1-Azinyl-1′-Alkenylferrocenes with Anticholinesterase, Antioxidant, and Antiaggregating Activities as Multifunctional Agents for Potential Treatment of Alzheimer’s Disease
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Products
2.2. Biological Investigations
2.2.1. Inhibition Studies of HsAChE, EcBChE, and SsCES. Structure-Activity Relationships
2.2.2. Kinetic Studies of HsAChE and EcBChE Inhibition
2.2.3. Molecular Docking of Fc Derivatives and Reference Compounds into HsAChE
2.2.4. Molecular Docking of Fc Derivatives and Reference Compounds into EcBChE
2.2.5. Molecular Docking of Fc Derivatives and Reference Compounds into SsCES1
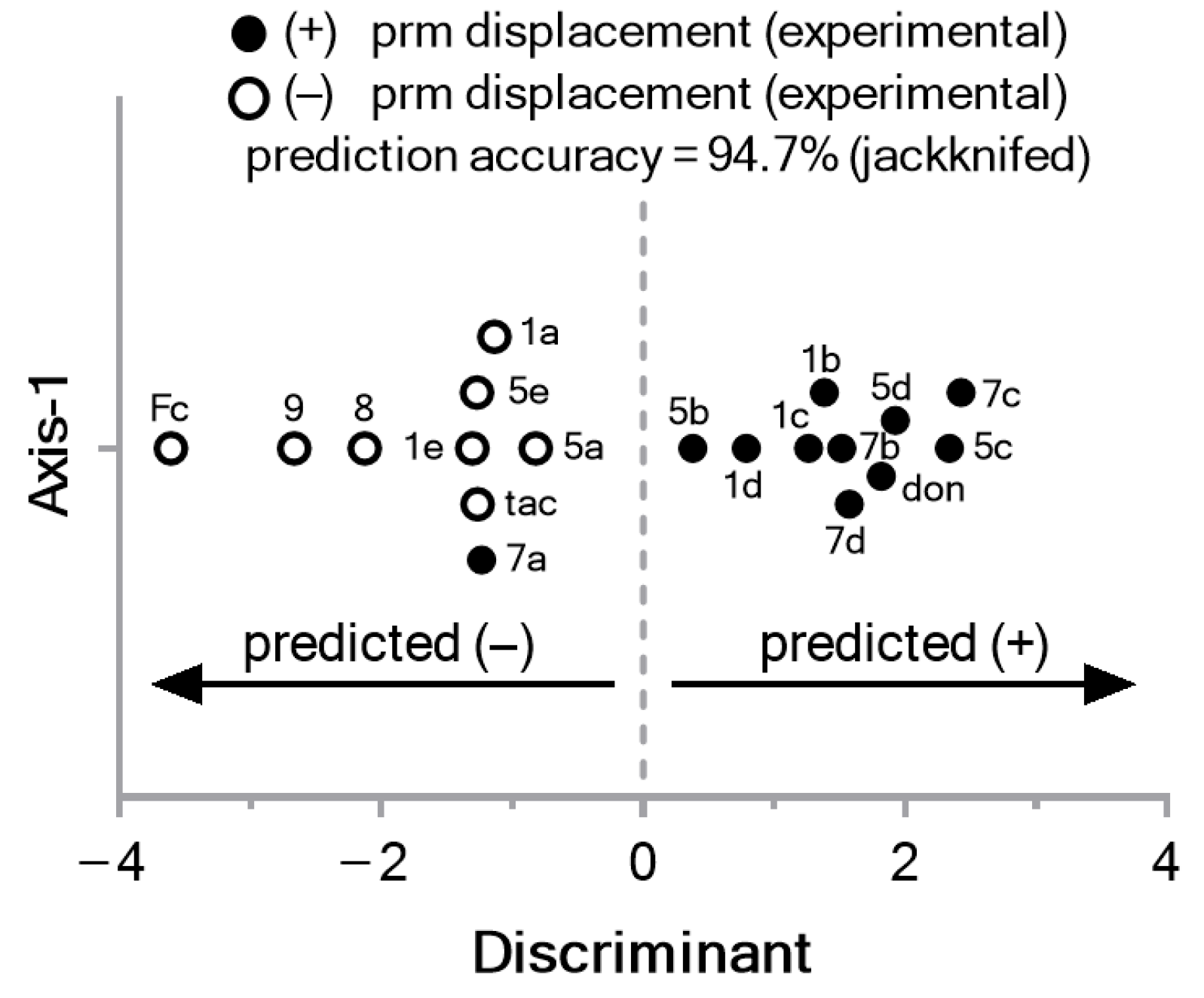
2.2.6. Displacement of Propidium from the EeAChE PAS
2.2.7. Molecular Docking of Fc Derivatives and Reference Compounds into EeAChE
2.2.8. Inhibition of HsAβ42 Self-Aggregation
2.2.9. Molecular Docking of Fc Derivatives and Reference Compounds to HsAβ42
2.2.10. Antioxidant Activity (AOA)
2.2.11. QC Analyses of Antioxidant Activity (AOA)
2.2.12. Cytotoxicity of Fc Derivatives
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 1-(Pyridazin-4-yl)Ferrocene 1e
3.1.2. Synthesis of 1-Acetyl-1′-(Pyridazin-4-yl)Ferrocene 2e
3.1.3. General Procedure for Formation of 1-Isopropenyl-1′-(Pyridazin-4-yl)Ferrocene 5e
3.1.4. Synthesis of 1-Azinyl-1′-(α-Hydroxyethyl)Ferrocenes 6a-e (General Procedure)
1-(Pyridin-2-yl)-1′-(α-Hydroxyethyl)Ferrocene (6a)
1-(Quinolin-2-yl)-1′-(α-Hydroxyethyl)Ferrocene (6b)
1-(Acridin-9-yl)-1′-(α-Hydroxyethyl)Ferrocene (6c)
1-(2,2′-Bipyridin-6-yl)-1′-(α-Hydroxyethyl)Ferrocene (6d)
1-(Pyridazin-4-yl)-1′-(α-Hydroxyethyl)Ferrocene (6e)
3.1.5. Synthesis of 1-Azinyl-1′-Vinylferrocenes 7a-e (General Procedure)
1-(Pyridin-2-yl)-1′-Vinylferrocene (7a)
1-(Quinolin-2-yl)-1′-Vinylferrocene (7b)
1-(Acridin-9-yl)-1′-Vinylferrocene (7c)
1-(2,2′-Bipyridin-6-yl)-1′-Vinylferrocene (7d)
3.1.6. Synthesis of Isopropenylferrocene 8 [60]
3.1.7. Synthesis of Vinylferrocene 9 [51]
3.2. Biological Investigations
3.2.1. In Vitro Inhibition of HsAChE, EcBChE, and SsCES1 Activities
3.2.2. Kinetic Study of HsAChE and EcBChE Inhibition. Determination of Steady-State Inhibition Constants
3.2.3. Propidium Displacement Studies
3.2.4. Inhibition of β-Amyloid (1–42) (HsAβ42) Self-Aggregation
3.2.5. Antioxidant Activity (AOA)
ABTS Radical Cation Scavenging Activity Assay
FRAP Assay
3.2.6. Cytotoxicity Study
3.3. Statistical Analyses
3.3.1. Experimental Data Presentation
3.3.2. Experimental Data Analyses
3.4. Molecular Modeling Studies
3.4.1. Preparation of Ligands for Molecular Docking, Tanimoto Similarity Coefficients (Tc), and Van Der Waals Volumes (Vn)
3.4.2. Quantum Chemical (QC) Calculations
3.4.3. Tc and Vn Calculations
3.4.4. Preparation of Protein Targets for Molecular Docking
3.4.5. Molecular Docking Procedure
3.4.6. Calculation of Overlap Volumes for Docked Ligands
3.4.7. Linear Discriminant Analysis (LDA) of Propidium Displacement from EeAChE PAS
3.4.8. Correlation of HsAβ42 Self-Aggregation % Inhibition and Docking Binding Energy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| AF3 | AlphaFold3 |
| AMD | American Micro Devices |
| Azinyl-Fc(s) | Azinylferrocene(s) |
| BChE | Butyrylcholinesterase |
| bnp | Bis(4-nitrophenyl) phosphate (BNPP) |
| BNPP | Bis(4-nitrophenyl) phosphate |
| CAS | Catalytic active site |
| CES | Carboxylesterase |
| ChE | Cholinesterase |
| CPU | Central processing unit |
| don | Donepezil |
| Eb | Binding energy |
| EcBChE | Equus caballus (equine; horse serum) butyrylcholinesterase |
| EeAChE | Electrophorus electricus (electric eel) acetylcholinesterase |
| FASTA | FAST-All file format for single-letter format sequences |
| Fc | Ferrocene |
| GB | Gigabyte |
| GPU | Graphical processing unit |
| HsAβ42 | Homo sapiens (human) amyloid-βpeptide (1–42) |
| HsAChE | Homo sapiens (human) acetylcholinesterase |
| LDA | Linear discriminant analysis |
| MSA | Multiple sequence alignment |
| myr | Myricetin |
| NCBI | National Center for Biotechnology Information |
| PAS | Peripheral anionic site |
| PAST | PAleontological STatistics |
| PDB | Protein Data Bank |
| prm | Propidium |
| QC | Quantum chemical; quantum-chemical |
| RAM | Random access memory |
| RTX | Ray tracing texel eXtreme |
| SCE | YASARA scene file |
| SDF | Structure-data format |
| SsCES | Sus scrofa (porcine; pig) carboxylesterase |
| SsCES1 | Sus scrofa (porcine; pig) carboxylesterase 1 |
| tac | Tacrine |
| Tc | Tanimoto coefficient |
| Vn | Normalized volume |
| YASARA | Yet another scientific artificial reality application |
| YOB | YASARA object file |
References
- Larik, F.A.; Saeed, A.; Fattah, T.A.; Muqadar, U.; Channar, P.A. Recent advances in the synthesis, biological activities and various applications of ferrocene derivatives. Appl. Organomet. Chem. 2016, 31, e3664. [Google Scholar] [CrossRef]
- Singh, A.; Lumb, I.; Mehra, V.; Kumar, V. Ferrocene-appended pharmacophores: An exciting approach for modulating the biological potential of organic scaffolds. Dalton Trans. 2019, 48, 2840–2860. [Google Scholar] [CrossRef]
- Sharma, S. A Short Review of Past, Present, and Future of Metallocene and Its Derivatives as an Effective Therapeutic Agent. J. Appl. Organomet. Chem. 2023, 3, 142–155. [Google Scholar] [CrossRef]
- Sharma, A.; Rana, R.; Kumar, N.; Kaur Gulati, H.; Jyoti; Khanna, A.; Sharma, S.; Pooja; Singh, J.V.; Bedi, P.M.S. Ferrocene-based compounds: Promising anticancer and antimalarial agents in modern therapeutics. Med. Chem. Res. 2025, 34, 1177–1199. [Google Scholar] [CrossRef]
- Snegur, L.V. Modern Trends in Bio-Organometallic Ferrocene Chemistry. Inorganics 2022, 10, 226. [Google Scholar] [CrossRef]
