Editorial: Radiolabeled Peptides in Cancer Imaging and Therapy—Emerging Isotopes
1. Overview—Precision with Purpose
2. Emerging Frontiers in TAT
3. Global Challenges
- 177Lu: Widely used for peptide receptor radionuclide therapy (PRRT), 177Lu is commercially available but subject to rising demand and regional shortages.Commercial Suppliers: Eckert and Ziegler (EZAG) (nuclear-reactor-based n.c.a. 177Lu); ITM (EndolucinBeta®); SHINE (fusion-driven neutron production via Cassiopeia); Isotopia (Global cGMP facility producing n.c.a. and c.a. 177Lu); Bruce Power + ITM / Framatome consortium (power-reactor-based 177Lu production); ANSTO (OPAL-reactor-based 177Lu supply); NTP Radioisotopes (with ITM tech transfer) (n.c.a. 177Lu); MONROL (reactor-based 177Lu supply).
- 68Ga: While widely accessible via generators, 68Ga is limited by its batch capacity, affecting its utility in high-throughput clinical settings.
- 64Cu, 203Pb, 89Zr: These isotopes require mid- to high-energy cyclotrons, making them inaccessible in many healthcare centers without access to cyclotron or cGMP radiochemistry facilities.
- 225Ac: A key radionuclide for TAT, 225Ac faces limited production, often relying on 229Th decay or high-energy proton spallation methods. Its global demand far exceeds current capacity. Several companies have announced scale-up efforts, but a large-scale stable supply remains a work in progress.Commercial Suppliers: Eckert & Ziegler (cyclotron-based irradiation onto 226Ra targets); NorthStar (electron-beam accelerator onto 226Ra targets); TerraPower (229Th decay harvesting in partnership with DOE); AlfaRim Medical/IONETIX Corporation (cyclotron-based 226Ra bombardment); PanTera Consortium (IBA and SCK CEN) (Rhodotron® electron accelerator production with industrial scale); Nusano (linear-accelerator-based, upcoming); ITM (Actineer™ JV with Canadian Nuclear Laboratories) (industrial-scale production via Actineer); BWXT Medical (DMF submitted to FDA for 225Ac API) in agreement with NorthStar supporting 225Ac production; SpectronRx recently announced that it has commenced mass production of high-purity Actinium-225 (225Ac)
- 211At: Although ideal for alpha therapy due to its short half-life and high LET, 211At production requires bismuth targetry and specialized cyclotrons, hindering scalability. The commercial-supplier statements below are either early-stage or under development, rather than long-established large-scale production practices.Commercial Suppliers: IONETIX Corporation (first commercial 211At cyclotron facility); Nusano (partnering with Atley to deliver large-scale 211At supply (~2025 rollout)); Framatome + IBA/PanTera (planning a European production plant (2027–2028) and to advance the industrial-scale production of 211At in Europe and the United States through a network of specialized cyclotrons).
- Investment in alternative production routes (e.g., cyclotron-based 225Ac, thorium target irradiation).
- Expansion of generator-based platforms, particularly for 68Ga and 203Pb.
- International collaboration and harmonization of isotope distribution and regulatory approvals.
- Development of flexible radiochemistry platforms to adapt tracers for multiple isotopes based on local availability.
4. Coda
Acknowledgments
Conflicts of Interest
List of Contributions
- Barta, P.; Nachtigal, P.; Maixnerova, J.; Zemankova, L.; Trejtnar, F. Validation of Freshly Isolated Rat Renal Cells as a Tool for Preclinical Assessment of Radiolabeled Receptor-Specific Peptide Uptake in the Kidney. Pharmaceuticals 2023, 16, 696. https://doi.org/10.3390/ph16050696.
- Chapeau, D.; Koustoulidou, S.; Handula, M.; Beekman, S.; de Ridder, C.; Stuurman, D.; de Blois, E.; Buchatskaya, Y.; van der Schilden, K.; de Jong, M.; Konijnenberg, M.; Seimbille, Y. [212Pb]Pb-eSOMA-01: A Promising Radioligand for Targeted Alpha Therapy of Neuroendocrine Tumors. Pharmaceuticals 2023, 16, 985. https://doi.org/10.3390/ph16070985.
