Advancements in Polysaccharide-Based Nanoparticles for the Treatment of Breast Cancer: A Comprehensive Review
Abstract
1. Introduction
2. Pathophysiology of Breast Cancer
3. Importance of Nanotechnology in Breast Cancer Treatment
4. Polysaccharides—A Multifunctional Polymer for Innovative Nanoparticle Design
5. Polysaccharide-Based Nanoparticles for Breast Cancer Therapy
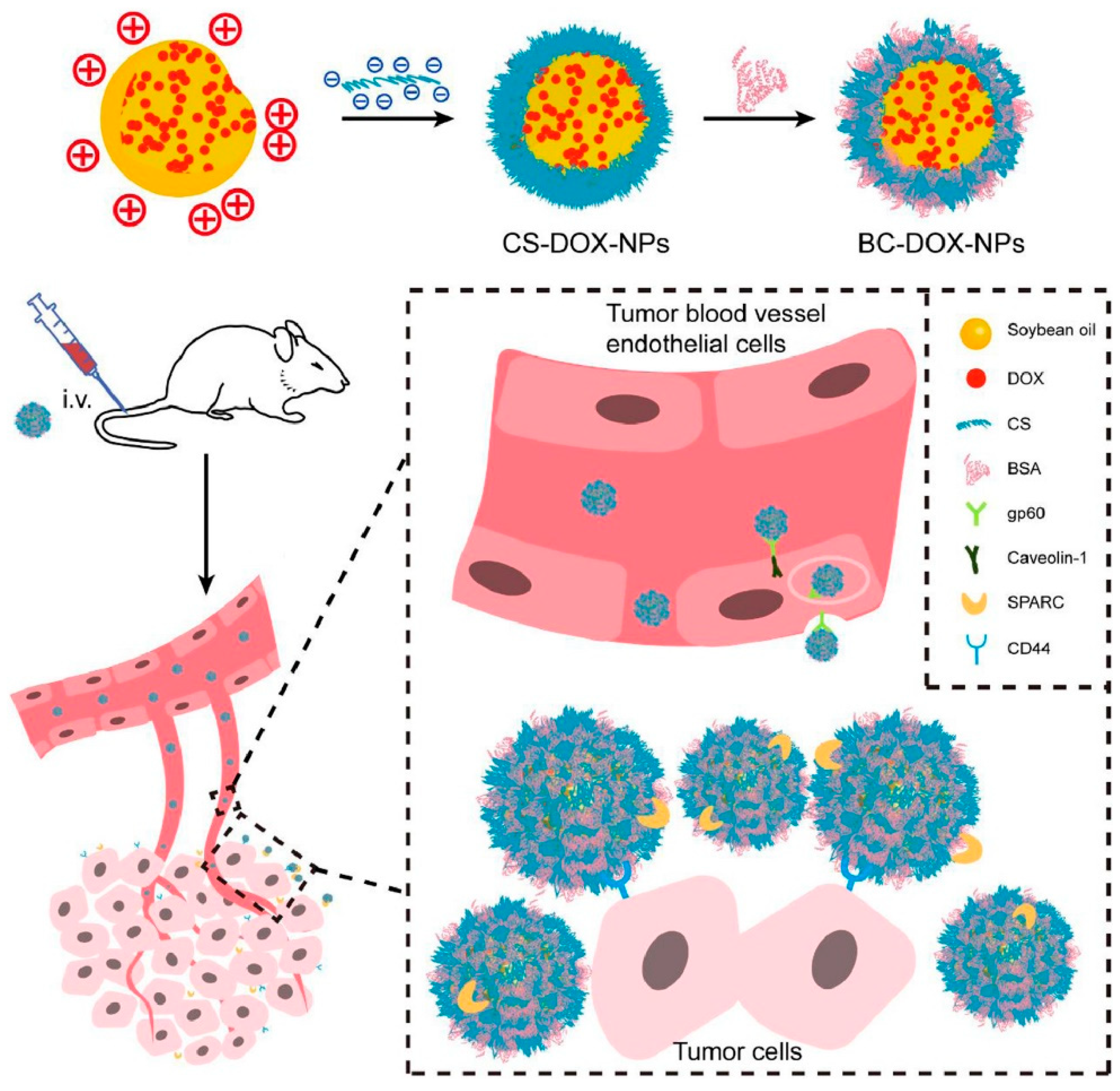
5.1. Chitosan
5.2. Alginate
5.3. Hyaluronic Acid
5.4. Dextran
5.5. Cellulose
5.6. Starch
5.7. Chondroitin Sulfate
5.8. Pullulan
6. Patents
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 23 July 2023).
- Indian Council of Medical Research. Indian Council of Medical Research National Cancer Registry Programme; Indian Council of Medical Research: Bangalore, India, 2020. [Google Scholar]
- Sathishkumar, K.; Sankarapillai, J.; Mathew, A.; Nair, R.A.; Gangane, N.; Khuraijam, S.; Barmon, D.; Pandya, S.; Majumdar, G.; Deshmane, V.; et al. Breast Cancer Survival in India across 11 Geographic Areas under the National Cancer Registry Programme. Cancer 2024, 130, 1816–1825. [Google Scholar] [CrossRef]
- Thakur, P.; Seam, R.K.; Gupta, M.K.; Gupta, M.; Sharma, M.; Fotedar, V. Breast Cancer Risk Factor Evaluation in a Western Himalayan State: A Case-Control Study and Comparison with the Western World. South Asian J. Cancer 2017, 6, 106–109. [Google Scholar] [CrossRef]
- Barrios, C.H. Global Challenges in Breast Cancer Detection and Treatment. Breast 2022, 62 (Suppl. S1), S3–S6. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the Mechanisms and Challenges of Cancer Drug Resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Hegde, G.V.; de la Cruz, C.; Eastham-Anderson, J.; Zheng, Y.; Sweet-Cordero, E.A.; Jackson, E.L. Residual Tumor Cells That Drive Disease Relapse after Chemotherapy Do Not Have Enhanced Tumor Initiating Capacity. PLoS ONE 2012, 7, e45647. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, R.; Gonçalves, A.; Bertucci, F. Personalized Medicine: Present and Future of Breast Cancer Management. Crit. Rev. Oncol. Hematol. 2014, 91, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Parveen, F.; Shah, H.; Yalamarty, S.S.K.; Ataide, J.A.; Torchilin, V.P. Recent Advances with Precision Medicine Treatment for Breast Cancer Including Triple-Negative Sub-Type. Cancers 2023, 15, 2204. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized Medicine: Motivation, Challenges, and Progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Mitra, O.; Tripathi, G.; Adur, I.; Mohanto, S.; Nama, M.; Samanta, S.; Gowda, B.J.; Subramaniyan, V.; Sundararajan, V.; et al. Nanomaterials-Assisted Photothermal Therapy for Breast Cancer: State-of-the Advances and Future Perspectives. Photodiagnosis Photodyn. Ther. 2024, 45, 103959. [Google Scholar] [CrossRef]
- Rehman, S.; Nabi, B.; Ahmad, S.; Baboota, S.; Ali, J. 10–Polysaccharide-Based Amorphous Solid Dispersions (ASDs) for Improving Solubility and Bioavailability of Drugs. In Polysaccharide Carriers for Drug Delivery; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 271–317. ISBN 978-0-08-102553-6. [Google Scholar]
- Kurczewska, J. Recent Reports on Polysaccharide-Based Materials for Drug Delivery. Polymers 2022, 14, 4189. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Silant’ev, V.E.; Shmelev, M.E.; Belousov, A.S.; Patlay, A.A.; Shatilov, R.A.; Farniev, V.M.; Kumeiko, V. V How to Develop Drug Delivery System Based on Carbohydrate Nanoparticles Targeted to Brain Tumors. Polymers 2023, 15, 2516. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Montero, M.P. Enhancement of Oral Bioavailability of Natural Compounds and Probiotics by Mucoadhesive Tailored Biopolymer-Based Nanoparticles: A Review. Food Hydrocoll. 2021, 118, 106772. [Google Scholar] [CrossRef]
- Moghaddam, F.D.; Heidari, G.; Zare, E.N.; Djatoubai, E.; Paiva-Santos, A.C.; Bertani, F.R.; Wu, A. Carbohydrate Polymer-Based Nanocomposites for Breast Cancer Treatment. Carbohydr. Polym. 2023, 304, 120510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-W.; Zhang, J.-G.; Yang, X.; Du, W.-L.; Yu, Z.-L.; Lv, Z.-Y.; Mou, X.-Z. Carbohydrates Based Stimulus Responsive Nanocarriers for Cancer-Targeted Chemotherapy: A Review of Current Practices. Expert Opin. Drug Deliv. 2022, 19, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. Immunosuppressive Effects of Vascular Endothelial Growth Factor. Oncol. Lett. 2022, 24, 369. [Google Scholar] [CrossRef] [PubMed]
- Hyun, G.H.; Jeong, D.-H.; Yang, Y.Y.; Cho, I.H.; Ha, Y.-J.; Xing, X.; Abbott, D.W.; Hsieh, Y.S.Y.; Kang, Y.P.; Cha, J.-H.; et al. Multivalent Carbohydrate Nanocomposites for Tumor Microenvironment Remodeling to Enhance Antitumor Immunity. ACS Nano 2023, 17, 11567–11582. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the Therapeutic Efficacy of Nanoparticles for Cancer Treatment Using Versatile Targeted Strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Cai, J.; Liao, W.; Wen, J.; Ye, F.; Nie, Q.; Chen, W.; Zhao, C. Algae-Derived Polysaccharides and Polysaccharide-Based Nanoparticles: A Natural Frontier in Breast Cancer Therapy. Int. J. Biol. Macromol. 2025, 297, 139936. [Google Scholar] [CrossRef] [PubMed]
- Shayegh, F.; Türk, Z.; Armani, A.; Zarghami, N. New Insights into Polysaccharide-Based Nanostructured Delivery Systems in Breast Cancer: Possible Application of Antisense Oligonucleotides in Breast Cancer Therapy. Int. J. Biol. Macromol. 2024, 272, 132890. [Google Scholar] [CrossRef]
- Bazzazan, M.A.; Fattollazadeh, P.; Shahbaz, S.K.; Rezaei, N. Polymeric Nanoparticles as a Promising Platform for Treating Triple-Negative Breast Cancer: Current Status and Future Perspectives. Int. J. Pharm. 2024, 664, 124639. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Emerging Role of Histone Deacetylase Inhibitors as Anti-Breast-Cancer Agents. Drug Discov. Today 2019, 24, 685–702. [Google Scholar] [CrossRef]
- Williams, C.; Lin, C.Y. Oestrogen Receptors in Breast Cancer: Basic Mechanisms and Clinical Implications. Ecancermedicalscience 2013, 7, 370. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Budzinska, A.; Mojzych, M.; Kontek, R. Metastasis and MAPK Pathways. Int. J. Mol. Sci. 2022, 23, 3847. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising Targets for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, A.; Veer, L.J.V.; Bissell, M.J. An “Elite Hacker”. Cell Adh. Migr. 2012, 6, 236–435. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Tang, F.; Wei, Y.Q.; Wei, X.W. Immunosuppressive Cells in Cancer: Mechanisms and Potential Therapeutic Targets. J. Hematol. Oncol. 2022, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Mitra, O.; P, S.; Bhattacharjee, A.; Mohanto, S.; Gowda, B.H.J.; Kar, S.; Ramaiah, S.; Anbarasu, A.; Ahmed, M.G. Exploring the Emerging Trends in the Synthesis and Theranostic Paradigms of Cerium Oxide Nanoparticles (CeONPs): A Comprehensive Review. Mater. Today Chem. 2024, 35, 101894. [Google Scholar] [CrossRef]
