Comparative Study on Extracts from Traditional Medicinal Plants Echinacea purpurea (L.) Moench and Onopordum acanthium (L.): Antioxidant Activity In Vitro and Anxiolytic Effect In Vivo
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of AOA of Extracts of EP and OA and Their Combinations In Vitro
2.2. Study of the Anxiolytic Effect of EP and OA Extracts and Their Combinations in Experimental Animals Subjected to an Acute Cold Stress Model
2.2.1. Confirmation of Stress Induction
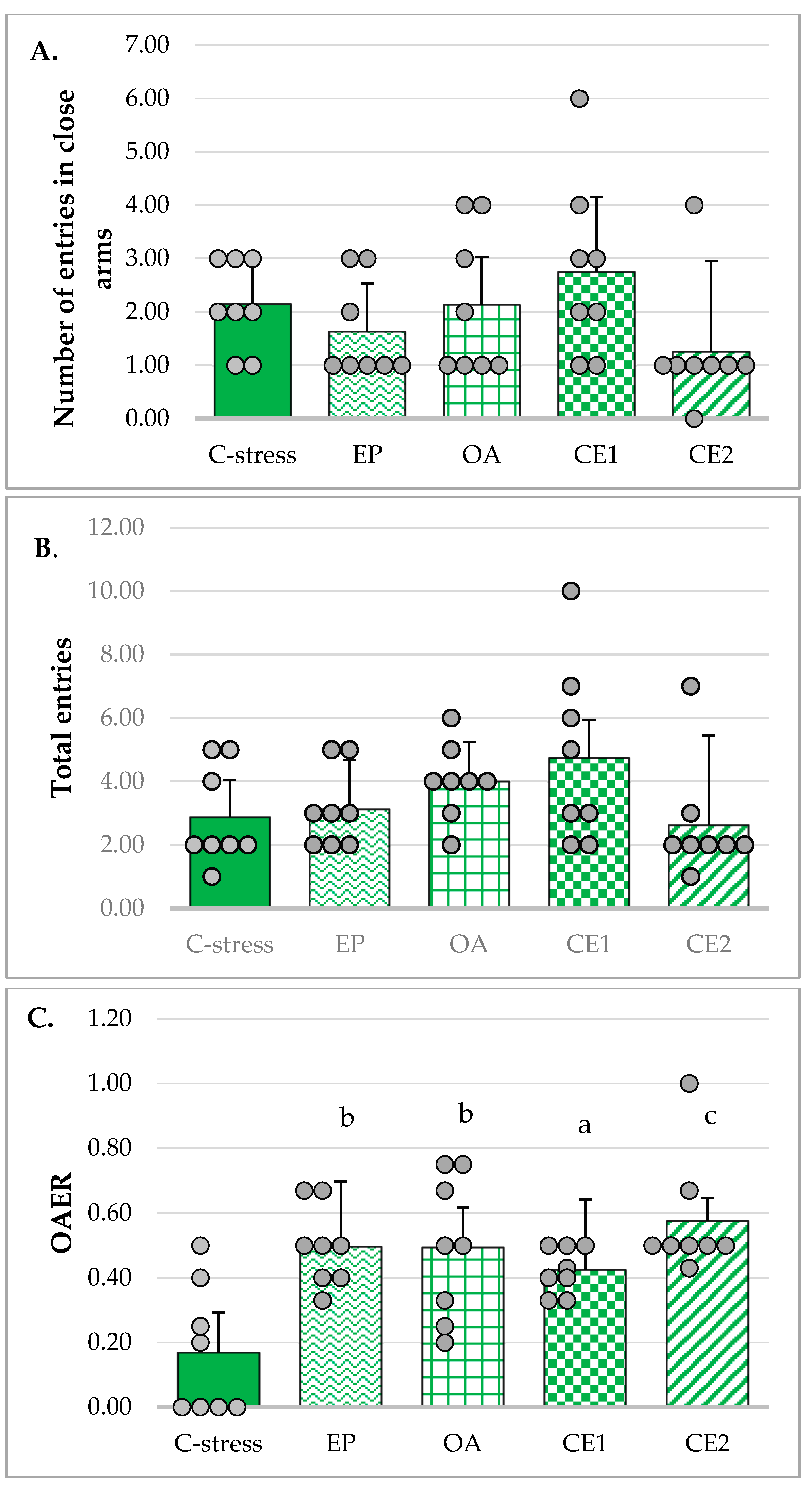
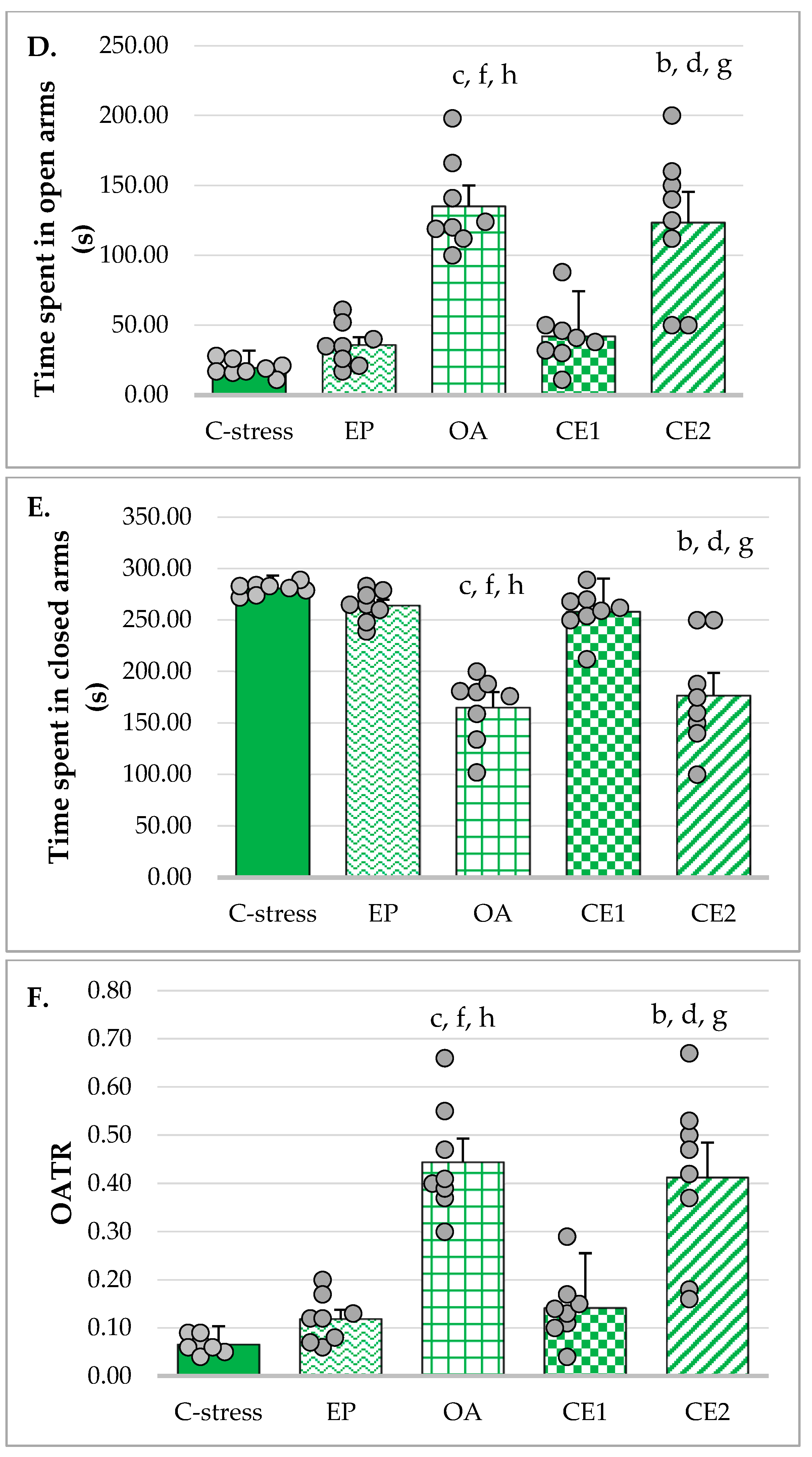
2.2.2. EPM
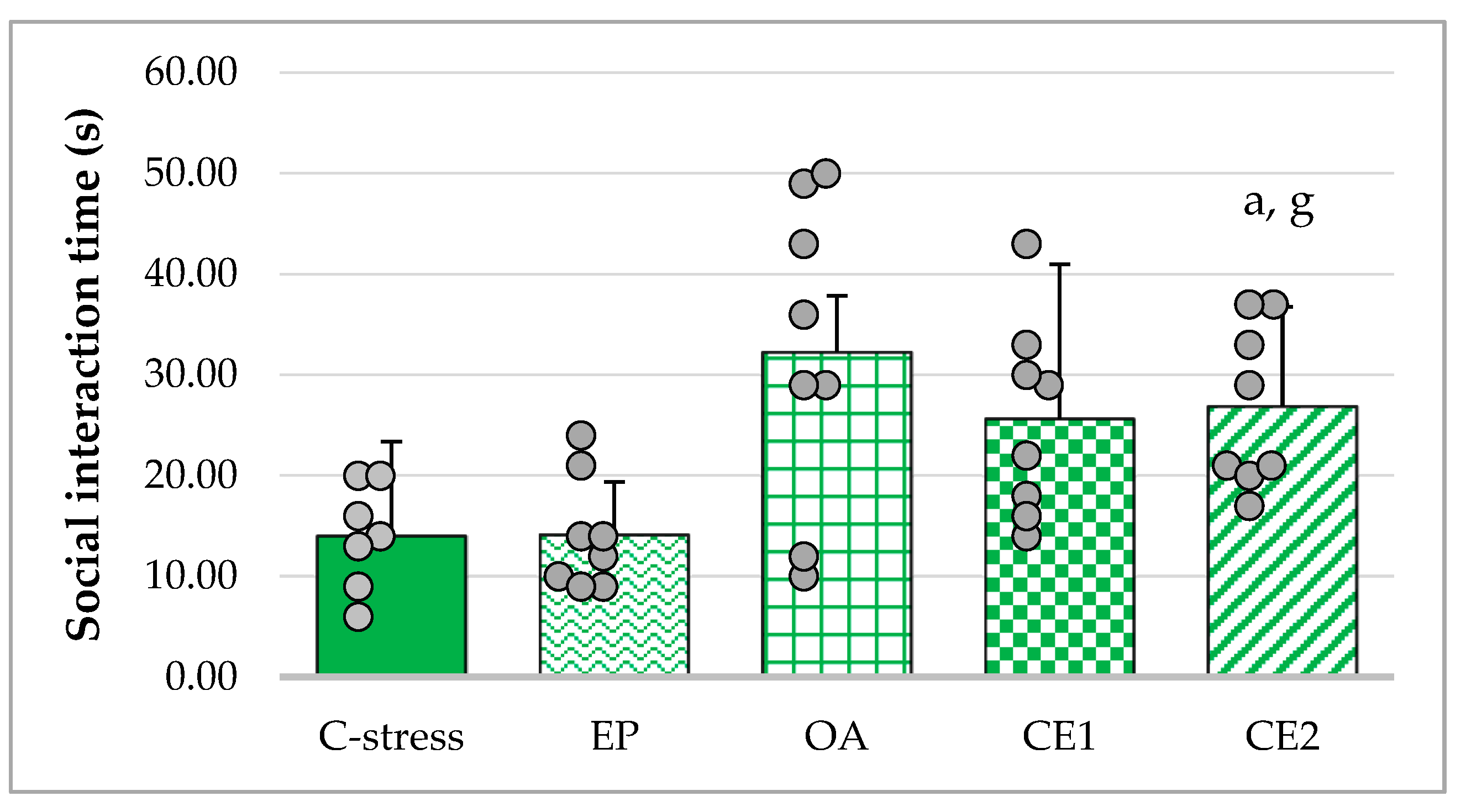
2.2.3. SIT
2.3. Effects of EP and OA Extracts and Their Combinations on Serum Cytokine Levels
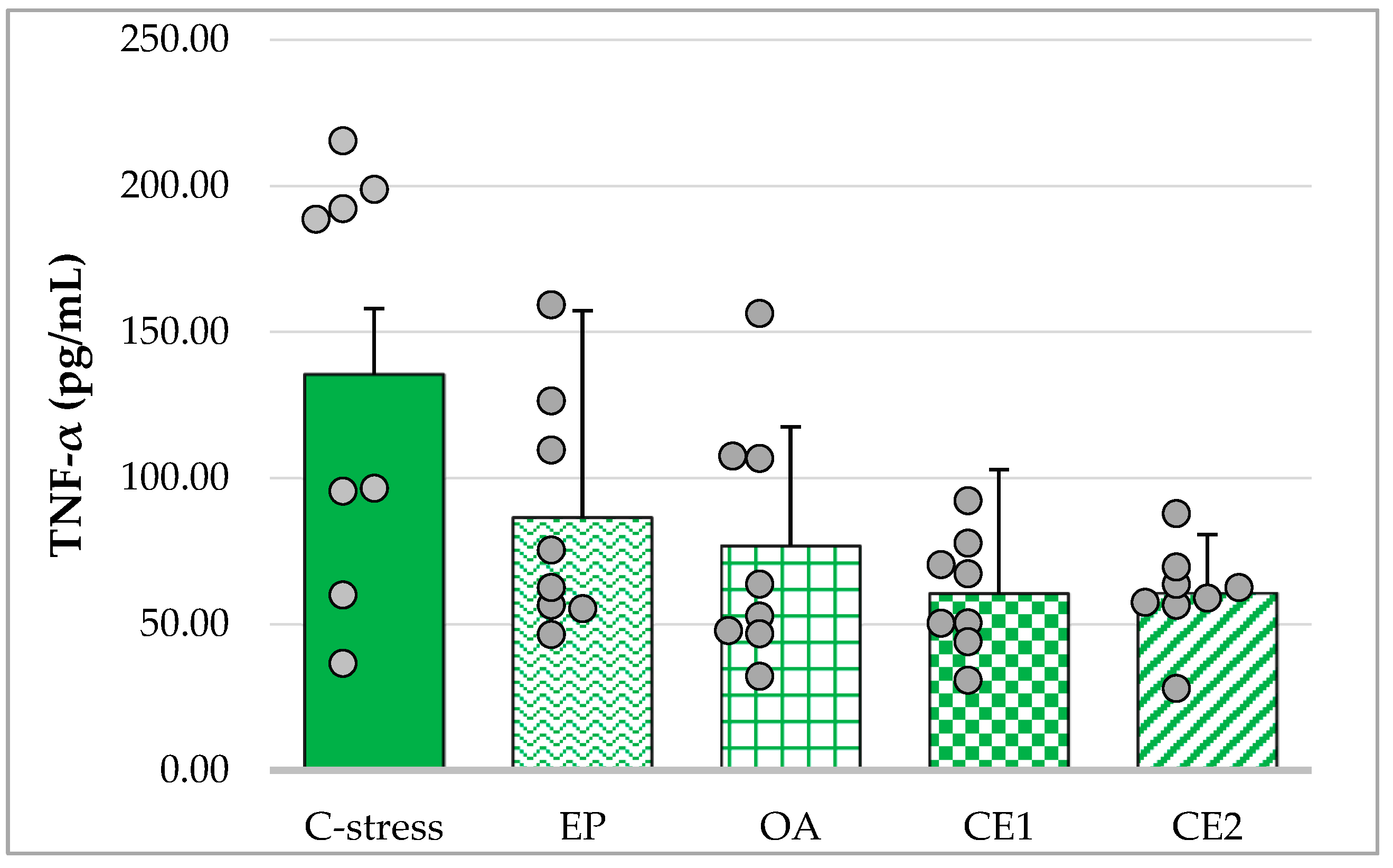
2.3.1. Changes in Serum TNF-α in Acute Cold Stress-Challenged Rats
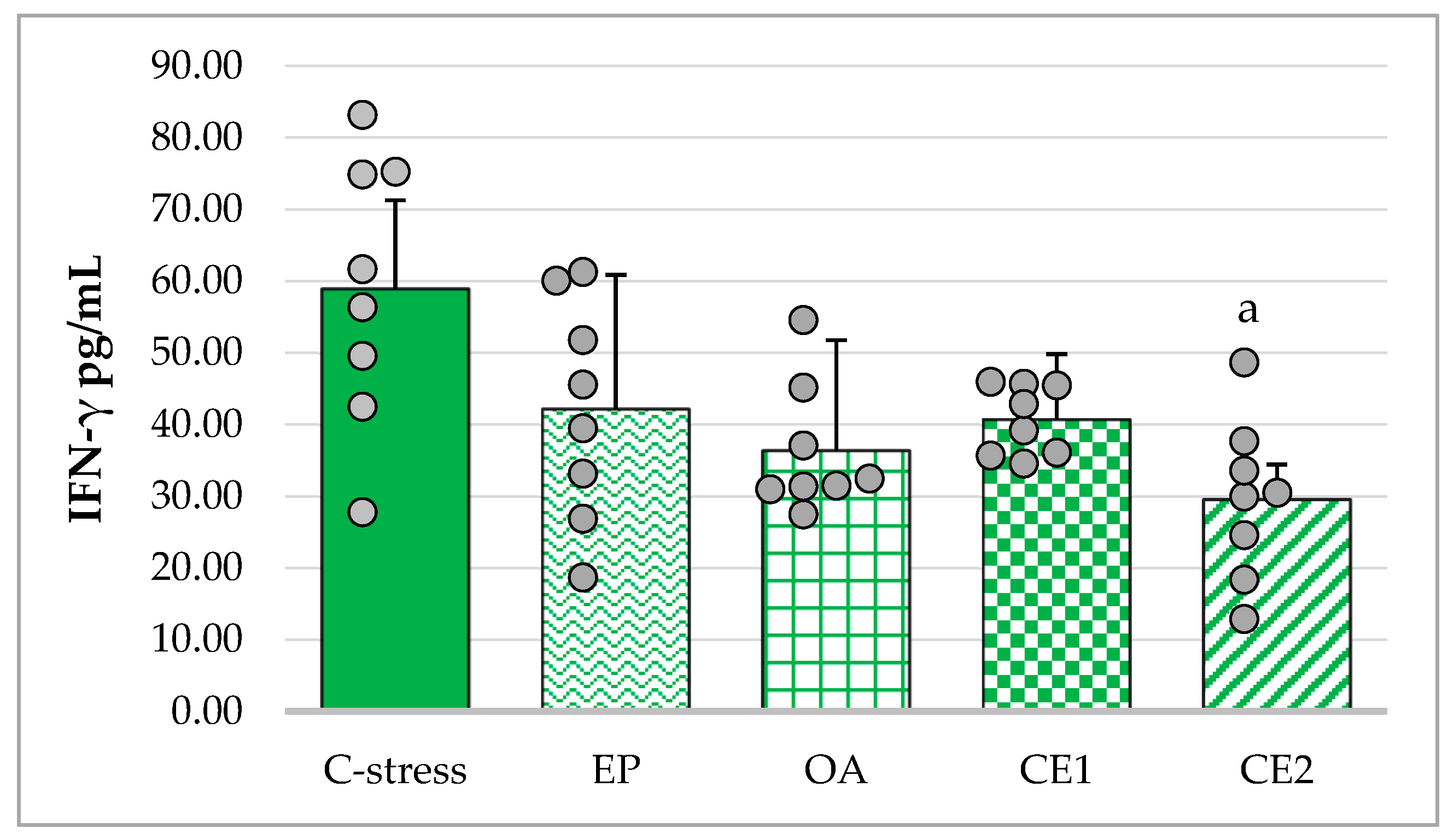
2.3.2. Changes in Serum IFN-γ in Acute Cold Stress-Challenged Rats
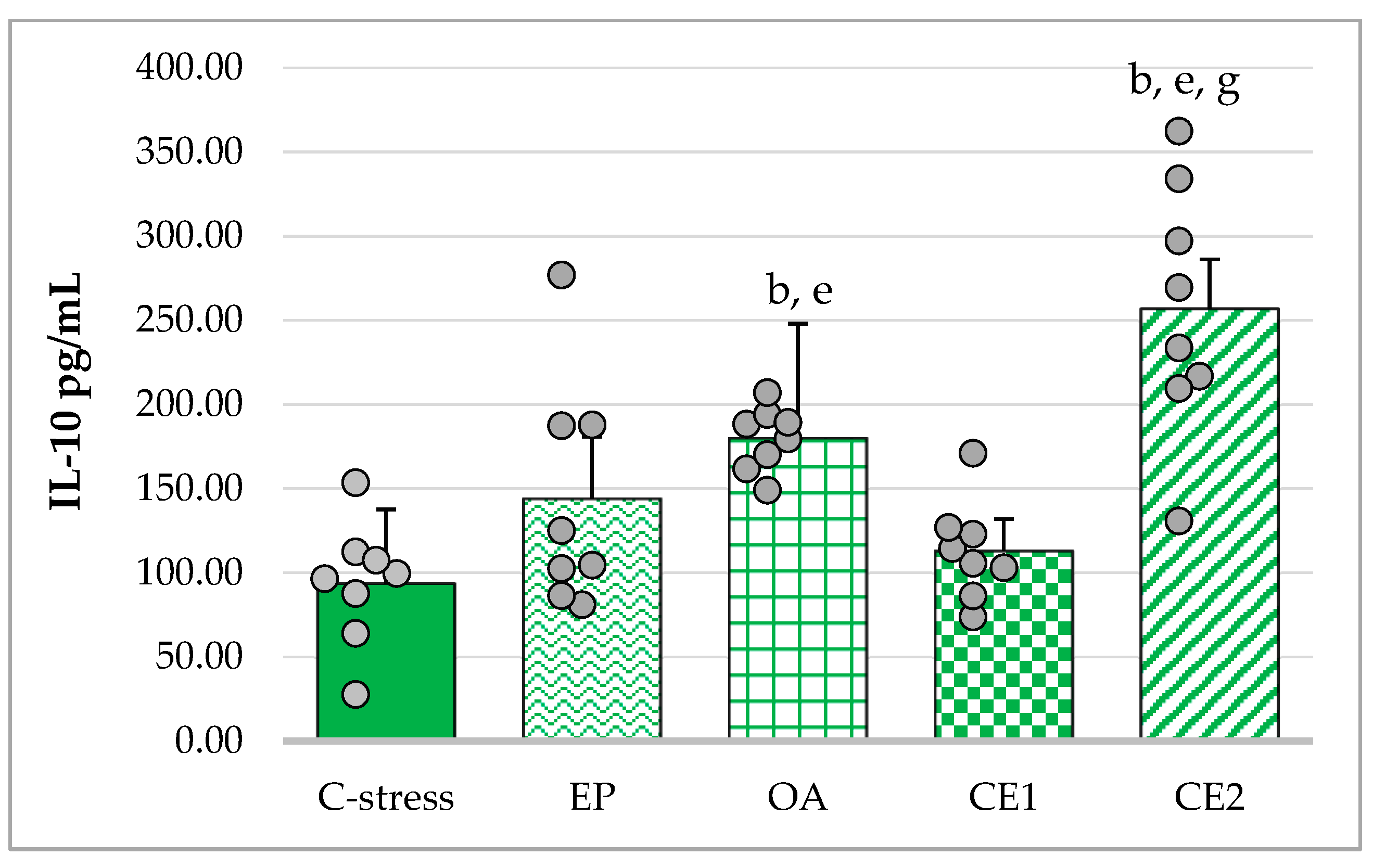
2.3.3. Changes in Serum IL-10 in Acute Cold Stress-Challenged Rats
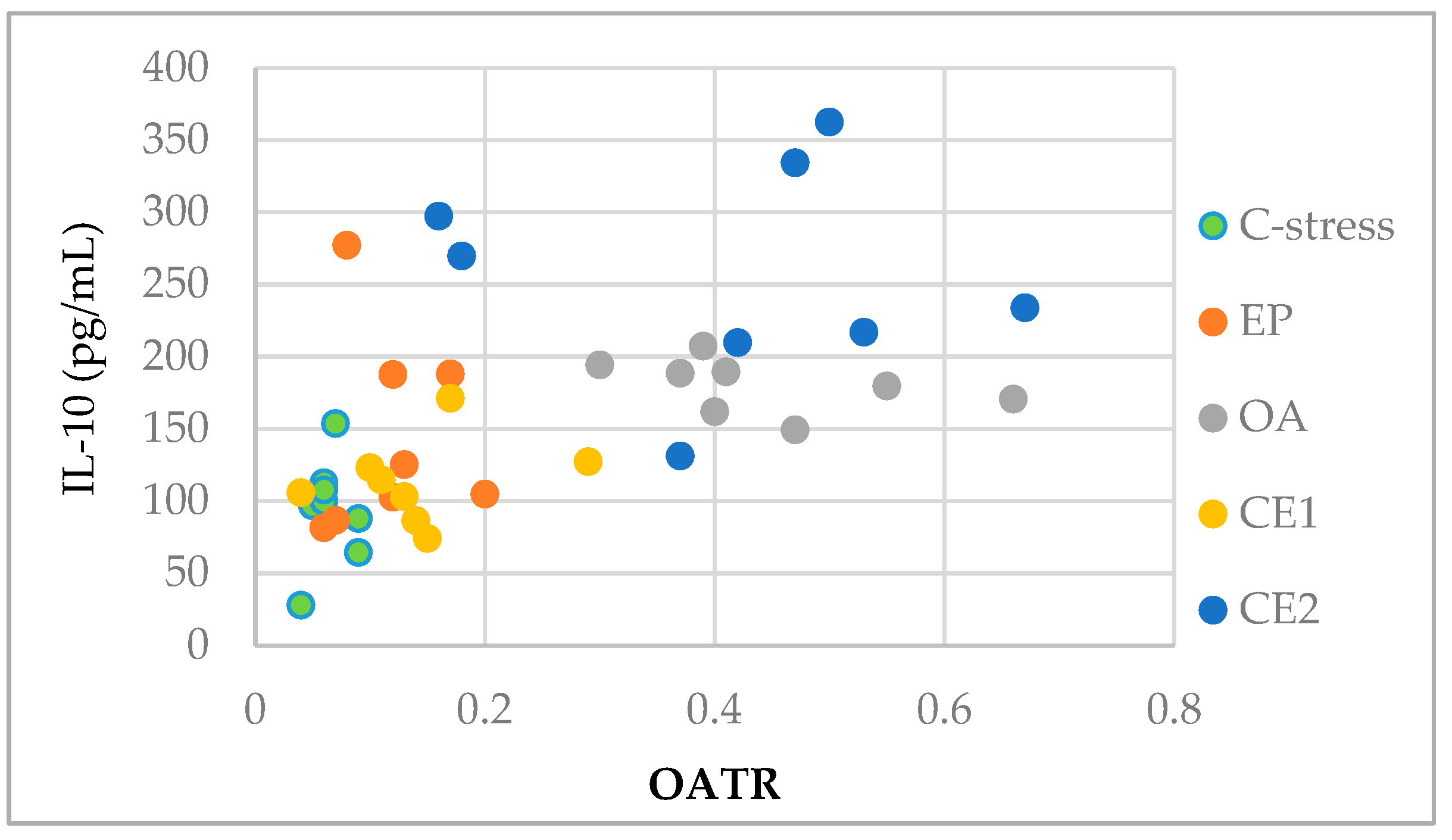
2.4. Correlations Between Behavioral and Immunological Parameters of EP and OA Extracts and Their Combinations
3. Materials and Methods
3.1. Plant Material from EP and OA
3.2. Extraction and HPLC Analysis of Individual Compounds
3.3. Determination of AOA In Vitro
3.3.1. Oxygen Radical Absorbance Capacity (ORAC) Assay
3.3.2. Hydroxyl Radical Averting Capacity (HORAC) Assay
3.3.3. Electrochemical Method for Determination of AOA
3.4. Experimental Animals and Treatment In Vivo
3.4.1. Experimental Animals
3.4.2. Experimental Groups and Treatments
3.4.3. Acute Cold Stress Model and Experimental Design
3.4.4. Behavioral Assessment of Anxiolytic Effects
Elevated Plus Maze (EPM)
Social Interaction Test (SIT)
Immunological Assays
- IFN-γ: sensitivity ~ 10 pg/mL; range 31.25–1000 pg/mL; intra-assay coefficient of variation (CV) < 5%, inter-assay CV < 10%
- IL-10: sensitivity 1.5 pg/mL; range 31.25–1000 pg/mL; intra-assay CV < 5%, inter-assay CV < 10%
- TNF-α: sensitivity 15 pg/mL; range 31.25–1000 pg/mL; intra-assay CV < 10%, inter-assay CV < 10%
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, M.G.; Goode, M.; Heinrich, M. Herbal medicines and botanicals for managing insomnia, stress, anxiety, and depression: A critical review of the emerging evidence focusing on the Middle East and Africa. PharmaNutrition 2024, 29, 100399. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.; Maes, M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012, 36, 764–785. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Pülschen, D.; Thome, J. The role of oxidative stress in depressive disorders. Curr. Pharm. Des. 2012, 18, 5890–5899. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression—Review. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Oršolić, N.; Karlović, D.; Peitl, V. Flavonols in action: Targeting oxidative stress and neuroinflammation in major depressive disorder. Int. J. Mol. Sci. 2023, 24, 6888. [Google Scholar] [CrossRef]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.; Dalton, B. Cytokine research in depression: Principles, challenges, and open questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef]
