Recent Progress on Affibody-Based Supramolecular Architectures: Moving from Monomeric Constructs to Multivalent Assemblies
Abstract
1. Introduction
2. Brief History and Development of Affibodies
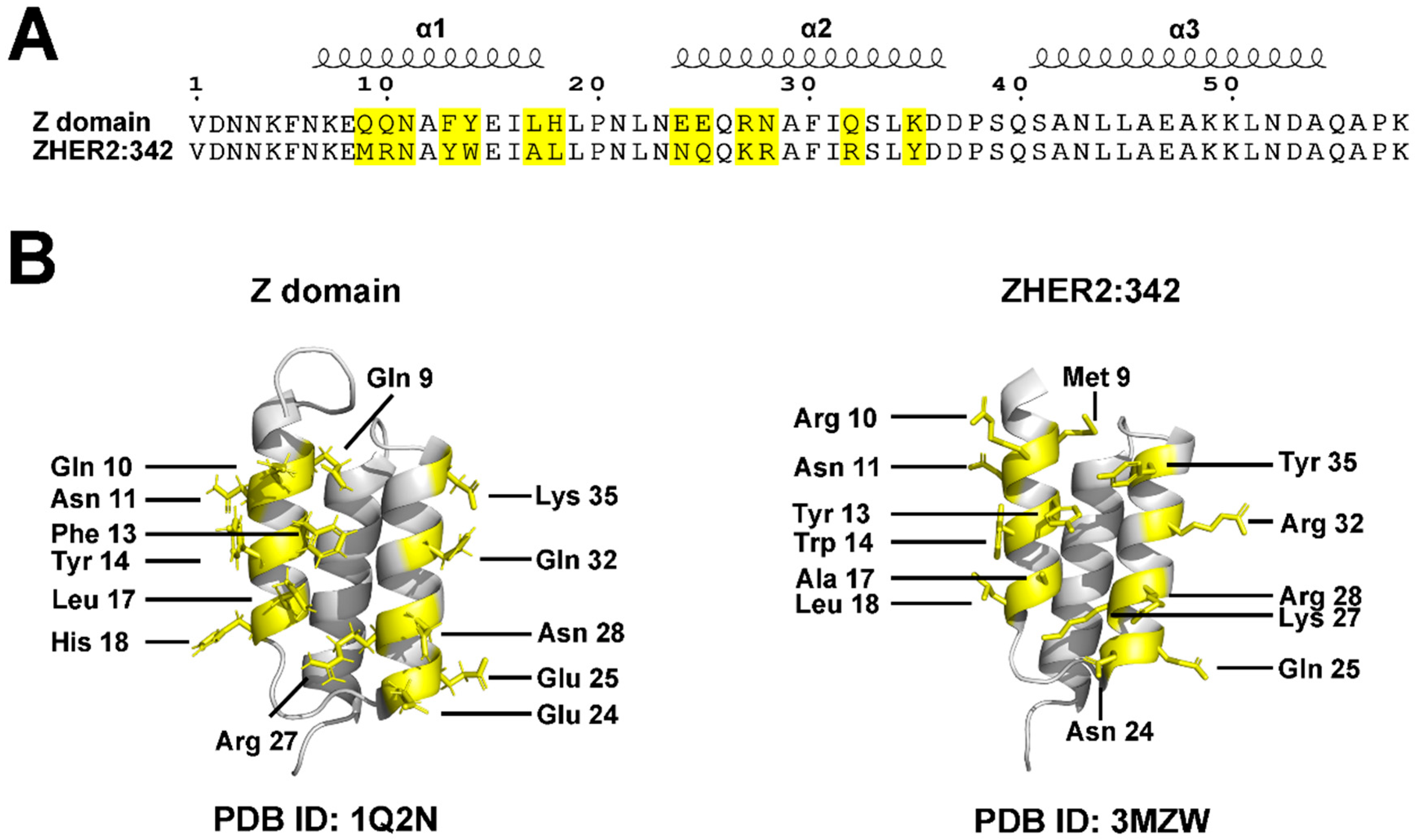
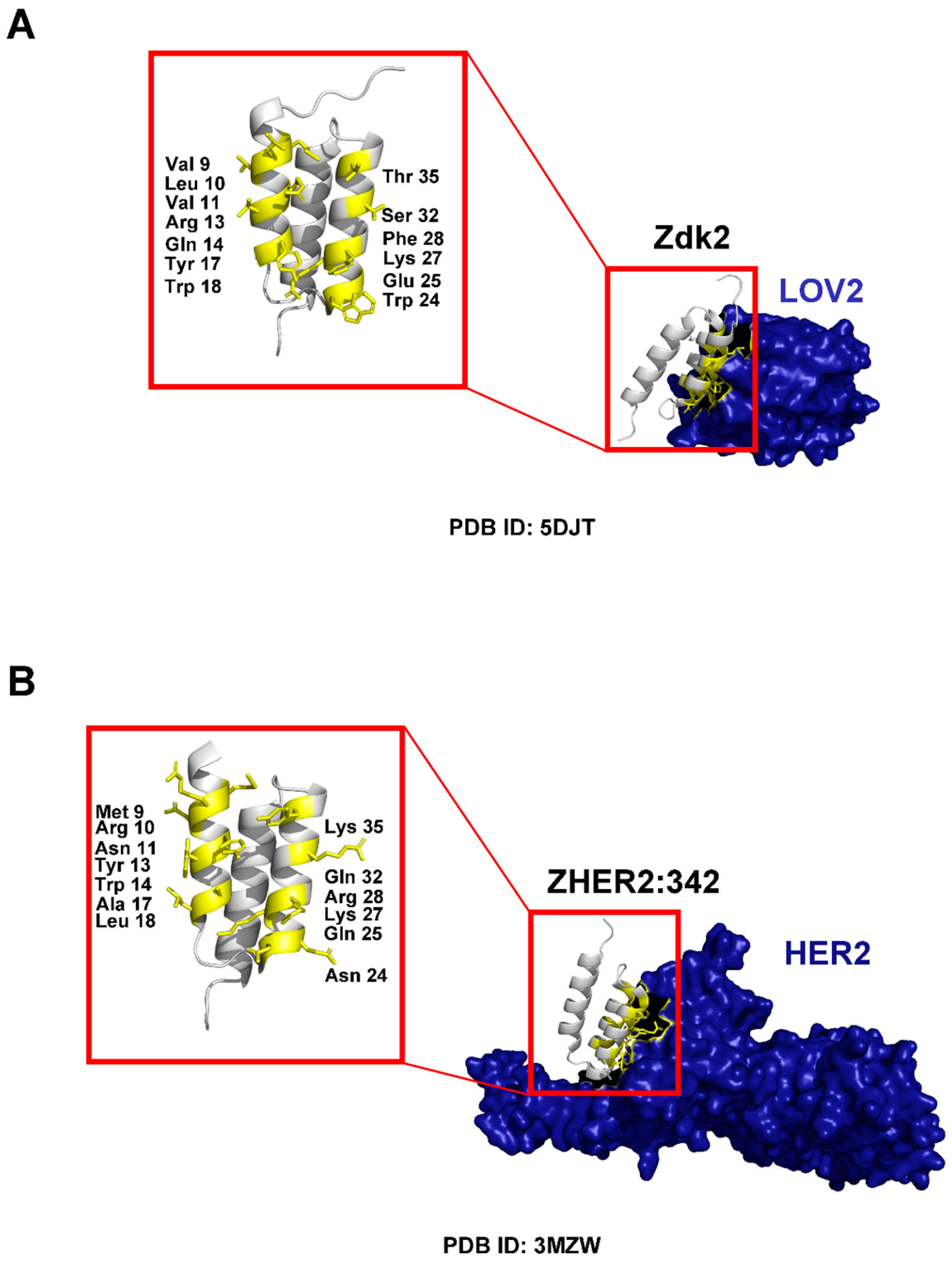
3. Molecular Structure and Scaffold Description
3.1. Binding Properties and Affinity
3.2. Stability
3.3. Half-Life
4. Basic Affibody Monomeric Constructs
5. Multivalent Assembly of Affibody Molecules
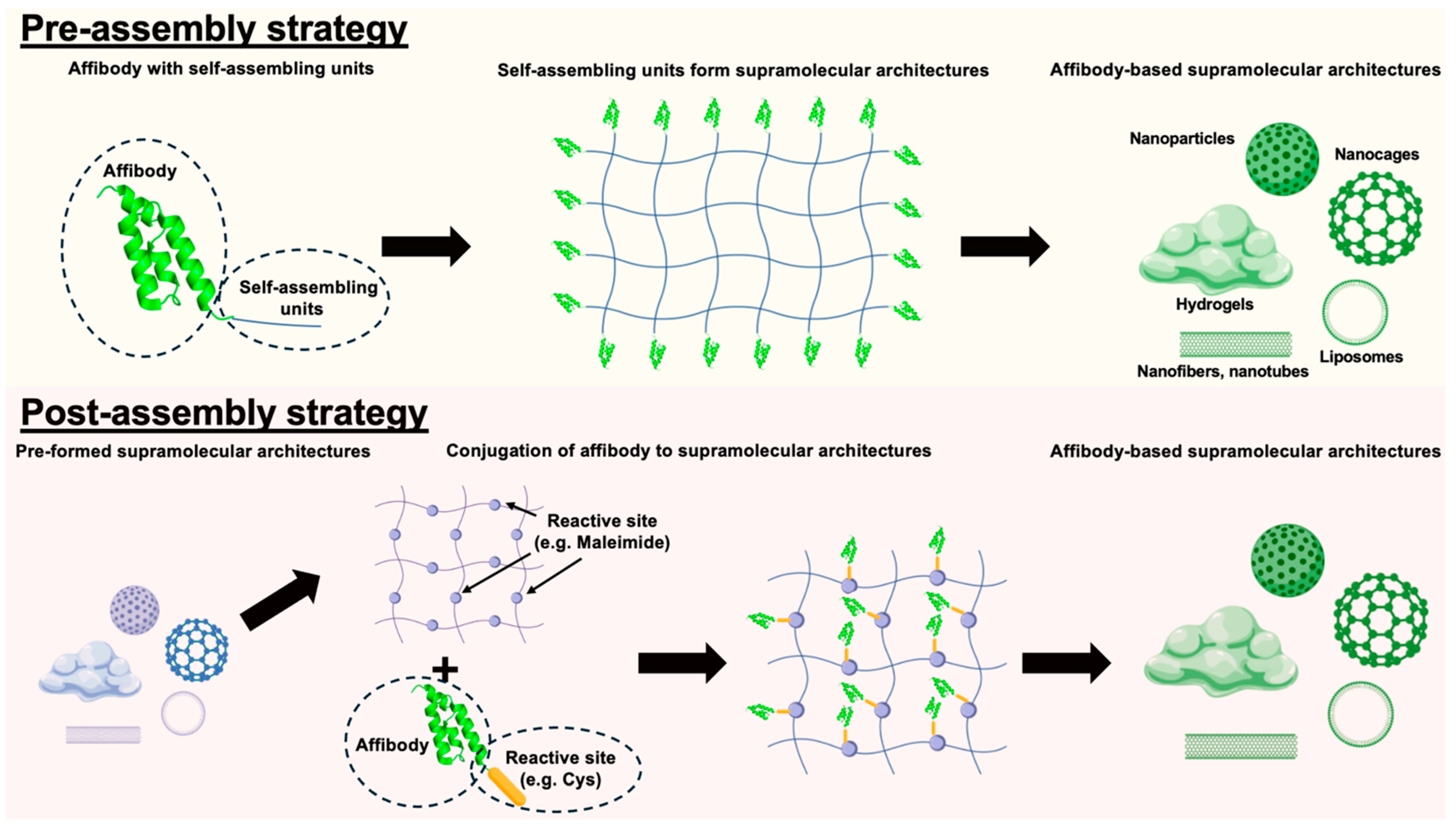
5.1. Supramolecular Incorporation Strategies
5.1.1. Pre-Assembly Incorporation Strategies
Examples of Pre-Assembly Incorporation in Affibody-Based Supramolecular Architectures
| Affibody Targets | Supramolecular Architectures | Self-Assembling Units | Payloads | Applications | Citation |
|---|---|---|---|---|---|
| HER2 | Virus-like particles | Hepatitis B core (HBc) proteins | Fluorescent probe (e.g., Alexa Fluor 488) | Targeted delivery | Nishimura et al. (2013) [49] |
| EGFR | Affibody-drug conjugate-based nanoagent (nanomicelles) | MMAE (amphiphile) | Anti-cancer drug (e.g., MMAE) & fluorescent probe (e.g., Cy5.5) | Targeted delivery | Yang et al. (2025) [44] |
| HER2 | Affibody-drug conjugate-based nanoagent (nanomicelles) | Epothilone B (amphiphile) | Anti-cancer drug (e.g., Epothilone B) & fluorescent probe (e.g., Cy5.5) | Targeted delivery | Xia et al. (2024) [43] |
| EGFR | Affibody-PROTAC conjugate-based nanoagent (nanomicelles) | PROTAC MS28 (amphiphile) | PROTAC (e.g., MS28) & fluorescent probe (e.g., Cy5.5) | Targeted delivery | Gao et al. (2024) [33] |
| HER2 | Affibody-PROTAC conjugate-based nanoagent (nanomicelles) | PROTAC MZ1 (amphiphile) | PROTAC (e.g., MZ1) & fluorescent probe (e.g., Cy5.5) | Targeted delivery | Li et al. (2024) [34] |
| HER2 | Nanomicelles | Elastin-like peptide (ELP)-MMAE | Anti-cancer drug (e.g., MMAE) & fluorescent probe (e.g., Cy5) | Targeted delivery | Li et al. (2024) [50] |
| AFP | Affibody aggregates | Self-assembly peptides | - | AFP detection | Liu et al. (2022) [51] |
| HER2 | Nanomicelles | G-quadruplex DNA | Anti-cancer drug (e.g., 5-FdUR and curcumin) & fluorescent probe (e.g., FAM) | Targeted delivery | Zhang et al. (2022) [52] |
| HER2 | Nanoparticles | RALA amphipathic peptides | Anti-cancer drug (e.g., FUdR15) & fluorescent probe (e.g., FAM) | Targeted delivery | Zhang et al. (2020) [53] |
| IGF-1 or PEDF | Hydrogel | Methylcellulose | IGF-1 or PEDF | Controlled release of therapeutic proteins | Teal et al. (2022) [54] |
5.1.2. Post-Assembly Incorporation Strategies
Examples of Post-Assembly Incorporation in Affibody-Based Supramolecular Architectures
| Affibody Targets | Supramolecular Architecture | Affibody Conjugation Method | Payloads | Applications | Citation |
|---|---|---|---|---|---|
| EGFR | Gold–silica nanoparticles | Thiol/maleimide | - | Targeted delivery for Raman imaging | Jokerst et al. (2011) [58] |
