Is Fentanyl Rebound an Intrinsic Feature of Naloxone Reversal?
Abstract
1. Introduction
2. Results
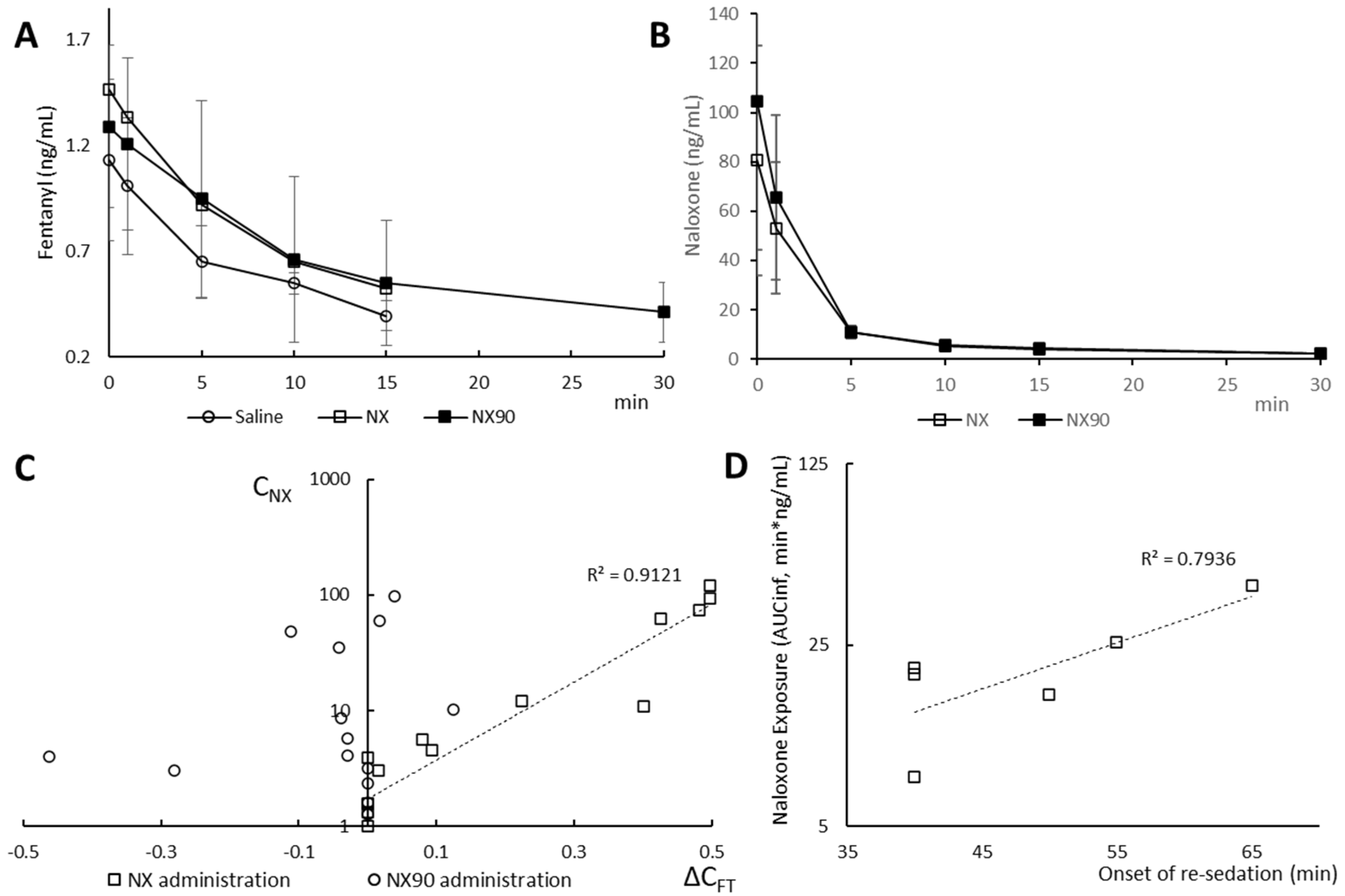
2.1. Pharmacokinetics
2.2. Clinical Follow-Up
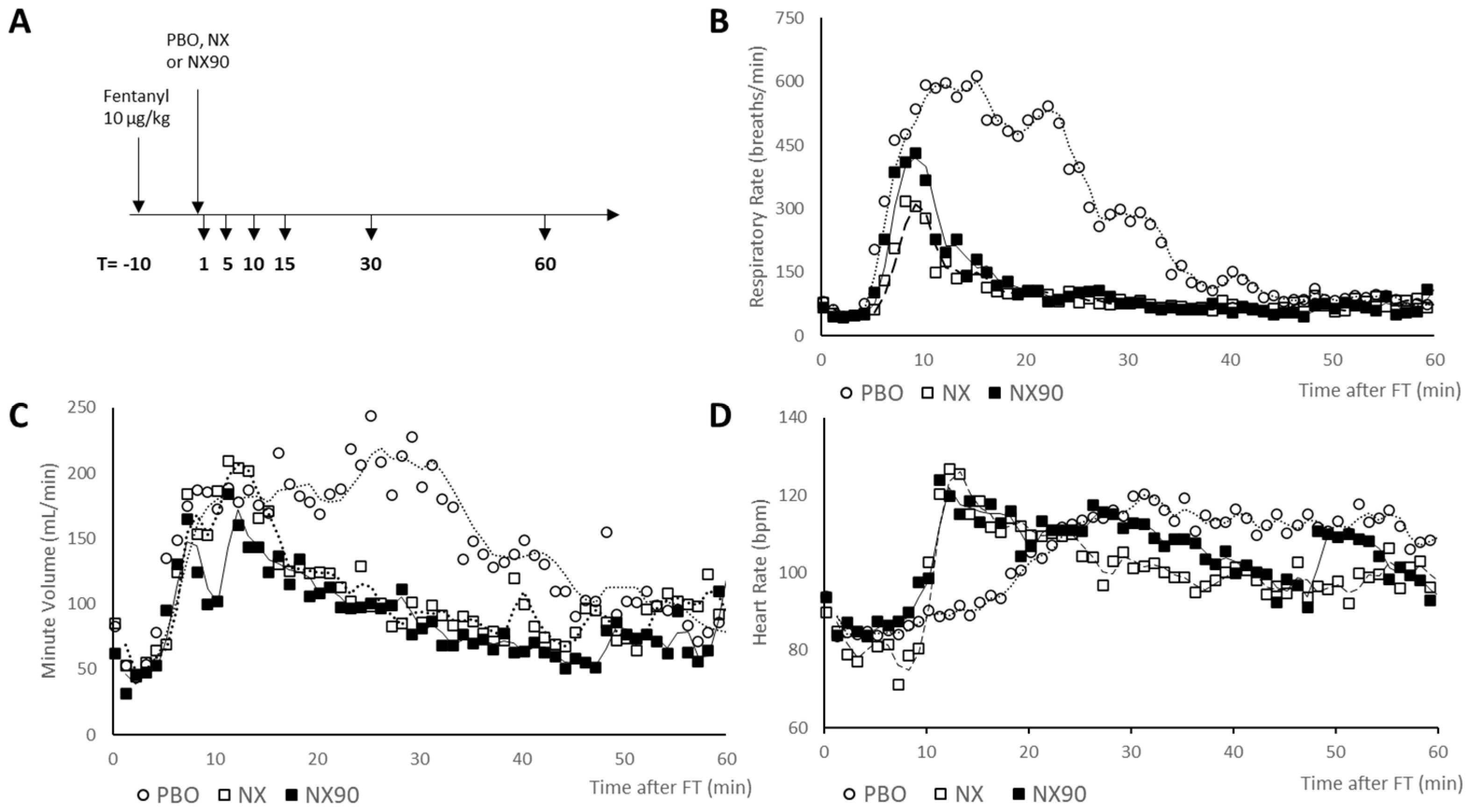
2.3. Respiratory Monitoring
2.4. EKG Monitoring
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Study Design
4.4. Pharmacokinetic Study
4.5. Experimental Procedure
4.6. Data Analysis
4.7. Statistical Analysis
5. Conclusions
- First in vivo evidence of naloxone-driven fentanyl redistribution
- Impact on Fentanyl Rebound
- Reduced Fentanyl Toxicity with NX90
- Future directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, E.; Sutcliffe, K.; Cavallo, D.; Ramos-Gonzalez, N.; Alhosan, N.; Henderson, G. The Anomalous Pharmacology of Fentanyl. Br. J. Pharmacol. 2023, 180, 797–812. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.J.; Webster, L.R.; Vortsman, E.; Ann LeQuang, J.; Raffa, R.B. Wooden Chest Syndrome: The Atypical Pharmacology of Fentanyl Overdose. J. Clin. Pharm. Ther. 2021, 46, 1505–1508. [Google Scholar] [CrossRef]
- Tschirhart, J.N.; Li, W.; Guo, J.; Zhang, S. Blockade of the Human Ether A-Go-Go–Related Gene (HERG) Potassium Channel by Fentanyl. Mol. Pharmacol. 2019, 95, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.; Escudero, C.; Carmona, J.A.; Silva, L.; Castillo-Olivares, J.L. Effect of Fentanyl on Cardiac Automaticity and Conduction: Direct or Mediated Action? Eur. Heart J. 1992, 13, 1277–1281. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Cisewski, J.A.; Rossen, L.M.; Sutton, P. Provisional Drug Overdose Death Counts; National Center for Health Statistics: Hyattsville, MD, USA, 2024. Available online: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data (accessed on 9 November 2024).