- Hani, U.; Gowda, B.H.J.; Haider, N.; Ramesh, K.; Paul, K.; Ashique, S.; Ahmed, M.G.; Narayana, S.; Mohanto, S.; Kesharwani, P. Nanoparticle-Based Approaches for Treatment of Hematological Malignancies: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 233. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; Meyers, C.; Jiang, Y.; Adair, J.H. The Use of Nanoparticulates to Treat Breast Cancer. Nanomedicine 2017, 12, 2367–2388. [Google Scholar] [CrossRef]
- Wu, D.; Si, M.; Xue, H.-Y.; Wong, H.L. Nanomedicine Applications in the Treatment of Breast Cancer: Current State of the Art. Int. J. Nanomed. 2017, 12, 5879–5892. [Google Scholar] [CrossRef]
- Alshareeda, A.T.; Nur Khatijah, M.Z.; Al-Sowayan, B.S. Nanotechnology: A Revolutionary Approach to Prevent Breast Cancer Recurrence. Asian J. Surg. 2023, 46, 13–17. [Google Scholar] [CrossRef]
- Tagde, P.; Najda, A.; Nagpal, K.; Kulkarni, G.T.; Shah, M.; Ullah, O.; Balant, S.; Rahman, M.H. Nanomedicine-Based Delivery Strategies for Breast Cancer Treatment and Management. Int. J. Mol. Sci. 2022, 23, 2856. [Google Scholar] [CrossRef]
- Khazaei, M.; Hosseini, M.S.; Haghighi, A.M.; Misaghi, M. Nanosensors and Their Applications in Early Diagnosis of Cancer. Sens. Bio-Sens. Res. 2023, 41, 100569. [Google Scholar] [CrossRef]
- Choi, K.Y.; Liu, G.; Lee, S.; Chen, X. Theranostic Nanoplatforms for Simultaneous Cancer Imaging and Therapy: Current Approaches and Future Perspectives. Nanoscale 2012, 4, 330–342. [Google Scholar] [CrossRef]
- Fan, Z.; Fu, P.P.; Yu, H.; Ray, P.C. Theranostic Nanomedicine for Cancer Detection and Treatment. J. Food Drug Anal. 2014, 22, 3–17. [Google Scholar] [CrossRef]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the Efficacy of Nanoparticle-Based Drug Delivery Systems for Cancer Treatment. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-Responsive Nanocarriers for Drug Delivery, Tumor Imaging, Therapy and Theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Davodabadi, F.; Sarhadi, M.; Arabpour, J.; Sargazi, S.; Rahdar, A.; Díez-Pascual, A.M. Breast Cancer Vaccines: New Insights into Immunomodulatory and Nano-Therapeutic Approaches. J. Control. Release 2022, 349, 844–875. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V.K.J. Polysaccharide Nanoparticles: From Fabrication to Applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef]
- Ahmad, A.; Gulraiz, Y.; Ilyas, S.; Bashir, S. Polysaccharide Based Nano Materials: Health Implications. Food Hydrocoll. Health 2022, 2, 100075. [Google Scholar] [CrossRef]
- Visan, A.I.; Cristescu, R. Polysaccharide-Based Coatings as Drug Delivery Systems. Pharmaceutics 2023, 15, 2227. [Google Scholar] [CrossRef] [PubMed]
- De Anda-Flores, Y.; Carvajal-Millan, E.; Campa-Mada, A.; Lizardi-Mendoza, J.; Rascon-Chu, A.; Tanori-Cordova, J.; Luisa Martínez-López, A.; Ribeiro, B.; Pinto, R.J.B.; Stana Kleinschek, K.; et al. Polysaccharide-Based Nanoparticles for Colon-Targeted Drug Delivery Systems. Polysaccharides 2021, 2, 626–647. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-Based Nanoparticles as Drug Delivery Systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, W.; Lin, X.; Feng, Y. Polysaccharide-Based Nanomaterials for Ocular Drug Delivery: A Perspective. Front. Bioeng. Biotechnol. 2020, 8, 601246. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiang, Y.; Sheng, R.; Tomás, H.; Rodrigues, J.; Gu, Z.; Zhang, H.; Gong, Q.; Luo, K. Polysaccharide-Based Nanomedicines for Cancer Immunotherapy: A Review. Bioact. Mater. 2021, 6, 3358–3382. [Google Scholar] [CrossRef]
- Dheer, D.; Arora, D.; Jaglan, S.; Rawal, R.K.; Shankar, R. Polysaccharides Based Nanomaterials for Targeted Anti-Cancer Drug Delivery. J. Drug Target. 2017, 25, 1–16. [Google Scholar] [CrossRef]
- Nandgude, T.; Pagar, R. Plausible Role of Chitosan in Drug and Gene Delivery against Resistant Breast Cancer Cells. Carbohydr. Res. 2021, 506, 108357. [Google Scholar] [CrossRef]
- de Oliveira Pedro, R.; Hoffmann, S.; Pereira, S.; Goycoolea, F.M.; Schmitt, C.C.; Neumann, M.G. Self-Assembled Amphiphilic Chitosan Nanoparticles for Quercetin Delivery to Breast Cancer Cells. Eur. J. Pharm. Biopharm. 2018, 131, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, S.; Yu, G.; Shen, L.; Fan, J.; Xu, L.; Zhang, H.; Zhao, N.; Zeng, Z.; Hu, T.; et al. Aptamer Internalization via Endocytosis Inducing S-Phase Arrest and Priming Maver-1 Lymphoma Cells for Cytarabine Chemotherapy. Theranostics 2017, 7, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Wood, E.; Dornish, M. Effect of Chitosan on Epithelial Cell Tight Junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, X.; Hunziker, P. Carbohydrate-Based Amphiphilic Nano Delivery Systems for Cancer Therapy. Nanoscale 2016, 8, 16091–16156. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Bhatt, T.; Agrawal, M.; Saha, R.N.; Saraf, S.; Saraf, S.; Alexander, A. Application of Chitosan Modified Nanocarriers in Breast Cancer. Int. J. Biol. Macromol. 2022, 194, 521–538. [Google Scholar] [CrossRef]
- Sheikh, A.; Md, S.; Alhakamy, N.A.; Kesharwani, P. Recent Development of Aptamer Conjugated Chitosan Nanoparticles as Cancer Therapeutics. Int. J. Pharm. 2022, 620, 121751. [Google Scholar] [CrossRef]
- Ekinci, M.; Koksal-Karayildirim, C.; Ilem-Ozdemir, D. Radiolabeled Methotrexate Loaded Chitosan Nanoparticles as Imaging Probe for Breast Cancer: Biodistribution in Tumor-Bearing Mice. J. Drug Deliv. Sci. Technol. 2023, 80, 104146. [Google Scholar] [CrossRef]
- Xu, X.; Li, Q.; Dong, W.; Zhao, G.; Lu, Y.; Huang, X.; Liang, X. Cinnamon Cassia Oil Chitosan Nanoparticles: Physicochemical Properties and Anti-Breast Cancer Activity. Int. J. Biol. Macromol. 2023, 224, 1065–1078. [Google Scholar] [CrossRef]
- Rajaei, M.; Rashedi, H.; Yazdian, F.; Navaei-Nigjeh, M.; Rahdar, A.; Díez-Pascual, A.M. Chitosan/Agarose/Graphene Oxide Nanohydrogel as Drug Delivery System of 5-Fluorouracil in Breast Cancer Therapy. J. Drug Deliv. Sci. Technol. 2023, 82, 104307. [Google Scholar] [CrossRef]
- Chang, H.; Yhee, J.Y.; Jeon, S.; Shim, M.K.; Yoon, H.Y.; Lee, S.; Kim, K. In Vivo Toxicity Evaluation of Tumor Targeted Glycol Chitosan Nanoparticles in Healthy Mice: Repeated High-Dose of Glycol Chitosan Nanoparticles Potentially Induce Cardiotoxicity. J. Nanobiotechnol. 2023, 21, 82. [Google Scholar] [CrossRef]
- Al-Othman, N.; Alhendi, A.; Ihbaisha, M.; Barahmeh, M.; Alqaraleh, M.; Al-Momany, B.Z. Role of CD44 in Breast Cancer. Breast Dis. 2020, 39, 1–13. [Google Scholar] [CrossRef]
- Louderbough, J.M.V.; Schroeder, J.A. Understanding the Dual Nature of CD44 in Breast Cancer Progression. Mol. Cancer Res. 2011, 9, 1573–1586. [Google Scholar] [CrossRef]
- Meylina, L.; Muchtaridi, M.; Joni, I.M.; Elamin, K.M.; Wathoni, N. Hyaluronic Acid-Coated Chitosan Nanoparticles as an Active Targeted Carrier of Alpha Mangostin for Breast Cancer Cells. Polymers 2023, 15, 1025. [Google Scholar] [CrossRef]
- Pooja, Y.S.; Rajana, N.; Yadav, R.; Naraharisetti, L.T.; Godugu, C.; Mehra, N.K. Design, Development, and Evaluation of CDK-4/6 Inhibitor Loaded 4-Carboxy Phenyl Boronic Acid Conjugated PH-Sensitive Chitosan Lecithin Nanoparticles in the Management of Breast Cancer. Int. J. Biol. Macromol. 2024, 258, 128821. [Google Scholar] [CrossRef] [PubMed]