| EGFR | Gold–iron oxide hetero-nanostructures | Thiol/maleimide | - | Targeted delivery for PET, Optical and MR Imaging | Yang et al. (2013) [48] |
| HER2 | Quantum dots and iron oxide nanoparticles | Thiol/maleimide | - | Targeted delivery for optical and MR Imaging | Gao et al. (2011) [38] |
| HER2 | Silver nanoparticles | NHS/EDC | - | Targeted delivery for photothermal therapy | Shipunova et al. (2022) [64] |
| HER2 | PLGA nanoparticles | NHS/EDC | Photosensitizer (e.g., Rose Bengal) | Targeted delivery for photosensitizer | Shipunova et al. (2021) [65] |
| HER2 | Phospholipid nanobubble | Biotin/streptavidin | - | Targeted delivery for ultrasound contrast agents | Yang et al. (2015) [66] |
| HER2 | Phospholipid nanobubble | Biotin/streptavidin | Photothermal agents (e.g., IR783) & photosensitizer (e.g., HPPH) | Targeted delivery for ultrasound contrast agents, photothermal therapy & photosensitizer | Cai et al. (2023) [67] |
| HER2/ EGFR | Lumazine synthase protein nanoparticles | SpyTag/SpyCatcher | Contrast agents (e.g., Gd(III)-DOTA) & fluorescent probe (e.g., Alexa680) | Targeted delivery for optical and MR Imaging | Kim et al. (2021) [68] |
| EGFR | SpyCatcher-mi3 protein nanoparticles | SpyTag/SpyCatcher | Anti-cancer drug (e.g., aldoxorubicin) & fluorescent probe (e.g., Fluorescein, Alexa647) | Targeted delivery | Eom et al. (2024) [69] |
| EGFR | Lumazine synthase protein nanoparticles | SpyTag/SpyCatcher | TRAIL | Targeted anti-cancer therapeutic | Jun et al. (2022) [62] |
| HER2/EGFR | Zr6-based MOF nanoparticles (PCN-224) | Adsorption | Anti-cancer drug (e.g., Camptothecin) | Targeted delivery | Oh et al. (2023) [70] |
| HER2 | DNA origami nanodevice | Thiol/maleimide/SMCC | Doxorubicin | Targeted delivery | Yu et al. (2023) [71] |
| BMP-2 | Polyethylene glycol–maleimide hydrogels | Thiol/maleimide | BMP-2 | Controlled release of therapeutic proteins | Dorogin et al. (2023) [72] |
| HER2 | Nanofiber | Affibody embedded during co-assembly | - | Targeted delivery | Liang et al. (2018) [40] |
6. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frejd, F.Y.; Kim, K.-T. Affibody molecules as engineered protein drugs. Exp. Mol. Med. 2017, 49, e306. [Google Scholar] [CrossRef]
- Löfblom, J.; Feldwisch, J.; Tolmachev, V.; Carlsson, J.; Ståhl, S.; Frejd, F.Y. Affibody molecules: Engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 2010, 584, 2670–2680. [Google Scholar] [CrossRef]
- Ståhl, S.; Gräslund, T.; Karlström, A.E.; Frejd, F.Y.; Nygren, P.-Å.; Löfblom, J. Affibody molecules in biotechnological and medical applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef]
- Ashford, M.B.; England, R.M.; Akhtar, N. Highway to success—Developing advanced polymer therapeutics. Adv. Ther. 2021, 4, 2000285. [Google Scholar] [CrossRef]