- France, C.P.; Ahern, G.P.; Averick, S.; Disney, A.; Enright, H.A.; Esmaeli-Azad, B.; Federico, A.; Gerak, L.R.; Husbands, S.M.; Kolber, B.; et al. Countermeasures for Preventing and Treating Opioid Overdose. Clin. Pharmacol. Ther. 2021, 109, 578–590. [Google Scholar] [CrossRef]
- Zuurmond, W.W.; Meert, T.F.; Noorduin, H. Partial versus Full Agonists for Opioid-Mediated Analgesia--Focus on Fentanyl and Buprenorphine. Acta Anaesthesiol. Belg. 2002, 53, 193–201. [Google Scholar]
- Yassen, A.; Olofsen, E.; van Dorp, E.; Sarton, E.; Teppema, L.; Danhof, M.; Dahan, A. Mechanism-Based Pharmacokinetic-Pharmacodynamic Modelling of the Reversal of Buprenorphine-Induced Respiratory Depression by Naloxone. Clin. Pharmacokinet. 2007, 46, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Bisaga, A. What Should Clinicians Do as Fentanyl Replaces Heroin? Addiction 2019, 114, 782–783. [Google Scholar] [CrossRef]
- Bird, H.E.; Huhn, A.S.; Dunn, K.E. Fentanyl Absorption, Distribution, Metabolism, and Excretion: Narrative Review and Clinical Significance Related to Illicitly Manufactured Fentanyl. J. Addict. Med. 2023, 17, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Huhn, A.S.; Hobelmann, J.G.; Oyler, G.A.; Strain, E.C. Protracted Renal Clearance of Fentanyl in Persons with Opioid Use Disorder. Drug Alcohol Depend. 2020, 214, 108147. [Google Scholar] [CrossRef]
- Hammarlund-Udenaes, M.; Fridén, M.; Syvänen, S.; Gupta, A. On the Rate and Extent of Drug Delivery to the Brain. Pharm. Res. 2008, 25, 1737–1750. [Google Scholar] [CrossRef]
- Hossain, J. Developing a PBPK Model for Intranasal Naloxone and Opioid Displacement from Brain Receptors. Ph.D. Thesis, Long Island University, Brooklyn, NY, USA, 2024. [Google Scholar]
- Olkkola, K.T.; Palkama, V.J.; Neuvonen, P.J. Ritonavir’s Role in Reducing Fentanyl Clearance and Prolonging Its Half-Life. Anesthesiology 1999, 91, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Ziesenitz, V.C.; König, S.K.; Mahlke, N.S.; Skopp, G.; Haefeli, W.E.; Mikus, G. Pharmacokinetic Interaction of Intravenous Fentanyl with Ketoconazole. J. Clin. Pharmacol. 2015, 55, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Lister, N.; Warrington, S.; Boyce, M.; Eriksson, C.; Tamaoka, M.; Kilborn, J. Pharmacokinetics, Safety, and Tolerability of Ascending Doses of Sublingual Fentanyl, with and without Naltrexone, in Japanese Subjects. J. Clin. Pharmacol. 2011, 51, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Krotulski, A.J.; Chapman, B.P.; Marks, S.J.; Ontiveros, S.T.; Devin-Holcombe, K.; Fogarty, M.F.; Trieu, H.; Logan, B.K.; Merchant, R.C.; Babu, K.M. Sentanyl: A Comparison of Blood Fentanyl Concentrations and Naloxone Dosing after Non-Fatal Overdose. Clin. Toxicol. 2022, 60, 197–204. [Google Scholar] [CrossRef]
- Takahashi, M.; Sugiyama, K.; Hori, M.; Chiba, S.; Kusaka, K. Naloxone Reversal of Opioid Anesthesia Revisited: Clinical Evaluation and Plasma Concentration Analysis of Continuous Naloxone Infusion after Anesthesia with High-Dose Fentanyl. J. Anesth. 2004, 18, 1–8. [Google Scholar] [CrossRef]