- Szücs, D.; Szabó, J.; Arató, V.; Gyuricza, B.; Szikra, D.; Tóth, I.; Képes, Z.; Trencsényi, G.; Fekete, A. Investigation of the Effect on the Albumin Binding Moiety for the Pharmacokinetic Properties of 68Ga-, 205/206Bi-, and 177Lu-Labeled NAPamide-Based Radiopharmaceuticals. Pharmaceuticals 2023, 16, 1280. https://doi.org/10.3390/ph16091280.
- Okarvi, S. Preparation, Radiolabeling with 68Ga/177Lu and Preclinical Evaluation of Novel Angiotensin Peptide Analog: A New Class of Peptides for Breast Cancer Targeting. Pharmaceuticals 2023, 16, 1550. https://doi.org/10.3390/ph16111550.
- Wang, L.; Chen, C.; Zhang, Z.; Kuo, H.; Zhang, C.; Colpo, N.; Merkens, H.; Bénard, F.; Lin, K. Synthesis and Evaluation of Novel 68Ga-Labeled [D-Phe6,Leu13ψThz14]bombesin(6-14) Analogs for Cancer Imaging with Positron Emission Tomography. Pharmaceuticals 2024, 17, 621. https://doi.org/10.3390/ph17050621.
- Koatale, P.; Welling, M.; Mdanda, S.; Mdlophane, A.; Takyi-Williams, J.; Durandt, C.; van den Bout, I.; Cleeren, F.; Sathekge, M.; Ebenhan, T. Evaluation of [68Ga]Ga-DOTA-AeK as a Potential Imaging Tool for PET Imaging of Cell Wall Synthesis in Bacterial Infections. Pharmaceuticals 2024, 17, 1150. https://doi.org/10.3390/ph17091150.
- Leier, S.; Wuest, F. Innovative Peptide Bioconjugation Chemistry with Radionuclides: Beyond Classical Click Chemistry. Pharmaceuticals 2024, 17, 1270. https://doi.org/10.3390/ph17101270.
- Ferreira, A.; Real, C.; Malafaia, O. Heterobivalent Dual-Target Peptide for Integrin-αvβ3 and Neuropeptide Y Receptors on Breast Tumor. Pharmaceuticals 2024, 17, 1328. https://doi.org/10.3390/ph17101328.
References
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical experience with alpha-particle emitting 211At: Treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J. Nucl. Med. 2008, 49, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Cederkrantz, E.; Bäck, T.; Divgi, C.; Elgqvist, J.; Himmelman, J.; Horvath, G.; Jacobsson, L.; Jensen, H.; Lindegren, S.; et al. Intraperitoneal alpha-radioimmunotherapy of ovarian cancer using 211At-MX35 F(ab’)2: Pharmacokinetics and dosimetry. J. Nucl. Med. 2009, 50, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Ye, T.; Qu, G.; Qin, Y.; Liao, J.; Yang, Y.; Zhang, W.; Liu, N.; Li, F. Locoregional radionuclide therapy of glioblastoma with [211At]At-PDA-FAPI. Sci. Rep. 2025, 15, 18248. [Google Scholar] [CrossRef] [PubMed]
- Stuparu, A.D.; Meyer, C.A.; Evans-Axelsson, S.L.; Lückerath, K.; Wei, L.H.; Kim, W.; Poddar, S.; Mona, C.E.; Dahlbom, M.; Girgis, M.D.; et al. Targeted alpha therapy in a systemic mouse model of prostate cancer—A feasibility study. Theranostics 2020, 10, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C.; Thiele, N.A.; Schlyer, D.; Wilson, J.J.; DiMagno, S.G.; Babich, J.W. A single dose of 225Ac-RPS-074 induces a complete tumor response in an LNCaP xenograft model. J. Nucl. Med. 2019, 60, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: Dosimetry estimate and empiric dose finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Wilson, J.; Andersson, J.D.; Wuest, F. Theranostic imaging surrogates for targeted alpha therapy: Progress in production, purification, and applications. Pharmaceuticals 2023, 16, 1622. [Google Scholar] [CrossRef] [PubMed]
