Longitudinal Cohort Event Monitoring of MMR and DT-IPV Vaccination at 9 Years of Age in The Netherlands
Abstract
1. Introduction
- What types of AEFIs occur, and at what frequency?
- Are any serious AEFIs reported that require medical intervention?
- What is the time to onset (TTO) and time to recovery (TTR) of AEFIs?
- Are there identifiable risk factors associated with the occurrence of AEFIs?
2. Results
2.1. Intake Questionnaire and General Participant Characteristics
2.2. Characteristics of Participating Children and Their Household Situation
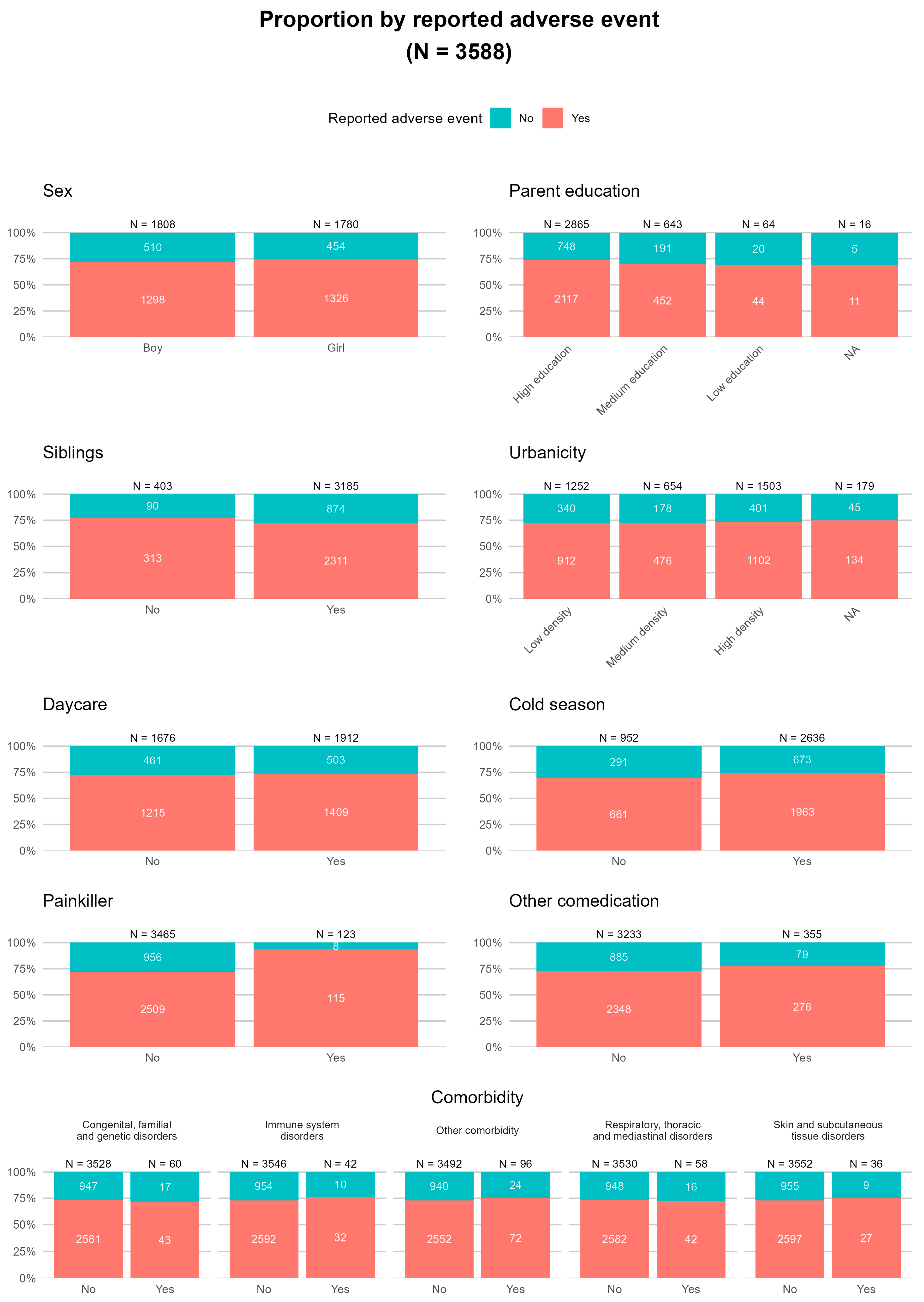
2.3. Reported Adverse Events Following Immunisation (AEFIs)
2.4. Consumption of Care
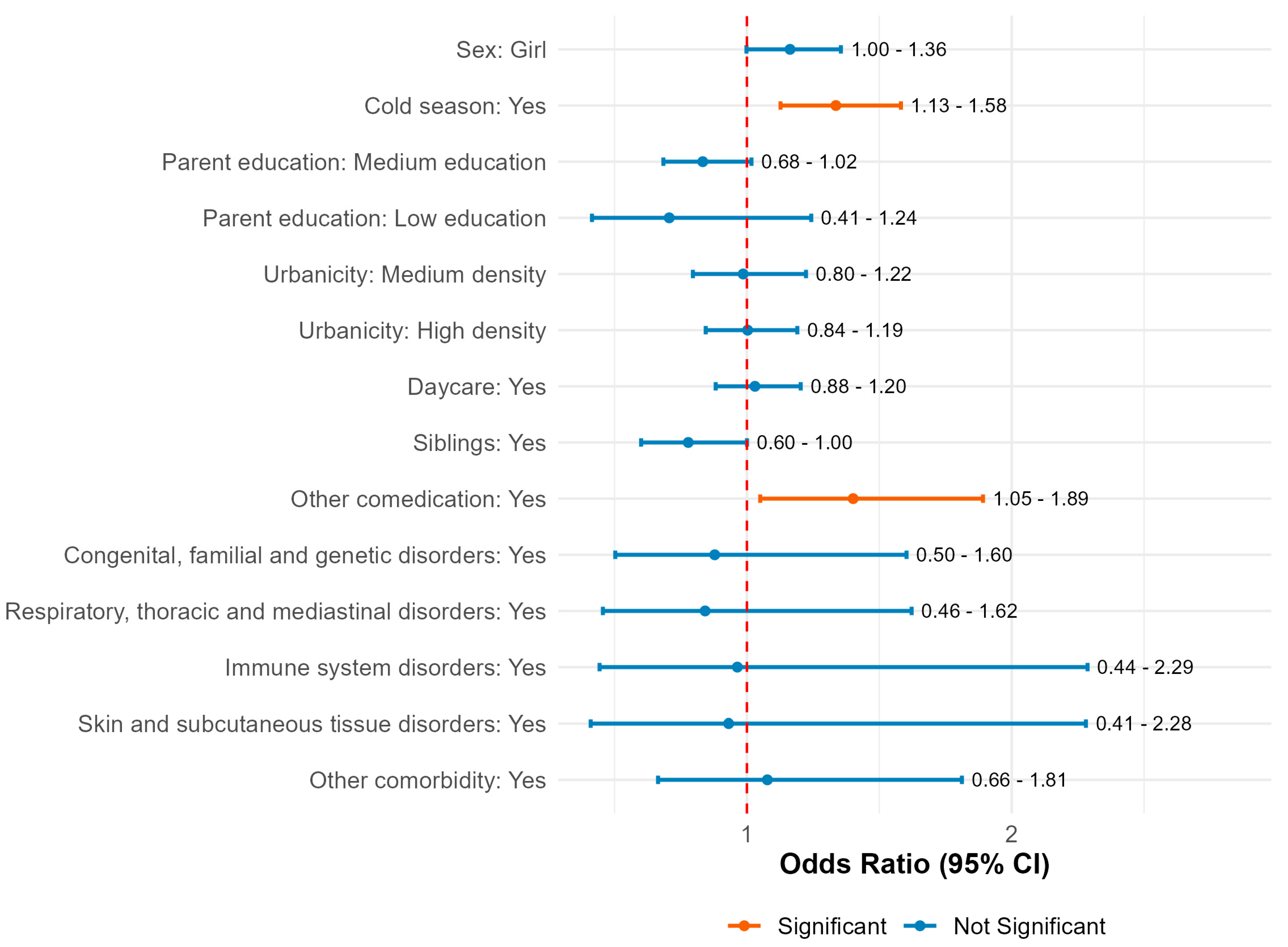
2.5. Prediction Model for Factors Contributing to Reported Adverse Events Following Immunisation (AEFIs)
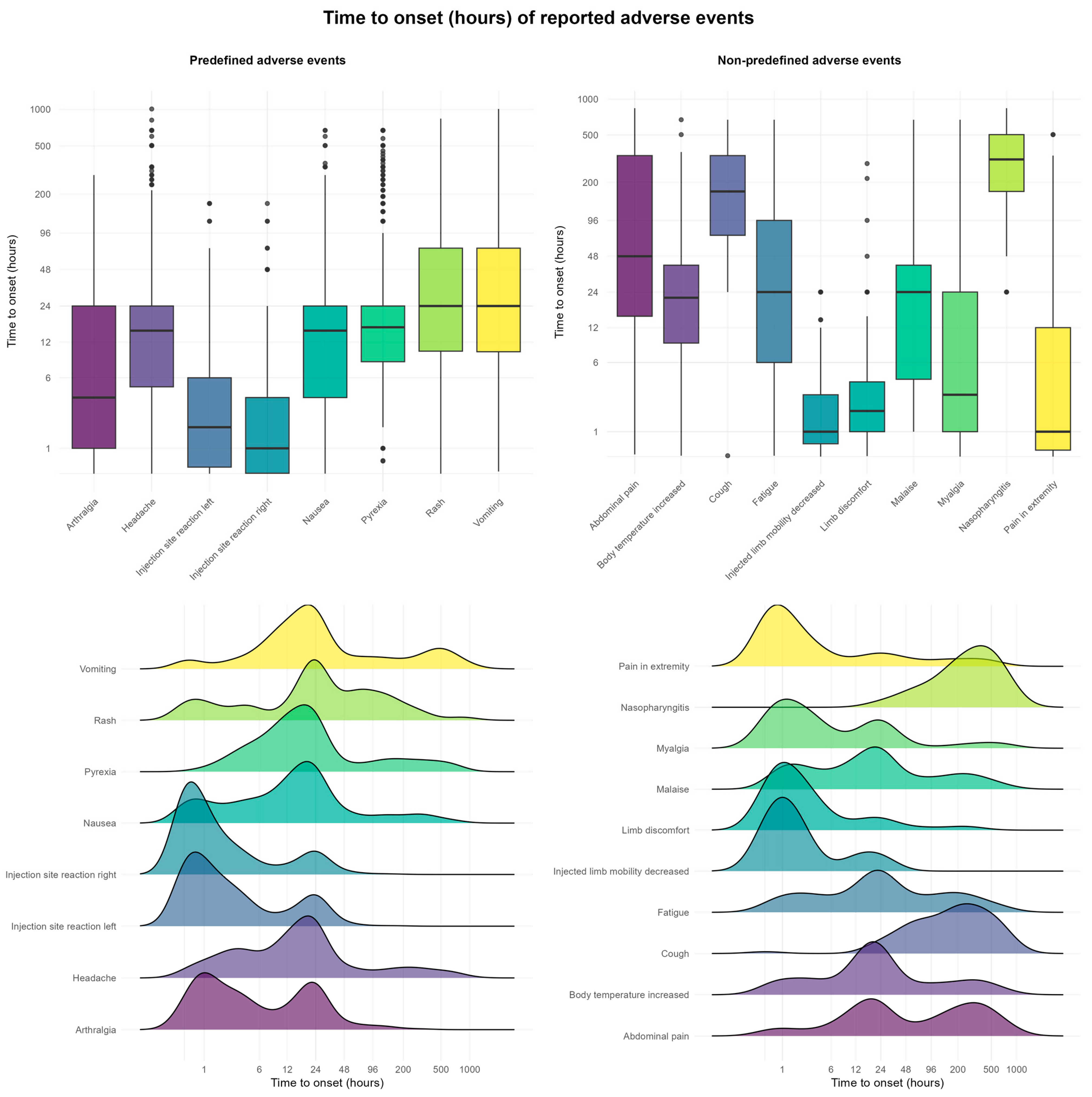
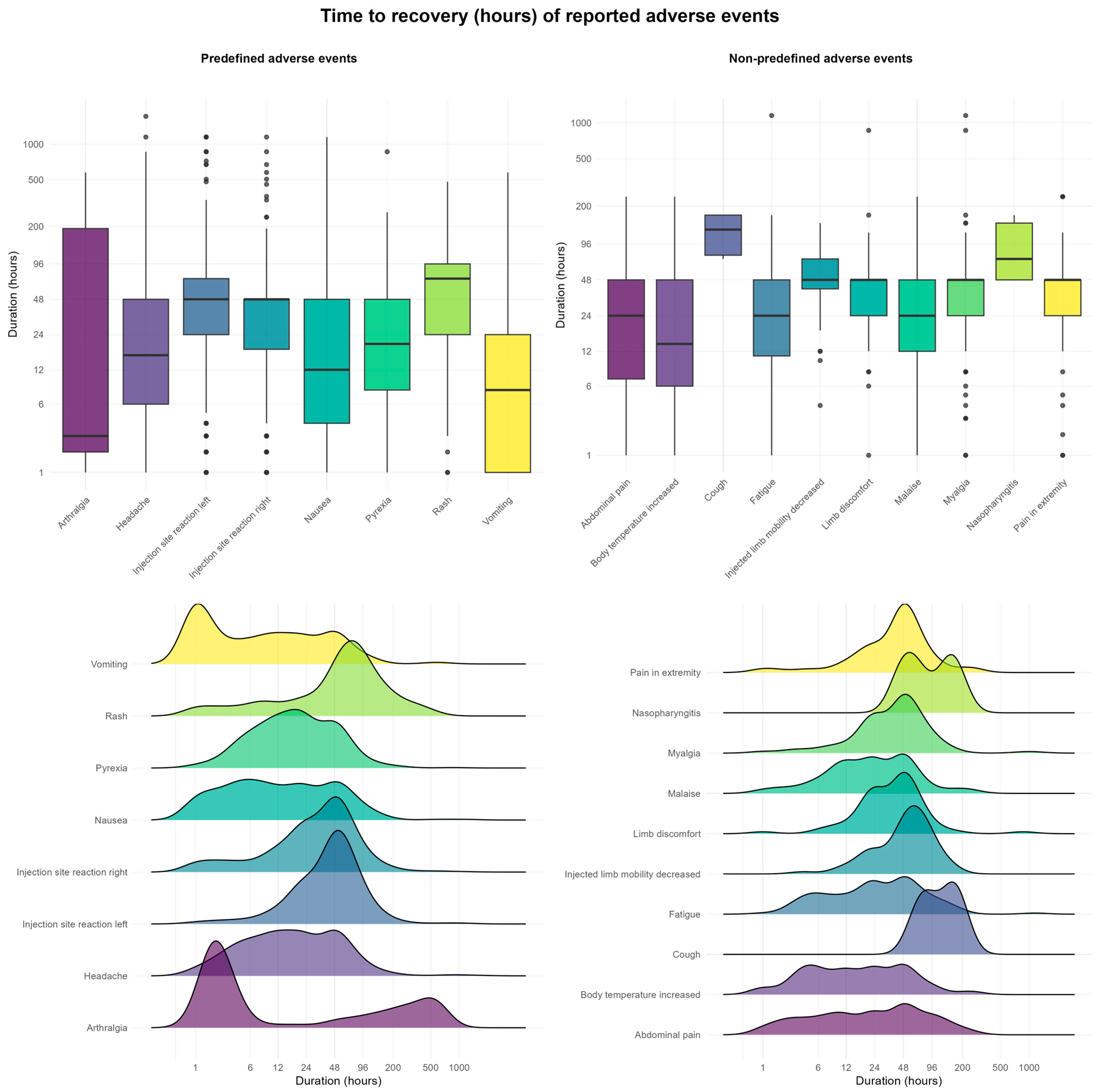
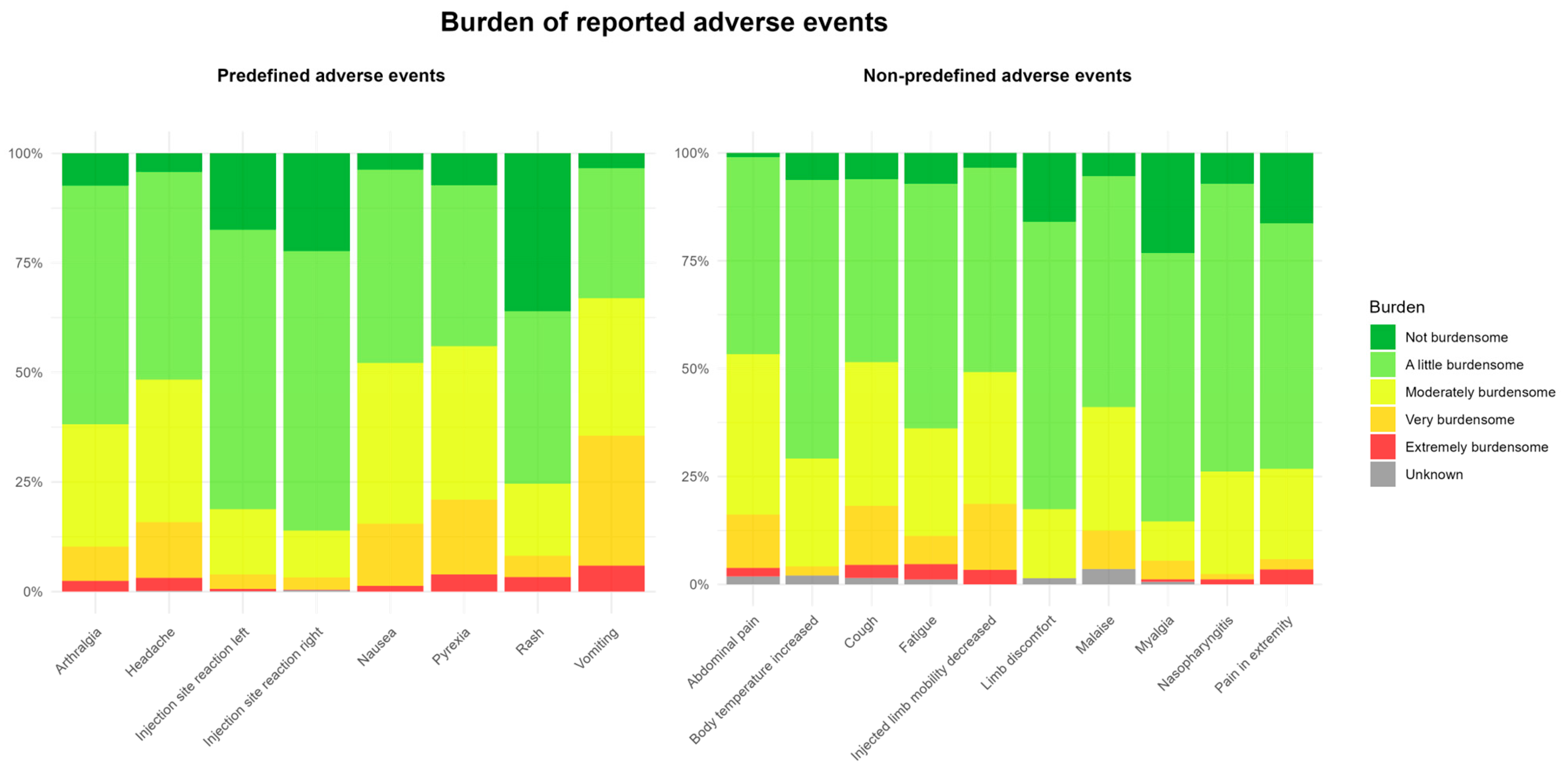
