Curcumin and Tetrahydrocurcumin as Multi-Organ Modulators of the Adipose Tissue–Gut–Liver Axis: Mechanistic Insights, Therapeutic Potential, and Translational Challenges
Abstract
1. Introduction
Methodology
2. Curcumin and Adipose Tissue: Remodeling and Metabolic Reprogramming
2.1. Adipose Tissue Dysfunction: From Storage to Endocrine Dysregulation
2.2. Modulation of Adipogenesis and Lipid Metabolism
2.3. Anti-Inflammatory and Anti-Fibrotic Actions
2.4. Browning of White Adipose Tissue and Mitochondrial Remodeling
2.5. Systemic Implications of Adipose Remodeling
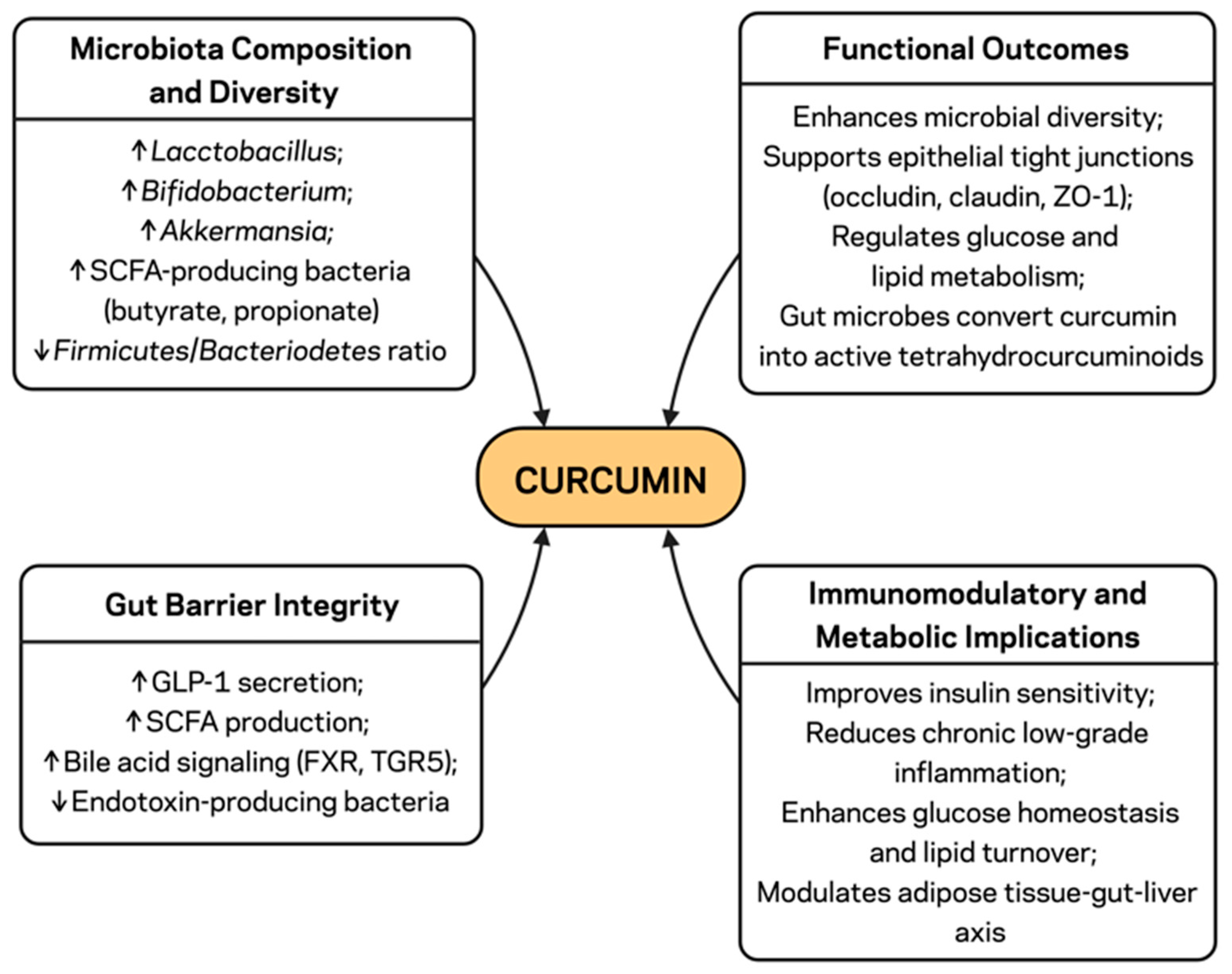
3. Curcumin and Gut Microbiota: Microbial and Barrier Effects
3.1. Gut Dysbiosis and Barrier Dysfunction: The Gateway to Metabolic Endotoxemia
3.2. Modulation of Microbiota Composition and Diversity
3.3. Enhancement of Gut Barrier Integrity
3.4. Immunomodulatory and Metabolic Implications
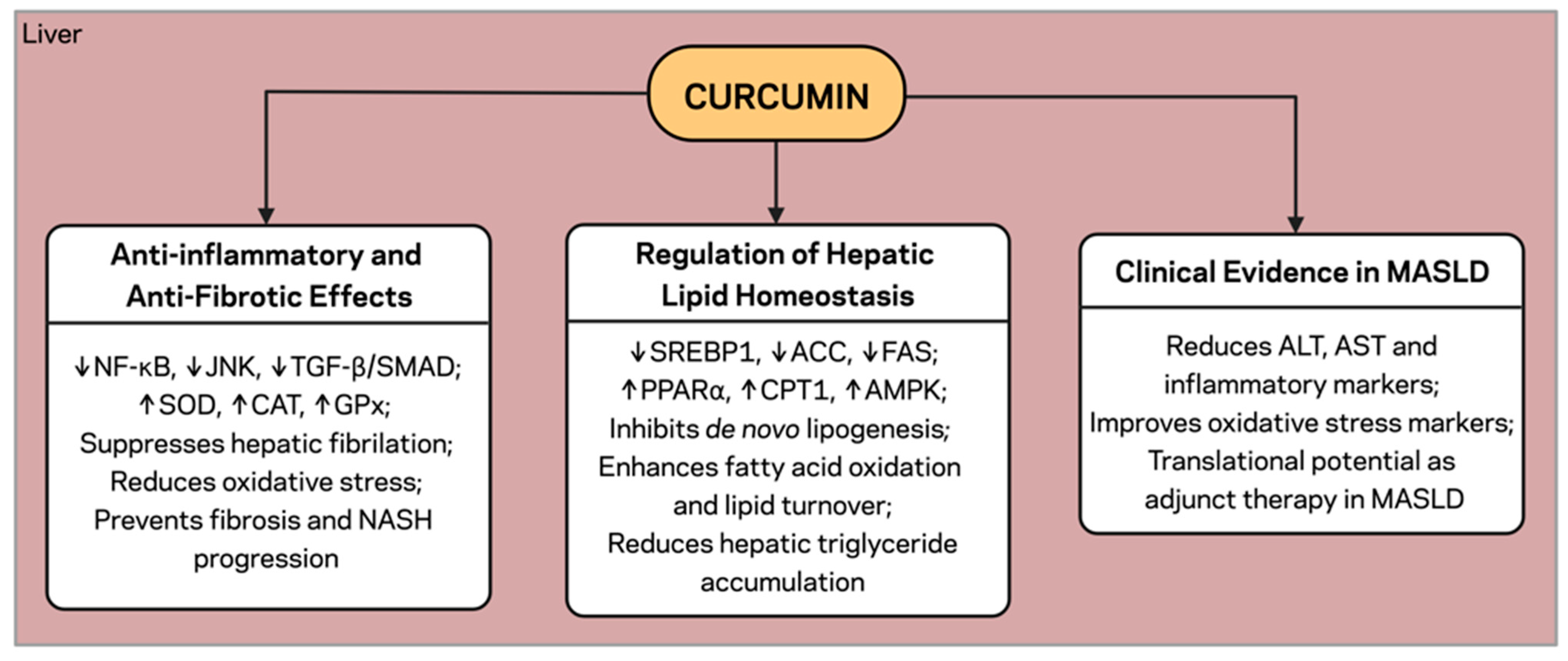
4. Curcumin and the Liver: Lipid Metabolism and MASLD
4.1. Hepatic Crosstalk: Convergence of Adipose and Gut-Derived Signals
4.2. Regulation of Hepatic Lipid Homeostasis
4.3. Anti-Inflammatory and Anti-Fibrotic Effects
4.4. Modulation of Insulin Sensitivity and Glucose Metabolism
4.5. Clinical Evidence in MASLD
5. Tetrahydrocurcumin and Curcumin Analogs: Overcoming Limitations
5.1. Bioavailability Challenges of Native Curcumin
5.2. Tetrahydrocurcumin: An Active Metabolite with Enhanced Pharmacokinetics
5.3. Monocarbonyl Curcumin Analogs: Structure–Activity Optimization
5.4. Advanced Delivery Systems for Targeted Multi-Organ Effects
6. Translational Evidence: From Mechanistic Studies to Clinical Application
6.1. Clinical Outcomes in MetS, MASLD, and Obesity
6.2. Tetrahydrocurcumin and Curcumin Analogs in Human Studies
6.3. Pharmacokinetic and Formulation Perspectives
6.4. Regulatory and Safety Considerations
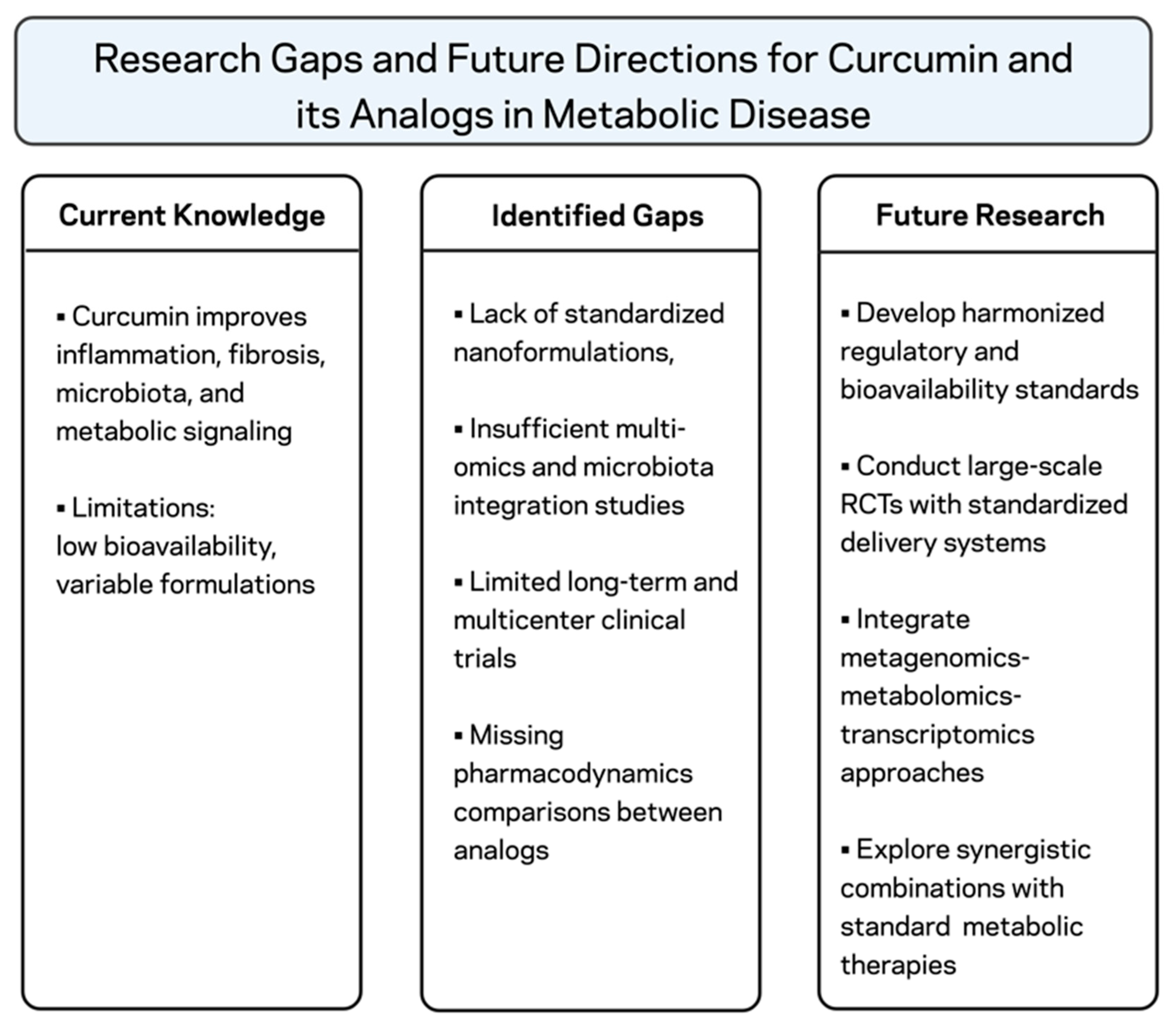
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA Carboxylase |
| AKT | Protein Kinase B |
| ALT | Alanine Aminotransferase |
| AMPK | AMP-Activated Protein Kinase |
| AST | Aspartate Aminotransferase |
| BMI | Body Mass Index |
| CAT | Catalase |
| CCAAT | CCAAT/Enhancer-Binding Protein |
| CD9 | Cluster of Differentiation 9 |
| CPT1 | Carnitine Palmitoyltransferase 1 |
| CRP | C-Reactive Protein |
| C/EBPα | CCAAT/Enhancer-Binding Protein Alpha |
| ER | Endoplasmic Reticulum |
| FAS | Fatty Acid Synthase |
| FFA | Free Fatty Acid |
| FXR | Farnesoid X Receptor |
| GI | Gastro-Intestinal |
| GLP-1 | Glucagon-Like Peptide 1 |
| GLP-2 | Glucagon-Like Peptide 2 |
| GPx | Glutathione Peroxidase |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
| IL-6 | Interleukin 6 |
| IR | Insulin Resistance |
| IRS-1 | Insulin Receptor Substrate 1 |
| JNK | c-Jun N-terminal Kinase |
| LPS | Lipopolysaccharide |
| MAFLD | Metabolic-Associated Fatty Liver Disease |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MCP-1 | Monocyte Chemoattractant Protein 1 |
| MetS | Metabolic Syndrome |
| NAD+ | Nicotinamide Adenine Dinucleotide |
| NASH | Non-Alcoholic Steatohepatitis |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| PDGFRα | Platelet-Derived Growth Factor Receptor Alpha |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha |
| PKA | Protein Kinase A |
| PPARα | Peroxisome Proliferator-Activated Receptor Alpha |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| SAR | Structure–Activity Relationship |
| SCFA | Short-Chain Fatty Acid |
| SIRT1 | Sirtuin 1 |
| SOD | Superoxide Dismutase |
| SREBP1c | Sterol Regulatory Element-Binding Protein 1c |
| T2DM | Type 2 Diabetes Mellitus |
| TG | Triglyceride |
| TGF-β | Transforming Growth Factor-Beta |
| TGR5 | Takeda G Protein-Coupled Receptor 5 |
| THC | Tetrahydrocurcumin |
| TLR4 | Toll-Like Receptor 4 |
| TNF | Tumor-Necrosis Factor |
| UCP1 | Uncoupling Protein 1 |
| VLDL | Very Low-Density Lipoprotein |
| ZO-1 | Zonula Occludens-1 |
References