- Jančić, A.M.; Katanić Stanković, J.S.; Srećković, N.; Mihailović, V.; Komatina, D.I.; Stevanović, D. Ferrocene-containing tetrahydropyrimidin-2(1H)-ones: Antioxidant and antimicrobial activity. J. Organomet. Chem. 2022, 967, 122335. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 66. [Google Scholar] [CrossRef]
- Fouda, M.F.R.; Abd-Elzaher, M.M.; Abdelsamaia, R.A.; Labib, A.A. On the medicinal chemistry of ferrocene. Appl. Organomet. Chem. 2007, 21, 613–625. [Google Scholar] [CrossRef]
- Yeganeh, M.S.; Abbasi, F.; Kazemizadeh, A.R. Recent Advances in the Synthesis of Ferrocene-Based Heterocycles by Multicomponent Reactions: A Review. Curr. Org. Chem. 2019, 22, 2555–2575. [Google Scholar] [CrossRef]
- Halder, B.; Mitra, A.; Dewangan, S.; Gazi, R.; Sarkar, N.; Jana, M.; Chatterjee, S. Solid state synthesis of bispyridyl-ferrocene conjugates with unusual site selective 1,4-Michael addition, as potential inhibitor and electrochemical probe for fibrillation in amyloidogenic protein. J. Mol. Struct. 2023, 1273, 134362. [Google Scholar] [CrossRef]
- Goyal, D.; Shuaib, S.; Mann, S.; Goyal, B. Rationally Designed Peptides and Peptidomimetics as Inhibitors of Amyloid-beta (Abeta) Aggregation: Potential Therapeutics of Alzheimer’s Disease. ACS Comb. Sci. 2017, 19, 55–80. [Google Scholar] [CrossRef]
- Yao, P.; Zhang, J.; You, S.; Qi, W.; Su, R.; He, Z. Ferrocene-modified peptides as inhibitors against insulin amyloid aggregation based on molecular simulation. J. Mater. Chem. B 2020, 8, 3076–3086. [Google Scholar] [CrossRef]
- Jawaria, R.; Hussain, M.; Ahmad, H.B.; Ashraf, M.; Hussain, S.; Naseer, M.M.; Khalid, M.; Hussain, M.A.; al-Rashida, M.; Tahir, M.N.; et al. Probing ferrocene-based thiosemicarbazones and their transition metal complexes as cholinesterase inhibitors. Inorg. Chim. Acta 2020, 508, 119658. [Google Scholar] [CrossRef]
- Almansour, A.I.; Ibrahim Al-Shemaimari, K.; Ibhrahim Alaqeel, S. Cholinesterase inhibitory activity and regioselective synthesis of spiropyrrolidinoindole integrated ferrocene hybrid heterocycles via multicomponent cycloaddition reaction. J. King Saud Univ. Sci. 2024, 36, 103027. [Google Scholar] [CrossRef]
- Altaf, A.A.; Kausar, S.; Hamayun, M.; Lal, B.; Tahir, M.N.; Badshah, A. Ferrocenylaniline based amide analogs of methoxybenzoic acids: Synthesis, structural characterization and butyrylcholinesterase (BChE) inhibition studies. J. Mol. Struct. 2017, 1146, 130–137. [Google Scholar] [CrossRef]
- Altaf, A.A.; Hamayun, M.; Lal, B.; Tahir, M.N.; Holder, A.A.; Badshah, A.; Crans, D.C. Ferrocene-based anilides: Synthesis, structural characterization and inhibition of butyrylcholinesterase. Dalton Trans. 2018, 47, 11769–11781. [Google Scholar] [CrossRef]
- Puranik, N.; Song, M. Therapeutic Role of Heterocyclic Compounds in Neurodegenerative Diseases: Insights from Alzheimer’s and Parkinson’s Diseases. Neurol. Int. 2025, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Waly, O.M.; Saad, K.M.; El-Subbagh, H.I.; Bayomi, S.M.; Ghaly, M.A. Synthesis, biological evaluation, and molecular modeling simulations of new heterocyclic hybrids as multi-targeted anti-Alzheimer’s agents. Eur. J. Med. Chem. 2022, 231, 114152. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Maddeboina, K.; Bachu, R.D.; Boddu, S.H.S.; Trippier, P.C.; Tiwari, A.K. Pivotal role of nitrogen heterocycles in Alzheimer’s disease drug discovery. Drug Discov. Today 2022, 27, 103322. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.D.; Alcantara, A.R.; Gonzalez, J.F.; Sanchez-Montero, J.M. Advances in the discovery of heterocyclic-based drugs against Alzheimer’s disease. Expert Opin. Drug Discov. 2023, 18, 1413–1428. [Google Scholar] [CrossRef]
- Ling, Y.; Hao, Z.-Y.; Liang, D.; Zhang, C.-L.; Liu, Y.-F.; Wang, Y. The Expanding Role of Pyridine and Dihydropyridine Scaffolds in Drug Design. Drug Des. Devel. Ther. 2021, 15, 4289–4338. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Kumar, S.K.A.; Shah, S.K.; Kazi, S.; Sarkar, N.; Banerjee, S.; Dey, S. Pyridine: The scaffolds with significant clinical diversity. RSC Adv. 2022, 12, 15385–15406. [Google Scholar] [CrossRef]
- Shaik, J.B.; Pinjari, M.K.M.; Gangaiah, D.A.; Nallagondu, C.G.R. Synthetic strategies of functionalized pyridines and their therapeutic potential as multifunctional anti-Alzheimer’s agents. In Recent Developments in the Synthesis and Applications of Pyridines; Singh, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 69–126. [Google Scholar] [CrossRef]
- Rammohan, A.; Bhaskar, B.V.; Zyryanov, G.V. Chapter 13—Recent developments in the synthesis of pyridine analogues as a potent anti-Alzheimer’s therapeutic leads. In Recent Developments in the Synthesis and Applications of Pyridines; Singh, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 411–444. [Google Scholar] [CrossRef]
- Kalhor, H.R.; Nazari Khodadadi, A. Synthesis and Structure Activity Relationship of Pyridazine-Based Inhibitors for Elucidating the Mechanism of Amyloid Inhibition. Chem. Res. Toxicol. 2018, 31, 1092–1104. [Google Scholar] [CrossRef]
- Alghamdi, S.; Asif, M. Role of pyridazine analogs as acetylcholinesterase inhibitor: An approach for management of Alzheimer’s disease. Eurasian Chem. Commun. 2021, 3, 435–442. [Google Scholar] [CrossRef]
- Tan, R.X.; Li, W.H.; Pang, J.M.; Zhong, S.M.; Huang, X.Y.; Deng, J.Z.; Zhou, L.Y.; Wu, J.Q.; Wang, X.Q. Design, synthesis, and evaluation of 2,2′-bipyridyl derivatives as bifunctional agents against Alzheimer’s disease. Mol. Divers. 2024, 28, 1225–1238. [Google Scholar] [CrossRef]