- Frame, E.; Bobba, K.; Gunter, D.; Mihailescu, L.; Bidkar, A.; Flavell, R.; Vetter, K. Coded aperture and Compton imaging for the development of 225 Ac-based radiopharmaceuticals. Med. Phys. 2023, 50, 6454–6468. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Mukai, K.; Naka, S.; Sasaki, H.; Kamiya, T.; Fukuhara, A.; Shirakami, Y.; Ooe, K.; Haba, H.; Toyoshima, A.; et al. Phase I investigator-initiated clinical trial of targeted alpha therapy using [At]NaAt for refractory thyroid cancer (Alpha-T1 trial). J. Nucl. Med. 2025, 66 (Suppl. S1), 251278. [Google Scholar]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Y.; Li, H.; Feng, Y.; Yang, X.; Chen, Y. Efficacy and safety of 225Ac-DOTATATE in the treatment of neuroendocrine neoplasms with high SSTR expression. Clin. Nucl. Med. 2024, 49, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Rinne, S.S.; Vargas, D.B.; Seo, S.; Veach, D.; McDevitt, M.R.; Vaughn, B.A.; Xu, H.; Guo, H.-F.; Fung, E.K.; de Stanchina, E.; et al. 225Ac α-Pretargeted radioimmunotherapy of human epidermal growth factor receptor 2-expressing breast cancer. J. Nucl. Med. 2025, 66, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Leier, S.; Wuest, F. Innovative peptide bioconjugation chemistry with radionuclides: Beyond classical click chemistry. Pharmaceuticals 2024, 17, 1270. [Google Scholar] [CrossRef] [PubMed]
- Fortune Business Insights. Lutetium-177 Market size, share, trends and forecast. Report. 2024. Available online: https://www.fortunebusinessinsights.com/lutetium-177-market-112740 (accessed on 19 November 2025).
- Verified Market Research. Actinium-225 Market size and forecast. Report. 2023. Available online: https://www.verifiedmarketresearch.com/product/actinium-225-market (accessed on 19 November 2025).
- Meta Tech Insights. Radiotheranostics market size and forecast (2025–2035). Report. 2024. Available online: https://www.metatechinsights.com/industry-insights/radiotheranostics-market-2969 (accessed on 19 November 2025).
- IAEA. Cyclotron Produced Radionuclides: Physical Characteristics and Production Methods; Technical Reports Series No. 468; International Atomic Energy Agency (IAEA): Vienna, Austria, 2009; Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/trs468_web.pdf (accessed on 19 November 2025).
- Jang, A.; Kendi, A.T.; Johnson, G.B.; Halfdanarson, T.R.; Sartor, O. Targeted alpha-particle therapy: A review of current trials. Int. J. Mol. Sci. 2023, 24, 11626. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, A.R.; Kleynhans, J.; Bouziotis, P.; Bruchertseifer, F.; Chakraborty, S.; De Blois, E.; Denecke, M.; Elboga, U.; Gizawy, M.; Horak, C.; et al. IAEA activities to support the member states in the production of targeted alpha therapy radiopharmaceuticals. Nucl. Med. Biol. 2025, 144–145, 109008. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research Report. Alpha emitter market size, share & trends analysis report by type of radionuclide (Astatine, Radium, Actinium, Lead, Bismuth), by medical application, by region, and segment forecasts, 2024–2030. Report ID: GVR-4-68040-177-8 2021, 110 Pages. Available online: https://www.grandviewresearch.com/industry-analysis/alpha-emitter-market-report# (accessed on 19 November 2025).