- World Health Organization. Obesity and Overweight–Key Facts; WHO Fact Sheet; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin. Mol. Hepatol. 2025, 31, S32–S50. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose Tissue Remodeling and Obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Stojchevski, R.; Chandrasekaran, P.; Hadzi-Petrushev, N.; Mladenov, M.; Avtanski, D. Adipose Tissue Dysfunction Related to Climate Change and Air Pollution: Understanding the Metabolic Consequences. Int. J. Mol. Sci. 2024, 25, 7849. [Google Scholar] [CrossRef]
- Avtanski, D.; Hadzi-Petrushev, N.; Josifovska, S.; Mladenov, M.; Reddy, V. Emerging Technologies in Adipose Tissue Research. Adipocyte 2023, 12, 2248673. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Leo, S.; Lazarevic, V.; Gaïa, N.; Estellat, C.; Girard, M.; Matheron, S.; Armand-Lefèvre, L.; Antoine Andremont The VOYAG-R Study Group; Schrenzel, J.; Ruppé, E. The Intestinal Microbiota Predisposes to Traveler’s Diarrhea and to the Carriage of Multidrug-Resistant Enterobacteriaceae after Traveling to Tropical Regions. Gut Microbes 2019, 10, 631–641. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Non-Alcoholic Fatty Liver Disease as a Cause and a Consequence of Metabolic Syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Glatthar, R.; Arch, R.H. Therapeutic Approaches for Nonalcoholic Steatohepatitis (NASH). In Cardiovascular, Endocrine and Metabolic Diseases; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Haas, J.T.; Francque, S.; Staels, B. Pathophysiology and Mechanisms of Nonalcoholic Fatty Liver Disease. Annu. Rev. Physiol. 2016, 78, 181–205. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and Anti-Inflammatory Effects of Curcuminoid-Piperine Combination in Subjects with Metabolic Syndrome: A Randomized Controlled Trial and an Updated Meta-Analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin Inhibits Adipogenesis in 3T3-L1 Adipocytes and Angiogenesis and Obesity in C57/BL Mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- Jalali, M.; Mahmoodi, M.; Mosallanezhad, Z.; Jalali, R.; Imanieh, M.H.; Moosavian, S.P. The Effects of Curcumin Supplementation on Liver Function, Metabolic Profile and Body Composition in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020, 48, 102283. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Daglia, M.; Hajizadeh Moghaddam, A.; Habtemariam, S.; Nabavi, S.M. Curcumin and Liver Disease: From Chemistry to Medicine. Compr. Rev. Food Sci. Food Saf. 2014, 13, 62–77. [Google Scholar] [CrossRef]
- Zhou, H.; Beevers, C.S.; Huang, S. The Targets of Curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Keremidarska-Markova, M.; Sazdova, I.; Mladenov, M.; Pilicheva, B.; Zagorchev, P.; Gagov, H. Sirtuin 1 and Hormonal Regulations in Aging. Appl. Sci. 2024, 14, 12051. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Lozada-García, M.C.; Enríquez, R.G.; Ramírez-Apán, T.O.; Nieto-Camacho, A.; Palacios-Espinosa, J.F.; Custodio-Galván, Z.; Soria-Arteche, O.; Pérez-Villanueva, J. Synthesis of Curcuminoids and Evaluation of Their Cytotoxic and Antioxidant Properties. Molecules 2017, 22, 633. [Google Scholar] [CrossRef] [PubMed]
- Selvam, C.; Jachak, S.M.; Thilagavathi, R.; Chakraborti, A.K. Design, Synthesis, Biological Evaluation and Molecular Docking of Curcumin Analogues as Antioxidant, Cyclooxygenase Inhibitory and Anti-Inflammatory Agents. Bioorg. Med. Chem. Lett. 2005, 15, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed]
- Mladenov, M.; Bogdanov, J.; Bogdanov, B.; Hadzi-Petrushev, N.; Kamkin, A.; Stojchevski, R.; Avtanski, D. Efficacy of the Monocarbonyl Curcumin Analog C66 in the Reduction of Diabetes-Associated Cardiovascular and Kidney Complications. Mol. Med. 2022, 28, 129. [Google Scholar] [CrossRef]
- Stojchevski, R.; Velichkovikj, S.; Bogdanov, J.; Hadzi-Petrushev, N.; Mladenov, M.; Poretsky, L.; Avtanski, D. Monocarbonyl Analogs of Curcumin C66 and B2BrBC Modulate Oxidative Stress, JNK Activity, and Pancreatic Gene Expression in Rats with Streptozotocin-Induced Diabetes. Biochem. Pharmacol. 2024, 229, 116491. [Google Scholar] [CrossRef]