- Nagani, A.; Shah, M.; Patel, S.; Patel, H.; Parikh, V.; Patel, A.; Patel, S.; Patel, K.; Parmar, H.; Bhimani, B.; et al. Unveiling piperazine-quinoline hybrids as potential multi-target directed anti-Alzheimer’s agents: Design, synthesis and biological evaluation. Mol. Divers. 2024, 29, 1453–1478. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Diassakou, A.; Kavetsou, E.; Kritsi, E.; Zoumpoulakis, P.; Pontiki, E.; Hadjipavlou-Litina, D.; Detsi, A. Novel quinolinone-pyrazoline hybrids: Synthesis and evaluation of antioxidant and lipoxygenase inhibitory activity. Mol. Divers. 2021, 25, 723–740. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Tzani, A.; Chronaki, K.; Prousis, K.C.; Pontiki, E.; Hadjiplavlou-Litina, D.; Detsi, A. Novel Multi-Target Agents Based on the Privileged Structure of 4-Hydroxy-2-quinolinone. Molecules 2023, 29, 190. [Google Scholar] [CrossRef]
- Yadav, V.; Reang, J.; Sharma, V.; Majeed, J.; Sharma, P.C.; Sharma, K.; Giri, N.; Kumar, A.; Tonk, R.K. Quinoline-derivatives as privileged scaffolds for medicinal and pharmaceutical chemists: A comprehensive review. Chem. Biol. Drug. Des. 2022, 100, 389–418. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.S.S.; Umar, T.; Aulakh, M.K. Quinolines: Privileged Scaffolds for Developing New Anti-neurodegenerative Agents. ChemistrySelect 2023, 8, e202204960. [Google Scholar] [CrossRef]
- Sharma, A.; Piplani, P. Acridine: A Scaffold for the Development of Drugs for Alzheimer’s Disease. Curr. Top. Med. Chem. 2023, 23, 1260–1276. [Google Scholar] [CrossRef]
- Mars, A.; Argoubi, W.; Ben Aoun, S.; Raouafi, N. Induced conformational change on ferrocenyl-terminated alkyls and their application as transducers for label-free immunosensing of Alzheimer’s disease biomarker. RSC Adv. 2016, 6, 2414–2421. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V.; Anand, P.; Kumar, V.; Ranjan Dwivedi, A.; Kumar, V. Advancements in the development of multi-target directed ligands for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2022, 61, 116742. [Google Scholar] [CrossRef]
- Wiles, A.A.; Zhang, X.; Fitzpatrick, B.; Long, D.L.; Macgregor, S.A.; Cooke, G. Redox-mediated reactions of vinylferrocene: Toward redox auxiliaries. Dalton Trans. 2016, 45, 7220–7225. [Google Scholar] [CrossRef]
- Chen, L.; Compton, R.G. Reference Electrodes for Electrochemical Sensors Based on Redox Couples Immobilized within Nafion Films. ACS Sens. 2019, 4, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Lee, W.; Song, J.Y.; Kang, E.; Joo, J.M. Nondirected Pd-catalyzed aerobic C-H alkenylation of ruthenocene and ferrocene. Chem. Commun. 2022, 58, 10809–10812. [Google Scholar] [CrossRef]
- Pi, C.; Li, Y.; Cui, X.; Zhang, H.; Han, Y.; Wu, Y. Redox of ferrocene controlled asymmetric dehydrogenative Heck reaction via palladium-catalyzed dual C–H bond activation. Chem. Sci. 2013, 4, 2675. [Google Scholar] [CrossRef]
- Lou, S.J.; Zhuo, Q.; Nishiura, M.; Luo, G.; Hou, Z. Enantioselective C-H Alkenylation of Ferrocenes with Alkynes by Half-Sandwich Scandium Catalyst. J. Am. Chem. Soc. 2021, 143, 2470–2476. [Google Scholar] [CrossRef]
- Jia, J.; Cui, Y.; Li, Y.; Sheng, W.; Han, L.; Gao, J. Synthesis, third-order nonlinear optical properties and theoretical analysis of vinylferrocene derivatives. Dyes Pigm. 2013, 98, 273–279. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Al Khalyfeh, K.; Schaarschmidt, D.; Korb, M. Multi-functionalized ferrocenes: –Synthesis and characterization –. J. Organomet. Chem. 2016, 804, 87–94. [Google Scholar] [CrossRef]
- Gormen, M.; Pigeon, P.; Top, S.; Hillard, E.A.; Huche, M.; Hartinger, C.G.; de Montigny, F.; Plamont, M.A.; Vessieres, A.; Jaouen, G. Synthesis, cytotoxicity, and COMPARE analysis of ferrocene and [3]ferrocenophane tetrasubstituted olefin derivatives against human cancer cells. ChemMedChem 2010, 5, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Idlas, P.; Ladaycia, A.; Nemati, F.; Lepeltier, E.; Pigeon, P.; Jaouen, G.; Decaudin, D.; Passirani, C. Ferrocifen stealth LNCs and conventional chemotherapy: A promising combination against multidrug-resistant ovarian adenocarcinoma. Int. J. Pharm. 2022, 626, 122164. [Google Scholar] [CrossRef]
- Zyryanova, E.Y.; Utepova, I.A.; Musikhina, A.A.; Boltneva, N.P.; Kovaleva, N.V.; Rudakova, E.V.; Serebryakova, O.G.; Makhaeva, G.F.; Kiskin, M.A.; Lazarev, V.F.; et al. 1,1′-Disubstituted azinylferrocenes: Synthesis, antiaggregation and antioxidant activity. Russ. Chem. Bull. 2024, 73, 2408–2421. [Google Scholar] [CrossRef]
- Fayolle, C.; Pigeon, P.; Fischer-Durand, N.; Salmain, M.; Buriez, O.; Vessieres, A.; Labbe, E. Synthesis, Electrochemical and Fluorescence Properties of the First Fluorescent Member of the Ferrocifen Family and of Its Oxidized Derivatives. Molecules 2022, 27, 6690. [Google Scholar] [CrossRef]
- Wang, Y.; Pigeon, P.; Li, W.; Yan, J.; Dansette, P.M.; Othman, M.; McGlinchey, M.J.; Jaouen, G. Diversity-oriented synthesis and bioactivity evaluation of N-substituted ferrocifen compounds as novel antiproliferative agents against TNBC cancer cells. Eur. J. Med. Chem. 2022, 234, 114202. [Google Scholar] [CrossRef]
- Wang, Y.; Pigeon, P.; Top, S.; Sanz Garcia, J.; Troufflard, C.; Ciofini, I.; McGlinchey, M.J.; Jaouen, G. Atypical Lone Pair-pi Interaction with Quinone Methides in a Series of Imido-Ferrociphenol Anticancer Drug Candidates. Angew. Chem. Int. Ed. Engl. 2019, 58, 8421–8425. [Google Scholar] [CrossRef]
- Jary, W.G.; Mahler, A.-K.; Purkathofer, T.; Baumgartner, J. A convenient synthesis of E-alkenylferrocenes. J. Organomet. Chem. 2001, 629, 208–212. [Google Scholar] [CrossRef]
- Shi, R.; Wang, H.; Tang, P.; Bin, Y. Synthesis of vinylferrocene and the ligand-exchange reaction between its copolymer and carbon nanotubes. Front. Chem. Sci. Eng. 2014, 8, 171–178. [Google Scholar] [CrossRef]
- Šebesta, R.; Plevová, K.; Mudráková, B. A Practical Three-Step Synthesis of Vinylferrocene. Synthesis 2017, 50, 760–763. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Utepova, I.A.; Kovalev, I.S.; Rusinov, V.L.; Starikova, Z.A. Direct C–C Coupling of Ferrocenyllithium and Azaheterocycles by Nucleophilic Substitution of Hydrogen—Synthesis of Mono- and 1,1′-Diazinylferrocenes. Eur. J. Org. Chem. 2007, 2007, 857–862. [Google Scholar] [CrossRef]