- Cole, J.T.; Holland, N.B. Multifunctional nanoparticles for use in theranostic applications. Drug Deliv. Transl. Res. 2015, 5, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, M. Nanoparticle-based theragnostics: Integrating diagnostic and therapeutic potentials in nanomedicine. J. Control. Release Off. J. Control. Release Soc. 2010, 146, 2. [Google Scholar] [CrossRef] [PubMed]
- Nord, K.; Gunneriusson, E.; Ringdahl, J.; Ståhl, S.; Uhlén, M.; Nygren, P.-Å. Binding proteins selected from combinatorial libraries of an α-helical bacterial receptor domain. Nat. Biotechnol. 1997, 15, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Feldwisch, J.; Tolmachev, V. Engineering of affibody molecules for therapy and diagnostics. In Therapeutic Proteins: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2012; pp. 103–126. [Google Scholar]
- Orlova, A.; Magnusson, M.; Eriksson, T.L.J.; Nilsson, M.; Larsson, B.; Höidén-Guthenberg, I.; Widström, C.; Carlsson, J.; Tolmachev, V.; Ståhl, S.; et al. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 2006, 66, 4339–4348. [Google Scholar] [CrossRef]
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J.; et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Nordberg, E.; Höidén-Guthenberg, I.; Brismar, H.; Adams, G.; Nilsson, F.; Carlsson, J.; Ståhl, S. Phage display selection of Affibody molecules with specific binding to the extracellular domain of the epidermal growth factor receptor. Protein Eng. Des. Sel. 2007, 20, 189–199. [Google Scholar] [CrossRef]
- Gast, V.; Sandegren, A.; Dunås, F.; Ekblad, S.; Güler, R.; Thorén, S.; Mohedano, M.T.; Molin, M.; Engqvist, M.K.M.; Siewers, V. Engineering Saccharomyces cerevisiae for the production and secretion of Affibody molecules. Microb. Cell Factories 2022, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, S.; Lindberg, H.; Hjelm, L.C.; Löfblom, J.; Leitao, C.D. Engineering of affibody molecules. Cold Spring Harb. Protoc. 2024, 2024, pdb-top107760. [Google Scholar] [CrossRef]
- Grimm, S.; Yu, F.; Nygren, P.-Å. Ribosome display selection of a murine IgG1 fab binding affibody molecule allowing species selective recovery of monoclonal antibodies. Mol. Biotechnol. 2011, 48, 263–276. [Google Scholar] [CrossRef]
- Ekerljung, L.; Steffen, A.-C.; Carlsson, J.; Lennartsson, J. Effects of HER2-binding affibody molecules on intracellular signaling pathways. Tumor Biol. 2006, 27, 201–210. [Google Scholar] [CrossRef]
- Altai, M.; Liu, H.; Orlova, A.; Tolmachev, V.; Gräslund, T. Influence of molecular design on biodistribution and targeting properties of an Affibody-fused HER2-recognising anticancer toxin. Int. J. Oncol. 2016, 49, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Orlova, A.; Feldwisch, J.; Abrahmsén, L.; Tolmachev, V. Update: Affibody molecules for molecular imaging and therapy for cancer. Cancer Biother. Radiopharm. 2007, 22, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga] ABY-025 Affibody PET/CT. Theranostics 2016, 6, 262. [Google Scholar] [CrossRef]
