; Li, Z. Resveratrol Alleviates 27-Hydroxycholesterol-Induced Senescence in Nerve Cells and Affects Zebrafish Locomotor Behavior via Activation of SIRT1-Mediated STAT3 Signaling. Oxidative Med. Cell. Longev. 2021, 2021, 6673343. [Google Scholar] [CrossRef]
- Ali, D.; Chen, L.; Kowal, J.M.; Okla, M.; Manikandan, M.; AlShehri, M.; AlMana, Y.; AlObaidan, R.; AlOtaibi, N.; Hamam, R.; et al. Resveratrol inhibits adipocyte differentiation and cellular senescence of human bone marrow stromal stem cells. Bone 2020, 133, 115252. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Lim, H.; Park, H.; Kim, H.P. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem. Pharmacol. 2015, 96, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Jiang, H.; Li, Y.; Gao, Q.; Xu, Y.N.; Kim, N. Kaempferol alleviates the reduction of developmental competence during aging of porcine oocytes. Anim. Sci. J. 2019, 90, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.-S.; Choi, D.-H.; Choi, J.; Cho, S.Y.; Kim, S.-H.; Baek, H.-S.; Yoon, K.D.; Son, S.W.; Son, E.D.; et al. Kaempferol tetrasaccharides restore skin atrophy via PDK1 inhibition in human skin cells and tissues: Bench and clinical studies. Biomed. Pharmacother. 2022, 156, 113864. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tan, Y.; Liu, F.; Wang, J.; Liu, F.; Zhang, Q.; Li, J. Pharmacological network analysis of the functions and mechanism of kaempferol from Du Zhong in intervertebral disc degeneration (IDD). J. Orthop. Transl. 2023, 39, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Zhang, F.; Wang, H.; Yao, J.; Chen, R.; Zhou, Z.; Yang, K.; Xie, Y.; Wan, T.; Ding, H. Apigenin exhibits protective effects in a mouse model ofd-galactose-induced aging via activating the Nrf2 pathway. Food Funct. 2017, 8, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Zohreh, B.; Masoumeh, V.; Fakhraddin, N.; Omrani, G.H.R. Apigenin-mediated Alterations in Viability and Senescence of SW480 Colorectal Cancer Cells Persist in The Presence of L-thyroxine. Anti-Cancer Agents Med. Chem. 2019, 19, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Perrott, K.M.; Wiley, C.D.; Desprez, P.-Y.; Campisi, J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. GeroScience 2017, 39, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Li, B.S.; Zhu, R.Z.; Lim, S.-H.; Seo, J.H.; Choi, B.-M. Apigenin Alleviates Oxidative Stress-Induced Cellular Senescence via Modulation of the SIRT1-NAD+-CD38 Axis. Am. J. Chin. Med. 2021, 49, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Kim, J.-R.; Choi, H.C. Genistein-induced LKB1–AMPK activation inhibits senescence of VSMC through autophagy induction. Vasc. Pharmacol. 2016, 81, 75–82. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Pang, X.; Zhao, Z.; Yu, H.; Zhou, H. Genistein protects against ox-LDL-induced senescence through enhancing SIRT1/LKB1/AMPK-mediated autophagy flux in HUVECs. Mol. Cell. Biochem. 2019, 455, 127–134. [Google Scholar] [CrossRef]
- Zhang, H.; Pang, X.; Yu, H.; Zhou, H. Genistein suppresses ox-LDL-elicited oxidative stress and senescence in HUVECs through the SIRT1-p66shc-Foxo3a pathways. J. Biochem. Mol. Toxicol. 2022, 36, e22939. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, S.; Qu, G.; Hua, J.; Zong, J.; Li, X.; Xu, F. Genistein alleviates H2O2 -induced senescence of human umbilical vein endothelial cells via regulating the TXNIP/NLRP3 axis. Pharm. Biol. 2021, 59, 1386–1399. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, Y.; Xue, K.; Li, J.; Son, G.; Wang, J.; Qian, W.; Wang, S.; Zheng, J.; Yang, C.; et al. Genistein mitigates senescence of bone marrow mesenchymal stem cells via ERRα-mediated mitochondrial biogenesis and mitophagy in ovariectomized rats. Redox Biol. 2023, 61, 102649. [Google Scholar] [CrossRef] [PubMed]