- Musikhina, A.A.; Serebrennikova, P.O.; Zabelina, O.N.; Utepova, I.A.; Chupakhin, O.N. Advanced Application of Planar Chiral Heterocyclic Ferrocenes. Inorganics 2022, 10, 152. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Serebrennikova, P.O.; Utepova, I.A.; Musikhina, A.A.; Suvorova, A.I.; Paznikova, Y.A. Two approaches to the synthesis of planar chiral quinolinyl cymantrene. Russ. Chem. Bull. 2020, 69, 458–460. [Google Scholar] [CrossRef]
- Musikhina, A.A.; Utepova, I.A.; Chupakhin, O.N.; Charushin, V.N.; Slepukhin, P.A. Transition metal-free regioselective cross-coupling of azine N-oxides with cymantrenyl lithium. J. Organomet. Chem. 2018, 870, 32–37. [Google Scholar] [CrossRef]
- Utepova, I.A.; Serebrennikova, P.O.; Streltsova, M.S.; Musikhina, A.A.; Fedorchenko, T.G.; Chupakhin, O.N.; Antonchick, A.P. Enantiomerically Enriched 1,2-P,N-Bidentate Ferrocenyl Ligands for 1,3-Dipolar Cycloaddition and Transfer Hydrogenation Reactions. Molecules 2018, 23, 1311. [Google Scholar] [CrossRef] [PubMed]
- Utepova, I.A.; Chupakhin, O.N.; Serebrennikova, P.O.; Musikhina, A.A.; Charushin, V.N. Two approaches in the synthesis of planar chiral azinylferrocenes. J. Org. Chem. 2014, 79, 8659–8667. [Google Scholar] [CrossRef]
- Musikhina, A.A.; Utepova, I.A.; Serebrennikova, P.O.; Chupakhin, O.N.; Charushin, V.N. Synthesis of chiral ferrocenylazines. Negishi cross-coupling or S N H reactions? Russ. J. Org. Chem. 2013, 49, 1191–1194. [Google Scholar] [CrossRef]
- Musikhina, A.A.; Utepova, I.A.; Chupakhin, O.N.; Suvorova, A.I.; Zyryanova, E.Y. Regioselective synthesis of 1-azinyl-1′-isopropenylferrocenes. Mend. Commun. 2020, 30, 209–210. [Google Scholar] [CrossRef]
- Miller, E.J.; Weigelt, C.A.; Serth, J.A.; Rusyid, R.; Brenner, J.; Luck, L.A.; Godlewski, M. The Wittig reaction in the generation of organometallic compounds containing alkenes as side groups. J. Organomet. Chem. 1992, 440, 91–101. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Rudakova, E.V.; Kovaleva, N.V.; Lushchekina, S.V.; Boltneva, N.P.; Proshin, A.N.; Shchegolkov, E.V.; Burgart, Y.V.; Saloutin, V.I. Cholinesterase and carboxylesterase inhibitors as pharmacological agents. Russ. Chem. Bull. 2019, 68, 967–984. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Radchenko, E.V.; Palyulin, V.A.; Rudakova, E.V.; Aksinenko, A.Y.; Sokolov, V.B.; Zefirov, N.S.; Richardson, R.J. Organophosphorus compound esterase profiles as predictors of therapeutic and toxic effects. Chem. Biol. Interact. 2013, 203, 231–237. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Rudakova, E.V.; Serebryakova, O.G.; Aksinenko, A.Y.; Lushchekina, S.V.; Bachurin, S.O.; Richardson, R.J. Esterase profiles of organophosphorus compounds in vitro predict their behavior in vivo. Chem. Biol. Interact. 2016, 259, 332–342. [Google Scholar] [CrossRef]
- Shesterenko, Y.; Shesterenko, Y.; Romanovska, I.; Semenishyn, N.; Smola, S.; Dekina, S. Pig Liver Cytosolic Carboxylesterase 1 and Its Inhibition by Remdesivir and Sofosbuvir. ChemistrySelect 2024, 9, e202305224. [Google Scholar] [CrossRef]
- Taylor, P.; Lappi, S. Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding. Biochemistry 1975, 14, 1989–1997. [Google Scholar] [CrossRef]
- De Ferrari, G.V.; Canales, M.A.; Shin, I.; Weiner, L.M.; Silman, I.; Inestrosa, N.C. A structural motif of acetylcholinesterase that promotes amyloid beta-peptide fibril formation. Biochemistry 2001, 40, 10447–10457. [Google Scholar] [CrossRef] [PubMed]
- Arce, M.P.; Rodriguez-Franco, M.I.; Gonzalez-Munoz, G.C.; Perez, C.; Lopez, B.; Villarroya, M.; Lopez, M.G.; Garcia, A.G.; Conde, S. Neuroprotective and cholinergic properties of multifunctional glutamic acid derivatives for the treatment of Alzheimer’s disease. J. Med. Chem. 2009, 52, 7249–7257. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, M.; Bertucci, C.; Cavrini, V.; Andrisano, V. β-Amyloid aggregation induced by human acetylcholinesterase: Inhibition studies. Biochem. Pharmacol. 2003, 65, 407–416. [Google Scholar] [CrossRef]
- Marco-Contelles, J.; Leon, R.; de los Rios, C.; Garcia, A.G.; Lopez, M.G.; Villarroya, M. New multipotent tetracyclic tacrines with neuroprotective activity. Bioorg. Med. Chem. 2006, 14, 8176–8185. [Google Scholar] [CrossRef] [PubMed]
- Makhaeva, G.F.; Fisenko, V.P.; Bachurin, S.O. New functions of cholinesterases in the development of Alzheimer’s disease as a target for neuroprotective drugs. Russ. Chem. Bull. 2025, 74, 2312–2331. [Google Scholar] [CrossRef]
- Keshet, B.; Gray, J.J.; Good, T.A. Structurally distinct toxicity inhibitors bind at common loci on beta-amyloid fibril. Protein Sci. 2010, 19, 2291–2304. [Google Scholar] [CrossRef]
- Afzal, W.; Afshan, H.; Inam, J.; Farman, S.; Khurshid, B. Targeting β-amyloid in Alzheimer’s disease: A molecular dynamics study of myricetin as a natural inhibitor. Pak. J. Med. Cardiol. Rev. 2025, 4, 2046–2074. [Google Scholar] [CrossRef]
- Itoh, S.G.; Yagi-Utsumi, M.; Kato, K.; Okumura, H. Key Residue for Aggregation of Amyloid-beta Peptides. ACS Chem. Neurosci. 2022, 13, 3139–3151. [Google Scholar] [CrossRef]
- Naldi, M.; Fiori, J.; Pistolozzi, M.; Drake, A.F.; Bertucci, C.; Wu, R.; Mlynarczyk, K.; Filipek, S.; De Simone, A.; Andrisano, V. Amyloid beta-peptide 25–35 self-assembly and its inhibition: A model undecapeptide system to gain atomistic and secondary structure details of the Alzheimer’s disease process and treatment. ACS Chem. Neurosci. 2012, 3, 952–962. [Google Scholar] [CrossRef]
- Ono, K.; Li, L.; Takamura, Y.; Yoshiike, Y.; Zhu, L.; Han, F.; Mao, X.; Ikeda, T.; Takasaki, J.; Nishijo, H.; et al. Phenolic compounds prevent amyloid beta-protein oligomerization and synaptic dysfunction by site-specific binding. J. Biol. Chem. 2012, 287, 14631–14643. [Google Scholar] [CrossRef]