- Eissler, N.; Altena, R.; Alhuseinalkhudhur, A.; Bragina, O.; Feldwisch, J.; Wuerth, G.; Loftenius, A.; Brun, N.; Axelsson, R.; Tolmachev, V.; et al. Affibody PET imaging of HER2-expressing cancers as a key to guide HER2-targeted therapy. Biomedicines 2024, 12, 1088. [Google Scholar] [CrossRef]
- Tolmachev, V.; Orlova, A. Affibody molecules as targeting vectors for PET imaging. Cancers 2020, 12, 651. [Google Scholar] [CrossRef]
- Wållberg, H.; Orlova, A.; Altai, M.; Hosseinimehr, S.J.; Widström, C.; Malmberg, J.; Ståhl, S.; Tolmachev, V. Molecular design and optimization of 99mTc-labeled recombinant affibody molecules improves their biodistribution and imaging properties. J. Nucl. Med. 2011, 52, 461–469. [Google Scholar] [CrossRef]
- Högbom, M.; Eklund, M.; Nygren, P.-Å.; Nordlund, P. Structural basis for recognition by an in vitro evolved affibody. Proc. Natl. Acad. Sci. USA 2003, 100, 3191–3196. [Google Scholar] [CrossRef] [PubMed]
- Lendel, C.; Dogan, J.; Härd, T. Structural basis for molecular recognition in an affibody: Affibody complex. J. Mol. Biol. 2006, 359, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Wahlberg, E.; Lendel, C.; Helgstrand, M.; Allard, P.; Dincbas-Renqvist, V.; Hedqvist, A.; Berglund, H.; Nygren, P.; Härd, T. An affibody in complex with a target protein: Structure and coupled folding. Proc. Natl. Acad. Sci. USA 2003, 100, 3185–3190. [Google Scholar] [CrossRef]
- Lindgren, J.; Wahlström, A.; Danielsson, J.; Markova, N.; Ekblad, C.; Gräslund, A.; Abrahmsen, L.; Karlström, A.E.; Wärmländer, S.K. N-terminal engineering of amyloid-β-binding Affibody molecules yields improved chemical synthesis and higher binding affinity. Protein Sci. 2010, 19, 2319–2329. [Google Scholar] [CrossRef]
- Engfeldt, T.; Renberg, B.; Brumer, H.; Nygren, P.Å.; Eriksson Karlström, A. Chemical synthesis of triple-labelled three-helix bundle binding proteins for specific fluorescent detection of unlabelled protein. ChemBioChem 2005, 6, 1043–1050. [Google Scholar] [CrossRef]
- Feldwisch, J.; Tolmachev, V.; Lendel, C.; Herne, N.; Sjöberg, A.; Larsson, B.; Rosik, D.; Lindqvist, E.; Fant, G.; Höidén-Guthenberg, I.; et al. Design of an optimized scaffold for affibody molecules. J. Mol. Biol. 2010, 398, 232–247. [Google Scholar] [CrossRef]
- Grönwall, C.; Ståhl, S. Engineered affinity proteins—Generation and applications. J. Biotechnol. 2009, 140, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, H. Recent advances of affibody molecules in biomedical applications. Bioorganic Med. Chem. 2024, 113, 117923. [Google Scholar] [CrossRef]
- Carter, P.; Presta, L.; Gorman, C.M.; Ridgway, J.B.; Henner, D.; Wong, W.L.; Rowland, A.M.; Kotts, C.; Carver, M.E.; Shepard, H.M. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA 1992, 89, 4285–4289. [Google Scholar] [CrossRef]
- Orlova, A.; Jonsson, A.; Rosik, D.; Lundqvist, H.; Lindborg, M.; Abrahmsen, L.; Ekblad, C.; Frejd, F.Y.; Tolmachev, V. Site-specific radiometal labeling and improved biodistribution using ABY-027, a novel HER2-targeting Affibody molecule–albumin-binding domain fusion protein. J. Nucl. Med. 2013, 54, 961–968. [Google Scholar] [CrossRef]