2.6. Time to Onset, Duration, and Burden of AEFIs
3. Discussion
3.1. Study Strengths and Limitations
3.2. Future Studies
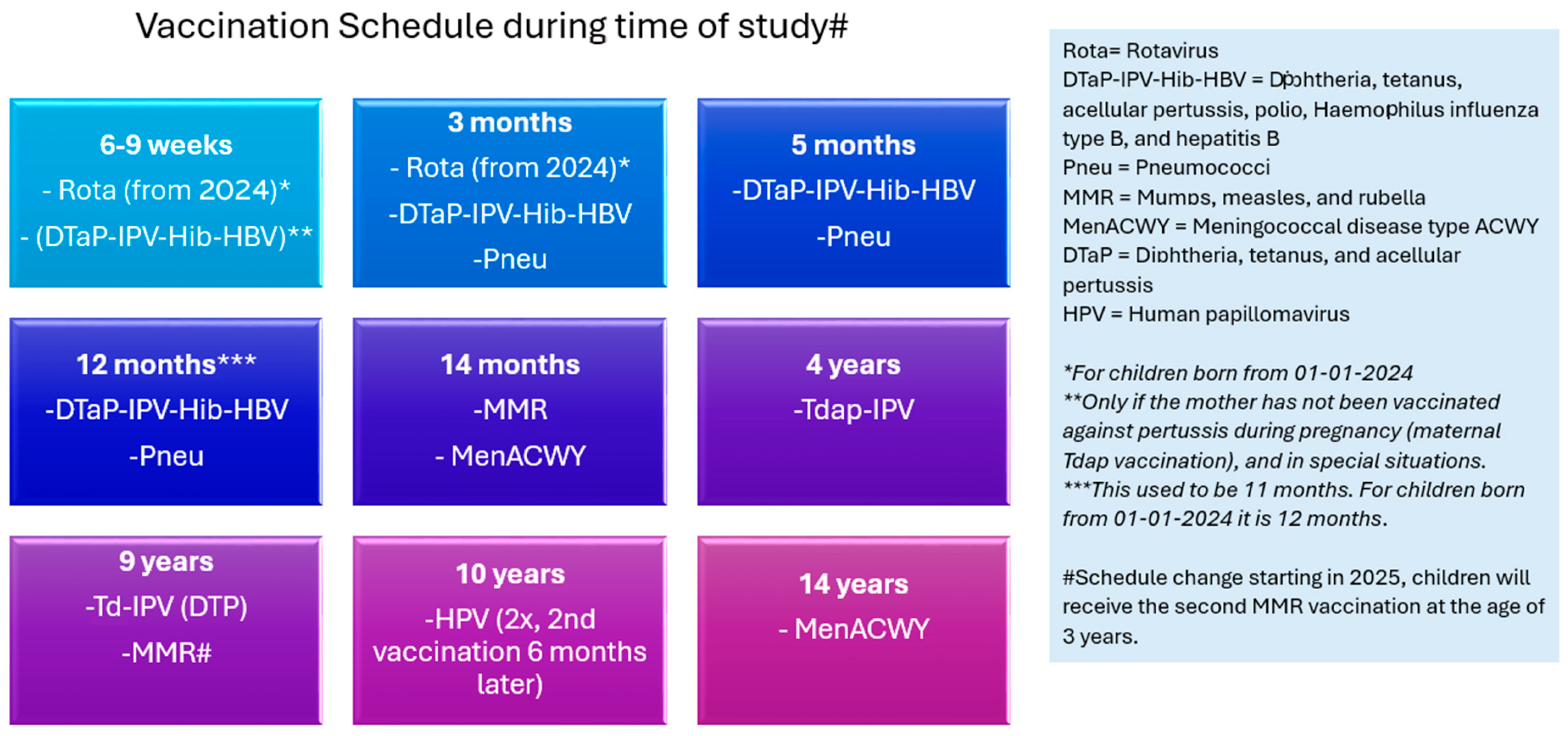
4. Materials and Methods
4.1. Registration and Intake Questionnaire
4.2. Questionnaire Timing
4.3. Questionnaire Coding
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEFI | Adverse event following immunisation |
| CEM | Cohort event monitoring |
| CIOMS | Council for International Organizations of Medical Sciences |
| DT-IPV | Diphtheria, pertussis, and inactivated poliomyelitis |
| Lareb | The Dutch Pharmacovigilance Centre |
| MedDRA | Medical Dictionary for Regulatory Activities |
| MMR | Measles, mumps, and rubella |
| NIP | National Immunisation Programme |
| PRO | Patient-reported outcome |
| RIVM | National Institute for Public Health and the Environment |
| TTO | Time to onset |
| TTR | Time to recovery |
References