- Quijia, C.R.; Ocaña, A.; Alonso-Moreno, C.; Galvão Frem, R.C.; Chorilli, M. Chitosan-Coated MIL-100(Fe) Nanoparticles for Enhanced Piperine Release in Breast Cancer Treatment. J. Mol. Struct. 2024, 1305, 137801. [Google Scholar] [CrossRef]
- Dastgir, M.; Ayyami, Y.; Pourfarshid, A.; Ghorbani, M.; Mortezazadeh, T. Evaluation of the Theranostic Characteristics of Chitosan-Decorated Bismuth Oxide Nanoparticles Conjugated Curcumin and 5-Aminolevulinic Acid as a Biocompatible Targeted Nanoplatform in CT and Breast Cancer Radiation Therapy. J. Drug Deliv. Sci. Technol. 2024, 92, 105279. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, X.; Shen, Y. Folate-Modified Carboxymethyl Chitosan-Based Drug Delivery System for Breast Cancer Specific Combination Therapy via Regulating Mitochondrial Calcium Concentration. Carbohydr. Polym. 2024, 323, 121434. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.Y.; Elshoky, H.A.; El-Sayed, N.M.; Fahmy, H.M.; Ali, M.A. Ameliorative Effect of Zinc Oxide-Chitosan Conjugates on the Anticancer Activity of Cisplatin: Approach for Breast Cancer Treatment. Int. J. Biol. Macromol. 2024, 257, 128597. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Dabiri, A.; Mashayekhi, F.; Rahimmanesh, I.; Bidram, E.; Karbasi, S.; Rafienia, M.; Javanmard, S.H.; Ertas, Y.N.; Zarrabi, A.; et al. Smart Co-Delivery of Plasmid DNA and Doxorubicin Using MCM-Chitosan-PEG Polymerization Functionalized with MUC-1 Aptamer against Breast Cancer. Biomed. Pharmacother. 2024, 173, 116465. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Manivasagan, P.; Zhang, X.; Jeong, M.S.; Jang, E.-S.; Wang, M.-H. Multifunctional Chitosan-Bimetallic Nanocarrier Deliver 5-Fluorouracil for Enhanced Treatment of Pancreatic and Triple-Negative Breast Cancer. Int. J. Biol. Macromol. 2024, 259, 129165. [Google Scholar] [CrossRef]
- Mohanto, S.; Chakraborty, P.; Pandian, C.S.; Manna, S.; Dutta, J. A Comprehensive Review On Alginate As Wound Dressing Biomaterial. Curr. Appl. Polym. Sci. 2020, 4, 3–14. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Solouk, A.; Saber-Samandari, S.; Akbari, S.; Ghanbari, H.; Brycki, B.E. Capsaicin-Loaded Alginate Nanoparticles Embedded Polycaprolactone-Chitosan Nanofibers as a Controlled Drug Delivery Nanoplatform for Anticancer Activity. J. Colloid Interface Sci. 2023, 638, 616–628. [Google Scholar] [CrossRef]
- Jayapal, J.J.; Dhanaraj, S. Exemestane Loaded Alginate Nanoparticles for Cancer Treatment: Formulation and in Vitro Evaluation. Int. J. Biol. Macromol. 2017, 105, 416–421. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Hu, X.; Lu, Y.; Zhang, J.; Jin, W.; Liu, W.; Shu, Y.; Cheng, Y.Y.; Li, W.; et al. Caffeic Acid-Grafted Chitosan/Sodium Alginate/Nanoclay-Based Multifunctional 3D-Printed Hybrid Scaffolds for Local Drug Release Therapy after Breast Cancer Surgery. Carbohydr. Polym. 2024, 324, 121441. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Varaprasad, K.; Kim, S.; Kumar Jangid, A.; Lee, W.; Syedahamed Haja Hameed, A.; Kim, K. Size-Dependent Cellular Uptakeof Sodium Alginate Passivated Tin Dioxide Nanoparticles in Triple-Negative Breast Cancercells. J. Ind. Eng. Chem. 2023, 123, 476–487. [Google Scholar] [CrossRef]
- Bulatao, B.P.; Nalinratana, N.; Jantaratana, P.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Lutein-Loaded Chitosan/Alginate-Coated Fe3O4 Nanoparticles as Effective Targeted Carriers for Breast Cancer Treatment. Int. J. Biol. Macromol. 2023, 242, 124673. [Google Scholar] [CrossRef]
- Alioghli Ziaei, A.; Erfan-Niya, H.; Fathi, M.; Amiryaghoubi, N. In Situ Forming Alginate/Gelatin Hybrid Hydrogels Containing Doxorubicin Loaded Chitosan/AuNPs Nanogels for the Local Therapy of Breast Cancer. Int. J. Biol. Macromol. 2023, 246, 125640. [Google Scholar] [CrossRef] [PubMed]
- Taran, Z.; Yektaniroumand Digehsaraei, S.; Salouti, M.; Amini, B.; Mahmazi, S.; Kalantari, M. Methotrexate Loaded in Alginate Beads for Controlled Drug Release against Breast Cancer. Gene 2023, 851, 146941. [Google Scholar] [CrossRef]
- Alzahrani, B.; Elderdery, A.Y.; Alsrhani, A.; Alzerwi, N.A.N.; Althobiti, M.M.; Elkhalifa, A.M.E.; Rayzah, M.; Idrees, B.; Kumar, S.S.; Mok, P.L. Sodium Alginate Encapsulated Iron Oxide Decorated with Thymoquinone Nanocomposite Induces Apoptosis in Human Breast Cancer Cells via PI3K-Akt-MTOR Pathway. Int. J. Biol. Macromol. 2023, 244, 125054. [Google Scholar] [CrossRef]
- Ibrahim, A.; Khalil, I.A.; Mahmoud, M.Y.; Bakr, A.F.; Ghoniem, M.G.; Al-Farraj, E.S.; El-Sherbiny, I.M. Layer-by-Layer Development of Chitosan/Alginate-Based Platelet-Mimicking Nanocapsules for Augmenting Doxorubicin Cytotoxicity against Breast Cancer. Int. J. Biol. Macromol. 2023, 225, 503–517. [Google Scholar] [CrossRef]
- Sharafshadeh, M.S.; Tafvizi, F.; Khodarahmi, P.; Ehtesham, S. Preparation and Physicochemical Properties of Cisplatin and Doxorubicin Encapsulated by Niosome Alginate Nanocarrier for Cancer Therapy. Int. J. Biol. Macromol. 2023, 235, 123686. [Google Scholar] [CrossRef]
- Loke, Y.L.; Beishenaliev, A.; Wang, P.-W.; Lin, C.-Y.; Chang, C.-Y.; Foo, Y.Y.; Faruqu, F.N.; Leo, B.F.; Misran, M.; Chung, L.Y.; et al. ROS-Generating Alginate-Coated Gold Nanorods as Biocompatible Nanosonosensitisers for Effective Sonodynamic Therapy of Cancer. Ultrason. Sonochem. 2023, 96, 106437. [Google Scholar] [CrossRef]
- Zaer, M.; Moeinzadeh, A.; Abolhassani, H.; Rostami, N.; Tavakkoli Yaraki, M.; Seyedi, S.A.; Nabipoorashrafi, S.A.; Bashiri, Z.; Moeinabadi-Bidgoli, K.; Moradbeygi, F.; et al. Doxorubicin-Loaded Niosomes Functionalized with Gelatine and Alginate as PH-Responsive Drug Delivery System: A 3D Printing Approach. Int. J. Biol. Macromol. 2023, 253, 126808. [Google Scholar] [CrossRef]
- Venkatesan, J.; Jeong Lee, S.; Hur, W.; Gupta, P.K.; Eun Son, S.; Been Lee, H.; Yeon Park, J.; Nyeon Kim, S.; Hun Seong, G. Preparation and Photothermal Effect of Chitosan-Alginate-Molybdenum Diselenide Nanocomposite Scaffolds for Cancer Therapy. J. Photochem. Photobiol. A Chem. 2023, 442, 114759. [Google Scholar] [CrossRef]
- Kreua-ongarjnukool, N.; Soomherun, N.; Niyomthai, S.T.; Soonklang, C. Turmeric-Loaded Alginate Particulate-Based Burst Release Delivery System Containing a Gas-Generating Agent. J. Drug Deliv. Sci. Technol. 2023, 87, 104850. [Google Scholar] [CrossRef]
- Dasuni Wasana, P.W.; Hasriadi; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P.; Rojsitthisak, P. Metformin and Curcumin Co-Encapsulated Chitosan/Alginate Nanoparticles as Effective Oral Carriers against Pain-like Behaviors in Mice. Int. J. Pharm. 2023, 640, 123037. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, S.; Wang, C.; Sun, T.; Yang, L. Hyaluronic acid-based nano drug delivery systems for breast cancer treatment: Recent advances. Front. Bioeng. Biotechnol. 2022, 10, 990145. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.; Fardous, R.; Alhindi, Y.M.; Aodah, A.H.; Alyami, M.; Alsuabeyl, M.S.; Alghamdi, W.M.; Alhasan, A.H.; Almalik, A. α(1)-Acid Glycoprotein-Decorated Hyaluronic Acid Nanoparticles for Suppressing Metastasis and Overcoming Drug Resistance Breast Cancer. Biomedicines 2022, 10, 414. [Google Scholar] [CrossRef]