- Zhang, J.; Bodenko, V.; Larkina, M.; Bezverkhniaia, E.; Xu, T.; Liao, Y.; Abouzayed, A.; Plotnikov, E.; Tretyakova, M.; Yuldasheva, F.; et al. Half-life extension via ABD-fusion leads to higher tumor uptake of an affibody-drug conjugate compared to PAS-and XTENylation. J. Control. Release 2024, 370, 468–478. [Google Scholar] [CrossRef]
- Gao, W.; Xia, X.; Yang, X.; Li, Q.; Xia, X.; Huang, W.; Yan, D. Amphiphilic Affibody-PROTAC conjugate self-assembled nanoagents for targeted cancer therapy. Chem. Eng. J. 2024, 495, 153437. [Google Scholar] [CrossRef]
- Li, Q.; Yang, X.; Zhao, M.; Xia, X.; Gao, W.; Huang, W.; Xia, X.; Yan, D. A self-assembled affibody-PROTAC conjugate nanomedicine for targeted cancer therapy. Nano Res. 2024, 17, 9954–9964. [Google Scholar] [CrossRef]
- Miao, H.; Sun, Y.; Jin, Y.; Hu, X.; Song, S.; Zhang, J. Application of a Novel 68Ga-HER2 Affibody PET/CT imaging in breast Cancer patients. Front. Oncol. 2022, 12, 894767. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Pan, D.; Yu, C.; Mi, B.; Huang, Q.; Sheng, J.; Yan, J.; Wang, X.; Yang, R.; et al. PET imaging of a 68Ga labeled modified HER2 affibody in breast cancers: From xenografts to patients. Br. J. Radiol. 2019, 92, 20190425. [Google Scholar] [CrossRef]
- Bragina, O.; Chernov, V.; Larkina, M.; Rybina, A.; Zelchan, R.; Garbukov, E.; Oroujeni, M.; Loftenius, A.; Orlova, A.; Sörensen, J.; et al. Phase I clinical evaluation of 99mTc-labeled Affibody molecule for imaging HER2 expression in breast cancer. Theranostics 2023, 13, 4858. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, K.; Miao, Z.; Ren, G.; Chen, X.; Gambhir, S.S.; Cheng, Z. Affibody-based nanoprobes for HER2-expressing cell and tumor imaging. Biomaterials 2011, 32, 2141–2148. [Google Scholar] [CrossRef]
- Schardt, J.S.; Oubaid, J.M.; Williams, S.C.; Howard, J.L.; Aloimonos, C.M.; Bookstaver, M.L.; Lamichhane, T.N.; Sokic, S.; Liyasova, M.S.; O’nEill, M.; et al. Engineered multivalency enhances affibody-based HER3 inhibition and downregulation in cancer cells. Mol. Pharm. 2017, 14, 1047–1056. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, L.; Zhao, W.; Xu, L.; Chen, Y.; Long, J.; Wang, F.; Wang, L.; Yang, Z. Supramolecular Nanofibers of Drug-Peptide Amphiphile and Affibody Suppress HER2+ Tumor Growth. Adv. Healthc. Mater. 2018, 7, 1800899. [Google Scholar] [CrossRef]
- Radford, D.C.; Yang, J.; Doan, M.C.; Li, L.; Dixon, A.S.; Owen, S.C.; Kopeček, J. Multivalent HER2-binding polymer conjugates facilitate rapid endocytosis and enhance intracellular drug delivery. J. Control. Release 2020, 319, 285–299. [Google Scholar] [CrossRef]
- Xia, X.; Yang, X.; Huang, W.; Xia, X.; Yan, D. Self-assembled nanomicelles of affibody-drug conjugate with excellent therapeutic property to cure ovary and breast cancers. Nano-Micro Lett. 2022, 14, 33. [Google Scholar] [CrossRef]