- Josifovska, S.; Panov, S.; Hadzi-Petrushev, N.; Mitrokhin, V.; Kamkin, A.; Stojchevski, R.; Avtanski, D.; Mladenov, M. Positive Tetrahydrocurcumin-Associated Brain-Related Metabolomic Implications. Molecules 2023, 28, 3734. [Google Scholar] [CrossRef]
- Petito, G.; Cioffi, F.; Magnacca, N.; de Lange, P.; Senese, R.; Lanni, A. Adipose Tissue Remodeling in Obesity: An Overview of the Actions of Thyroid Hormones and Their Derivatives. Pharmaceuticals 2023, 16, 572. [Google Scholar] [CrossRef]
- McLaughlin, T.; Liu, L.-F.; Lamendola, C.; Shen, L.; Morton, J.; Rivas, H.; Winer, D.; Tolentino, L.; Choi, O.; Zhang, H.; et al. T-Cell Profile in Adipose Tissue Is Associated with Insulin Resistance and Systemic Inflammation in Humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2637–2643. [Google Scholar] [CrossRef]
- Marcelin, G.; Ferreira, A.; Liu, Y.; Atlan, M.; Aron-Wisnewsky, J.; Pelloux, V.; Botbol, Y.; Ambrosini, M.; Fradet, M.; Rouault, C.; et al. A PDGFRα-Mediated Switch toward CD9 high Adipocyte Progenitors Controls Obesity-Induced Adipose Tissue Fibrosis. Cell Metab. 2017, 25, 673–685. [Google Scholar] [CrossRef]
- Li, X.; Ren, Y.; Chang, K.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose Tissue Macrophages as Potential Targets for Obesity and Metabolic Diseases. Front. Immunol. 2023, 14, 1153915. [Google Scholar] [CrossRef]
- Tian, L.; Song, Z.; Shao, W.; Du, W.W.; Zhao, L.R.; Zeng, K.; Yang, B.B.; Jin, T. Curcumin Represses Mouse 3T3-L1 Cell Adipogenic Differentiation via Inhibiting miR-17-5p and Stimulating the Wnt Signalling Pathway Effector Tcf7l2. Cell Death Dis. 2017, 8, e2559. [Google Scholar] [CrossRef] [PubMed]
- Obrzut, O.; Gostyńska-Stawna, A.; Kustrzyńska, K.; Stawny, M.; Krajka-Kuźniak, V. Curcumin: A Natural Warrior against Inflammatory Liver Diseases. Nutrients 2025, 17, 1373. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Luo, Y.; Wang, L.; Zhang, K.; Peng, J.; Fan, G. Therapeutic Effect of Curcumin on Metabolic Diseases: Evidence from Clinical Studies. Int. J. Mol. Sci. 2023, 24, 3323. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Ye, Z.; Xu, C.; Zhang, M.; Ruan, B.; Wei, M.; Jiang, Y.; Zhang, Y.; Wang, L.; et al. Curcumin Promotes Browning of White Adipose Tissue in a Norepinephrine-Dependent Way. Biochem. Biophys. Res. Commun. 2015, 466, 247–253. [Google Scholar] [CrossRef]
- Zhao, D.; Pan, Y.; Yu, N.; Bai, Y.; Ma, R.; Mo, F.; Zuo, J.; Chen, B.; Jia, Q.; Zhang, D.; et al. Curcumin Improves Adipocytes Browning and Mitochondrial Function in 3T3-L1 Cells and Obese Rodent Model. R. Soc. Open Sci. 2021, 8, 200974. [Google Scholar] [CrossRef]
- Dahdah, N.; Tercero-Alcázar, C.; Malagón, M.M.; Garcia-Roves, P.M.; Guzmán-Ruiz, R. Interrelation of Adipose Tissue Macrophages and Fibrosis in Obesity. Biochem. Pharmacol. 2024, 225, 116324. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Smirnova, E.; Tuong, D.T.C.; Vinod, S.; Chin, S.; Moniruzzaman, M.; Senthil, K.; Lee, D.I.; Adhimoolam, K.; Min, T. Unraveling Curcumin’s Molecular Targets and Its Potential in Suppressing Skin Inflammation Using Network Pharmacology and In Vitro Studies. Drug Dev. Res. 2025, 86, e70058. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Su, Y.-F.; Yang, H.-W.; Lee, Y.-H.; Chou, J.I.; Ueng, K.-C. Lipid-Lowering Effects of Curcumin in Patients with Metabolic Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Phytother. Res. 2014, 28, 1770–1777. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The Effect of Curcumin Supplementation on Anthropometric Indices, Insulin Resistance and Oxidative Stress in Patients with Type 2 Diabetes: A Randomized, Double-Blind Clinical Trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in Gut Microbiota Control Inflammation in Obese Mice through a Mechanism Involving GLP-2-Driven Improvement of Gut Permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcoholado, L.; Ordóñez, R.; Otero, A.; Plaza-Andrade, I.; Laborda-Illanes, A.; Medina, J.A.; Ramos-Molina, B.; Gómez-Millán, J.; Queipo-Ortuño, M.I. Gut Microbiota-Mediated Inflammation and Gut Permeability in Patients with Obesity and Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 6782. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 Links Innate Immunity and Fatty Acid-Induced Insulin Resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Fleishman, J.S.; Kumar, S. Bile Acid Metabolism and Signaling in Health and Disease: Molecular Mechanisms and Therapeutic Targets. Signal Transduct. Target. Ther. 2024, 9, 97. [Google Scholar] [CrossRef]