- ByteDance, A.M.L.A.I.S.T.; Chen, X.; Zhang, Y.; Lu, C.; Ma, W.; Guan, J.; Gong, C.; Yang, J.; Zhang, H.; Zhang, K.; et al. Protenix—Advancing Structure Prediction Through a Comprehensive AlphaFold3 Reproduction. bioRxiv 2025. [Google Scholar] [CrossRef]
- Crescenzi, O.; Tomaselli, S.; Guerrini, R.; Salvadori, S.; D’Ursi, A.M.; Temussi, P.A.; Picone, D. Solution structure of the Alzheimer amyloid beta-peptide (1-42) in an apolar microenvironment. Similarity with a virus fusion domain. Eur. J. Biochem. 2002, 269, 5642–5648. [Google Scholar] [CrossRef]
- Santoro, A.; Grimaldi, M.; Buonocore, M.; Stillitano, I.; D’Ursi, A.M. Exploring the Early Stages of the Amyloid Abeta(1-42) Peptide Aggregation Process: An NMR Study. Pharmaceuticals 2021, 14, 732. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Grishchenko, M.V.; Lushchekina, S.V.; Astakhova, T.Y.; Serebryakova, O.G.; Timokhina, E.N.; Zhilina, E.F.; et al. Conjugates of Tacrine and Salicylic Acid Derivatives as New Promising Multitarget Agents for Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 2285. [Google Scholar] [CrossRef] [PubMed]
- Makhaeva, G.F.; Grishchenko, M.V.; Kovaleva, N.V.; Boltneva, N.P.; Rudakova, E.V.; Astakhova, T.Y.; Timokhina, E.N.; Pronkin, P.G.; Lushchekina, S.V.; Khudina, O.G.; et al. Conjugates of amiridine and salicylic derivatives as promising multifunctional CNS agents for potential treatment of Alzheimer’s disease. Arch. Pharm. 2025, 358, e2400819. [Google Scholar] [CrossRef]
- Di Cera, E. Mechanisms of ligand binding. Biophys. Rev. 2020, 1, 11303. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D. Methods for determination of antioxidant capacity: A review. Int. J. Pharm. Sci. Res. 2015, 6, 546–566. [Google Scholar] [CrossRef]
- Shenderovich, I.G. The Partner Does Matter: The Structure of Heteroaggregates of Acridine Orange in Water. Molecules 2019, 24, 2816. [Google Scholar] [CrossRef]
- Jaouen, G.; Top, S. The Ferrocifen Family as Potent and Selective Antitumor Compounds: Mechanisms of Action. In Advances in Organometallic Chemistry and Catalysis; Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 563–580. [Google Scholar] [CrossRef]
- Jaouen, G.; Vessieres, A.; Top, S. Ferrocifen type anti cancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S. Expanding the chemical space of hydrophobic pharmacophores: The role of hydrophobic substructures in the development of novel transcription modulators. MedChemComm 2016, 7, 1082–1092. [Google Scholar] [CrossRef]
- Pigeon, P.; Gormen, M.; Kowalski, K.; Muller-Bunz, H.; McGlinchey, M.J.; Top, S.; Jaouen, G. Atypical McMurry cross-coupling reactions leading to a new series of potent antiproliferative compounds bearing the key [ferrocenyl-ene-phenol] motif. Molecules 2014, 19, 10350–10369. [Google Scholar] [CrossRef] [PubMed]
- Borys, A.M. An Illustrated Guide to Schlenk Line Techniques. Organometallics 2023, 42, 182–196. [Google Scholar] [CrossRef]
- Gao, J.; Martin, A.; Yatvin, J.; White, E.; Locklin, J. Permanently grafted icephobic nanocomposites with high abrasion resistance. J. Mater. Chem. A 2016, 4, 11719–11728. [Google Scholar] [CrossRef]
- Drouin, B.J.; Lavaty, T.G.; Cassak, P.A.; Kukolich, S.G. Measurements of structural and quadrupole coupling parameters for bromoferrocene using microwave spectroscopy. J. Chem. Phys. 1997, 107, 6541–6548. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Lushchekina, S.V.; Boltneva, N.P.; Serebryakova, O.G.; Rudakova, E.V.; Ustyugov, A.A.; Bachurin, S.O.; Shchepochkin, A.V.; Chupakhin, O.N.; Charushin, V.N.; et al. 9-Substituted acridine derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors possessing antioxidant activity for Alzheimer’s disease treatment. Bioorg. Med. Chem. 2017, 25, 5981–5994. [Google Scholar] [CrossRef]
- Taylor, P.; Lwebuga-Mukasa, J.; Lappi, S.; Rademacher, J. Propidium—A fluorescence probe for a peripheral anionic site on acetylcholinesterase. Mol. Pharmacol. 1974, 10, 703–708. [Google Scholar] [CrossRef]
- Konagurthu, A.S.; Whisstock, J.C.; Stuckey, P.J.; Lesk, A.M. MUSTANG: A multiple structural alignment algorithm. Proteins 2006, 64, 559–574. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Lushchekina, S.V.; Faingold, I.I.; Poletaeva, D.A.; Soldatova, Y.V.; Kotelnikova, R.A.; Serkov, I.V.; et al. New Multifunctional Agents Based on Conjugates of 4-Amino-2,3-polymethylenequinoline and Butylated Hydroxytoluene for Alzheimer’s Disease Treatment. Molecules 2020, 25, 5891. [Google Scholar] [CrossRef]
- LeVine, H., 3rd. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Meth. Enzymol. 1999, 309, 274–284. [Google Scholar] [CrossRef]
- Munoz-Ruiz, P.; Rubio, L.; Garcia-Palomero, E.; Dorronsoro, I.; del Monte-Millan, M.; Valenzuela, R.; Usan, P.; de Austria, C.; Bartolini, M.; Andrisano, V.; et al. Design, synthesis, and biological evaluation of dual binding site acetylcholinesterase inhibitors: New disease-modifying agents for Alzheimer’s disease. J. Med. Chem. 2005, 48, 7223–7233. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Elkina, N.A.; Shchegolkov, E.V.; Boltneva, N.P.; Lushchekina, S.V.; Serebryakova, O.G.; Rudakova, E.V.; Kovaleva, N.V.; Radchenko, E.V.; Palyulin, V.A.; et al. Synthesis, molecular docking, and biological evaluation of 3-oxo-2-tolylhydrazinylidene-4,4,4-trifluorobutanoates bearing higher and natural alcohol moieties as new selective carboxylesterase inhibitors. Bioorg. Chem. 2019, 91, 103097. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth. Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Dutysheva, E.A.; Kuznetcova, L.S.; Utepova, I.A.; Margulis, B.A.; Guzhova, I.V.; Lazarev, V.F. Induction of Chaperone Synthesis in Human Neuronal Cells Blocks Oxidative Stress-Induced Aging. Acta Naturae 2025, 17, 29–35. [Google Scholar] [CrossRef]
- PubChem. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 10 October 2025).