- Thummarati, P.; Suksiriworapong, J.; Sakchaisri, K.; Nawroth, T.; Langguth, P.; Roongsawang, B.; Junyaprasert, V.B. Comparative Study of Dual Delivery of Gemcitabine and Curcumin Using CD44 Targeting Hyaluronic Acid Nanoparticles for Cancer Therapy. J. Drug Deliv. Sci. Technol. 2022, 77, 103883. [Google Scholar] [CrossRef]
- Gao, N.; Fu, Y.; Gong, H.; Liu, H.; Li, W. Hyaluronic Acid and Cholecalciferol Conjugate Based Nanomicelles: Synthesis, Characterization, and Cytotoxicity against MCF-7 Breast Cancer Cells. Carbohydr. Res. 2022, 522, 108706. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wang, H.; Zhang, Y.; Xu, X.; Yuan, X.; Li, J. PH-Responsive Hyaluronic Acid Nanoparticles for Enhanced Triple Negative Breast Cancer Therapy. Int. J. Nanomed. 2022, 17, 1437–1457. [Google Scholar] [CrossRef]
- Ghalehkhondabi, V.; Soleymani, M.; Fazlali, A. Synthesis of Quercetin-Loaded Hyaluronic Acid-Conjugated PH/Redox Dual-Stimuli Responsive Poly(Methacrylic Acid)/Mesoporous Organosilica Nanoparticles for Breast Cancer Targeted Therapy. Int. J. Biol. Macromol. 2024, 263, 130168. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, Q.; Wang, Z.; Liu, Y.; Yang, S.; Zhao, X.; Peng, J. A Chemo/Chemodynamic Nanoparticle Based on Hyaluronic Acid Induces Ferroptosis and Apoptosis for Triple-Negative Breast Cancer Therapy. Carbohydr. Polym. 2024, 329, 121795. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W.; Lei, L.; Tong, F.; Zhang, H.; Zhang, Y.; Yang, W.; Tang, Y.; Lin, R.; Xia, X.; et al. Combination Losartan with Hyaluronic Acid Modified Diethyldithiocarbamate Loaded Hollow Copper Sulfide Nanoparticles for the Treatment of Breast Cancer and Metastasis. Chin. Chem. Lett. 2024, 35, 108765. [Google Scholar] [CrossRef]
- Gao, F.; Wu, Y.; Wang, R.; Yao, Y.; Liu, Y.; Fan, L.; Xu, J.; Zhang, J.; Han, X.; Guan, X. Precise Nano-System-Based Drug Delivery and Synergistic Therapy against Androgen Receptor-Positive Triple-Negative Breast Cancer. Acta Pharm. Sin. B 2024, 14, 2685–2697. [Google Scholar] [CrossRef]
- Lima-Sousa, R.; Melo, B.L.; Mendonça, A.G.; Correia, I.J.; de Melo-Diogo, D. Hyaluronic Acid-Functionalized Graphene-Based Nanohybrids for Targeted Breast Cancer Chemo-Photothermal Therapy. Int. J. Pharm. 2024, 651, 123763. [Google Scholar] [CrossRef]
- Shah, H.S.; Zaib, S.; Usman, F.; Sarfraz, M.; Faiz, R.; Rehman, S.A.; Khan, A.A.; Alanazi, A.M.; Khan, R.; Nasrullah, U.; et al. Synthesis, Characterization, Pharmacological and Computational Evaluation of Hyaluronic Acid Modified Chebulinic Acid Encapsulated Chitosan Nanocomposite for Cancer Therapy. Int. J. Biol. Macromol. 2024, 263, 130160. [Google Scholar] [CrossRef]
- Sun, J.; Ye, T.; Chen, X.; Li, B.; Wei, Y.; Zheng, H.; Piao, J.-G.; Li, F. A Self-Assembly Active Nanomodulator Based on Berberine for Photothermal Immunotherapy of Breast Cancer via Dual Regulation of Immune Suppression. Int. J. Pharm. 2024, 653, 123898. [Google Scholar] [CrossRef]
- Bakrania, A.K.; Variya, B.C.; Rathod, L.V.; Patel, S.S. DEAE-Dextran Coated Paclitaxel Nanoparticles Act as Multifunctional Nano System for Intranuclear Delivery to Triple Negative Breast Cancer through VEGF and NOTCH1 Inhibition. Eur. J. Pharm. Biopharm. 2018, 122, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, J.; Lv, H.; Huang, X.; Dong, P.; Wang, Q.; Yang, H.; Wang, S.; Li, X.; Hu, J.; et al. Complete Regression of Xenografted Breast Tumors by Dextran-Based Dual Drug Conjugates Containing Paclitaxel and Docosahexaenoic Acid. Eur. J. Med. Chem. 2022, 240, 114567. [Google Scholar] [CrossRef]
- Li, M.; Tang, Z.; Zhang, Y.; Lv, S.; Li, Q.; Chen, X. Targeted Delivery of Cisplatin by LHRH-Peptide Conjugated Dextran Nanoparticles Suppresses Breast Cancer Growth and Metastasis. Acta Biomater. 2015, 18, 132–143. [Google Scholar] [CrossRef]
- Li, Y.; Jia, F.; Gao, Y.; Wang, X.; Cui, X.; Pan, Z.; Wang, W.; Li, M.; Lu, J.; Wu, Y. Self-Assembled Nanocomposites of Carboxymethyl β-Dextran/Protamine Sulfate for Enhanced Chemotherapeutic Drug Sensitivity of Triple-Negative Breast Cancer by Autophagy Inhibition via a Ternary Collaborative Strategy. Int. J. Biol. Macromol. 2023, 233, 123663. [Google Scholar] [CrossRef]
- Lewińska, A.; Radoń, A.; Gil, K.; Błoniarz, D.; Ciuraszkiewicz, A.; Kubacki, J.; Kądziołka-Gaweł, M.; Łukowiec, D.; Gębara, P.; Krogul-Sobczak, A.; et al. Carbon-Coated Iron Oxide Nanoparticles Promote Reductive Stress-Mediated Cytotoxic Autophagy in Drug-Induced Senescent Breast Cancer Cells. ACS Appl. Mater. Interfaces 2024, 16, 15457–15478. [Google Scholar] [CrossRef]
- Lopes, F.A.C.; Fernandes, A.V.F.; Rodrigues, J.M.; Queiroz, M.-J.R.P.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Castanheira, E.M.S.; Rodrigues, A.R.O.; et al. Magnetoliposomes Containing Multicore Nanoparticles and a New Antitumor Thienopyridine Compound with Potential Application in Chemo/Thermotherapy. Biomedicines 2022, 10, 1547. [Google Scholar] [CrossRef]
- Kurihara, Y.; Yokota, H.; Takahashi, M. Water-Dispersible Carboxymethyl Dextran-Coated Melamine Nanoparticles for Biosensing Applications. ACS Omega 2022, 7, 41641–41650. [Google Scholar] [CrossRef]
- Zhou, J.; Meli, V.S.; Yu-Tin Chen, E.; Kapre, R.; Nagalla, R.; Xiao, W.; Borowsky, A.D.; Lam, K.S.; Liu, W.F.; Louie, A.Y. Magnetic Resonance Imaging of Tumor-Associated-Macrophages (TAMs) with a Nanoparticle Contrast Agent. RSC Adv. 2022, 12, 7742–7756. [Google Scholar] [CrossRef] [PubMed]
- Yousefvand, M.; Mohammadi, Z.; Ghorbani, F.; Irajirad, R.; Abedi, H.; Seyedi, S.; Papi, A.; Montazerabadi, A. Investigation of Specific Targeting of Triptorelin-Conjugated Dextran-Coated Magnetite Nanoparticles as a Targeted Probe in GnRH(+) Cancer Cells in MRI. Contrast Media Mol. Imaging 2021, 2021, 5534848. [Google Scholar] [CrossRef] [PubMed]
- Sampath, M.; Pichaimani, A.; Kumpati, P.; Sengottuvelan, B. The Remarkable Role of Emulsifier and Chitosan, Dextran and PEG as Capping Agents in the Enhanced Delivery of Curcumin by Nanoparticles in Breast Cancer Cells. Int. J. Biol. Macromol. 2020, 162, 748–761. [Google Scholar] [CrossRef]
- Nikkhoo, A.; Rostami, N.; Farhadi, S.; Esmaily, M.; Moghadaszadeh Ardebili, S.; Atyabi, F.; Baghaei, M.; Haghnavaz, N.; Yousefi, M.; Aliparasti, M.R.; et al. Codelivery of STAT3 SiRNA and BV6 by Carboxymethyl Dextran Trimethyl Chitosan Nanoparticles Suppresses Cancer Cell Progression. Int. J. Pharm. 2020, 581, 119236. [Google Scholar] [CrossRef]
- Zeng, X.; Cheng, X.; Zheng, Y.; Yan, G.; Wang, X.; Wang, J.; Tang, R. Indomethacin-Grafted and PH-Sensitive Dextran Micelles for Overcoming Inflammation-Mediated Multidrug Resistance in Breast Cancer. Carbohydr. Polym. 2020, 237, 116139. [Google Scholar] [CrossRef] [PubMed]