- Berger, A. Th1 and Th2 responses: What are they? BMJ 2000, 321, 424. [Google Scholar] [CrossRef]
- Freire, M.A.M. Natural compounds in neuroprotection and nervous system regeneration: A narrative review. Regen. Med. Rep. 2026, 3. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 13–33. [Google Scholar]
- Phootha, N.; Yongparnichkul, N.; Fang, Z.; Gan, R.-Y.; Zhang, P. Plants and phytochemicals potentials in tackling anxiety: A systematic review. Phytomed. Plus 2022, 2, 100375. [Google Scholar] [CrossRef]
- Kenda, M.; Kočevar Glavač, N.; Nagy, M.; Sollner Dolenc, M. Medicinal Plants Used for Anxiety, depression, or stress treatment: An Update. Molecules 2022, 27, 6021. [Google Scholar] [CrossRef]
- Chaves, A.N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxid. Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef] [PubMed]
- Seymenska, D.; Shishkova, K.; Hinkov, A.; Benbassat, N.; Teneva, D.; Denev, P. Comparative study on phytochemical composition, antioxidant, and anti-HSV-2 activities of Sambucus nigra L. and Sambucus ebulus L. extracts. Appl. Sci. 2023, 13, 12593. [Google Scholar] [CrossRef]
- Fedotova, J.; Kubatka, P.; Büsselberg, D.; Shleikin, A.G.; Caprnda, M.; Dragasek, J.; Rodrigo, L.; Pohanka, M.; Gasparova, I.; Nosal, V.; et al. Therapeutical strategies for anxiety and anxiety-like disorders using plant-derived natural compounds and plant extracts. Biomed. Pharmacother. 2017, 95, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Kandilarov, I.; Zlatanova, H.; Delev, D.; Kostadinova, I.; Katsarova, M.; Dimitrova, S.; Lukanov, L.; Sadakov, F. Anxiolytic effect and acute toxicity of two novel combinations of plant extracts—Antistress I and Antistress II on rats. Int. J. Toxicol. Pharmacol. Res. 2017, 9, 39–47. [Google Scholar]
- Burlou-Nagy, C.; Bănică, F.; Jurca, T.; Vicaș, L.G.; Marian, E.; Muresan, M.E.; Bácskay, I.; Kiss, R.; Fehér, P.; Pallag, A. Echinacea purpurea (L.) Moench: Biological and pharmacological properties. A review. Plants 2022, 11, 1244. [Google Scholar] [CrossRef]
- Haller, J.; Faludi, G.; Kovacs, G.; Purebl, G.; Füzesi, Z.; Freund, T.F. The Effects of Echinacea (EP107TM) on Anxiety: A Comparison of Anxiety Measures in a Randomized, Double-Blind, Placebo-Controlled Study. Pharmaceuticals 2025, 18, 264. [Google Scholar] [CrossRef]
- Haller, J.; Hohmann, J.; Freund, T.F. The effect of Echinacea preparations in three laboratory tests of anxiety: Comparison with chlordiazepoxide. Phytother. Res. 2010, 24, 1605–1613. [Google Scholar] [CrossRef]