- Curay, C.M.; Irwin, M.R.; Kiyatkin, E.A. The Pattern of Brain Oxygen Response Induced by Intravenous Fentanyl Limits the Time Window of Therapeutic Efficacy of Naloxone. Neuropharmacology 2023, 231, 109507. [Google Scholar] [CrossRef]
- Moore, P.G.; Quail, A.W.; Cottee, D.B.; McIlveen, S.A.; White, S.W. Effect of Fentanyl on Baroreflex Control of Circumflex Coronary Conductance. Clin. Exp. Pharmacol. Physiol. 2000, 27, 1028–1033. [Google Scholar] [CrossRef]
- Chang, S.W.; Voelkel, N.F. Actions of Opiate Agonists, Naloxone, and Paraben Preservatives in the Rat Lung Circulation. Proc. Soc. Exp. Biol. Med. Soc. 1986, 181, 404–410. [Google Scholar] [CrossRef]
- Scott, P.J.H.; Koeppe, R.A.; Shao, X.; Rodnick, M.E.; Sowa, A.R.; Henderson, B.D.; Stauff, J.; Sherman, P.S.; Arteaga, J.; Carlo, D.J.; et al. The Effects of Intramuscular Naloxone Dose on Mu Receptor Displacement of Carfentanil in Rhesus Monkeys. Molecules 2020, 25, 1360. [Google Scholar] [CrossRef]
- Voronkov, M.; Nikonov, G.; Ataiants, J.; Isakulyan, L.; Stefanut, C.; Cernea, M.; Abernethy, J. Modifying Naloxone to Reverse Fentanyl-Induced Overdose. Int. J. Pharm. 2022, 611, 121326. [Google Scholar] [CrossRef]
- Marucio, R.L.; Monteiro, E.R.; Moroz, L.R.; Fantoni, D.T. Postoperative Analgesic Effects of Epidural Administration of Neostigmine Alone or in Combination with Morphine in Dogs Undergoing Orthopedic Surgery of the Pelvic Limbs. Am. J. Vet. Res. 2014, 75, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.A.; Haughan, J.; Gianotti, G.; Varner, K.; Drobatz, K.J.; Stefanovski, D.; Robinson, M.; Pennington, M.; McGuire, A.; Otto, C.M. Pharmacokinetics and Pharmacodynamics of Intranasal and Intramuscular Administration of Naloxone in Working Dogs Administered Fentanyl. J. Vet. Intern. Med. 2023, 37, 2422–2428. [Google Scholar] [CrossRef] [PubMed]
- Mills, T.; Robinson, M.A.; Barr, C.; Stefanovski, D.; You, Y.; Proctor, R.; Seizova, K.; Mallikarjun, A.; Otto, C.M. A Randomized, Blinded, Placebo-Controlled Crossover Study of the Pharmacokinetics and Pharmacodynamics of Naloxone, Naltrexone, and Nalmefene in Methadone-Sedated Working Dogs. J. Vet. Pharmacol. Ther. 2025, 48, 359–367. [Google Scholar] [CrossRef]
- Baehr, C.A.; Wu, M.M.; Pandit, S.G.; Arias-Umana, J.; AuCoin, D.; Pravetoni, M. Pharmacological Profiling of Antifentanyl Monoclonal Antibodies in Combination with Naloxone in Pre- and Postexposure Models of Fentanyl Toxicity. J. Pharmacol. Exp. Ther. 2022, 381, 129–136. [Google Scholar] [CrossRef]
- Bremer, P.T.; Burke, E.L.; Barrett, A.C.; Desai, R.I. Investigation of Monoclonal Antibody CSX-1004 for Fentanyl Overdose. Nat. Commun. 2023, 14, 7700. [Google Scholar] [CrossRef]
- Li, X.; Zakin, M.; Shetty, C.; Oliver, P.; Wallach, D. Sequestration Compounds for Treatment of Substance Use Disorder and Uses Thereof. US17/669,122, 4 August 2022. Available online: https://lens.org/039-781-970-247-873 (accessed on 9 November 2024).