- Watson, A.J.M.; Duckworth, C.A. Gut Microbiota Control Gut Permeability through GLP-2. Gastroenterology 2010, 138, 779–781. [Google Scholar] [CrossRef]
- Różański, G.; Kujawski, S.; Newton, J.L.; Zalewski, P.; Słomko, J. Curcumin and Biochemical Parameters in Metabolic-Associated Fatty Liver Disease (MAFLD)—A Review. Nutrients 2021, 13, 2654. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Wu, K.; Chen, H.; Wang, Q.; Xiao, Y.; Yao, S.; Hong, A.; Zhang, M.; Lei, S.; et al. Exploring the Impact of Short-Term Travel on Gut Microbiota and Probiotic Bacteria-Mediated Stability. Biomedicines 2024, 12, 1378. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short-Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; El Rayess, Y.; Abi Rizk, A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Atanasova-Panchevska, N.; Stojchevski, R.; Hadzi-Petrushev, N.; Mitrokhin, V.; Avtanski, D.; Mladenov, M. Antibacterial and Antiviral Properties of Tetrahydrocurcumin-Based Formulations: An Overview of Their Metabolism in Different Microbiotic Compartments. Life 2022, 12, 1708. [Google Scholar] [CrossRef]
- Gaskell, C.; MacDonald, R.; Aleem, E.; Bendriss, G. Obesity and Cancer: Unravelling the Microbiome’s Hidden Role. Front. Nutr. 2025, 12, 1602603. [Google Scholar] [CrossRef]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of Gut Microbiota Contributes to Curcumin-Mediated Attenuation of Hepatic Steatosis in Rats. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Sears, D.D. TLR4 and Insulin Resistance. Gastroenterol. Res. Pract. 2010, 2010, 212563. [Google Scholar] [CrossRef] [PubMed]
- Feng, J. Role of Curcumin in Altering Gut Microbiota for Anti-Obesity and Anti-Hyperlipidemic Effects. Front. Microbiol. 2025, 16, 1625098. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Lee, J.-H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial Metabolic Effects of a Probiotic via Butyrate-Induced GLP-1 Hormone Secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Zhu, J.; He, L. The Modulatory Effects of Curcumin on the Gut Microbiota: A Potential Strategy for Disease Treatment and Health Promotion. Microorganisms 2024, 12, 642. [Google Scholar] [CrossRef]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile Acid Metabolism and Signaling, the Microbiota, and Metabolic Disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef]
- Radu, F.; Potcovaru, C.-G.; Salmen, T.; Filip, P.V.; Pop, C.; Fierbințeanu-Braticievici, C. The Link between NAFLD and Metabolic Syndrome. Diagnostics 2023, 13, 614. [Google Scholar] [CrossRef]
- Fang, Y.-L.; Chen, H.; Wang, C.-L.; Liang, L. Pathogenesis of Non-Alcoholic Fatty Liver Disease in Children and Adolescence: From “Two-Hit Theory” to “Multiple Hit Model”. World J. Gastroenterol. 2018, 24, 2974–2983. [Google Scholar] [CrossRef]
- Favero, G.; Krajčíková, K.; Bonomini, F.; Rodella, L.F.; Tomečková, V.; Rezzani, R. Browning of Adipose Tissue and Sirtuin Involvement. In Adipose Tissue; Szablewski, L., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Basha, A.; May, S.C.; Anderson, R.M.; Samala, N.; Mirmira, R.G. Non-Alcoholic Fatty Liver Disease: Translating Disease Mechanisms into Therapeutics Using Animal Models. Int. J. Mol. Sci. 2023, 24, 9996. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate Mediates a Microbiome–Brain–β-Cell Axis to Promote Metabolic Syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, Y.; Shao, C.; Jiang, C.; Wu, L.; Xiao, J.; Tang, L. Gut Microbiota-Targeted Therapeutics for Metabolic Disorders: Mechanistic Insights into the Synergy of Probiotic-Fermented Herbal Bioactives. Int. J. Mol. Sci. 2025, 26, 5486. [Google Scholar] [CrossRef]