- Haller, J.; Freund, T.F.; Pelczer, K.G.; Füredi, J.; Krecsak, L.; Zámbori, J. The anxiolytic potential and psychotropic side effects of an echinacea preparation in laboratory animals and healthy volunteers. Phytother. Res. 2013, 27, 54–61. [Google Scholar] [CrossRef]
- Liu, R.; Caram-Salas, N.L.; Li, W.; Wang, L.; Arnason, J.T.; Harris, C.S. Interactions of Echinacea spp. root extracts and alkylamides with the endocannabinoid system and peripheral inflammatory pain. Front. Pharmacol. 2021, 12, 651292. [Google Scholar]
- Costa, A.; Micheli, L.; Sordi, V.; Ciampi, C.; Lucci, J.; Passani, M.B.; Provensi, G. Preventing social defeat stress-induced behavioural and neurochemical alterations by repeated treatment with a mix of Centella asiatica, Echinacea purpurea and Zingiber officinale standardized extracts. Front. Pharmacol. 2024, 15, 1439811. [Google Scholar] [CrossRef] [PubMed]
- Garsiya, E.R.; Konovalov, D.A.; Shamilov, A.A.; Glushko, M.P.; Orynbasarova, K.K. Traditional medicine plant, Onopordum acanthium L. (Asteraceae): Chemical composition and pharmacological research. Plants 2019, 8, 40. [Google Scholar] [CrossRef]
- Sharef, A.Y.; Hamdi, B.A.; Alrawi, R.A.; Ahmad, H.O. Onopordum acanthium L. extract attenuates pancreatic β-cells and cardiac inflammation in streptozotocin-induced diabetic rats. PLoS ONE 2023, 18, e0280351. [Google Scholar] [CrossRef] [PubMed]
- Amarakoon, D.; Lee, W.J.; Tamia, G.; Lee, S.H. Indole-3-Carbinol: Occurrence, Health-Beneficial Properties, and Cellular/Molecular Mechanisms. Annu. Rev. Food Sci. Technol. 2023, 14, 347–366. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, Y.; Wu, Y.; Yang, H.; Zhu, P.; Yan, F.; Zhao, R.; Tian, P.; Wang, T.; Fan, Q.; et al. Medicinal herbs for the treatment of anxiety: A systematic review and network meta-analysis. Pharmacol. Res. 2022, 179, 106204. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Lister, R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 1987, 92, 180–185. [Google Scholar] [CrossRef]
- Kumar, D.; Bhat, Z.; Kumar, V.; Khan, N.; Chashoo, I.; Zargar, M.; Shah, M.Y. Effects of Stachys tibetica essential oil in anxiety. Eur. J. Integr. Med. 2012, 4, e169–e176. [Google Scholar] [CrossRef]
- Belzung, C.; Lemoine, M. Criteria of validity for animal models of psychiatric disorders: Focus on anxiety disorders and depression. Biol. Mood Anxiety Disord. 2011, 1, 9. [Google Scholar] [CrossRef]
- Atrooz, F.; Alkadhi, K.; Salim, S. Understanding stress: Insights from rodent models. Curr. Res. Neurobiol. 2021, 2, 100013. [Google Scholar] [CrossRef] [PubMed]