- Lindegren, S.; Albertsson, P.; Bäck, T.; Jensen, H.; Palm, S.; Aneheim, E. Realizing clinical trials with Astatine-211: The chemistry infrastructure. Cancer Biother. Radiopharm. 2020, 35, 425–436. [Google Scholar] [CrossRef] [PubMed]
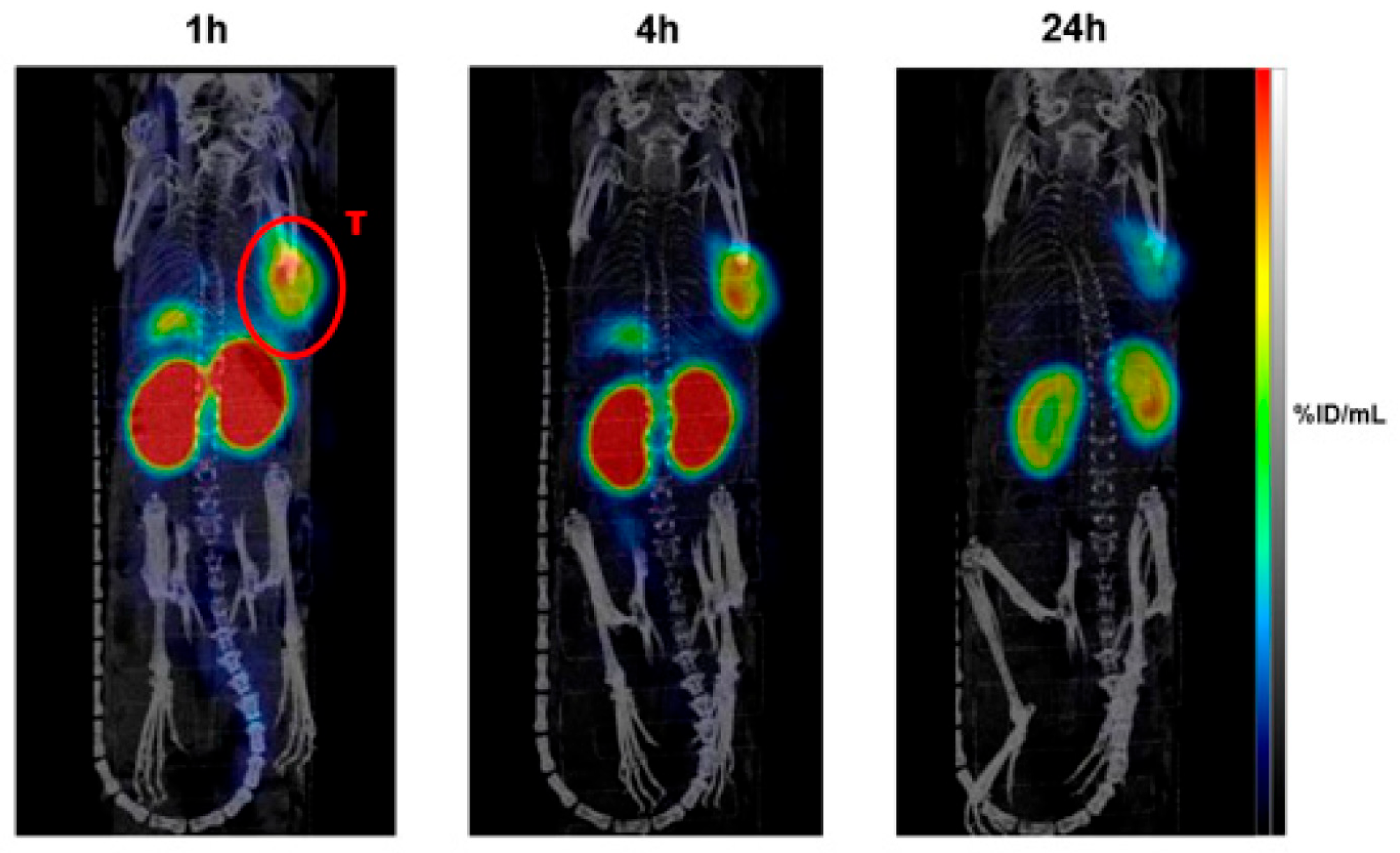
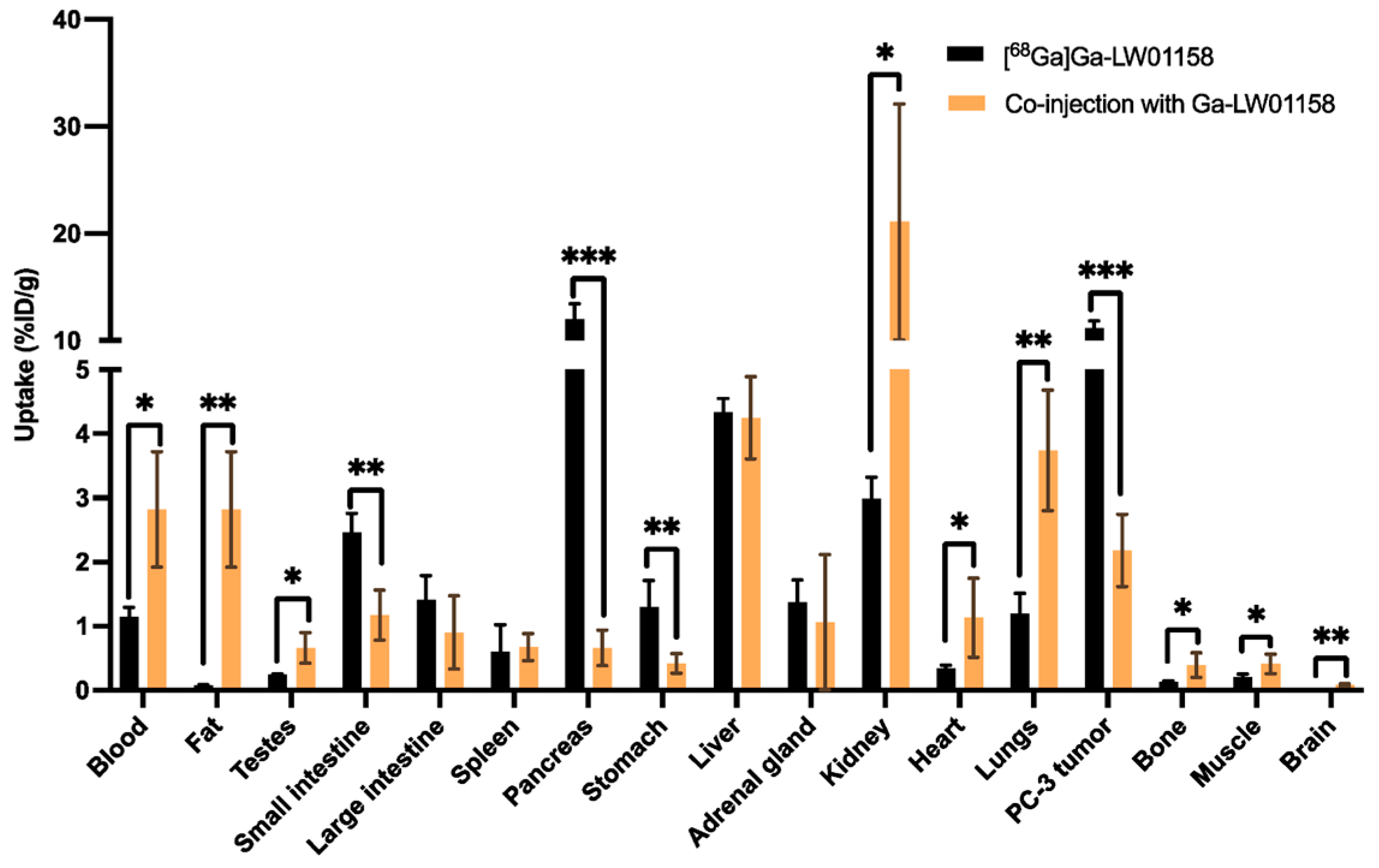
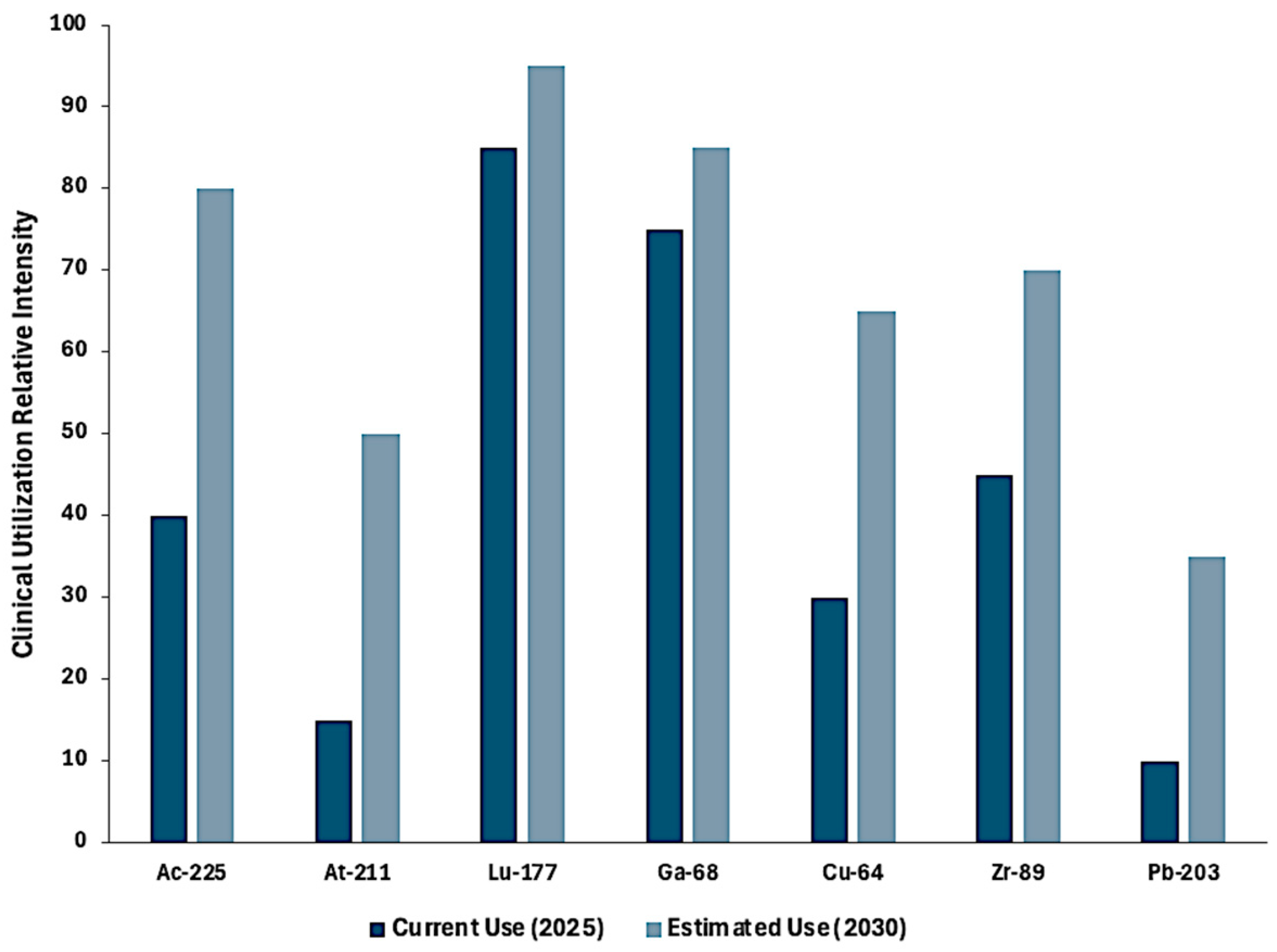
| Radionuclide | Market Outlook/Forecast | Clinical Activity Level |
|---|---|---|
| 177Lu | Market projected to grow from USD 2.73 billion (2025) to USD 10.84 billion (2032) (~21.8% CAGR) | Highest; FDA-approved for NETs (Lutathera) and prostate cancer (Pluvicto) |
| 225Ac | Market forecast to grow from ~USD 0.7 million (2023) to USD 1.7 billion (2031) per verified market research (2023); numerous Phase I/II trials | High and expanding rapidly |