- Karimi, Z.; Taymouri, S.; Minaiyan, M.; Mirian, M. Evaluation of Thermosensitive Chitosan Hydrogel Containing Gefitinib Loaded Cellulose Acetate Butyrate Nanoparticles in a Subcutaneous Breast Cancer Model. Int. J. Pharm. 2022, 624, 122036. [Google Scholar] [CrossRef] [PubMed]
- El Fawal, G.; Abu-Serie, M.M.; El-Gendi, H.; El-Fakharany, E.M. Fabrication, Characterization and in Vitro Evaluation of Disulfiram-Loaded Cellulose Acetate/Poly(Ethylene Oxide) Nanofiber Scaffold for Breast and Colon Cancer Cell Lines Treatment. Int. J. Biol. Macromol. 2022, 204, 555–564. [Google Scholar] [CrossRef]
- Atallah, M.A.; Sallam, M.A.; Abdelmoneem, M.A.; Teleb, M.; Elkhodairy, K.A.; Bekhit, A.A.; Khafaga, A.F.; Noreldin, A.E.; Elzoghby, A.O.; Khattab, S.N. Green Self-Assembled Lactoferrin Carboxymethyl Cellulose Nanogels for Synergistic Chemo/Herbal Breast Cancer Therapy. Colloids Surf. B Biointerfaces 2022, 217, 112657. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, N.; Akelah, A.; Elnagar, M.; Abdelwahab, M.A. Antibacterial and Cytotoxicity of Methylene Blue Loaded-Cellulose Nanocarrier on Breast Cancer Cell Line. Carbohydr. Polym. Technol. Appl. 2021, 2, 100138. [Google Scholar] [CrossRef]
- Pathania, K.; Sah, S.P.; Salunke, D.B.; Jain, M.; Yadav, A.K.; Yadav, V.G.; Pawar, S. V Green Synthesis of Lignin-Based Nanoparticles as a Bio-Carrier for Targeted Delivery in Cancer Therapy. Int. J. Biol. Macromol. 2023, 229, 684–695. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Murugesan, M.; Huong, H.; Le, T.-T.; Phan, T.-H.; Manivasagan, P.; Mathiyalagan, R.; Jang, E.-S.; Yang, D.C.; Li, Y.; et al. Cellulose Nanocrystals-Incorporated Thermosensitive Hydrogel for Controlled Release, 3D Printing, and Breast Cancer Treatment Applications. ACS Appl. Mater. Interfaces 2022, 14, 42812–42826. [Google Scholar] [CrossRef]
- Jaisankar, E.; Azarudeen, R.S.; Thirumarimurugan, M. A Study on the Effect of Nanoscale MgO and Hydrogen Bonding in Nanofiber Mats for the Controlled Drug Release along with In Vitro Breast Cancer Cell Line and Antimicrobial Studies. ACS Appl. Bio. Mater. 2022, 5, 4327–4341. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Alsharidah, M.; Al Rugaie, O.; Tawfeek, H.M.; Tolba, N.S. Silver Nanoparticle-Coated Ethyl Cellulose Inhibits Tumor Necrosis Factor-α of Breast Cancer Cells. Drug Des. Devel. Ther. 2021, 15, 2035–2046. [Google Scholar] [CrossRef]
- Arumugam, M.; Murugesan, B.; Pandiyan, N.; Chinnalagu, D.K.; Rangasamy, G.; Mahalingam, S. Electrospinning Cellulose Acetate/Silk Fibroin/Au-Ag Hybrid Composite Nanofiber for Enhanced Biocidal Activity against MCF-7 Breast Cancer Cell. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112019. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Manjusha, V.; Chithra Sekhar, V. A New Biodegradable Nano Cellulose-Based Drug Delivery System for PH-Controlled Delivery of Curcumin. Int. J. Biol. Macromol. 2021, 183, 2044–2054. [Google Scholar] [CrossRef]
- Hussein, Y.; Loutfy, S.A.; Kamoun, E.A.; El-Moslamy, S.H.; Radwan, E.M.; Elbehairi, S.E.I. Enhanced Anti-Cancer Activity by Localized Delivery of Curcumin Form PVA/CNCs Hydrogel Membranes: Preparation and in Vitro Bioevaluation. Int. J. Biol. Macromol. 2021, 170, 107–122. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Karthikkumar, V.; Wang, M.-H. Smart Drug Delivery of P-Coumaric Acid Loaded Aptamer Conjugated Starch Nanoparticles for Effective Triple-Negative Breast Cancer Therapy. Int. J. Biol. Macromol. 2022, 195, 22–29. [Google Scholar] [CrossRef]
- He, C.; Guo, Y.; Karmakar, B.; El-kott, A.; Ahmed, A.E.; Khames, A. Decorated Silver Nanoparticles on Biodegradable Magnetic Chitosan/Starch Composite: Investigation of Its Cytotoxicity, Antioxidant and Anti-Human Breast Cancer Properties. J. Environ. Chem. Eng. 2021, 9, 106393. [Google Scholar] [CrossRef]
- Xu, C.; Li, S.; Chen, J.; Wang, H.; Li, Z.; Deng, Q.; Li, J.; Wang, X.; Xiong, Y.; Zhang, Z.; et al. Doxorubicin and Erastin Co-Loaded Hydroxyethyl Starch-Polycaprolactone Nanoparticles for Synergistic Cancer Therapy. J. Control. Release 2023, 356, 256–271. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Wang, H.; Li, Z.; Chen, J.; Zhang, Z.; Zeng, H.; Yu, X.; Yang, X.; Yang, X.; et al. Hydroxyethyl Starch-Folic Acid Conjugates Stabilized Theranostic Nanoparticles for Cancer Therapy. J. Control. Release 2023, 353, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Alp, E.; Damkaci, F.; Guven, E.; Tenniswood, M. Starch Nanoparticles for Delivery of the Histone Deacetylase Inhibitor CG-1521 in Breast Cancer Treatment. Int. J. Nanomed. 2019, 14, 1335–1346. [Google Scholar] [CrossRef]
- Steeves, M.; Combita, D.; Whelan, W.; Ahmed, M. Chemotherapeutics Loaded Poly(Dopamine) Core-Shell Nanoparticles for Breast Cancer Treatment. J. Pharmacol. Exp. Ther. 2024, 390, 78–87. [Google Scholar] [CrossRef]
- Healy, S.; Henderson, E.T.; Ke, S.; Kelly, J.; Simons, B.W.; Hu, C.; Ivkov, R.; Korangath, P. Spatial Analysis of Nanoparticle Distribution in Human Breast Xenografts Reveals Nanoparticles Targeted to Cancer Cells Localized with Tumor-Associated Stromal Cells. Nanotheranostics 2023, 7, 393–411. [Google Scholar] [CrossRef]
- Eskandani, M.; Derakhshankhah, H.; Zare, S.; Jahanban-Esfahlan, R.; Jaymand, M. Enzymatically Crosslinked Magnetic Starch-Grafted Poly(Tannic Acid) Hydrogel for “Smart” Cancer Treatment: An in Vitro Chemo/Hyperthermia Therapy Study. Int. J. Biol. Macromol. 2023, 253, 127214. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, C.; Zhang, F.; Zhou, Z.; Sun, M. “Attractive/Adhesion Force” Dual-Regulatory Nanogels Capable of CXCR4 Antagonism and Autophagy Inhibition for the Treatment of Metastatic Breast Cancer. J. Control. Release 2022, 341, 892–903. [Google Scholar] [CrossRef]
- Mousazadeh, H.; Bonabi, E.; Zarghami, N. Stimulus-Responsive Drug/Gene Delivery System Based on Polyethylenimine Cyclodextrin Nanoparticles for Potential Cancer Therapy. Carbohydr. Polym. 2022, 276, 118747. [Google Scholar] [CrossRef]
- Tan, T.; Yang, Q.; Chen, D.; Zhao, J.; Xiang, L.; Feng, J.; Song, X.; Fu, Y.; Gong, T. Chondroitin Sulfate-Mediated Albumin Corona Nanoparticles for the Treatment of Breast Cancer. Asian J. Pharm. Sci. 2021, 16, 508–518. [Google Scholar] [CrossRef]
- Shi, X.; Yang, X.; Liu, M.; Wang, R.; Qiu, N.; Liu, Y.; Yang, H.; Ji, J.; Zhai, G. Chondroitin Sulfate-Based Nanoparticles for Enhanced Chemo-Photodynamic Therapy Overcoming Multidrug Resistance and Lung Metastasis of Breast Cancer. Carbohydr. Polym. 2021, 254, 117459. [Google Scholar] [CrossRef] [PubMed]
- Setayesh, A.; Bagheri, F.; Boddohi, S. Self-Assembled Formation of Chondroitin Sulfate-Based Micellar Nanogel for Curcumin Delivery to Breast Cancer Cells. Int. J. Biol. Macromol. 2020, 161, 771–778. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Ji, J.; Li, L.; Zhai, G. Redox-Responsive Nanoparticles Based on Chondroitin Sulfate and Docetaxel Prodrug for Tumor Targeted Delivery of Docetaxel. Carbohydr. Polym. 2021, 255, 117393. [Google Scholar] [CrossRef]