- El Marzouki, H.; Aboussaleh, Y.; Najimi, M.; Chigr, F.; Ahami, A. Effect of cold stress on neurobehavioral and physiological parameters in rats. Front. Physiol. 2021, 12, 660124. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, Y.; Li, S. Effect of Acute Cold Stress on Neuroethology in Mice and Establishment of Its Model. Animals 2022, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Vlasheva, M.; Katsarova, M.; Kandilarov, I.; Zlatanova-Tenisheva, H.; Gardjeva, P.; Denev, P.; Sadakova, N.; Filipov, V.; Kostadinov, I.; Dimitrova, S. Echinacea purpurea and Onopordum acanthium Combined Extracts Cause Immunomodulatory Effects in Lipopolysaccharide-Challenged Rats. Plants 2024, 13, 3397. [Google Scholar] [CrossRef]
- Ou, B.; Hampsh-Woodill, M.; Prior, R. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.; Prior, R.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef]
- Korotkova, E.; Karbainov, Y.; Shevchuk, A. Study of antioxidant properties by voltammetry. J. Electroanal. Chem. 2002, 518, 56–60. [Google Scholar] [CrossRef]
- Morandi Vuolo, M.; Silva Lima, V.; Maróstica Junior, M.R. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Segura Campos, M.R., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. [Google Scholar]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Singla, R.; Kamboj, S.; Duvey, B.K.; Bhargava, A.; Chaudhary, J. Flavonoids and anxiety: Decoding their role in brain function and pathophysiology. Med. Drug Discov. 2025, 27, 100214. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, P.; Deng, Z.; Jin, G.; Guo, Y.; Zhang, Y. Diversity of antioxidant ingredients among Echinacea species. Ind. Crops Prod. 2021, 170, 113699. [Google Scholar] [CrossRef]
- Parzhanova, A.; Yanakieva, V.; Vasileva, I.; Momchilova, M.; Dimitrov, D.; Ivanova, P.; Tumbarski, Y. Physicochemical, Antioxidant, and Antimicrobial Properties of Three Medicinal Plants from the Western Part of the Rhodope Mountains, Bulgaria. Life 2023, 13, 2237. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.M.; Enache, T.A.; Gil, E.S.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Electrochemical methods to evaluate the antioxidant activity and capacity of foods: A review. Electroanalysis 2021, 33, 1419–1435. [Google Scholar] [CrossRef]
- Motta, S.; Cassino, C.; Bosso, A.; Lopresti, M.; Messina, S.; Calegari, G.; Basana, A.; Ravera, M. Characterization of 37 enological tannins using a multiple technique approach: Linear sweep voltammetry as a rapid method both for classification and determination of antioxidant properties. Food Chem. 2025, 463, 141475. [Google Scholar] [CrossRef]
- Banica, F.; Bungau, S.; Tit, D.M.; Behl, T.; Otrisal, P.; Nechifor, A.C.; Gitea, D.; Pavel, F.-M.; Nemeth, S. Determination of the Total Polyphenols Content and Antioxidant Activity of Echinacea Purpurea Extracts Using Newly Manufactured Glassy Carbon Electrodes Modified with Carbon Nanotubes. Processes 2020, 8, 833. [Google Scholar] [CrossRef]
- Newair, E.F.; Abdel-Hamid, R.; Kilmartin, P.A. Electrochemical Determination of the Antioxidant Activity in Echinacea Purpurea Roots Using Square Wave Voltammetry. Electroanalysis 2017, 29, 1131–1140. [Google Scholar] [CrossRef]