- Qiu, L.; Gao, C.; Wang, H.; Ren, Y.; Li, J.; Li, M.; Du, X.; Li, W.; Zhang, J. Effects of Dietary Polyphenol Curcumin Supplementation on Metabolic, Inflammatory, and Oxidative Stress Indices in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2023, 14, 1216708. [Google Scholar] [CrossRef]
- Qadir, M.I.; Naqvi, S.T.Q.; Muhammad, S.A. Curcumin: A Polyphenol with Molecular Targets for Cancer Control. Asian Pac. J. Cancer Prev. 2016, 17, 2735–2739. [Google Scholar]
- Al-Suhaimi, E.A. Curcumin Induces Apoptosis of 3T3-L1 Adipocytes and Affects Molecular Signals of Adiponectin, AMPK and PKA. An. Real Acad. Farm. 2014, 80, 720–734. [Google Scholar]
- Alam, M.S.; Anwar, M.J.; Maity, M.K.; Azam, F.; Jaremko, M.; Emwas, A.-H. The Dynamic Role of Curcumin in Mitigating Human Illnesses: Recent Advances in Therapeutic Applications. Pharmaceuticals 2024, 17, 1674. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-Alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-Controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Sun, H.; Liu, T.; Wang, Z.; Shen, W.; Yuan, X.; Xie, J.; Zhang, Y. Role of Curcumin in Chronic Liver Diseases: A Comprehensive Review. Drug Des. Devel. Ther. 2025, 19, 3395–3406. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, Y.; Zhang, Q.; Xiao, X. Crosstalk between Glucagon-Like Peptide 1 and Gut Microbiota in Metabolic Diseases. mBio 2024, 15, e02032-23. [Google Scholar] [CrossRef] [PubMed]
- Różański, G.; Tabisz, H.; Zalewska, M.; Niemiro, W.; Kujawski, S.; Newton, J.; Zalewski, P.; Słomko, J. Meta-Analysis of Exploring the Effect of Curcumin Supplementation with or without Other Advice on Biochemical and Anthropometric Parameters in Patients with Metabolic-Associated Fatty Liver Disease (MAFLD). Int. J. Environ. Res. Public Health 2023, 20, 4266. [Google Scholar] [CrossRef] [PubMed]
- Nurcahyanti, A.D.R.; Cokro, F.; Wulanjati, M.P.; Mahmoud, M.F.; Wink, M.; Sobeh, M. Curcuminoids for Metabolic Syndrome: Meta-Analysis Evidences Toward Personalized Prevention and Treatment Management. Front. Nutr. 2022, 9, 891339. [Google Scholar] [CrossRef]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and Inflammation in Non-Alcoholic Fatty Liver Disease: A Randomized, Placebo-Controlled Clinical Trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T., IV; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose Escalation of a Curcuminoid Formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of Curcumin in Buffer Solutions and Characterization of Its Degradation Products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Pari, L.; Murugan, P. Tetrahydrocurcumin Prevents Brain Lipid Peroxidation in Streptozotocin-Induced Diabetic Rats. J. Med. Food 2007, 10, 323–329. [Google Scholar] [CrossRef]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Donpunha, W.; Kukongviriyapan, V.; Kongyingyoes, B.; Sompamit, K.; Phisalaphong, C. Antioxidant and Vascular Protective Effects of Curcumin and Tetrahydrocurcumin in Rats with L-NAME-Induced Hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 519–529. [Google Scholar] [CrossRef]
- Murugan, P.; Pari, L. Antioxidant Effect of Tetrahydrocurcumin in Streptozotocin–Nicotinamide Induced Diabetic Rats. Life Sci. 2006, 79, 1720–1728. [Google Scholar] [CrossRef]
- Yin, S.; Zheng, X.; Yao, X.; Wang, Y.; Liao, D. Synthesis and Anticancer Activity of Mono-Carbonyl Analogues of Curcumin. J. Cancer Ther. 2013, 4, 113–123. [Google Scholar] [CrossRef]