- Singhai, N.J.; Maheshwari, R.; Jain, N.K.; Ramteke, S. Chondroitin Sulphate and α-Tocopheryl Succinate Tethered Multiwalled Carbon Nanotubes for Dual-Action Therapy of Triple-Negative Breast Cancer. J. Drug Deliv. Sci. Technol. 2020, 60, 102080. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhao, J.; Rong, H.; Chen, Y.; Xiong, M.; Ye, X.; Yu, S.; Hu, H. Coordinated Regulation of BACH1 and Mitochondrial Metabolism through Tumor-Targeted Self-Assembled Nanoparticles for Effective Triple Negative Breast Cancer Combination Therapy. Acta Pharm. Sin. B 2022, 12, 3934–3951. [Google Scholar] [CrossRef]
- Liu, M.; Fu, M.; Yang, X.; Jia, G.; Shi, X.; Ji, J.; Liu, X.; Zhai, G. Paclitaxel and Quercetin Co-Loaded Functional Mesoporous Silica Nanoparticles Overcoming Multidrug Resistance in Breast Cancer. Colloids Surf. B Biointerfaces 2020, 196, 111284. [Google Scholar] [CrossRef] [PubMed]
- Oommen, O.P.; Duehrkop, C.; Nilsson, B.; Hilborn, J.; Varghese, O.P. Multifunctional Hyaluronic Acid and Chondroitin Sulfate Nanoparticles: Impact of Glycosaminoglycan Presentation on Receptor Mediated Cellular Uptake and Immune Activation. ACS Appl. Mater. Interfaces 2016, 8, 20614–20624. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Chung, S.-J.; Cho, H.-J.; Kim, D.-D. Bile Acid-Conjugated Chondroitin Sulfate A-Based Nanoparticles for Tumor-Targeted Anticancer Drug Delivery. Eur. J. Pharm. Biopharm. 2015, 94, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Lee, H.S.; Kang, N.-W.; Lee, S.Y.; Kim, D.H.; Kim, S.; Yoon, I.-S.; Cho, H.-J.; Kim, D.-D. Blood Component Ridable and CD44 Receptor Targetable Nanoparticles Based on a Maleimide-Functionalized Chondroitin Sulfate Derivative. Carbohydr. Polym. 2020, 230, 115568. [Google Scholar] [CrossRef]
- Liu, M.; Du, H.; Zhai, G. Self-Assembled Nanoparticles Based on Chondroitin Sulfate-Deoxycholic Acid Conjugates for Docetaxel Delivery: Effect of Degree of Substitution of Deoxycholic Acid. Colloids Surf. B Biointerfaces 2016, 146, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, J.; Liu, C.; Jia, Y.; Wu, Y.; Shan, Y.; Qian, Z.; Liao, J. Graphene-Nanoparticle-Based Self-Healing Hydrogel in Preventing Postoperative Recurrence of Breast Cancer. ACS Biomater. Sci. Eng. 2019, 5, 768–779. [Google Scholar] [CrossRef] [PubMed]
- AbdElhamid, A.S.; Zayed, D.G.; Helmy, M.W.; Ebrahim, S.M.; Bahey-El-Din, M.; Zein-El-Dein, E.A.; El-Gizawy, S.A.; Elzoghby, A.O. Lactoferrin-Tagged Quantum Dots-Based Theranostic Nanocapsules for Combined COX-2 Inhibitor/Herbal Therapy of Breast Cancer. Nanomedicine 2018, 13, 2637–2656. [Google Scholar] [CrossRef]
- Bonzi, G.; Salmaso, S.; Scomparin, A.; Eldar-Boock, A.; Satchi-Fainaro, R.; Caliceti, P. Novel Pullulan Bioconjugate for Selective Breast Cancer Bone Metastases Treatment. Bioconjug. Chem. 2015, 26, 489–501. [Google Scholar] [CrossRef]
- Xu, R.; Sui, J.; Zhao, M.; Yang, Y.; Tong, L.; Liu, Y.; Sun, Y.; Fan, Y.; Liang, J.; Zhang, X. Targeted Inhibition of HER-2 Positive Breast Cancer Cells by Trastuzumab Functionalized Pullulan-Doxorubicin Nanoparticles. Polym. Test. 2022, 113, 107669. [Google Scholar] [CrossRef]
- Chen, L.; Ji, F.; Bao, Y.; Xia, J.; Guo, L.; Wang, J.; Li, Y. Biocompatible Cationic Pullulan-g-Desoxycholic Acid-g-PEI Micelles Used to Co-Deliver Drug and Gene for Cancer Therapy. Mater. Sci. Eng. C 2017, 70, 418–429. [Google Scholar] [CrossRef]
- Asgari, S.; Pourjavadi, A.; Setayeshmehr, M.; Boisen, A.; Ajalloueian, F. Encapsulation of Drug-Loaded Graphene Oxide-Based Nanocarrier into Electrospun Pullulan Nanofibers for Potential Local Chemotherapy of Breast Cancer. Macromol. Chem. Phys. 2021, 222, 2100096. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhang, C.; Zhou, P.; Liu, Y.; An, T.; Sun, D.; Zhang, N.; Wang, Y. Core-Shell Nanoparticles Based on Pullulan and Poly(β-Amino) Ester for Hepatoma-Targeted Codelivery of Gene and Chemotherapy Agent. ACS Appl. Mater. Interfaces 2014, 6, 18712–18720. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, Y.; Wang, Y.; Wu, J.; Yang, X.; Li, R.; Wang, Y.; Zhang, N. PH-Sensitive Pullulan-Based Nanoparticle Carrier for Adriamycin to Overcome Drug-Resistance of Cancer Cells. Carbohydr. Polym. 2014, 111, 908–917. [Google Scholar] [CrossRef]
- Pranatharthiharan, S.; Patel, M.D.; Malshe, V.C.; Devarajan, P. V Polyethylene Sebacate Doxorubicin Nanoparticles: Role of Carbohydrate Anchoring on in Vitro and in Vivo Anticancer Efficacy. Drug Deliv. 2016, 23, 2980–2989. [Google Scholar] [CrossRef]
- Na, K.; Lee, E.S.; Bae, Y.H. Self-Organized Nanogels Responding to Tumor Extracellular PH: PH-Dependent Drug Release and in Vitro Cytotoxicity against MCF-7 Cells. Bioconjug. Chem. 2007, 18, 1568–1574. [Google Scholar] [CrossRef]
- De, A.; Wadhwani, A.; Sauraj; Roychowdhury, P.; Kang, J.H.; Ko, Y.T.; Kuppusamy, G. WZB117 Decorated Metformin-Carboxymethyl Chitosan Nanoparticles for Targeting Breast Cancer Metabolism. Polymers 2023, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Mehata, A.K.; Bharti, S.; Singh, P.; Viswanadh, M.K.; Kumari, L.; Agrawal, P.; Singh, S.; Koch, B.; Muthu, M.S. Trastuzumab Decorated TPGS-g-Chitosan Nanoparticles for Targeted Breast Cancer Therapy. Colloids Surf. B Biointerfaces 2019, 173, 366–377. [Google Scholar] [CrossRef]
- Castro, F.; Pinto, M.L.; Pereira, C.L.; Serre, K.; Barbosa, M.A.; Vermaelen, K.; Gärtner, F.; Gonçalves, R.M.; De Wever, O.; Oliveira, M.J. Chitosan/γ-PGA Nanoparticles-Based Immunotherapy as Adjuvant to Radiotherapy in Breast Cancer. Biomaterials 2020, 257, 120218. [Google Scholar] [CrossRef]
- Pandya, A.D.; Øverbye, A.; Sahariah, P.; Gaware, V.S.; Høgset, H.; Masson, M.; Høgset, A.; Mælandsmo, G.M.; Skotland, T.; Sandvig, K.; et al. Drug-Loaded Photosensitizer-Chitosan Nanoparticles for Combinatorial Chemo- and Photodynamic-Therapy of Cancer. Biomacromolecules 2020, 21, 1489–1498. [Google Scholar] [CrossRef]
- Oliveira, C.; Gonçalves, C.S.; Martins, E.P.; Neves, N.M.; Reis, R.L.; Costa, B.M.; Silva, T.H.; Martins, A. Fucoidan/Chitosan Nanoparticles Functionalized with Anti-ErbB-2 Target Breast Cancer Cells and Impair Tumor Growth in Vivo. Int. J. Pharm. 2021, 600, 120548. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Su, X.; Gregory, D.A.; Li, W.; Cai, Z.; Zhao, X. Magnetic Alginate/Chitosan Nanoparticles for Targeted Delivery of Curcumin into Human Breast Cancer Cells. Nanomaterials 2018, 8, 907. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Q.; Zhou, Z.; He, N.; Li, S.; Zhao, C. Co-Delivery of Doxorubicin and Hydroxychloroquine via Chitosan/Alginate Nanoparticles for Blocking Autophagy and Enhancing Chemotherapy in Breast Cancer Therapy. Front. Pharmacol. 2023, 14, 1176232. [Google Scholar] [CrossRef]
- Rosch, J.G.; Winter, H.; DuRoss, A.N.; Sahay, G.; Sun, C. Inverse-Micelle Synthesis of Doxorubicin-Loaded Alginate/Chitosan Nanoparticles and in Vitro Assessment of Breast Cancer Cytotoxicity. Colloid Interface Sci. Commun. 2019, 28, 69–74. [Google Scholar] [CrossRef]
- Osanloo, M.; Pishamad, S.; Ghanbariasad, A.; Zarenezhad, E.; Alipanah, M.; Alipanah, H. Comparison Effects of Ferula Gummosa Essential Oil and Beta-Pinene Alginate Nanoparticles on Human Melanoma and Breast Cancer Cells Proliferation and Apoptotic Index in Short Term Normobaric Hyperoxic Model. BMC Complement. Med. Ther. 2023, 23, 428. [Google Scholar] [CrossRef]