- RIVM. Rijksvaccinatieprogramma. 2025. Available online: https://rijksvaccinatieprogramma.nl/ (accessed on 10 February 2025).
- van Wijhe, M.; McDonald, S.A.; de Melker, H.E.; Postma, M.J.; Wallinga, J. Effect of vaccination programmes on mortality burden among children and young adults in The Netherlands during the 20th century: A historical analysis. Lancet Infect. Dis. 2016, 16, 592–598. [Google Scholar] [CrossRef] [PubMed]
- van Wijhe, M.; McDonald, S.A.; de Melker, H.E.; Postma, M.J.; Wallinga, J. Estimating the Population-Level Effectiveness of Vaccination Programs in The Netherlands. Epidemiology 2018, 29, 215–223. [Google Scholar] [CrossRef] [PubMed]
- de Greeff, S.C.; de Melker, H.E.; Spanjaard, L.; van den Hof, S.; Dankert, J. The first effect of the national vaccination campaign against meningococcal-C disease: A rapid and sharp decrease in the number of patients. Ned. Tijdschr. Geneeskd. 2003, 147, 1132–1135. [Google Scholar] [PubMed]
- Bijlsma, M.W.; Brouwer, M.C.; Spanjaard, L.; van de Beek, D.; van der Ende, A. A decade of herd protection after introduction of meningococcal serogroup C conjugate vaccination. Clin. Infect. Dis. 2014, 59, 1216–1221. [Google Scholar] [CrossRef]
- Pluijmaekers, A.J.M.; Steens, A.; Houweling, H.; Rots, N.Y.; Benschop, K.S.M.; van Binnendijk, R.S.; Bodewes, R.; Brouwer, J.G.M.; Buisman, A.; Duizer, E.; et al. A literature review and evidence-based evaluation of the Dutch national immunisation schedule yield possibilities for improvements. Vaccine X 2024, 20, 100556. [Google Scholar] [CrossRef]
- RIVM. Rijksvaccinatie Programma—Nieuw Vaccinatieschema Vanaf 2025 per Geboortejaar. 2025. Available online: https://rijksvaccinatieprogramma.nl/nieuw-vaccinatieschema-2025 (accessed on 31 March 2025).
- Swedish Council on Health Technology A. SBU Systematic Review Summaries. In Vaccines to Children: Protective Effect and Adverse Events: A Systematic Review; Swedish Council on Health Technology Assessment (SBU): Stockholm, Sweden, 2009. [Google Scholar]
- Bonhoeffer, J.; Kohl, K.; Chen, R.T.; Duclos, P.; Heijbel, H.; Heininger, U. Standardized case definitions of adverse events following immunization (AEFI). Vaccine 2004, 22, 547–550. [Google Scholar] [CrossRef]
- Netherlands Pharmacovigilance Centre Lareb. About Lareb. 2025. Available online: https://www.lareb.nl/en/pages/about-lareb (accessed on 16 June 2025).
- Kemmeren, J.M.; van der Maas, N.A.; de Melker, H.E. Parental reports of adverse events following simultaneously given dT-IPV and MMR vaccines in healthy 9-year-old children. Eur. J. Pediatr. 2011, 170, 339–345. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). About V-Safe. 2025. Available online: https://www.cdc.gov/vaccine-safety-systems/v-safe/index.html#:~:text=Overview,COVID%2D19%20or%20RSV%20vaccine (accessed on 12 September 2025).