- Liang, G.; Li, X.; Chen, L.; Yang, S.; Wu, X.; Studer, E.; Gurley, E.; Hylemon, P.B.; Ye, F.; Li, Y.; et al. Synthesis and Anti-Inflammatory Activities of Mono-Carbonyl Analogues of Curcumin. Bioorg. Med. Chem. Lett. 2008, 18, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Cao, W.; Zhao, L.; Han, Q.; Yang, S.; Yang, K.; Pan, X.; Wang, Q.; Wang, Y. Design, Synthesis, and Antitumor Evaluation of Novel Mono-Carbonyl Curcumin Analogs in Hepatocellular Carcinoma Cell. Pharmaceuticals 2022, 15, 950. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.J.; Choudhary, K.; Ali, A.; Ali, A.; Azam, F.; Almalki, A.H.; Santali, E.Y.; Bakht, M.A.; Tahir, A.; Salahuddin. Synthesis, DFT Analyses, Antiproliferative Activity, and Molecular Docking Studies of Curcumin Analogues. Plants 2022, 11, 2835. [Google Scholar] [CrossRef]
- Negi, N.; Chand, G.; Kholia, D.; Anand, R.; Upadhyay, S.K.; Tewari, G.; Joshi, P. Recent Development in the Structural Modifications of Monocarbonyl Analogues of Curcumin and Their Improved Biological Activities: A Review. Pharmacogn. Rev. 2023, 17, 247–254. [Google Scholar] [CrossRef]
- Tu, Z.-S.; Wang, Q.; Sun, D.-D.; Dai, F.; Zhou, B. Design, Synthesis, and Evaluation of Curcumin Derivatives as Nrf2 Activators and Cytoprotectors against Oxidative Death. Eur. J. Med. Chem. 2017, 134, 72–85. [Google Scholar] [CrossRef]
- Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Limami, Y.; Duval, R.E. Curcumin-Based Nanoparticles: Advancements and Challenges in Tumor Therapy. Pharmaceutics 2025, 17, 114. [Google Scholar] [CrossRef]
- Godse, S.; Zhou, L.; Sakshi, S.; Singla, B.; Singh, U.P.; Kumar, S. Nanocarrier-Mediated Curcumin Delivery: An Adjuvant Strategy for CNS Disease Treatment. Exp. Biol. Med. 2023, 248, 2151–2166. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, C.; Zhou, Y.; Xu, F.; Xu, Y. Synthesis and Cytotoxic Evaluation of Monocarbonyl Analogs of Curcumin as Potential Anti-Tumor Agents. Drug Dev. Res. 2016, 77, 43–49. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin Extract for Prevention of Type 2 Diabetes in Prediabetic Subjects: A Randomized Controlled Trial. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Na, L.X.; Li, Y.; Pan, H.Z.; Zhou, X.L.; Sun, D.J.; Meng, M.; Li, X.X.; Sun, C.H. Curcuminoids Decrease Serum Free Fatty Acids and Improve Glycemic Control in Type 2 Diabetes: A Randomized Double-Blind Trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Curcumin Lowers Serum Lipids and Uric Acid in Subjects with NAFLD: A Randomized Controlled Trial. J. Cardiovasc. Pharmacol. 2016, 68, 223–229. [Google Scholar] [CrossRef]
- Chashmniam, S.; Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Azimi Nezhad, M.; Nobakht, F.M. A Pilot Study of the Effect of Phospholipid Curcumin on Serum Metabolomic Profile in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Eur. J. Clin. Nutr. 2019, 73, 1224–1235. [Google Scholar] [CrossRef]
- Gerami, H.; Mozaffari-Khosravi, H.; Mansour, A.; Sohrabpour, A.A.; Poustchi, H.; Hashemi Taheri, A.P.; Jaafari, M.R.; Jambarsang, S.; Khayyatzadeh, S.S. Nano-Curcumin Supplementation Improves Liver Fibrosis in NAFLD: A Double-Blind Randomized Trial. Sci. Rep. 2025, 15, 21862. [Google Scholar] [CrossRef]
- Bateni, Z.; Rahimi, H.R.; Hedayati, M.; Afsharian, S.; Goudarzi, R.; Sohrab, G. The Effects of Nano-Curcumin Supplementation on Glycemic Control, Blood Pressure, Lipid Profile, and Insulin Resistance in Patients with Metabolic Syndrome: A Randomized, Double-Blind Clinical Trial. Phytother. Res. 2021, 35, 3945–3953. [Google Scholar] [CrossRef] [PubMed]
- Sedighiyan, M.; Jafari, E.; Athar, S.S.; Yekaninejad, M.S.; Alvandi, E.; Abdolahi, M.; Djalali, M. The Effects of Nano-Curcumin Supplementation on Leptin and Adiponectin in Migraine Patients: A Double-Blind Clinical Trial Study from Gene Expression to Clinical Symptoms. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. The Effect of Curcumin on Reducing Atherogenic Risks in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2024, 16, 2441. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Jacob, J.; Amalraj, A.; Raj, K.J.; Divya, C.; Kunnumakkara, A.B.; Gopi, S. A Novel Bioavailable Hydrogenated Curcuminoids Formulation (CuroWhite™) Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients—A Randomized, Double-Blind and Placebo-Controlled Study. J. Tradit. Complement. Med. 2018, 9, 346–352. [Google Scholar] [CrossRef]