- Weis, K.E.; Raetzman, L.T. Genistein inhibits proliferation and induces senescence in neonatal mouse pituitary gland explant cultures. Toxicology 2019, 427, 152306. [Google Scholar] [CrossRef]
- Jenie, R.I.; Amalina, N.D.; Ilmawati, G.P.N.; Utomo, R.Y.; Ikawati, M.; Khumaira, A.; Kato, J.Y.; Meiyanto, E. Cell Cycle Modulation of CHO-K1 Cells Under Genistein Treatment Correlates with Cells Senescence, Apoptosis and ROS Level but in a Dose-Dependent Manner. Adv. Pharm. Bull. 2019, 9, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Lin, C. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors 2018, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, N.; Liang, B.; Liu, Q.; Zhang, E.; Peng, L.; Deng, H.; Li, R.; Li, Z.; Zhu, H. Pterostilbene protects against UVB-induced photo-damage through a phosphatidylinositol-3-kinase-dependent Nrf2/ARE pathway in human keratinocytes. Redox Rep. 2017, 22, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-J.; Chiu, H.-W.; Lee, Y.-L.; Li, C.-Y.; Wang, Y.-J.; Lee, Y.-H. Pterostilbene Attenuates Hexavalent Chromium-Induced Allergic Contact Dermatitis by Preventing Cell Apoptosis and Inhibiting IL-1β-Related NLRP3 Inflammasome Activation. J. Clin. Med. 2018, 7, 489. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.-L.; Huang, P.-H.; Wang, H.-C.; Tseng, C.-H.; Yen, F.-L. Pterostilbene Attenuates Particulate Matter-Induced Oxidative Stress, Inflammation and Aging in Keratinocytes. Antioxidants 2021, 10, 1552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, J.; Tie, X.; Lin, T.; Yang, W.; Li, Z.; Zou, Y.; Guan, G.; Liu, P.; Luo, W.; et al. Pterostilbene and its nicotinate derivative ameliorated vascular endothelial senescence and elicited endothelium-dependent relaxations via activation of sirtuin 1. Can. J. Physiol. Pharmacol. 2021, 99, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, X.; He, X.; Ma, S.; Sun, C.; Zhang, J.; Xu, X.; Jin, W.; Yan, J.; Lin, P.; et al. Suppressive effects of pterostilbene on human cytomegalovirus (HCMV) infection and HCMV-induced cellular senescence. Virol. J. 2022, 19, 224. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, Y.; Xu, W.; Wang, X.; Jin, H.; Bao, X.; Lu, C. Induction of Sestrin2 by pterostilbene suppresses ethanol-triggered hepatocyte senescence by degrading CCN1 via p62-dependent selective autophagy. Cell Biol. Toxicol. 2023, 39, 729–749. [Google Scholar] [CrossRef]
- Giovannelli, L. Beneficial effects of olive oil phenols on the aging process: Experimental evidence and possible mechanisms of action. Nutr. Aging 2012, 1, 207–223. [Google Scholar] [CrossRef]
- Menicacci, B.; Cipriani, C.; Margheri, F.; Mocali, A.; Giovannelli, L. Modulation of the Senescence-Associated Inflammatory Phenotype in Human Fibroblasts by Olive Phenols. Int. J. Mol. Sci. 2017, 18, 2275. [Google Scholar] [CrossRef] [PubMed]