- Di Pietrantonj, C.; Rivetti, A.; Marchione, P.; Debalini, M.G.; Demicheli, V. Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Database Syst. Rev. 2020, 4, CD004407. [Google Scholar] [CrossRef]
- RIVM. Rijksvaccinatie Programma—DTP-Vaccinatie. 2025. Available online: https://rijksvaccinatieprogramma.nl/vaccinaties/dtp (accessed on 31 March 2025).
- RIVM. Rijksvaccinatie Programma—De BMR-Prik in Het Kort. 2025. Available online: https://rijksvaccinatieprogramma.nl/vaccinaties/bmr (accessed on 31 March 2025).
- van der Kooi, D.; van Balveren, L.; Boetzkes, S.; van Hunsel, F. Latentietijden van vermoede bijwerkingen na BMR-vaccinatie in de spontane meldingen van Bijwerkingencentrum Lareb. In JGZ Tijdschrift voor Jeugdgezondheidszorg; Springer: Berlin/Heidelberg, Germany, 2025; ISSN 1876-598X. [Google Scholar] [CrossRef]
- LeBaron, C.W.; Bi, D.; Sullivan, B.J.; Beck, C.; Gargiullo, P. Evaluation of potentially common adverse events associated with the first and second doses of measles-mumps-rubella vaccine. Pediatrics 2006, 118, 1422–1430. [Google Scholar] [CrossRef]
- Virtanen, M.; Peltola, H.; Paunio, M.; Heinonen, O.P. Day-to-day reactogenicity and the healthy vaccinee effect of measles-mumps-rubella vaccination. Pediatrics 2000, 106, E62. [Google Scholar] [CrossRef]
- Kuter, B.J.; Brown, M.; Wiedmann, R.T.; Hartzel, J.; Musey, L. Safety and Immunogenicity of M-M-RII (Combination Measles-Mumps-Rubella Vaccine) in Clinical Trials of Healthy Children Conducted Between 1988 and 2009. Pediatr. Infect. Dis. J. 2016, 35, 1011–1020. [Google Scholar] [CrossRef]
- Kant, A.; van Hunsel, F. Authors’ Reply to Mungmunpuntipantip et al.’s Comment on “Description of Frequencies of Reported Adverse Events Following Immunization Among Four Different COVID-19 Vaccine Brands”. Drug Saf. 2022, 45, 925–926. [Google Scholar] [CrossRef]
- Colloca, L.; Miller, F.G. The nocebo effect and its relevance for clinical practice. Psychosom. Med. 2011, 73, 598–603. [Google Scholar] [CrossRef]
- Raethke, M.; Gorter, J.; Kalf, R.; van Balveren, L.; Jajou, R.; van Hunsel, F. Frequency, Timing, Burden and Recurrence of Adverse Events Following Immunization After HPV Vaccine Based on a Cohort Event Monitoring Study in The Netherlands. Vaccines 2025, 13, 812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adab, N.; Jacoby, A.; Smith, D.; Chadwick, D. Additional educational needs in children born to mothers with epilepsy. J. Neurol. Neurosurg. Psychiatry 2001, 70, 15–21. [Google Scholar] [CrossRef]
- Harper, A.; Flanagan, K.L. Effect of sex on vaccination outcomes: Important but frequently overlooked. Curr. Opin. Pharmacol. 2018, 41, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Ducharme, R.; Ward, B.; Hawken, S. Increased emergency room visits or hospital admissions in females after 12-month MMR vaccination, but no difference after vaccinations given at a younger age. Vaccine 2014, 32, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Bartz, D.; Chitnis, T.; Kaiser, U.B.; Rich-Edwards, J.W.; Rexrode, K.M.; Pennell, P.B.; Goldstein, J.M.; O’Neal, M.A.; LeBoff, M.; Behn, M.; et al. Clinical Advances in Sex- and Gender-Informed Medicine to Improve the Health of All: A Review. JAMA Intern. Med. 2020, 180, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Woestenberg, P.J.; van Hunsel, F.; Maas, V.Y.F. Reply to Correspondence on “Comparison of Perceived Adverse Events After COVID-19 Vaccination Between Pregnant and NonPregnant Women Using Two Cohort Studies in The Netherlands”. Birth Defects Res. 2025, 117, e2511. [Google Scholar] [CrossRef]
- Statistics Netherlands. How Many Residents of The Netherlands Have a Non-Dutch Background? 2024. Available online: https://www.cbs.nl/en-gb/dossier/asylum-migration-and-integration/how-many-residents-of-the-netherlands-have-a-non-dutch-background-#:~:text=A%20total%20of%2016.2%20percent,in%20the%20Netherlands%20as%20immigrants (accessed on 17 October 2025).
- Statistics Netherlands. Wat Is Het Onderwijs-Niveau van Nederland? 2024. Available online: https://longreads.cbs.nl/nederland-in-cijfers-2024/wat-is-het-onderwijsniveau-van-nederland/ (accessed on 31 March 2025).
- RIVM. Klachten en Kwalen Bij Kinderen in Nederland—Omvang en Gevolgen Geïnventariseerd; Rijksinstituut voor Volksgezondheid en Miliei: Bilthoven, The Netherlands, 2010; Report No.: RIVM Rapport 260136001/2010. [Google Scholar]
- Shrank, W.H.; Patrick, A.R.; Brookhart, M.A. Healthy user and related biases in observational studies of preventive interventions: A primer for physicians. J. Gen. Intern. Med. 2011, 26, 546–550. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Causality assessment of an adverse event following immunization (AEFI). In User Manual for the Revised WHO Classification; 2019 Update; World Health Organization (WHO): Geneva, Switzerland, 2019. [Google Scholar]
- Harmark, L. Web-Based Intensive Monitoring; A Patient Based Pharmacovigilance Tool: Rijksuniversiteit Groningen. 2012. Available online: https://www.rug.nl/news/2012/06/22_harmark?lang=en (accessed on 14 July 2025).