- He, Y.; Wang, H.; Lin, S.; Chen, T.; Chang, D.; Sun, Y.; Wang, C.; Liu, Y.; Lu, Y.; Song, J.; et al. Advanced Effect of Curcumin and Resveratrol on Mitigating Hepatic Steatosis in Metabolic Associated Fatty Liver Disease via the PI3K/AKT/mTOR and HIF-1/VEGF Cascade. Biomed. Pharmacother. 2023, 165, 115279. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Davis, J.; Zhang, A.J.; He, X.; Mathews, S.T. Curcumin Activates AMPK and Suppresses Gluconeogenic Gene Expression in Hepatoma Cells. Biochem. Biophys. Res. Commun. 2009, 388, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Lukkunaprasit, T.; Tansawet, A.; Boonmanunt, S.; Sobhonslidsuk, A.; McKay, G.J.; Attia, J.; Thakkinstian, A. An Updated Meta-Analysis of Effects of Curcumin on Metabolic Dysfunction-Associated Fatty Liver Disease Based on Available Evidence from Iran and Thailand. Sci. Rep. 2023, 13, 5824. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Chen, A. Curcumin Suppresses Expression of Low-Density Lipoprotein (LDL) Receptor, Leading to the Inhibition of LDL-Induced Activation of Hepatic Stellate Cells. Br. J. Pharmacol. 2009, 157, 1354–1367. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-Malignant Lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Hadži-Petrushev, N.; Angelovski, M.; Rebok, K.; Mitrokhin, V.; Kamkin, A.; Mladenov, M. Antioxidant and Anti-Inflammatory Effects of the Monocarbonyl Curcumin Analogs B2BrBC and C66 in Monocrotaline-Induced Right Ventricular Hypertrophy. J. Biochem. Mol. Toxicol. 2019, 33, e22353. [Google Scholar] [CrossRef]
- Colino, C.I.; Lanao, J.M.; Gutierrez-Millan, C. Targeting of Hepatic Macrophages by Therapeutic Nanoparticles. Front. Immunol. 2020, 11, 218. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K. Efficacy and Safety of Tetrahydrocurcuminoids for the Treatment of Canker Sore and Gingivitis. Evid. Based Complement. Alternat. Med. 2020, 2020, 6611877. [Google Scholar] [CrossRef]

| Aspect | Key Features/Mechanism | Functional Outcomes | References |
|---|---|---|---|
| Bioavailability challenges of native curcumin | Poor solubility; rapid metabolism (glucuronidation, sulfation); instability at physiological pH; low membrane permeability | Nanomolar plasma concentrations even at high doses; limited tissue penetration; weak sustained biological activity | [22,78,79,80] |
| Tetrahydrocurcumin (THC) | Hydrogenated metabolite; ↑ solubility and stability; better absorption; activates AMPK, PGC-1α, mitochondrial biogenesis | Stronger antioxidant and anti-inflammatory vs. curcumin; ↓ LDL; improved insulin sensitivity; ↓ steatosis and systemic inflammation; supports adipose browning and hepatic lipid oxidation; potential to modulate liver-related endotoxemia | [81,82,83,84] |
| Monocarbonyl analogs (e.g., C66, B2BrBC) | Stable linker replacing dienone bridge; ↑ chemical stability and pharmacokinetics; favorable SAR profile | Potent NF-κB inhibition; ↓ cytokines; ↓ oxidative stress; better efficacy in insulin resistance and hepatic injury; enhanced target engagement in metabolic regulation | [85,86,87,88,89] |
| Advanced delivery systems | Liposomes, polymeric nanoparticles, micelles, phospholipid complexes; prebiotic-functionalized bioactive carriers | ↑ Intestinal absorption and systemic bioavailability; targeted delivery to liver, gut, adipose tissue; improved efficacy in MASLD models; microbiota and epithelial barrier modulation; systemic formulations act on adipose and liver | [90,91,92] |
| Condition/Target | Curcumin Formulations | Sample Size/Duration/Dose | Population | Study Design | Primary Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Prediabetes | Curcumin extract | 240/9 months/1.5 g/day | Adults with prediabetes | RCT, double-blind, placebo-controlled | Prevention of T2DM; ↓ HbA1c; ↑ β-cell function | [93] |
| Type 2 Diabetes | Curcuminoids | 100/12 weeks/300 mg/day | Adults with T2DM | RCT, double-blind, placebo-controlled | ↓ FFA; ↓ HbA1c; ↓ FPG | [94] |