- Xia, X.; Yang, X.; Gao, W.; Huang, W.; Xia, X.; Yan, D. A novel HER2 targeting nanoagent self-assembled from affibody-epothilone B conjugate for cancer therapy. J. Nanobiotechnology 2024, 22, 502. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xia, X.; Li, Q.; Zhao, M.; Gao, W.; Xia, X.-X.; Huang, W.; Yan, D. An Affibody-Cytotoxin Conjugate-Based Nanoagent for EGFR Targeting and Precise Therapy of Epidermal Squamous Cell Carcinoma. ACS Appl. Nano Mater. 2025, 8, 2826–2835. [Google Scholar] [CrossRef]
- Talreja, S.; Tiwari, S. Supramolecular chemistry: Unveiling the fascinating world of non-covalent interactions and complex assemblies. J. Pharm. Pharmacol. Res. 2023, 7, 133–139. [Google Scholar] [CrossRef]
- Fu, R.; Carroll, L.; Yahioglu, G.; Aboagye, E.O.; Miller, P.W. Antibody fragment and affibody immunoPET imaging agents: Radiolabelling strategies and applications. ChemMedChem 2018, 13, 2466–2478. [Google Scholar] [CrossRef]
- Persson, J.; Puuvuori, E.; Zhang, B.; Velikyan, I.; Åberg, O.; Müller, M.; Nygren, P.; Ståhl, S.; Korsgren, O.; Eriksson, O.; et al. Discovery, optimization and biodistribution of an Affibody molecule for imaging of CD69. Sci. Rep. 2021, 11, 19151. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, K.; Qi, S.; Liu, H.; Jiang, Y.; Jiang, H.; Li, J.; Chen, K.; Zhang, H.; Cheng, Z. Affibody modified and radiolabeled gold–iron oxide hetero-nanostructures for tumor PET, optical and MR imaging. Biomaterials 2013, 34, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Mimura, W.; Suffian, I.F.M.; Amino, T.; Ishii, J.; Ogino, C.; Kondo, A. Granting specificity for breast cancer cells using a hepatitis B core particle with a HER2-targeted affibody molecule. J. Biochem. 2013, 153, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, X.; Xia, X.; Xia, X.-X.; Yan, D. Affibody-Functionalized Elastin-like Peptide–Drug Conjugate Nanomicelle for Targeted Ovarian Cancer Therapy. Biomacromolecules 2024, 25, 6474–6484. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Y.; Chen, X.; Chen, L.; Zhang, X.; Cui, D.; Li, Y.; Liu, Z.; Zhao, Q.; Diao, A. Development of active affibody aggregates induced by a self-assembling peptide for high sensitive detection of alpha-fetoprotein. Chem. Eng. J. 2022, 436, 135208. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, S.; Zhang, F.; Han, M.; Wang, X.; Du, J.; Zhang, H.; Li, W. Affibody modified G-quadruplex DNA micelles incorporating polymeric 5-Fluorodeoxyuridine for targeted delivery of curcumin to enhance synergetic therapy of HER2 positive gastric cancer. Nanomaterials 2022, 12, 696. [Google Scholar] [CrossRef]
- Zhang, F.; Yin, J.; Zhang, C.; Han, M.; Wang, X.; Fu, S.; Du, J.; Zhang, H.; Li, W. Affibody-conjugated RALA polymers delivering oligomeric 5-fluorodeoxyuridine for targeted therapy of HER2 overexpressing gastric cancer. Macromol. Biosci. 2020, 20, 2000083. [Google Scholar] [CrossRef]