- RIVM. Rijksvaccinatie Programma—Onderbouwing en Wijzigingen in Historisch Overzicht. 2025. Available online: https://rijksvaccinatieprogramma.nl/professionals/richtlijnen/uitvoering/3-onderbouwing-en-wijzigingen (accessed on 31 March 2025).
- The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Welcome to MedDRA. 2021. Available online: https://www.meddra.org/ (accessed on 14 July 2025).
- KNMP. The G-Standaard: The Medicines Standard in Healthcare. 2025. Available online: https://www.knmp.nl/over-de-knmp/producten-en-diensten/wat-is-de-g-standaard (accessed on 12 September 2025).
- CIOMS Working Group VIII. Practical Aspects of Signal Detection in Pharmacovigilance. In Report of CIOMS Working Group VIII; CIOMS Working Group VIII: Geneva, Switzerland, 2010; Report No.: 9290360828. [Google Scholar]
- RIVM. Vaccinatietechniek. 2025. Available online: https://rijksvaccinatieprogramma.nl/professionals/richtlijnen/uitvoering/9-vaccinatietechniek (accessed on 15 July 2025).
- Statistics Netherlands. Opleidingsniveau. 2025. Available online: https://www.cbs.nl/nl-nl/nieuws/2023/48/hoge-tevredenheid-ondanks-lage-materiele-welvaart-in-caribisch-nederland/opleidingsniveau (accessed on 12 September 2025).
- Statistics Netherlands. Microdata: Zelf Onderzoek Doen. 2025. Available online: https://www.cbs.nl/nl-nl/onze-diensten/maatwerk-en-microdata/microdata-zelf-onderzoek-doen (accessed on 12 September 2025).
- Senaviratna, N.A.M.R.; Cooray, T.M.J.A. Diagnosing Multicollinearity of Logistic Regression Model. Asian J. Probab. Stat. 2019, 5, 1–9. [Google Scholar] [CrossRef]
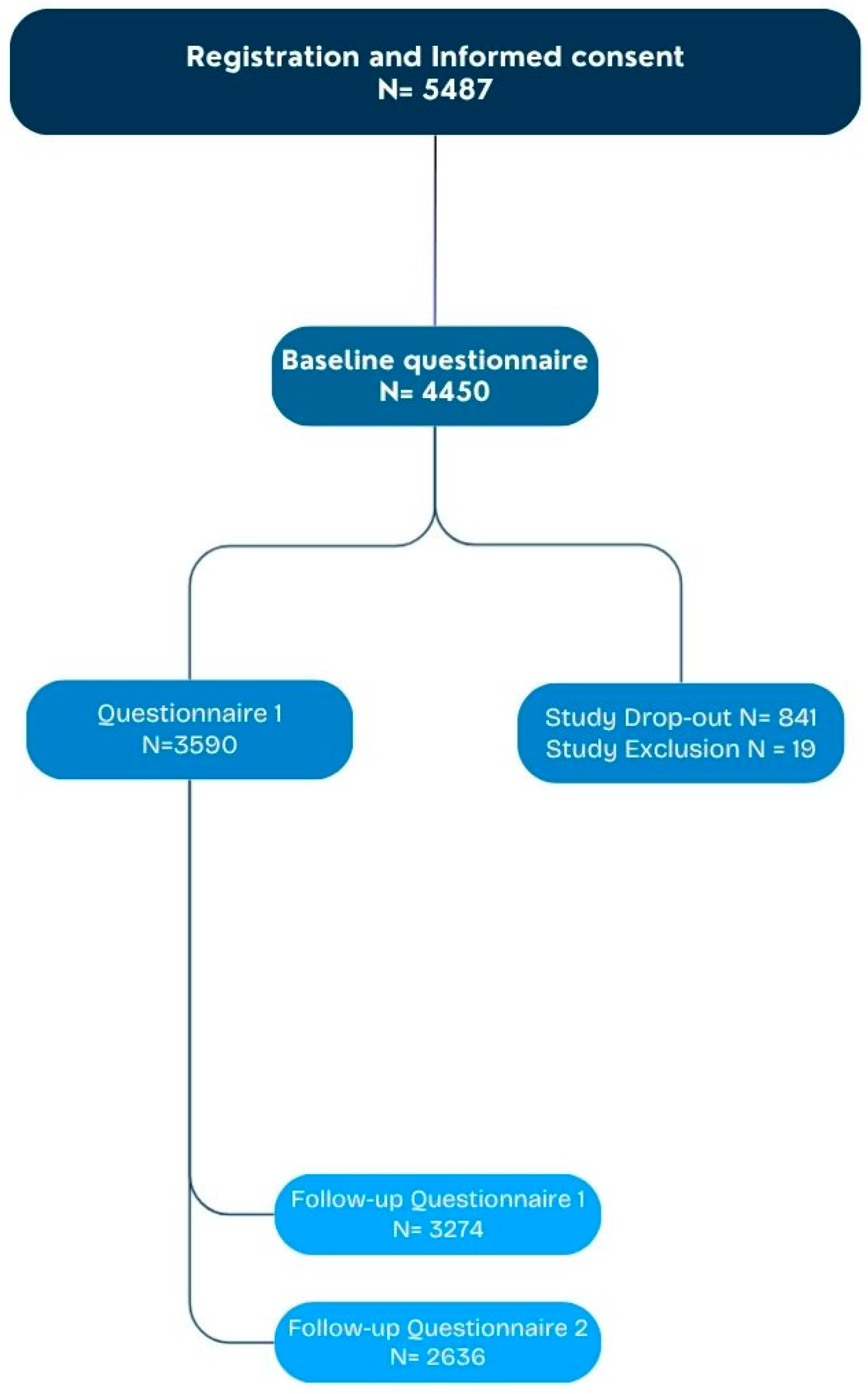
| Characteristics of Participants (N = 3590 Questionnaire 1) | |
|---|---|
| Questionnaires filled in by unique participants, n (%) | |
| Baseline questionnaire | 3590 (100) |
| First questionnaire (7 days after vaccination) | 3274 (91.2) |
| Follow-up questionnaire (14 days after vaccination) | 2636 (73.4) |
| Sex, n (%) | |
| Boy | 1808 (50.4) |
| Girl | 1780 (49.6) |
| Missing | 2 (0.1) |
| Birth cohort, n (%) | |
| 2013 | 756 (21.1) |
| 2014 | 2730 (76.0) |
| 2015 | 104 (2.9) |
| Existing medical conditions (MedDRA System Organ Class level), n (%) | |
| Congenital, familial, and genetic disorders | 60 (1.7) |
| Respiratory, thoracic, and mediastinal disorders | 58 (1.6) |
| Immune system disorders | 42 (1.2) |
| Skin and subcutaneous disorders | 36 (1.0) |
| Psychiatric disorders | 20 (0.6) |
| Nervous system disorders | 18 (0.5) |
| Gastrointestinal disorders | 16 (0.4) |
| Cardiac disorders | 11 (0.3) |
| Eye disorders | 6 (0.2) |
| Metabolism and nutrition disorders | 6 (0.2) |
| Musculoskeletal and connective tissue disorders | 5 (0.1) |