- Sonam, K.S.; Guleria, S. Synergistic antioxidant activity of natural products. Ann. Pharmacol. Pharm. 2017, 2, 1086. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- López-Crespo, G.A.; Flores, P.; Sánchez-Santed, F.; Sánchez-Amate, M.C. Acute high dose of chlorpyrifos alters performance of rats in the elevated plus-maze and the elevated T-maze. Neurotoxicology 2009, 30, 1025–1029. [Google Scholar] [CrossRef]
- Haller, J.; Krecsak, L.; Zámbori, J. Double-blind placebo-controlled trial of the anxiolytic effects of a standardized Echinacea extract. Phytother. Res. 2020, 34, 660–668. [Google Scholar] [CrossRef]
- Abdel-Wahhab, K.G.; Sayed, R.S.; El-Sahra, D.G.; Hassan, L.K.; Elqattan, G.M.; Mannaa, F.A. Echinacea purpurea extract intervention for counteracting neurochemical and behavioral changes induced by bifenthrin. Metab. Brain Dis. 2024, 39, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Tang, J.J.; Feng, S.; Huang, H.; Lu, F.N.; Lu, X.M.; Wang, Y.T. Chlorogenic Acid Improves PTSD-like Symptoms and Associated Mechanisms. Curr. Neuropharmacol. 2021, 19, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Haranishi, Y.; Kataoka, K.; Takahashi, Y.; Terada, T.; Nakamura, M.; Sata, T. Chlorogenic acid administered intrathecally alleviates mechanical and cold hyperalgesia in a rat neuropathic pain model. Eur. J. Pharmacol. 2014, 15, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Rohleder, N. Stress and inflammation—The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology 2019, 105, 164–171. [Google Scholar] [CrossRef]
- Srivastava, K.K.; Kumar, R. Stress, oxidative injury and disease. Indian J. Clin. Biochem. 2015, 30, 3–10. [Google Scholar] [CrossRef]
- Tang, L.; Cai, N.; Zhou, Y.; Liu, Y.; Hu, J.; Li, Y.; Yi, S.; Song, W.; Kang, L.; He, H. Acute stress induces an inflammation dominated by innate immunity represented by neutrophils in mice. Front. Immunol. 2022, 13, 1014296. [Google Scholar] [CrossRef]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef]
- Park, S.; Lee, M.S.; Jung, S.; Lee, S.; Kwon, O.; Kreuter, M.H.; Perrinjaquet-Moccetti, T.; Min, B.; Yun, S.H.; Kim, Y. Echinacea purpurea Protects Against Restraint Stress-Induced Immunosuppression in BALB/c Mice. J. Med. Food. 2018, 21, 261–268. [Google Scholar] [CrossRef]
- Mandolesi, G.; Bullitta, S.; Fresegna, D.; Gentile, A.; De Vito, F.; Dolcetti, E.; Rizzo, F.R.; Strimpakos, G.; Centonze, D.; Musella, A. Interferon-γ causes mood abnormalities by altering cannabinoid CB1 receptor function in the mouse striatum. Neurobiol. Dis. 2017, 108, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, V.; Klein, R.G.; Alonso, C.M.; Babb, J.S.; Nishawala, M.; De Jesus, G.; Hirsch, G.S.; Hottinger-Blanc, P.M.; Gonzalez, C.J. Immune system dysregulation in adolescent major depressive disorder. J. Affect. Disord. 