- Teal, C.J.; Hettiaratchi, M.H.; Ho, M.T.; Ortin-Martinez, A.; Ganesh, A.N.; Pickering, A.J.; Golinski, A.W.; Hackel, B.J.; Wallace, V.A.; Shoichet, M.S. Directed evolution enables simultaneous controlled release of multiple therapeutic proteins from biopolymer-based hydrogels. Adv. Mater. 2022, 34, 2202612. [Google Scholar] [CrossRef]
- Justino, C.I.; Duarte, A.C.; Rocha-Santos, T.A. Analytical applications of affibodies. TrAC Trends Anal. Chem. 2015, 65, 73–82. [Google Scholar] [CrossRef]
- Abd Ellah, N.H.; Abouelmagd, S.A. Surface functionalization of polymeric nanoparticles for tumor drug delivery: Approaches and challenges. Expert Opin. Drug Deliv. 2017, 14, 201–214. [Google Scholar] [CrossRef]
- Distaffen, H.E.; Jones, C.W.; Abraham, B.L.; Nilsson, B.L. Multivalent display of chemical signals on self-assembled peptide scaffolds. Pept. Sci. 2021, 113, e24224. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Miao, Z.; Zavaleta, C.; Cheng, Z.; Gambhir, S.S. Affibody-functionalized gold–silica nanoparticles for Raman molecular imaging of the epidermal growth factor receptor. Small 2011, 7, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Park, H.; Choi, W.I.; Sung, D.; Lee, J.H.; Lee, K.Y.; Kim, S. Sensitive detection of dengue virus NS1 by highly stable affibody-functionalized gold nanoparticles. New J. Chem. 2018, 42, 12607–12614. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, N.; Qin, Y.; Wu, F.; Xu, Z.; Lan, T.; Cheng, Z.; Zhao, P.; Liu, H. Affibody-functionalized Ag2S quantum dots for photoacoustic imaging of epidermal growth factor receptor overexpressed tumors. Nanoscale 2018, 10, 16581–16590. [Google Scholar] [CrossRef]
- Al-Ani, A.W.; Zamberlan, F.; Ferreira, L.; Bradshaw, T.D.; Thomas, N.R.; Turyanska, L. Near-infrared PbS quantum dots functionalized with affibodies and ZnPP for targeted imaging and therapeutic applications. Nano Express 2021, 2, 040005. [Google Scholar] [CrossRef]
- Jun, H.; Jang, E.; Kim, H.; Yeo, M.; Park, S.G.; Lee, J.; Shin, K.J.; Chae, Y.C.; Kang, S.; Kim, E. TRAIL & EGFR affibody dual-display on a protein nanoparticle synergistically suppresses tumor growth. J. Control. Release 2022, 349, 367–378. [Google Scholar] [CrossRef]
- Kim, K.R.; Lee, A.S.; Kim, S.M.; Heo, H.R.; Kim, C.S. Virus-like nanoparticles as a theranostic platform for cancer. Front. Bioeng. Biotechnol. 2023, 10, 1106767. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Belova, M.M.; Kotelnikova, P.A.; Shilova, O.N.; Mirkasymov, A.B.; Danilova, N.V.; Komedchikova, E.N.; Popovtzer, R.; Deyev, S.M.; Nikitin, M.P. Photothermal therapy with HER2-targeted silver nanoparticles leading to cancer remission. Pharmaceutics 2022, 14, 1013. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Sogomonyan, A.S.; Zelepukin, I.V.; Nikitin, M.P.; Deyev, S.M. PLGA Nanoparticles decorated with anti-HER2 affibody for targeted delivery and photoinduced cell death. Molecules 2021, 26, 3955. [Google Scholar] [CrossRef]