- Frediani, E.; Scavone, F.; Laurenzana, A.; Chillà, A.; Tortora, K.; Cimmino, I.; Leri, M.; Bucciantini, M.; Mangoni, M.; Fibbi, G.; et al. Olive phenols preserve lamin B1 expression reducing cGAS/STING/NFκB-mediated SASP in ionizing radiation-induced senescence. J. Cell. Mol. Med. 2022, 26, 2337–2350. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Choi, M. Anti-inflammatory and anti-aging effects of hydroxytyrosol on human dermal fibroblasts (HDFs). Biomed. Dermatol. 2018, 2, 21. [Google Scholar] [CrossRef]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as Neuroprotective Agent: From Bench to Bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Dizaj, S.M.; Saadat, Y.R.; Khezri, K.; Jafari, S.; Ahmadian, E.; Jahandizi, N.G.; Raeesi, S. Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Wufuer, H.; Li, Z.; Sun, R.; Jiang, Z.; Dou, X.; Fu, Q.; Campisi, J.; Sun, Y. Rutin is a potent senomorphic agent to target senescent cells and can improve chemotherapeutic efficacy. Aging Cell 2024, 23, e13921. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. BioFactors 2021, 47, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Younis, R.L.; El-Gohary, R.M.; Ghalwash, A.A.; Hegab, I.I.; Ghabrial, M.M.; Aboshanady, A.M.; Mostafa, R.A.; El-Azeem, A.H.A.; Farghal, E.E.; Belal, A.A.; et al. Luteolin Mitigates D-Galactose-Induced Brain Ageing in Rats: SIRT1-Mediated Neuroprotection. Neurochem. Res. 2024, 49, 2803–2820. [Google Scholar] [CrossRef] [PubMed]
- Mbara, K.C.; Devnarain, N.; Owira, P.M.O. Potential Role of Polyphenolic Flavonoids as Senotherapeutic Agents in Degenerative Diseases and Geroprotection. Pharm. Med. 2022, 36, 331–352. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Sacco, S.M.; Gil-Izquierdo, A.; Trzeciakiewicz, A.; Morand, C.; Barron, D.; Pinaud, S.; Offord, E.; Horcajada, M.-N. Differential effects of two citrus flavanones on bone quality in senescent male rats in relation to their bioavailability and metabolism. Bone 2011, 49, 1108–1116. [Google Scholar] [CrossRef]
- Tsai, Y.-F.; Chen, Y.-R.; Chen, J.-P.; Tang, Y.; Yang, K.-C. Effect of hesperidin on anti-inflammation and cellular antioxidant capacity in hydrogen peroxide-stimulated human articular chondrocytes. Process. Biochem. 2019, 85, 175–184. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.-A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus Polyphenol Hesperidin Stimulates Production of Nitric Oxide in Endothelial Cells while Improving Endothelial Function and Reducing Inflammatory Markers in Patients with Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Chen, Z.; Xie, Q.; Lei, H.; Xiang, S. Hesperidin protects against IL-1β-induced inflammation in human osteoarthritis chondrocytes. Exp. Ther. Med. 2018, 16, 3721–3727. [Google Scholar] [CrossRef]
- Choi, E.M.; Lee, Y.S. Effects of hesperetin on the production of inflammatory mediators in IL-1β treated human synovial cells. Cell. Immunol. 2010, 264, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadou, K.; Vauzour, D.; Lee, H.Y.; Rodriguez-Mateos, A.; Williams, R.J.; Spencer, J.P.E. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch. Biochem. Biophys. 2009, 484, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Nyane, N.A.; Tlaila, T.B.; Malefane, T.G.; Ndwandwe, D.E.; Owira, P.M.O. Metformin-like antidiabetic, cardio-protective and non-glycemic effects of naringenin: Molecular and pharmacological insights. Eur. J. Pharmacol. 2017, 803, 103–111. [Google Scholar] [CrossRef]
- Da Pozzo, E.; Costa, B.; Cavallini, C.; Testai, L.; Martelli, A.; Calderone, V.; Martini, C. The Citrus Flavanone Naringenin Protects Myocardial Cells against Age-Associated Damage. Oxidative Med. Cell. Longev. 2017, 2017, 9536148. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wu, Y.; He, D.; Zhu, X.; Li, H.; Liu, H.; Liu, H. Anti-aging effects of Ribes meyeri anthocyanins on neural stem cells and aging mice. Aging 2020, 12, 17738–17753. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Kim, G.R. Inhibitory effect of naringenin on LPS-induced skin senescence by SIRT1 regulation in HDFs. Biomed. Dermatol. 2018, 2, 26. [Google Scholar] [CrossRef]