- Espinosa-Cano, E.; Huerta-Madroñal, M.; Cámara-Sánchez, P.; Seras-Franzoso, J.; Schwartz, S.J.; Abasolo, I.; San Román, J.; Aguilar, M.R. Hyaluronic Acid (HA)-Coated Naproxen-Nanoparticles Selectively Target Breast Cancer Stem Cells through COX-Independent Pathways. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112024. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.; Liang, X.; Yin, Y.; Zuo, T.; Chen, D.; Shen, Q. Hyaluronic Acid-Modified Cationic Nanoparticles Overcome Enzyme CYP1B1-Mediated Breast Cancer Multidrug Resistance. Nanomedicine 2019, 14, 447–464. [Google Scholar] [CrossRef]
- Alves, C.G.; de Melo-Diogo, D.; Lima-Sousa, R.; Costa, E.C.; Correia, I.J. Hyaluronic Acid Functionalized Nanoparticles Loaded with IR780 and DOX for Cancer Chemo-Photothermal Therapy. Eur. J. Pharm. Biopharm. 2019, 137, 86–94. [Google Scholar] [CrossRef]
- Wang, R.; Yang, H.; Khan, A.R.; Yang, X.; Xu, J.; Ji, J.; Zhai, G. Redox-Responsive Hyaluronic Acid-Based Nanoparticles for Targeted Photodynamic Therapy/Chemotherapy against Breast Cancer. J. Colloid Interface Sci. 2021, 598, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Jia, W.; Dong, P.; Liu, J.; Wang, S.; Li, X.; Hu, J.; Zhao, L.; Shi, Y. Improved Antitumor Efficacy of a Dextran-Based Docetaxel-Coupled Conjugate against Triple-Negative Breast Cancer. Curr. Drug Deliv. 2024, 21, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Liu, J.; Lv, H.; Wu, J.; Zhang, N.; Wang, S.; Li, X.; Hu, J.; Wang, A.; Li, D.J.; et al. The Enhanced Antitumor Activity of the Polymeric Conjugate Covalently Coupled with Docetaxel and Docosahexaenoic Acid. Biomater. Sci. 2022, 10, 3454–3465. [Google Scholar] [CrossRef]
- Askar, M.A.; El Shawi, O.E.; Abou Zaid, O.A.R.; Mansour, N.A.; Hanafy, A.M. Breast Cancer Suppression by Curcumin-Naringenin-Magnetic-Nano-Particles: In Vitro and in Vivo Studies. Tumor Biol. 2021, 43, 225–247. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.S.; Sajid, M.I.; Parang, K.; Tiwari, R.K. Synthesis, Characterization, and Cytotoxicity Evaluation of Dextran-Myristoyl-ECGKRK Peptide Conjugate. Int. J. Biol. Macromol. 2021, 191, 1204–1211. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, S. A Convergent Fabrication of PH and Redox Dual-Responsive Hybrids of Mesoporous Silica Nanoparticles for the Treatment of Breast Cancer. J. Biomater. Sci. Polym. Ed. 2023, 34, 147–165. [Google Scholar] [CrossRef]
- Allahyari, M.; Motavalizadeh-Kakhky, A.R.; Mehrzad, J.; Zhiani, R.; Chamani, J. Cellulose Nanocrystals Derived from Chicory Plant: An Un-Competitive Inhibitor of Aromatase in Breast Cancer Cells via PI3K/AKT/MTOP Signalling Pathway. J. Biomol. Struct. Dyn. 2023, 42, 5575–5589. [Google Scholar] [CrossRef]
- Jutakridsada, P.; Suwannaruang, T.; Kasemsiri, P.; Weerapreeyakul, N.; Knijnenburg, J.T.N.; Theerakulpisut, S.; Kamwilaisak, K.; Chindaprasirt, P. Controllability, Antiproliferative Activity, Ag(+) Release, and Flow Behavior of Silver Nanoparticles Deposited onto Cellulose Nanocrystals. Int. J. Biol. Macromol. 2023, 225, 899–910. [Google Scholar] [CrossRef]
- Khella, K.F.; Abd El Maksoud, A.I.; Hassan, A.; Abdel-Ghany, S.E.; Elsanhoty, R.M.; Aladhadh, M.A.; Abdel-Hakeem, M.A. Carnosic Acid Encapsulated in Albumin Nanoparticles Induces Apoptosis in Breast and Colorectal Cancer Cells. Molecules 2022, 27, 4102. [Google Scholar] [CrossRef]
- Omrani, Z.; Pourmadadi, M.; Yazdian, F.; Rashedi, H. Preparation and Characterization of PH-Sensitive Chitosan/Starch/MoS(2) Nanocomposite for Control Release of Curcumin Macromolecules Drug Delivery; Application in the Breast Cancer Treatment. Int. J. Biol. Macromol. 2023, 250, 125897. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Tajiki, A.; Abdouss, M. A Green Approach for Preparation of Polyacrylic Acid/Starch Incorporated with Titanium Dioxide Nanocomposite as a Biocompatible Platform for Curcumin Delivery to Breast Cancer Cells. Int. J. Biol. Macromol. 2023, 242, 124785. [Google Scholar] [CrossRef] [PubMed]
- Azimijou, N.; Karimi-Soflou, R.; Karkhaneh, A. CD44 Targeted-Chondroitin Sulfate Nanoparticles: Fine-Tuning Hydrophobic Groups to Enhance in Vitro PH-Responsiveness and in Vivo Efficacy for Advanced Breast Cancer Treatment. Biomater. Adv. 2024, 158, 213776. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, J.; Jiang, R.; Yuan, Q.; Ding, Y.; Ren, J.; Jiang, D.; Wang, Y.; Wang, L.; Chen, P.; et al. Versatile Chondroitin Sulfate-Based Nanoplatform for Chemo-Photodynamic Therapy against Triple-Negative Breast Cancer. Int. J. Biol. Macromol. 2024, 265, 130709. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, L.; Ling, Y.; Xiao, X.; Gong, J.; Jin, H.; Xu, J.; Chen, P.; Xie, X.; Zhang, L. Peptide-Modified Bioresponsive Chondroitin Sulfate Micelles for Targeted Doxorubicin Delivery in Triple-Negative Breast Cancer. Colloids Surf. B Biointerfaces 2023, 227, 113381. [Google Scholar] [CrossRef]
- Yu, J.; Wang, L.; Xie, X.; Zhu, W.; Lei, Z.; Lv, L.; Yu, H.; Xu, J.; Ren, J. Multifunctional Nanoparticles Codelivering Doxorubicin and Amorphous Calcium Carbonate Preloaded with Indocyanine Green for Enhanced Chemo-Photothermal Cancer Therapy. Int. J. Nanomed. 2023, 18, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Xu, F.; Yao, T.; Zhu, J.; Yu, H.; Wang, G.; Li, J. TPGS and Chondroitin Sulfate Dual-Modified Lipid-Albumin Nanosystem for Targeted Delivery of Chemotherapeutic Agent against Multidrug-Resistant Cancer. Int. J. Biol. Macromol. 2021, 183, 1270–1282. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Y.; Wen, S.; Wen, Y.; Wang, R.; Zhang, Q.; Qin, G.; Yi, H.; Wu, M.; Lu, L.; et al. Synergistically Enhanced Inhibitory Effects of Pullulan Nanoparticle-Mediated Co-Delivery of Lovastatin and Doxorubicin to Triple-Negative Breast Cancer Cells. Nanoscale Res. Lett. 2019, 14, 314. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wei, X.; Qin, J.; Chen, H.; Shen, Z.; Lv, F.; Nan, W.; Wang, Y.; Li, Q.; Zhang, Q. Synthesis, Characterization and In Vivo Efficacy of Biotin-Conjugated Pullulan Acetate Nanoparticles as a Novel Anticancer Drug Carrier. J. Biomed. Nanotechnol. 2017, 13, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/079907316/publication/US2022047559A1?q=pn%3DUS2022047559A1 (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/068180514/publication/CN110339372A?q=pn%3DCN110339372A (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/062593328/publication/CN108186607A?q=pn%3DCN108186607A (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/063206357/publication/CN108434123A?q=pn%3DCN108434123A (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/072666554/publication/US12377117B2?q=pn%3DUS12377117B2 (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/089532444/publication/CN117414346A?q=pn%3DCN117414346A (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/062240944/publication/WO2018098705A1?q=pn%3DWO2018098705A1 (accessed on 20 October 2025).