2009, 115, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, C.; Li, W.; Ma, Y.; Huo, S.; Ozathaley, A.; Ren, J.; Yuan, W.; Ni, H.; Li, D.; et al. Depression-like behavior associated with E/I imbalance of mPFC and amygdala without TRPC channels in mice of knockout IL-10 from microglia. Brain Behav. Immun. 2021, 97, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S.; Burke, H.M.; Epel, E.S.; Mellon, S.H.; Rosser, R.; Reus, V.I.; Wolkowitz, O.M. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J. Psychiatr. Res. 2009, 43, 962–969. [Google Scholar] [CrossRef]
- Camara, M.L.; Corrigan, F.; Jaehne, E.J.; Jawahar, M.C.; Anscomb, H.; Koerner, H.; Baune, B.T. TNF-α and its receptors modulate complex behaviours and neurotrophins in transgenic mice. Psychoneuroendocrinology 2013, 38, 3102–3114. [Google Scholar] [CrossRef]
- Shah, M.A.; Kang, J.B.; Park, D.J.; Kim, M.O.; Koh, P.O. Chlorogenic acid alleviates cerebral ischemia-induced neuroinflammation via attenuating nuclear factor kappa B activation. Neurosci. Lett. 2022, 773, 136495. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Kumar, G.; Gedda, M.R.; Tiwari, N.; Patnaik, R.; Singh, R.K.; Singh, S.P. Effect of chlorogenic acid supplementation in MPTP-intoxicated mouse. Front. Pharmacol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Rahmani, A.H. Unlocking the Pharmacological Potential of Myricetin Against Various Pathogenesis. Int. J. Mol. Sci. 2025, 26, 4188. [Google Scholar] [CrossRef]
- Asgari, Z.; Iranzadeh, S.; Roghani, M. Myricetin alleviates learning and memory deficits in trimethyltin Alzheimer’s phenotype via attenuating hippocampal endoplasmic reticulum stress and regulating inflammation and oxidative stress. Brain Res. Bull. 2025, 227, 111382. [Google Scholar] [CrossRef]
- Hyam, S.R.; Lee, I.A.; Gu, W.; Kim, K.A.; Jeong, J.J.; Jang, S.E.; Han, M.J.; Kim, D.H. Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing M1 macrophages to M2-like macrophages. Eur. J. Pharmacol. 2013, 708, 21–29. [Google Scholar] [CrossRef]
- Xu, X.; Piao, H.N.; Aosai, F.; Zeng, X.Y.; Cheng, J.H.; Cui, Y.X.; Li, J.; Ma, J.; Piao, H.R.; Jin, X.; et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br. J. Pharmacol. 2020, 177, 5224–5245. [Google Scholar] [CrossRef]
- Castro, M.F.V.; Assmann, C.E.; Stefanello, N.; Reichert, K.P.; Palma, T.V.; da Silva, A.D.; Miron, V.V.; Mostardeiro, V.B.; Morsch, V.V.M.; Schetinger, M.R.C. Caffeic acid attenuates neuroinflammation and cognitive impairment in streptozotocin-induced diabetic rats: Pivotal role of the cholinergic and purinergic signaling pathways. J. Nutr. Biochem. 2023, 115, 109280. [Google Scholar] [CrossRef] [PubMed]