- Farsad-Naeimi, A.; Alizadeh, M.; Esfahani, A.; Aminabad, E.D. Effect of fisetin supplementation on inflammatory factors and matrix metalloproteinase enzymes in colorectal cancer patients. Food Funct. 2018, 9, 2025–2031. [Google Scholar] [CrossRef]
- Wang, L.; Cao, D.; Wu, H.; Jia, H.; Yang, C.; Zhang, L. Fisetin prolongs therapy window of brain ischemic stroke using tissue plasminogen activator: A double-blind randomized placebo-controlled clinical trial. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619871359. [Google Scholar] [CrossRef] [PubMed]
- Hambright, W.S.; Duke, V.R.; Goff, A.D.; Goff, A.W.; Minas, L.T.; Kloser, H.; Gao, X.; Huard, C.; Guo, P.; Lu, A.; et al. Clinical validation of C12FDG as a marker associated with senescence and osteoarthritic phenotypes. Aging Cell 2024, 23, e14113. [Google Scholar] [CrossRef] [PubMed]
- Tavenier, J.; Nehlin, J.O.; Houlind, M.B.; Rasmussen, L.J.; Tchkonia, T.; Kirkland, J.L.; Andersen, O.; Rasmussen, L.J.H. Fisetin as a senotherapeutic agent: Evidence and perspectives for age-related diseases. Mech. Ageing Dev. 2024, 222, 111995. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, R.; Sharma, A.; Goel, A.; Padwad, Y. Long term consumption of green tea EGCG enhances healthspan and lifespan in mice by mitigating multiple aspects of cellular senescence in mitotic and post-mitotic tissues, gut dysbiosis and immunosenescence. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Kumari, A.; Gulati, A.; Padwad, Y.; Sharma, R. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology 2019, 20, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Han, D.-W.; Lee, M.H.; Kim, B.; Lee, J.J.; Hyon, S.-H.; Park, J.-C. Preventive effects of epigallocatechin-3-O-gallate against replicative senescence associated with p53 acetylation in human dermal fibroblasts. Oxidative Med. Cell. Longev. 2012, 2012, 850684. [Google Scholar] [CrossRef]
- Lambert, J.D.; Lee, M.-J.; Lu, H.; Meng, X.; Hong, J.J.J.; Seril, D.N.; Yang, C.S.; Sturgill, M.G. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr. 2003, 133, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, E.O.W.; Zhu, Y.; Tchkonia, T.; Kirkland, J.L. Discovery, development, and future application of senolytics: Theories and predictions. FEBS J. 2020, 287, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef]
- Meiners, F.; Hinz, B.; Boeckmann, L.; Secci, R.; Sueto, S.; Kuepfer, L.; Fuellen, G.; Barrantes, I. Computational identification of natural senotherapeutic compounds that mimic dasatinib based on gene expression data. Sci. Rep. 2024, 14, 6286. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fu, Q.; Li, Z.; Liu, H.; Wang, Y.; Lin, X.; He, R.; Zhang, X.; Ju, Z.; Campisi, J.; et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 2021, 3, 1706–1726. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, P.; Ling, Y.; Song, X.; Lu, Z.; He, Q.; Li, Z.; Lu, N.; Guo, Q. Inhibitory effects of GL-V9 on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-2/9. Eur. J. Pharm. Sci. 2011, 43, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Ren, C.; Kong, Y.; Ni, Q.; Wang, Z.; Zhao, D.; Li, N.; Chen, X.; Lu, Y. Determination of GL-V9, a derivative of wogonin, in rat plasma by UPLC–MS/MS and its application to a pharmacokinetic study after oral and pulmonary administration. Biomed. Chromatogr. 2019, 33, e4556. [Google Scholar] [CrossRef]