- Espacenet–Search Results. Available online: https://worldwide.espacenet.com/patent/search/family/067793550/publication/CN110201181A?q=pn%3DCN110201181A (accessed on 20 October 2025).
| Type of NPs | Composition of Delivery System | In Vitro Cell Line, In Vivo Model | Key Features/Outcome of the Study | Ref. |
|---|---|---|---|---|
| Chitosan-based NPs | WZB117/-O-carboxymethyl-chitosan (OCMC) NPs/Metformin (WZB117-OCMC-MET) | MCF-7 |
| [158] |
| DOX/D-α-tocopherol polyethylene glycol 1000 succinate/chitosan (TPGS-g-chitosan) NPs | SK-BR-3; SK-BR-3 induced mouse |
| [159] | |
| Chitosan/poly (γ-glutamic acid) NPs (Ch/γ-PGA NPs) + radiotherapy (RT) | 4T1 orthotopic breast tumor mouse |
| [160] | |
| Mertansine (MRT)/cabazitaxel (CBZ)/chitosan (CS)/tetraphenylchlorin (TPC) nanoconjugate | MDA-MB-468 or MDA-MB-231; Mouse model |
| [161] | |
| Fucoidan/chitosan NPs/gemcitabine/ErbB-2 antibody (NPs + Gem + Ab) | SKBR3 cells |
| [162] | |
| Curcumin/magnetic alginate/chitosan NPs | MDA-MB-231 |
| [163] | |
| Alginate-based NPs | 2,3-dimercaptosuccinic acid (DMSA)/Fe3O4 NPs/chitosan/alginate NPs (CANPs)/doxorubicin (DOX)/hydroxychloroquine (HCQ) | MCF-7, and MDA-MB-231 cell line |
| [164] |
| Alginate/chitosan/doxorubicin (DOX) NPs | Murine breast cancer cell line 4T1 |
| [165] | |
| Alginate NPs/β-pinene/Ferula gummosa essential oil | A-375 and MDA-MB-231 cells |
| [166] | |
| Hyaluronic acid (HA)-based NPs | Naproxen (NAP)/hyaluronic acid (HA) NPs | MCF-7 breast cancer cells |
| [167] |
| HA/polyethyleneimine NPs [HA/PEI NPs])/DTX/α-napthtoflavone | MDR in breast cancer induced by CYP1B1 |
| [168] | |
| HA/IR780/Doxorubicin (DOX) (HPN) | 2D/3D models |
| [169] | |
| Docosahexaenoic acid (DHA)/chlorin e6 (Ce6)/HA [HA-cys-DHA/Ce6 (CHD)]/docetaxel (DTX) | MCF-7 cells |
| [170] | |
| α1-acid glycoprotein (AGP)/HA NPs (AGP-HA NPs) | MCF-7 cells |
| [93] | |
| Dextran-based NPs | Dextran/docetaxel (DTX)/docosahexaenoic acid (DHA) (DDD) NPs | MCF-7, and 4T1-tumor-bearing mice |
| [171] |
| DTX/docosahexaenoic acid (DHA)/biofunctionalized dextran NPs | MCF-7 cells, Nude mice |
| [172] | |
| Curcumin/naringenin/dextran-coated magnetic NPs (CUR-NAR-D-MNPs) | MCF-7 cells |
| [173] | |
| Dextran/myristoyl-ECGKRK peptide NPs | MDA-MB-231, and MCF-7 |
| [174] | |
| Cellulose-based NPs | MSN/sodium hyaluronate (SH)/SS/oxidized sodium carboxymethyl cellulose (O-CMC) nanohybrids | MCF-7, and MDA-MB-231 cells, Mouse model |
| [175] |
| Cellulose Nanocrystals (CNCs) from Chicory plant waste | MCF-7 cells with CYP19 |
| [176] | |
| Silver NPs (AgNPs)/carboxylated cellulose nanocrystals (Ag-cCNC) from Eucalyptus pulp | MCF-7 cells |
| [177] | |
| Carnosic acid (CA)/bovine serum albumin (BSA)/chitosan (CH)/cellulose (CL) NPs (CA-BSA-NPs) | MCF-7 and Caco-2 cells |
| [178] | |
| Starch-based NPs | Curcumin/chitosan/starch/MoS2 nanocomposite | MCF-7 cell |
| [179] |
| Curcumin/polyacrylic acid (PAA)/starch/titanium dioxide (TiO2) nanocomposite | MCF-7 cell line |
| [180] | |
| Starch NP/CG-1521 | MCF-7 cells |
| [131] | |
| Chondroitin sulfate-cholesterol (ChS-Chol) nano-assemblies/doxorubicin (Dox) | 4T1, MCF-7, and MDA-MB-231 cells |
| [181] | |
| Chondroitin sulfate A (CSA)/chlorin e6 (Ce6)/doxorubicin (DOX) NPs | 4T1, and MDA-MB-231 cells, Balb/c mice |
| [182] | |
| Peptide-grafted chondroitin sulfate A-ss-deoxycholic acid (TCSSD)/DOX TCSSD (TCSSD-D) micelles | MDA-MB-231 cells |
| [183] | |
| Indocyanine green (ICG)/calcium-carbonate (ICG@) NP/poly (lactic-co-glycolic acid)-ss-chondroitin sulfate A (PSC) NPs | 4T1 cells, and 4T1-bearing Balb/c mice |
| [184] | |
| d-α-tocopherol polyethylene 1000 glycol succinate (TPGS)/chondroitin sulfate (CS)/paclitaxel (PTX) | MDR breast cancer (MCF-7/MDR) cells |
| [185] | |
| Pullulan-based NPs | Lovastatin (LV)/pullulan (PLV) NPs | MDA-MB-231, MB-453 cells |
| [186] |
| Biotin/pullulan acetate (Bio-PA) NPs/Epirubicin (EPI) | MCF-7 cells, Nude mice |
| [187] |
| Patent No. | Type of NPs | Title of Invention | Year | Ref. |
|---|---|---|---|---|
| US2022047559A1 | Chitosan-based NPs | Drug composition for treating breast cancer and method for manufacturing the same | 2022 | [188] |
| CN110339372A | Chitosan-based NPs | Novel RGD-chitosan oligosaccharide silicon oxide/BCSG1-siRNA nanoparticle breast cancer targeted therapy method | 2019 | [189] |
| CN108186607A | Chitosan-based NPs | Preparation method of breast cancer targeted chitosan grafted polymer medicine-carrying composite material | 2018 | [190] |
| CN108434123A | Chitosan-based NPs | Preparation method of L-peptide modified chitosan drug-loading nanoparticles with breast cancer targeting function | 2018 | [191] |
| US12377117B2 | Hyaluronic acid-based NPs | Hyaluronic acid nanoparticles comprising NADPH oxidase inhibitors and their uses in treating cancer | 2025 | [192] |
| CN117414346A | Hyaluronic acid-based NPs | Metal polyphenol nanoparticle, preparation method and application in preparation of TMEM16A and/or EGFR inhibitor | 2024 | [193] |
| WO2018098705A1 | Dextran-based NPs | Dextran-magnetic iron oxide nanoparticle, preparation and use in treating cancer and as a contrast | 2018 | [194] |
| CN110201181A | Pullulan-based NPs | Pullulan nanoparticles with co-supported lovastatin and doxorubicin, and preparation method thereof | 2019 | [195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanto, S.; Gowda, B.H.J.; Hani, U.; Narayana, S.; Ahmed, M.G.; Fatima, F.; Paul, K. Advancements in Polysaccharide-Based Nanoparticles for the Treatment of Breast Cancer: A Comprehensive Review. Pharmaceuticals 2025, 18, 1712. https://doi.org/10.3390/ph18111712
Mohanto S, Gowda BHJ, Hani U, Narayana S, Ahmed MG, Fatima F, Paul K. Advancements in Polysaccharide-Based Nanoparticles for the Treatment of Breast Cancer: A Comprehensive Review. Pharmaceuticals. 2025; 18(11):1712. https://doi.org/10.3390/ph18111712
Chicago/Turabian StyleMohanto, Sourav, Benachakal Honnegowda Jaswanth Gowda, Umme Hani, Soumya Narayana, Mohammed Gulzar Ahmed, Farhat Fatima, and Karthika Paul. 2025. "Advancements in Polysaccharide-Based Nanoparticles for the Treatment of Breast Cancer: A Comprehensive Review" Pharmaceuticals 18, no. 11: 1712. https://doi.org/10.3390/ph18111712
APA StyleMohanto, S., Gowda, B. H. J., Hani, U., Narayana, S., Ahmed, M. G., Fatima, F., & Paul, K. (2025). Advancements in Polysaccharide-Based Nanoparticles for the Treatment of Breast Cancer: A Comprehensive Review. Pharmaceuticals, 18(11), 1712. https://doi.org/10.3390/ph18111712