| Type 2 Diabetes | Curcuminoids + piperine | 100/12 weeks/500 mg/day + 5 mg/day piperine | Adults with T2DM | RCT, double-blind | Improved glycemic control; ↓ ALT/AST; ↓ hs-CRP | [95] |
| NAFLD/MASLD | Curcumin | 80/8 weeks/500 mg/day | Adults with NAFLD | RCT, double-blind | ↓ hepatic steatosis; ↓ ALT/AST | [72] |
| NAFLD/MASLD | Curcumin | 102/8 weeks/1000 mg/day | Adults with NAFLD | RCT | ↓ LDL; ↓ TG; ↓ uric acid | [96] |
| NAFLD/MASLD | Curcumin | 50/12 weeks/1500 mg/day | Adults with NAFLD | RCT, double-blind | ↓ liver inflammation; ↓ cytokines | [77] |
| NAFLD/MASLD (adjunct) | Phospholipid curcumin | 58/8 weeks/250 mg/day | Adults with NAFLD | RCT, double-blind | ↓ hepatic fat content; ↓ ALT | [97] |
| NAFLD fibrosis | Nano-curcumin | 55/16 weeks/80 mg/day | Adults with NAFLD + fibrosis | RCT, double-blind | ↓ liver stiffness; ↓ GGT; improved fibrosis scores | [98] |
| Metabolic Syndrome | Nano-curcumin | 50/12 weeks/80 mg/day | Adults with MetS | RCT, double-blind | ↓ TG; ↓ insulin; improved HOMA-IR | [99] |
| Migraine/metabolic markers | Nano-curcumin | 44/8 weeks/80 mg/day | Adults with migraine | RCT, double-blind | ↓ leptin; ↑ adiponectin; ↓ migraine burden | [100] |
| T2DM + Obesity | Curcumin | 227/12 months/1500 mg/day | Overweight/obese adults with T2DM | RCT, double-blind | ↓ atherogenic indices; improved metabolic profile | [101] |
| Metabolic Syndrome | Curcumin extract | 65/12 weeks/1890 mg/day | Adults with MetS | RCT, double-blind | ↓ TG; ↓ LDL-C; improved non-HDL-C | [40] |
| Metabolic Syndrome | Curcuminoids + piperine | 117/8 weeks/1 g/day + 10 mg/day | Adults with MetS | RCT, double-blind | ↑ SOD; ↓ MDA; ↓ CRP | [15] |
| Type 2 Diabetes | Curcumin | 53/10 weeks/1500 mg/day | Adults with T2DM | RCT, double-blind | ↓ fasting glucose; ↓ HOMA-IR; ↓ oxidative stress | [41] |
| Safety/Pharmacokinetics | Curcuminoid formulation | 24/3 months/500 mg–12 g/day | Healthy volunteers | Phase I dose escalation | Safety established; Pharmacokinetics characterized | [79] |
| PK/Bioavailability | Curcumin ± piperine | 10/Single dose/2 g ± 20 mg piperine | Healthy volunteers | Randomized cross-over | ↑ bioavailability ~20-fold with piperine | [102] |
| Rheumatoid Arthritis | Bioavailable curcuminoid | 24/12 weeks/250–500 mg/day | Adults with Rheumatoid Arthritis | RCT, double-blind | ↓ disease activity; ↓ inflammatory markers | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konaktchieva, M.; Stojchevski, R.; Hadzi-Petrushev, N.; Gagov, H.; Konakchieva, R.; Mitrokhin, V.; Kungulovski, G.; Mladenov, M.; Avtanski, D. Curcumin and Tetrahydrocurcumin as Multi-Organ Modulators of the Adipose Tissue–Gut–Liver Axis: Mechanistic Insights, Therapeutic Potential, and Translational Challenges. Pharmaceuticals 2025, 18, 1791. https://doi.org/10.3390/ph18121791
Konaktchieva M, Stojchevski R, Hadzi-Petrushev N, Gagov H, Konakchieva R, Mitrokhin V, Kungulovski G, Mladenov M, Avtanski D. Curcumin and Tetrahydrocurcumin as Multi-Organ Modulators of the Adipose Tissue–Gut–Liver Axis: Mechanistic Insights, Therapeutic Potential, and Translational Challenges. Pharmaceuticals. 2025; 18(12):1791. https://doi.org/10.3390/ph18121791
Chicago/Turabian StyleKonaktchieva, Marina, Radoslav Stojchevski, Nikola Hadzi-Petrushev, Hristo Gagov, Rositza Konakchieva, Vadim Mitrokhin, Gjoko Kungulovski, Mitko Mladenov, and Dimiter Avtanski. 2025. "Curcumin and Tetrahydrocurcumin as Multi-Organ Modulators of the Adipose Tissue–Gut–Liver Axis: Mechanistic Insights, Therapeutic Potential, and Translational Challenges" Pharmaceuticals 18, no. 12: 1791. https://doi.org/10.3390/ph18121791
APA StyleKonaktchieva, M., Stojchevski, R., Hadzi-Petrushev, N., Gagov, H., Konakchieva, R., Mitrokhin, V., Kungulovski, G., Mladenov, M., & Avtanski, D. (2025). Curcumin and Tetrahydrocurcumin as Multi-Organ Modulators of the Adipose Tissue–Gut–Liver Axis: Mechanistic Insights, Therapeutic Potential, and Translational Challenges. Pharmaceuticals, 18(12), 1791. https://doi.org/10.3390/ph18121791