- Yang, D.; Tian, X.; Ye, Y.; Liang, Y.; Zhao, J.; Wu, T.; Lu, N. Identification of GL-V9 as a novel senolytic agent against senescent breast cancer cells. Life Sci. 2021, 272, 119196. [Google Scholar] [CrossRef]
- Slika, L.; Patra, D. Traditional Uses, Therapeutic Effects and Recent Advances of Curcumin: A Mini-Review. Mini Rev. Med. Chem. 2020, 20, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, J.; Wang, X.; Zhang, R. Curcumin Alleviates D-Galactose-Induced Cardiomyocyte Senescence by Promoting Autophagy via the SIRT1/AMPK/mTOR Pathway. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Deng, J.; Ouyang, P.; Li, W.; Zhong, L.; Gu, C.; Shen, L.; Cao, S.; Yin, L.; Ren, Z.; Zuo, Z.; et al. Curcumin Alleviates the Senescence of Canine Bone Marrow Mesenchymal Stem Cells during In Vitro Expansion by Activating the Autophagy Pathway. Int. J. Mol. Sci. 2021, 22, 11356. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Lee, S.-J.; Chandrasekaran, P.; Lamichhane, G.; O’connell, J.F.; Egan, J.M.; Kim, Y. Dietary Curcumin Attenuates Hepatic Cellular Senescence by Suppressing the MAPK/NF-κB Signaling Pathway in Aged Mice. Antioxidants 2023, 12, 1165. [Google Scholar] [CrossRef]
- Li, J.-H.; Wei, T.-T.; Guo, L.; Cao, J.-H.; Feng, Y.-K.; Guo, S.-N.; Liu, G.-H.; Ding, Y.; Chai, Y.-R. Curcumin protects thymus against D-galactose-induced senescence in mice. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394, 411–420. [Google Scholar] [CrossRef]
- Serati-Nouri, H.; Rasoulpoor, S.; Pourpirali, R.; Sadeghi-Soureh, S.; Esmaeilizadeh, N.; Dadashpour, M.; Roshangar, L.; Zarghami, N. In vitro expansion of human adipose-derived stem cells with delayed senescence through dual stage release of curcumin from mesoporous silica nanoparticles/electrospun nanofibers. Life Sci. 2021, 285, 119947. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.K.; Ferstl, E.M.; Davis, M.C.; Herold, M.; Kurtkaya, S.; Camalier, R.F.; Hollingshead, M.G.; Kaur, G.; Sausville, E.A.; Rickles, F.R.; et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorganic Med. Chem. 2004, 12, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, Y.; Zhang, R.; Zheng, G.; Zhou, D. The curcumin analog EF24 is a novel senolytic agent. Aging 2019, 11, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Yue, J.; Sims, M.; Pfeffer, L.M. The curcumin analog EF24 targets NF-κB and miRNA-21, and has potent anticancer activity in vitro and in vivo. PLoS ONE 2013, 8, e71130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Zheng, R.; Hou, K.; Zhang, Y.; Jia, T.; Lu, X.; Samarawickrama, P.N.; Jia, S.; He, Y.; et al. Curcumin analogue EF24 prevents alveolar epithelial cell senescence to ameliorate idiopathic pulmonary fibrosis via activation of PTEN. Phytomedicine 2024, 133, 155882. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M.; Bracci, M.; Santarelli, L.; Sayeed, M.A.; Pierpaoli, E.; Giacconi, R.; Costarelli, L.; Piacenza, F.; Basso, A.; Cardelli, M.; et al. Inducers of Senescence, Toxic Compounds, and Senolytics: The Multiple Faces of Nrf2-Activating Phytochemicals in Cancer Adjuvant Therapy. Mediat. Inflamm. 2018, 2018, 4159013. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, S.; Liu, X.; Wang, Y.; Chang, J.; Zhang, X.; Mackintosh, S.G.; Tackett, A.J.; He, Y.; Lv, D.; et al. Oxidation resistance 1 is a novel senolytic target. Aging Cell 2018, 17, e12780. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Liu, X.; Zhang, X.; Shi, P.; Wang, Y.; Zhou, D.; Zheng, G. Design and optimization of piperlongumine analogs as potent senolytics. Bioorganic Med. Chem. Lett. 2024, 98, 129593. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Patra, J.K.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Stinco, C.M.; Heredia, F.J.; Vicario, I.M.; Meléndez-Martínez, A.J. In vitro antioxidant capacity of tomato products: Relationships with their lycopene, phytoene, phytofluene and alpha-tocopherol contents, evaluation of interactions and correlation with reflectance measurements. LWT Food Sci. Technol. 2016, 65, 718–724. [Google Scholar] [CrossRef]