| Renal and urinary disorders | 5 (0.1) |
| Ear and labyrinth disorders | 4 (0.1) |
| Blood and lymphatic system disorders | 3 (0.1) |
| Endocrine disorders | 2 (0.1) |
| Vascular disorders | 1 (0.0) |
| General disorders and administration site conditions | 1 (0.0) |
| Infections and infestations | 1 (0.0) |
| Investigations | 1 (0.0) |
| Neoplasms—benign, malignant, and unspecified (incl. cysts and polyps) | 1 (0.0) |
| Surgical and medical procedures | 1 (0.0) |
| Reported AEFIs | Total Participants N = 3590 | |
|---|---|---|
| At least one AEFI | 2625 (73.1) | |
| At least one predefined AEFI | 2322 (64.7) | |
| At least one other AEFI | 969 (27.0) | |
| At least one serious AEFI | 4 (0.1) | |
| Predefined AEFIs (MedDRA PT) | ||
| Injection site reaction (grouped per child) | 1872 (52.1) | |
| Headache | 646 (18.0) | |
| Pyrexia | 495 (13.8) | |
| Nausea | 293 (8.2) | |
| Arthralgia | 244 (6.8) | |
| Vomiting | 117 (3.3) | |
| Rash | 60 (1.7) | |
| Top 10 Non-Predefined AEFIs (MedDRA PT) | ||
| Fatigue | 166 (4.6) | |
| Myalgia | 162 (4.5) | |
| Abdominal pain | 103 (2.9) | |
| Pain in limb | 85 (2.4) | |
| Naso-pharyngitis | 84 (2.3) | |
| Limb discomfort | 68 (1.9) | |
| Cough | 66 (1.8) | |
| Limb mobility decreased | 58 (1.6) | |
| Malaise | 56 (1.5) | |
| Body temperature increased | 48 (1.3) |
| Injection Site Reactions: MMR (Right Side) and DT-IPV (Left Side) (N = 3590) (n, %) | |
|---|---|
| Left | 1585 (44.2) * |
| Right | 843 (23.5) * |
| Unknown | 120 (3.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raethke, M.; Gorter, J.; Kalf, R.; Balveren, L.v.; Boetzkes, S.; Jajou, R.; van Hunsel, F. Longitudinal Cohort Event Monitoring of MMR and DT-IPV Vaccination at 9 Years of Age in The Netherlands. Pharmaceuticals 2025, 18, 1635. https://doi.org/10.3390/ph18111635
Raethke M, Gorter J, Kalf R, Balveren Lv, Boetzkes S, Jajou R, van Hunsel F. Longitudinal Cohort Event Monitoring of MMR and DT-IPV Vaccination at 9 Years of Age in The Netherlands. Pharmaceuticals. 2025; 18(11):1635. https://doi.org/10.3390/ph18111635
Chicago/Turabian StyleRaethke, Monika, Jeroen Gorter, Rachel Kalf, Leontine van Balveren, Sanne Boetzkes, Rana Jajou, and Florence van Hunsel. 2025. "Longitudinal Cohort Event Monitoring of MMR and DT-IPV Vaccination at 9 Years of Age in The Netherlands" Pharmaceuticals 18, no. 11: 1635. https://doi.org/10.3390/ph18111635
APA StyleRaethke, M., Gorter, J., Kalf, R., Balveren, L. v., Boetzkes, S., Jajou, R., & van Hunsel, F. (2025). Longitudinal Cohort Event Monitoring of MMR and DT-IPV Vaccination at 9 Years of Age in The Netherlands. Pharmaceuticals, 18(11), 1635. https://doi.org/10.3390/ph18111635