- Ozvoldik, K.; Stockner, T.; Krieger, E. YASARA Model-Interactive Molecular Modeling from Two Dimensions to Virtual Realities. J. Chem. Inf. Model. 2023, 63, 6177–6182. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- CCDC. 2025. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 15 October 2025).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16 Revision C. 01. 2016; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Petersson, G.A.; Al-Laham, M.A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Hawkins, P.C.; Skillman, A.G.; Nicholls, A. Comparison of shape-matching and docking as virtual screening tools. J. Med. Chem. 2007, 50, 74–82. [Google Scholar] [CrossRef] [PubMed]
- OpenEye. ROCS 3.8.0.1; Cadence Molecular Sciences: Santa Fe, NM, USA, 2025; Available online: http://www.eyesopen.com (accessed on 15 October 2025).
- Schrödinger, LLC. The Schrödinger Suite, Including Phase (Used for Volume Calculations) (Release 2025-2); Schrödinger, LLC: New York, NY, USA, 2025. [Google Scholar]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Lexa, K.W.; Carlson, H.A. Protein flexibility in docking and surface mapping. Q. Rev. Biophys. 2012, 45, 301–343. [Google Scholar] [CrossRef]
- UniProt. 2025. Available online: https://www.uniprot.org/ (accessed on 11 October 2025).
- NCBI-Protein. 2025. Available online: https://www.ncbi.nlm.nih.gov/protein/ (accessed on 11 October 2025).
- Protenix. 2025. Available online: https://github.com/bytedance/Protenix (accessed on 10 October 2025).
- Krieger, E.; Dunbrack, R.L., Jr.; Hooft, R.W.; Krieger, B. Assignment of protonation states in proteins and ligands: Combining pKa prediction with hydrogen bonding network optimization. Methods Mol. Biol. 2012, 819, 405–421. [Google Scholar] [CrossRef]
- Chen, X.; Fang, L.; Liu, J.; Zhan, C.G. Reaction pathway and free energy profiles for butyrylcholinesterase-catalyzed hydrolysis of acetylthiocholine. Biochemistry 2012, 51, 1297–1305. [Google Scholar] [CrossRef]
- Golicnik, M.; Sinko, G.; Simeon-Rudolf, V.; Grubic, Z.; Stojan, J. Kinetic model of ethopropazine interaction with horse serum butyrylcholinesterase and its docking into the active site. Arch. Biochem. Biophys. 2002, 398, 23–31. [Google Scholar] [CrossRef]
- Kovarik, Z.; Radic, Z.; Grgas, B.; Skrinjaric-Spoljar, M.; Reiner, E.; Simeon-Rudolf, V. Amino acid residues involved in the interaction of acetylcholinesterase and butyrylcholinesterase with the carbamates Ro 02-0683 and bambuterol, and with terbutaline. Biochim. Biophys. Acta 1999, 1433, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Froment, M.T.; Fortier, P.L.; Visicchio, J.E.; Bartels, C.F.; Lockridge, O. Butyrylcholinesterase-catalysed hydrolysis of aspirin, a negatively charged ester, and aspirin-related neutral esters. Biochim. Biophys. Acta 1998, 1387, 41–52. [Google Scholar] [CrossRef]
- Moorad, D.; Chunyuan, L.; Ashima, S.; Bhupendra, D.; Gregory, G. Purification and Determination of the Amino Acid Sequence of Equine Serum Butyrylcholinesterase. Toxicol. Meth. 2008, 9, 219–227. [Google Scholar] [CrossRef]
- Rosenberry, T.L.; Brazzolotto, X.; Macdonald, I.R.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef]
- Bourne, Y.; Grassi, J.; Bougis, P.E.; Marchot, P. Conformational flexibility of the acetylcholinesterase tetramer suggested by x-ray crystallography. J. Biol. Chem. 1999, 274, 30370–30376. [Google Scholar] [CrossRef] [PubMed]
- Salih, E.; Chishti, S.B.; Vicedomine, P.; Cohen, S.G.; Chiara, D.C.; Cohen, J.B. Active-site peptides of acetylcholinesterase of Electrophorus electricus: Labelling of His-440 by 1-bromo-[2-14C] pinacolone and Ser-200 by tritiated diisopropyl fluorophosphate. Biochim. Biophys. Acta 1994, 1208, 324–331. [Google Scholar] [CrossRef]
- Johnson, G.; Moore, S.W. The peripheral anionic site of acetylcholinesterase: Structure, functions and potential role in rational drug design. Curr. Pharm. Des. 2006, 12, 217–225. [Google Scholar] [CrossRef]
- Shafferman, A.; Velan, B.; Ordentlich, A.; Kronman, C.; Grosfeld, H.; Leitner, M.; Flashner, Y.; Cohen, S.; Barak, D.; Ariel, N. Substrate inhibition of acetylcholinesterase: Residues affecting signal transduction from the surface to the catalytic center. EMBO J. 1992, 11, 3561–3568. [Google Scholar] [CrossRef]
- Wiesner, J.; Kriz, Z.; Kuca, K.; Jun, D.; Koca, J. Acetylcholinesterases--the structural similarities and differences. J. Enzyme Inhib. Med. Chem. 2007, 22, 417–424. [Google Scholar] [CrossRef]
- Wang, D.; Zou, L.; Jin, Q.; Hou, J.; Ge, G.; Yang, L. Human carboxylesterases: A comprehensive review. Acta Pharm. Sin. B 2018, 8, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.; Rabeler, C.; Rao, S.; Richardson, R.J.; Collins, E.S. Distinguishing classes of neuroactive drugs based on computational physicochemical properties and experimental phenotypic profiling in planarians. PLoS ONE 2025, 20, e0315394. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.J.; Meira, W., Jr. Linear discriminant analysis. In Data Mining and Machine Learning: Fundamental Concepts and Algorithms, 2nd ed.; Cambridge University Press: Cambridge, UK, 2020; pp. 501–516. [Google Scholar]
- PAST. 2025. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 15 October 2025).
- Lachenbruch, P.A.; Mickey, M.R. Estimation of Error Rates in Discriminant Analysis. Technometrics 1968, 10, 1–11. [Google Scholar] [CrossRef]
- CRC Handbook of Chemistry and Physics, 97th ed.; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; p. 2670. [Google Scholar] [CrossRef]
- Brown, H.C. Determination of Organic Structures by Physical Methods; Braude, E.A., Nachod, F.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1955. [Google Scholar]
- CRC Handbook of Chemistry and Physics, 90th ed.; Lide, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB5195697.htm (accessed on 1 October 2025).