- Mishra, A.K.; Singh, R.; Rawat, H.; Kumar, V.; Jagtap, C.; Jain, A. The influence of food matrix on the stability and bioavailability of phytochemicals: A comprehensive review. Food Humanit. 2024, 2, 100202. [Google Scholar] [CrossRef]
- De Luca, F.; Gola, F.; Azzalin, A.; Casali, C.; Gaiaschi, L.; Milanesi, G.; Vicini, R.; Rossi, P.; Bottone, M.G. A Lombard Variety of Sweet Pepper Regulating Senescence and Proliferation: The Voghera Pepper. Nutrients 2024, 16, 1681. [Google Scholar] [CrossRef]
- Chen, X.; Wu, F.; Chen, C.; Ren, Q.; Zhang, A. Ginkgo Biloba Extract Can Antagonize Subchronic Arsenite Exposure-Induced Hepatocyte Senescence by Inhibiting Oxidative Damage and Inflammation in Rats. Biol. Trace Elem. Res. 2024, 202, 4596–4604. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleky, H.M.; Zhou, J.-R. Underlying Mechanisms of Brain Aging and Neurodegenerative Diseases as Potential Targets for Preventive or Therapeutic Strategies Using Phytochemicals. Nutrients 2023, 15, 3456. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis Mediates Acquired Resilience: Using Plant-Derived Chemicals to Enhance Health. Annu. Rev. Food Sci. Technol. 2021, 12, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.-M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Rodier, F.; Patil, C.K.; Freund, A.; Desprez, P.-Y.; Campisi, J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 2011, 286, 36396–36403. [Google Scholar] [CrossRef]
- Watanabe, S.; Kawamoto, S.; Ohtani, N.; Hara, E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017, 108, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Favari, C.; de Alvarenga, J.F.R.; Sánchez-Martínez, L.; Tosi, N.; Mignogna, C.; Cremonini, E.; Manach, C.; Bresciani, L.; Del Rio, D.; Mena, P. Factors driving the inter-individual variability in the metabolism and bioavailability of (poly)phenolic metabolites: A systematic review of human studies. Redox Biol. 2024, 71, 103095. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Vedova, L.; Baron, G.; Morazzoni, P.; Aldini, G.; Gado, F. The Potential of Polyphenols in Modulating the Cellular Senescence Process: Implications and Mechanism of Action. Pharmaceuticals 2025, 18, 138. https://doi.org/10.3390/ph18020138
Della Vedova L, Baron G, Morazzoni P, Aldini G, Gado F. The Potential of Polyphenols in Modulating the Cellular Senescence Process: Implications and Mechanism of Action. Pharmaceuticals. 2025; 18(2):138. https://doi.org/10.3390/ph18020138
Chicago/Turabian StyleDella Vedova, Larissa, Giovanna Baron, Paolo Morazzoni, Giancarlo Aldini, and Francesca Gado. 2025. "The Potential of Polyphenols in Modulating the Cellular Senescence Process: Implications and Mechanism of Action" Pharmaceuticals 18, no. 2: 138. https://doi.org/10.3390/ph18020138
APA StyleDella Vedova, L., Baron, G., Morazzoni, P., Aldini, G., & Gado, F. (2025). The Potential of Polyphenols in Modulating the Cellular Senescence Process: Implications and Mechanism of Action. Pharmaceuticals, 18(2), 138. https://doi.org/10.3390/ph18020138