- Robinson, T.M.; Kruse-Elliott, K.T.; Markel, M.D. A Comparison of Transdermal Fentanyl versus Epidural Morphine for Analgesia in Dogs Undergoing Major Orthopedic Surgery. J. Am. Anim. Hosp. Assoc. 1999, 35, 95–100. [Google Scholar] [CrossRef]
- Declaration of Transparency and Scientific Rigour: Revised Checklist for Animal Experimentation. Br. J. Pharmacol. 2020, 177, 4313. [CrossRef]
- Flacke, J.W.; Flacke, W.E.; Bloor, B.C.; Olewine, S. Effects of Fentanyl, Naloxone, and Clonidine on Hemodynamics and Plasma Catecholamine Levels in Dogs. Anesth. Analg. 1983, 62, 305–313. [Google Scholar] [CrossRef]
- Bosquez-Berger, T.; Gudorf, J.A.; Kuntz, C.P.; Desmond, J.A.; Schlebach, J.P.; VanNieuwenhze, M.S.; Straiker, A. Structure-Activity Relationship Study of Cannabidiol-Based Analogs as Negative Allosteric Modulators of the μ-Opioid Receptor. J. Med. Chem. 2023, 66, 9466–9494. [Google Scholar] [CrossRef]
- O’Brien, E.S.; Rangari, V.A.; El Daibani, A.; Eans, S.O.; Hammond, H.R.; White, E.; Wang, H.; Shiimura, Y.; Krishna Kumar, K.; Jiang, Q.; et al. A Μ-Opioid Receptor Modulator That Works Cooperatively with Naloxone. Nature 2024, 631, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Cernea, M.; Nikonov, G.; Ataiants, J.; Ştefănuţ, C.; Abernethy, J.; Voronkov, M. Nalbuphine Potentiates Reversal of Fentanyl Overdose by Naloxone. Pharmaceuticals 2024, 17, 866. [Google Scholar] [CrossRef] [PubMed]
| Intervention | t1/2 | C0 | AUClINF | MRT | Vss | CL |
|---|---|---|---|---|---|---|
| (min) | (ng/mL) | (min·ng/mL) | (min) | (L/kg) | (mL/min/kg) | |
| NaCl (0.9%, IV) | 14.3 ± 1.9 | 1.1 ± 0.4 | 17.3 ± 3.9 | 19.9 ± 1.8 | 11.8 ± 1.7 | 594.9 ± 134.9 |
| NX (0.12 µmol/kg, IV) | 13.0 ± 4.9 | 1.4 ± 0.4 | 21.7 ± 3.4 | 18.6 ± 7.2 | 8.4 ± 2.4 | 467.1 ± 67.4 |
| NX90 (0.12 µmol/kg, IV) | 11.2 ± 6.1 | 1.2 ± 0.4 | 22.1 ± 18.1 | 16.2 ± 8.8 | 8.7 ± 2.6 | 713.7 ± 531.7 |
| Intervention | t1/2 | C0 | AUCINF | MRT | Vss | CL |
|---|---|---|---|---|---|---|
| (min) | (ng/mL) | (min·ng/mL) | (min) | (L/kg) | (mL/min/kg) | |
| NX (0.12 µmol/kg, IV) | 22.3 ± 1.4 | 80.6 ± 46.8 | 389.4 ± 38.9 | 18.0 ± 8.1 | 1.9 ± 1.1 | 103.4 ± 10.9 |
| NX90 (0.12 µmol/kg, IV) | 21.5 ± 1.9 | 104.6 ± 60.3 | 439.3 ± 177.7 | 16.0 ± 2.3 | 2.1 ± 0.8 | 132.5 ± 44.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronkov, M.; Nikonov, G.; Uzbil, M.; Milevich, G.; Abernethy, J.; Barthélémy, I. Is Fentanyl Rebound an Intrinsic Feature of Naloxone Reversal? Pharmaceuticals 2025, 18, 1634. https://doi.org/10.3390/ph18111634
Voronkov M, Nikonov G, Uzbil M, Milevich G, Abernethy J, Barthélémy I. Is Fentanyl Rebound an Intrinsic Feature of Naloxone Reversal? Pharmaceuticals. 2025; 18(11):1634. https://doi.org/10.3390/ph18111634
Chicago/Turabian StyleVoronkov, Michael, Georgiy Nikonov, Melda Uzbil, George Milevich, John Abernethy, and Inès Barthélémy. 2025. "Is Fentanyl Rebound an Intrinsic Feature of Naloxone Reversal?" Pharmaceuticals 18, no. 11: 1634. https://doi.org/10.3390/ph18111634
APA StyleVoronkov, M., Nikonov, G., Uzbil, M., Milevich, G., Abernethy, J., & Barthélémy, I. (2025). Is Fentanyl Rebound an Intrinsic Feature of Naloxone Reversal? Pharmaceuticals, 18(11), 1634. https://doi.org/10.3390/ph18111634