- Portenkirchner, E.; Enengl, C.; Enengl, S.; Hinterberger, G.; Schlager, S.; Apaydin, D.; Neugebauer, H.; Knör, G.; Sariciftci, N.S. A Comparison of Pyridazine and Pyridine as Electrocatalysts for the Reduction of Carbon Dioxide to Methanol. ChemElectroChem 2014, 1, 1543–1548. [Google Scholar] [CrossRef]
- Fujiki, R.; Matsui, T.; Shigeta, Y.; Nakano, H.; Yoshida, N. Recent Developments of Computational Methods for pKa Prediction Based on Electronic Structure Theory with Solvation Models. J 2021, 4, 849–864. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Y.; Zhang, X.; Liu, H.; Zhang, S.-T.; Li, W.; Yang, B. Noncovalent π–π dimerization based on acridine and acid-responsive luminescence switching. Dyes Pigm. 2022, 205, 110527. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Chitra, R. Stacking interaction between homostacks of simple aromatics and the factors influencing these interactions. CrystEngComm 2010, 12, 2113–2121. [Google Scholar] [CrossRef]
- Paik, D.; Lee, H.; Kim, H.; Choi, J.M. Thermodynamics of pi-pi Interactions of Benzene and Phenol in Water. Int. J. Mol. Sci. 2022, 23, 9811. [Google Scholar] [CrossRef]
- Srivastava, A.; Garg, A.; Das, D.; Debnath, A. Molecular dynamics simulations of a stacked π-conjugated soft material: Binding energy and preferential geometry for self-assembly. Bull. Mater. Sci. 2020, 43, 181. [Google Scholar] [CrossRef]
- Lessa, M.D.; Stoyanov, S.R.; de Carneiro, J.W.M.; da Costa, L.M. Density functional theory investigation of the contributions of pi-pi stacking and hydrogen bonding with water to the supramolecular aggregation interactions of model asphaltene heterocyclic compounds. J. Mol. Model. 2024, 30, 145. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, H.; Iura, R.; Kawabata, H. Water-accelerated pi-Stacking Reaction in Benzene Cluster Cation. Sci. Rep. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Pawledzio, S.; Ziemniak, M.; Trzybinski, D.; Arhangelskis, M.; Makal, A.; Wozniak, K. Influence of N-protonation on electronic properties of acridine derivatives by quantum crystallography. RSC Adv. 2024, 14, 5340–5350. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Bell, E.W.; Zhang, Y. DockRMSD: An open-source tool for atom mapping and RMSD calculation of symmetric molecules through graph isomorphism. J. Cheminform. 2019, 11, 40. [Google Scholar] [CrossRef]
- Jung, J.; Lee, B. Protein structure alignment using environmental profiles. Protein Eng. 2000, 13, 535–543. [Google Scholar] [CrossRef]











| Azinyl Substituent | Yield 1a-e (%) | Yield 2a-e (%) | Yield 5a-e (%) | Yield 6a-e (%) | Yield 7a-e (%) |
|---|---|---|---|---|---|
| Pyridin-2-yl (a) | 67 [52] | 67 | 72 | 93 | 90 |
| Quinolin-2-yl (b) | 67 [52] | 55 | 81 | 98 | 93 |
| Acridin-9-yl (c) | 80 [52] | 52 | 75 | 86 | 88 |
| 2,2′-Bipyridin-6-yl (d) | 78 [52] | 62 | 68 | 98 | 95 |
| Pyridazin-4-yl (e) | 53 | 58 | 63 | 82 | - |
 | ||||||
| No |  | Inhibition of Hs AChE, EcBChE and SsCES IC50, μM or % Inhibition at 20 μM | Propidium Displacement, (%) 1 | HsAβ42 self-Aggregation Inhibition, (%) 2 | ||
| HsAChE | EcBChE | SsCES | ||||
| 1-azinyl-Fcs, R1=H | ||||||
| 1a |  | n.a. | 20.3 ± 1.4% | 17.5 ± 1.3% | n.a. | 43.4 ± 3.4 |
| 1b |  | 68.1 ± 3.4 μM | 2.70 ± 0.19 μM | 29.8 ± 2.0% | 10.2 ± 0.8 | 79.5 ± 6.3 |
| 1c |  | 6.5 ± 0.8% | 21.2 ± 1.6% | n.a. | 11.2 ± 0.9 | 71.0 ± 4.9 |
| 1d |  | 7.89 ± 0.63 μM | 13.8 ± 1.2 μM | 20.9 ± 1.6% | 11.1 ± 0.9 | 63.7 ± 5.1 |
| 1e |  | n.a. | 13.1 ± 1.4% | n.a. | n.a. | 53.3 ± 3.7 |
| Fc | - | 9.6 ± 1.4% | 6.7 ± 1.0% | 4.2 ± 0.7% | n.a. | 3.5 ± 0.4 |
| 1-azinyl-1′-isopropenyl-Fcs, R1 = –C(CH3)=CH2 | ||||||
| 5a |  | 4.4 ± 1.1% | 18.3 ± 1.6% | n.a. | n.a. | 62.4 ± 4.9 |
| 5b |  | 4.01 ± 0.28 μM | 9.91 ± 0.69 μM | 35.4 ± 2.8% | 11.5 ± 0.8 | 82.9 ± 6.6 |
| 5c |  | 36.7 ± 3.3% | 96.7 ± 6.6 μM | n.a. | 10.5 ± 0.9 | 90.1 ± 7.2 |
| 5d |  | 5.12 ± 0.41 μM | 4.86 ± 0.39 μM | n.a. | 8.2 ± 0.6 | 83.8 ± 4.2 |
| 5e |  | n.a. | n.a. | n.a. | n.a. | 48.6 ± 3.5 |
| 8 | - | n.a. | 3.9 ± 0.8% | 2.9 ± 0.6% | n.a. | n.a. |
| 1-azinyl-1′-vinyl-Fcs, R1 = –CH=CH2 | ||||||
| 7a |  | 16.5 ± 1.3 μM | 20.1 ± 1.6 μM | 18.5 ± 1.6% | 9.6 ± 0.7 | 84.6 ± 7.5 |
| 7b |  | 3.32 ± 0.23 μM | 3.68 ± 0.29 μM | 29.8 ± 2.6% | 7.7 ± 0.6 | 89.6 ± 6.2 |
| 7c |  | 22.8 ± 1.8% | 7.6 ± 1.1% | 14.1 ± 1.5% | 10.4 ± 0.8 | 86.9 ± 6.1 |
| 7d |  | 6.34 ± 0.51 μM | 7.94 ± 0.55 μM | 5.8 ± 1.3% | 9.7 ± 0.6 | 81.5 ± 4.9 |
| 9 | - | 4.2 ± 0.9% | n.a. | 23.4 ± 2.1% | n.a. | 44.9 ± 3.1 |
| BNPP | n.a. | n.a. | 99.1 ± 0.9% 1.80 ± 0.11 µM | n.d. | n.d. | |
| Tacrine | 0.601 ± 0.047 μM | 0.0295 ± 0.0002 μM | n.a. | 3.1 ± 0.2 | 5.9 ± 0.5 | |
| Donepezil | 0.040 ± 0.004 μM | 19.2 ± 3.0 μM | n.a. | 11.9 ± 0.9 | n.d. | |
| Myricetin | n.d. | n.d. | n.d. | n.d. | 79.4 ± 6.3 | |