- Sadigh-Eteghad, S.; Khayat-Nuri, H.; Abadi, N.; Ghavami, S.; Golabi, M.; Shanebandi, D. Synergetic effects of oral administration of levamisole and Echinacea purpurea on immune response in Wistar rat. Res. Vet. Sci. 2011, 91, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.Z.; Yang, S.J.; Hu, W.X.; Wen, Z.; He, D.; Zeng, L.F.; Xiang, Q.; Wu, X.M.; Zhou, W.Y.; Zhu, Q.X. Effect of cold stress on immunity in rats. Exp. Ther. Med. 2016, 11, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Chan, J.N.; Lee, J.C.; Lee, S.S.; Hui, K.K.; Chan, A.H.; Fung, T.K.; Sánchez-Vidaña, D.I.; Lau, B.W.; Ngai, S.P. Interaction effect of social isolation and high-dose corticosteroid on neurogenesis and emotional behavior. Front. Behav. Neurosci. 2017, 11, 18. [Google Scholar] [CrossRef]
- Kumar, V.; Bhat, Z.A.; Kumar, D. Animal models of anxiety: A comprehensive review. J. Pharmacol. Toxicol. Methods 2013, 68, 175–183. [Google Scholar] [CrossRef]

| Extracts | ORAC, µmol TE/g | HORAC, µmol GAE/g | Electrochemical Method | |
|---|---|---|---|---|
| K, μmol/L·min ± SD | AOA | |||
| EP | 1417.2 ± 6.2 | 181.1 ± 10.4 | 14.0 ± 0.8 | 22.0 |
| OA | 368.4 ± 0.5 | 60.0 ± 1.0 | 8.5 ± 0.04 | 13.4 |
| CE1 | 736.5 ± 6.3 | 174.4 ± 1.7 | 17.1 ± 0.9 | 27.0 |
| CE2 | 1841.7 ± 75.0 | 277.2 ± 4.2 | 25.1 ± 1.3 | 39.6 |
| Trolox | - | - | 0.6 ± 0.001 | 1.0 |
| Extracts | EP | OA | CE1 | CE2 | |
|---|---|---|---|---|---|
| Analyte, μg/g | |||||
| Ferulic acid | 770.7 ± 44.9 | nd | 471.9 ± 28.26 | 726.0 ± 43.56 | |
| Caffeic acid | 1115.0 ± 55.0 | 265.0 ± 14.1 | 696.0 ± 42.9 | 839.0 ± 24.9 | |
| Caftaric acid | 3060.0 ± 142.3 | nd | 1450.0 ± 74.9 | 2748.0 ± 147.5 | |
| Chicoric acid | 12,915.7 ± 773.2 | nd | 6505.0 ± 390.3 | 8350.0 ± 441.2 | |
| Cynarin | 39.3 ± 2.1 | nd | nd | trace | |
| Echinacoside | 55.4 ± 2.3 | nd | nd | trace | |
| Neochlorogenic acid | 301.0 ± 27.3 | 596.0 ± 35.3 | 662.7 ± 49.8 | 443.8 ± 24.5 | |
| Chlorogenic acid | 904.7 ± 54.1 | 661.0 ± 37.3 | 967.0 ± 55.5 | 330.6 ± 17.5 | |
| Quercetin | 270.0 ± 13.3 | 584.6 ± 33.3 | 338.0 ± 3.3 | 98.5 ± 5.1 | |
| Apigenin | nd | 280.0 ± 3.7 | 173.2 ± 2.7 | 57.5 ± 2.9 | |
| Rutin | 2300.0 ± 132.1 | nd | 1340.0 ± 76.5 | 1837.0 ± 115.3 | |
| Myricetin | nd | 1322.0 ± 66.3 | 1006.0 ± 65.7 | 361.0 ± 24.7 | |
| Epicatechin | 142.3 ± 6.7 | 139.0 ± 1.2 | 856.0 ± 51.3 | 239.0 ± 15.3 | |
| Scutellarin | nd | 35.0 ± 1.3 | trace | nd | |
| Arctigenin | nd | 555 ± 32.7 | 225.2 ± 12.3 | 108.0 ± 13.2 | |
| Parameter | Non-Stressed Control (n = 8) Mean ± SD | Stressed Control (n = 8) Mean ± SD | t(df) | p-Value |
|---|---|---|---|---|
| Total entries | 3.75 ± 1.16 | 2.88 ± 1.55 | t(14) = 1.28 | p = 0.22 a |
| Closed arms entries | 2.00 ± 0.76 | 2.13 ± 0.83 | t(14) = −0.31 | p = 0.76 a |
| OAER | 0.47 ± 0.12 | 0.17 ± 0.2 | t(14) = 3.56 | p = 0.003 **,a |
| Time spent in open arms (s) | 54.38 ± 12.37 | 19.38 ± 5.53 | t(9.69) = 7.30 | p < 0.001 ***,b |
| Time spent in closed arms (s) | 245.62 ± 12.37 | 280.62 ± 5.53 | t(9.69) = −7.30 | p < 0.001 ***,b |
| OATR | 0.18 ± 0.04 | 0.07 ± 0.02 | t(14) = 8.05 | p < 0.001 ***,a |