| 68Ga | Rising PET theranostic use (e.g., PSMA-11, DOTATATE); integral to expanding radiotheranostics market, projected to reach USD 31.8 billion by 2035 | High; widely used in PET imaging |
| 89Zr | Niche but expanding for immuno-PET; several active antibody-tracking trials | Moderate; used in clinical trials |
| 64Cu | Used in diagnostics (64Cu-DOTATATE, 64Cu-PSMA) and as theranostic pair with 67Cu; several trials | Moderate; expanding theranostic interest |
| 203Pb | Diagnostic surrogate for 212Pb; early clinical evaluations as imaging companion in TAT development | Low to moderate; companion imaging use |
| 211At | Early-stage α-emitter under evaluation in first-in-human glioblastoma and thyroid cancer trials; not yet in routine clinical use | Low; not yet in routine clinical use |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, N. Editorial: Radiolabeled Peptides in Cancer Imaging and Therapy—Emerging Isotopes. Pharmaceuticals 2025, 18, 1836. https://doi.org/10.3390/ph18121836
Malik N. Editorial: Radiolabeled Peptides in Cancer Imaging and Therapy—Emerging Isotopes. Pharmaceuticals. 2025; 18(12):1836. https://doi.org/10.3390/ph18121836
Chicago/Turabian StyleMalik, Noeen. 2025. "Editorial: Radiolabeled Peptides in Cancer Imaging and Therapy—Emerging Isotopes" Pharmaceuticals 18, no. 12: 1836. https://doi.org/10.3390/ph18121836
APA StyleMalik, N. (2025). Editorial: Radiolabeled Peptides in Cancer Imaging and Therapy—Emerging Isotopes. Pharmaceuticals, 18(12), 1836. https://doi.org/10.3390/ph18121836