 | ||||
| No |  | ABTS•+-Scavenging Activity | Ferric Reducing Antioxidant Power | |
| TEAC 1 | IC50, µM | TE 2 | ||
| 1-azinyl-Fcs, R1=H | ||||
| 1a | Pyridyl-2 | 2.1 ± 0.1 | 10.8 ± 0.6 | 2.05 ± 0.02 |
| 1b | Quinolin-2-yl | 4.2 ± 0.2 | 6.6 ± 0.3 | 2.21 ± 0.01 |
| 1c | Acridin-9-yl | 1.50 ± 0.08 | 14.6 ± 0.3 | 1.59 ± 0.17 |
| 1d | 2,2′-Bipyridin-6-yl | 1.00 ± 0.04 | 20.6 ± 1.5 | 1.77 ± 0.04 |
| 1e | Pyridazin-4-yl | 2.3 ± 0.1 | 9.2 ± 0.5 | 1.72 ± 0.02 |
| Fc | - | 0.57 ± 0.03 | 32.9 ± 1.9 | 0.79 ± 0.02 |
| 1-azinyl-1′-isopropenyl-Fcs, R1 = –C(CH3)=CH2 | ||||
| 5a | Pyridyl-2 | 2.2 ± 0.1 | 9.4 ± 0.5 | 2.85 ± 0.13 |
| 5b | Quinolin-2-yl | 4.3 ± 0.2 | 5.6 ± 0.3 | 3.13 ± 0.17 |
| 5c | Acridin-9-yl | 1.35 ± 0.06 | 15.8 ± 0.8 | 2.45 ± 0.14 |
| 5d | 2,2′-Bipyridin-6-yl | 0.94 ± 0.05 | 20.8 ± 0.1 | 1.47 ± 0.06 |
| 5e | Pyridazin-4-yl | 2.5 ± 0.2 | 9.3 ± 0.4 | 2.65 ± 0.02 |
| 8 | - | 0.73 ± 0.04 | 26.3 ± 1.5 | 1.1 ± 0.1 |
| 1-azinyl-1′-vinyl-Fcs, R1 = –CH=CH2 | ||||
| 7a | Pyridyl-2 | 2.60 ± 0.08 | 8.4 ± 0.3 | 2.13 ± 0.03 |
| 7b | Quinolin-2-yl | 4.4 ± 0.2 | 5.3 ± 0.2 | 2.21 ± 0.02 |
| 7c | Acridin-9-yl | 2.9 ± 0.1 | 6.9 ± 0.3 | 1.36 ± 0.01 |
| 7d | 2,2′-Bipyridin-6-yl | 1.36 ± 0.06 | 15.6 ± 0.7 | 1.93 ± 0.06 |
| 9 | - | 1.00 ± 0.04 | 19.4 ± 0.1 | 1.04 ± 0.02 |
| Trolox | 1.0 | 20.1 ± 1.2 | 1.0 | |
| Compound | BDE, kcal/mol | IP, kcal/mol | Exp. FRAP, TE | Exp. ABTS, TEAC | ||
|---|---|---|---|---|---|---|
| Water | Ethanol | Water | Ethanol | Water | Ethanol | |
| Fc | 111.6 1 | 111.2 1 | 74.9 | 82.6 | 0.79 ± 0.02 | 0.57 ± 0.03 |
| 1a | - not calculated | 105.5 5 | - not calculated | 83.1 | - | 2.10 ± 0.10 |
| 1ap | 67.5 4 | 67.1 4 | 81.0 | 90.6 | 2.05 ± 0.02 | |
| 1bp | 69.1 4 | 68.7 4 | 81.6 | 91.3 | 2.21 ± 0.01 | 4.20 ± 0.20 |
| 1cp | 68.8 4 | 69.5 4 | 78.8 | 88.4 | 1.59 ± 0.17 | 1.50 ± 0.08 |
| 8 | 88.1 3 | 87.0 3 | 74.0 | 82.0 | 1.10 ± 0.10 | 0.73 ± 0.04 |
| 5a | - not calculated | 86.9 3 | - not calculated | 82.0 | - | 2.20 ± 0.10 |
| 5ap | 66.8 4 | 66.6 4 | 79.9 | 89.4 | 2.85 ± 1.3 | |
| 5bp | 68.9 4 | 68.4 4 | 80.5 | 90.2 | 3.13 ± 1.7 | 4.30 ± 0.20 |
| 5cp | 68.5 4 | 69.1 4 | 80.3 | 89.2 | 2.45 ± 0.14 | 1.35 ± 0.06 |
| 9 | 103.2 2 | 102.0 2 | 74.7 | 82.2 | 1.04 ± 0.02 | 1.00 ± 0.04 |
| 7a | - not calculated | 101.5 2 | - not calculated | 82.4 | - | 2.60 ± 0.03 |
| 7ap | 67.1 4 | 66.8 4 | 80.9 | 90.6 | 2.13 ± 0.03 | |
| 7bp | 68.6 4 | 68.3 4 | 81.1 | 90.8 | 2.21 ± 0.02 | 4.40 ± 0.03 |
| 7cp | 68.5 4 | 69.0 4 | 77.8 | 87.4 | 1.36 ± 0.01 | 2.90 ± 0.03 |
| 1b | 1d | 5b | 5d | 7b | 7d | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|
| MSC-Neu | 393.1 ± 13.2 | 632.3 ± 22.8 | 564.3 ± 31.8 | 413.9 ± 18.6 | 277 ± 14.8 | 299.2 ± 21.9 | 103.6 ± 12.3 | 138.9 ± 15.7 |
| SH-SY5Y | 242.7 ± 27.4 | 152.4 ± 21.2 | 281 ± 30.1 | 188.8 ± 19.3 | 287.4 ± 31.7 | 158.1 ± 19.8 | 43.4 ± 6.7 | 57.8 ± 8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhaeva, G.F.; Utepova, I.A.; Rudakova, E.V.; Kovaleva, N.V.; Boltneva, N.P.; Zyryanova, E.Y.; Musikhina, A.A.; Lazarev, V.F.; Vladimirova, S.A.; Guzhova, I.V.; et al. 1-Azinyl-1′-Alkenylferrocenes with Anticholinesterase, Antioxidant, and Antiaggregating Activities as Multifunctional Agents for Potential Treatment of Alzheimer’s Disease. Pharmaceuticals 2025, 18, 1862. https://doi.org/10.3390/ph18121862
Makhaeva GF, Utepova IA, Rudakova EV, Kovaleva NV, Boltneva NP, Zyryanova EY, Musikhina AA, Lazarev VF, Vladimirova SA, Guzhova IV, et al. 1-Azinyl-1′-Alkenylferrocenes with Anticholinesterase, Antioxidant, and Antiaggregating Activities as Multifunctional Agents for Potential Treatment of Alzheimer’s Disease. Pharmaceuticals. 2025; 18(12):1862. https://doi.org/10.3390/ph18121862
Chicago/Turabian StyleMakhaeva, Galina F., Irina A. Utepova, Elena V. Rudakova, Nadezhda V. Kovaleva, Natalia P. Boltneva, Elena Yu. Zyryanova, Alexandra A. Musikhina, Vladimir F. Lazarev, Snezhana A. Vladimirova, Irina V. Guzhova, and et al. 2025. "1-Azinyl-1′-Alkenylferrocenes with Anticholinesterase, Antioxidant, and Antiaggregating Activities as Multifunctional Agents for Potential Treatment of Alzheimer’s Disease" Pharmaceuticals 18, no. 12: 1862. https://doi.org/10.3390/ph18121862
APA StyleMakhaeva, G. F., Utepova, I. A., Rudakova, E. V., Kovaleva, N. V., Boltneva, N. P., Zyryanova, E. Y., Musikhina, A. A., Lazarev, V. F., Vladimirova, S. A., Guzhova, I. V., Ganebnykh, I. N., Astakhova, T. Y., Timokhina, E. N., Chupakhin, O. N., Charushin, V. N., & Richardson, R. J. (2025). 1-Azinyl-1′-Alkenylferrocenes with Anticholinesterase, Antioxidant, and Antiaggregating Activities as Multifunctional Agents for Potential Treatment of Alzheimer’s Disease. Pharmaceuticals, 18(12), 1862. https://doi.org/10.3390/ph18121862