| Social interaction time (s) | 32.25 ± 8.17 | 14.00 ± 4.87 | t(14) = 5.43 | p < 0.001 ***,a |
| Cytokine | Non-Stressed Control (mean ± SD) | Stressed Control (mean ± SD) | t(df) | p-Value |
|---|---|---|---|---|
| TNF-α (pg/mL) | 93.23 ± 22.46 | 135.55 ± 70.75 | t(8.40) = 1.61 | p = 0.14 b |
| IFN-γ (pg/mL) | 39.23 ± 12.33 | 58.93 ± 18.72 | t(14) = 2.49 | p = 0.026 *,a |
| IL-10 (pg/mL) | 164.34 ± 43.96 | 93.74 ± 36.71 | t(14)= −3.49 | p = 0.004 **,a |
| Variables | INF-γ (pg/mL) | IL-10 (pg/mL) |
|---|---|---|
| OAER | r(38) = −0.103 p = 0.529 | r(38) = −0.002 p = 0.999 |
| OATR | r(38) = −0.299 p = 0.061 | r(38) = 0.552 p < 0.001 *** |
| Social interaction time (s) | r(38) = −0.094 p = 0.563 | r(38) = 0.255; p = 0.113 |
| Group | Legend | Description | Acute Cold Stress Model |
|---|---|---|---|
| 1 | C-stress | Distilled water, 10 mL/kg body weight | Yes |
| 2 | C0 | Distilled water, 10 mL/kg body weight | No |
| 3 | EP | E. purpurea, 500 mg/kg body weight | Yes |
| 4 | OA | O. acanthium, 500 mg/kg body weight | Yes |
| 5 | CE1 | Combination 1, 500 mg/kg body weight | Yes |
| 6 | CE2 | Combination 2, 500 mg/kg body weight | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasheva, M.; Katsarova, M.; Kandilarov, I.; Zlatanova-Tenisheva, H.; Gardjeva, P.; Denev, P.; Atliev, K.; Sadakova, N.; Dimitrova, M.; Kostadinov, I.; et al. Comparative Study on Extracts from Traditional Medicinal Plants Echinacea purpurea (L.) Moench and Onopordum acanthium (L.): Antioxidant Activity In Vitro and Anxiolytic Effect In Vivo. Pharmaceuticals 2025, 18, 1801. https://doi.org/10.3390/ph18121801
Vlasheva M, Katsarova M, Kandilarov I, Zlatanova-Tenisheva H, Gardjeva P, Denev P, Atliev K, Sadakova N, Dimitrova M, Kostadinov I, et al. Comparative Study on Extracts from Traditional Medicinal Plants Echinacea purpurea (L.) Moench and Onopordum acanthium (L.): Antioxidant Activity In Vitro and Anxiolytic Effect In Vivo. Pharmaceuticals. 2025; 18(12):1801. https://doi.org/10.3390/ph18121801
Chicago/Turabian StyleVlasheva, Maria, Mariana Katsarova, Ilin Kandilarov, Hristina Zlatanova-Tenisheva, Petya Gardjeva, Petko Denev, Kiril Atliev, Nora Sadakova, Maria Dimitrova, Ilia Kostadinov, and et al. 2025. "Comparative Study on Extracts from Traditional Medicinal Plants Echinacea purpurea (L.) Moench and Onopordum acanthium (L.): Antioxidant Activity In Vitro and Anxiolytic Effect In Vivo" Pharmaceuticals 18, no. 12: 1801. https://doi.org/10.3390/ph18121801
APA StyleVlasheva, M., Katsarova, M., Kandilarov, I., Zlatanova-Tenisheva, H., Gardjeva, P., Denev, P., Atliev, K., Sadakova, N., Dimitrova, M., Kostadinov, I., & Dimitrova, S. (2025). Comparative Study on Extracts from Traditional Medicinal Plants Echinacea purpurea (L.) Moench and Onopordum acanthium (L.): Antioxidant Activity In Vitro and Anxiolytic Effect In Vivo. Pharmaceuticals, 18(12), 1801. https://doi.org/10.3390/ph18121801