- Yang, H.; Cai, W.; Xu, L.; Lv, X.; Qiao, Y.; Li, P.; Wu, H.; Yang, Y.; Zhang, L.; Duan, Y. Nanobubble–Affibody: Novel ultrasound contrast agents for targeted molecular ultrasound imaging of tumor. Biomaterials 2015, 37, 279–288. [Google Scholar] [CrossRef]
- Cai, W.; Lv, W.; Meng, L.; Duan, Y.; Zhang, L. The combined effect of nanobubble-ir783-hpph-affibody complex and laser on her2-positive breast cancer. Int. J. Nanomed. 2023, 18, 339–351. [Google Scholar] [CrossRef]
- Kim, H.; Jin, S.; Choi, H.; Kang, M.; Park, S.G.; Jun, H.; Cho, H.; Kang, S. Target-switchable Gd (III)-DOTA/protein cage nanoparticle conjugates with multiple targeting affibody molecules as target selective T1 contrast agents for high-field MRI. J. Control. Release 2021, 335, 269–280. [Google Scholar] [CrossRef]
- Eom, S.; Jun, H.; Kim, E.; Min, D.; Kim, H.; Kang, S. Developing Porous Protein Cage Nanoparticles as Cargo-Loadable and Ligand-Displayable Modular Delivery Nanoplatforms. ACS Appl. Mater. Interfaces 2024, 16, 58464–58476. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Choi, E.; Jana, B.; Go, E.M.; Jin, E.; Jin, S.; Lee, J.; Bae, J.; Yang, G.; Kwak, S.K.; et al. Protein-precoated surface of metal-organic framework nanoparticles for targeted delivery. Small 2023, 19, 2300218. [Google Scholar] [CrossRef]
- Yu, L.; Xu, Y.; Amin, A.; Jiang, S.; Sample, M.; Prasad, A.; Stephanopoulos, N.; Šulc, P.; Yan, H. CytoDirect: A nucleic acid nanodevice for specific and efficient delivery of functional payloads to the cytoplasm. J. Am. Chem. Soc. 2023, 145, 27336–27347. [Google Scholar] [CrossRef]
- Dorogin, J.; Hochstatter, H.B.; Shepherd, S.O.; Svendsen, J.E.; Benz, M.A.; Powers, A.C.; Fear, K.M.; Townsend, J.M.; Prell, J.S.; Hosseinzadeh, P.; et al. Moderate-Affinity affibodies modulate the delivery and bioactivity of bone morphogenetic Protein-2. Adv. Healthc. Mater. 2023, 12, 2300793. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wei, L.; Du, C.; Kam, A.; Loo, S. Recent Progress on Affibody-Based Supramolecular Architectures: Moving from Monomeric Constructs to Multivalent Assemblies. Pharmaceuticals 2025, 18, 1669. https://doi.org/10.3390/ph18111669
Wang H, Wei L, Du C, Kam A, Loo S. Recent Progress on Affibody-Based Supramolecular Architectures: Moving from Monomeric Constructs to Multivalent Assemblies. Pharmaceuticals. 2025; 18(11):1669. https://doi.org/10.3390/ph18111669
Chicago/Turabian StyleWang, Hongfei, Liqiang Wei, Chunyue Du, Antony Kam, and Shining Loo. 2025. "Recent Progress on Affibody-Based Supramolecular Architectures: Moving from Monomeric Constructs to Multivalent Assemblies" Pharmaceuticals 18, no. 11: 1669. https://doi.org/10.3390/ph18111669
APA StyleWang, H., Wei, L., Du, C., Kam, A., & Loo, S. (2025). Recent Progress on Affibody-Based Supramolecular Architectures: Moving from Monomeric Constructs to Multivalent Assemblies. Pharmaceuticals, 18(11), 1669. https://doi.org/10.3390/ph18111669








