Treosulfan-Based Conditioning Regimens for Allogeneic Hematopoietic Cell Transplantation in Acute Myeloid Leukemia and Other Myeloid Malignancies
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Engraftment, Chimerism, and Early Toxicity
2.3. Graft-Versus-Host Disease
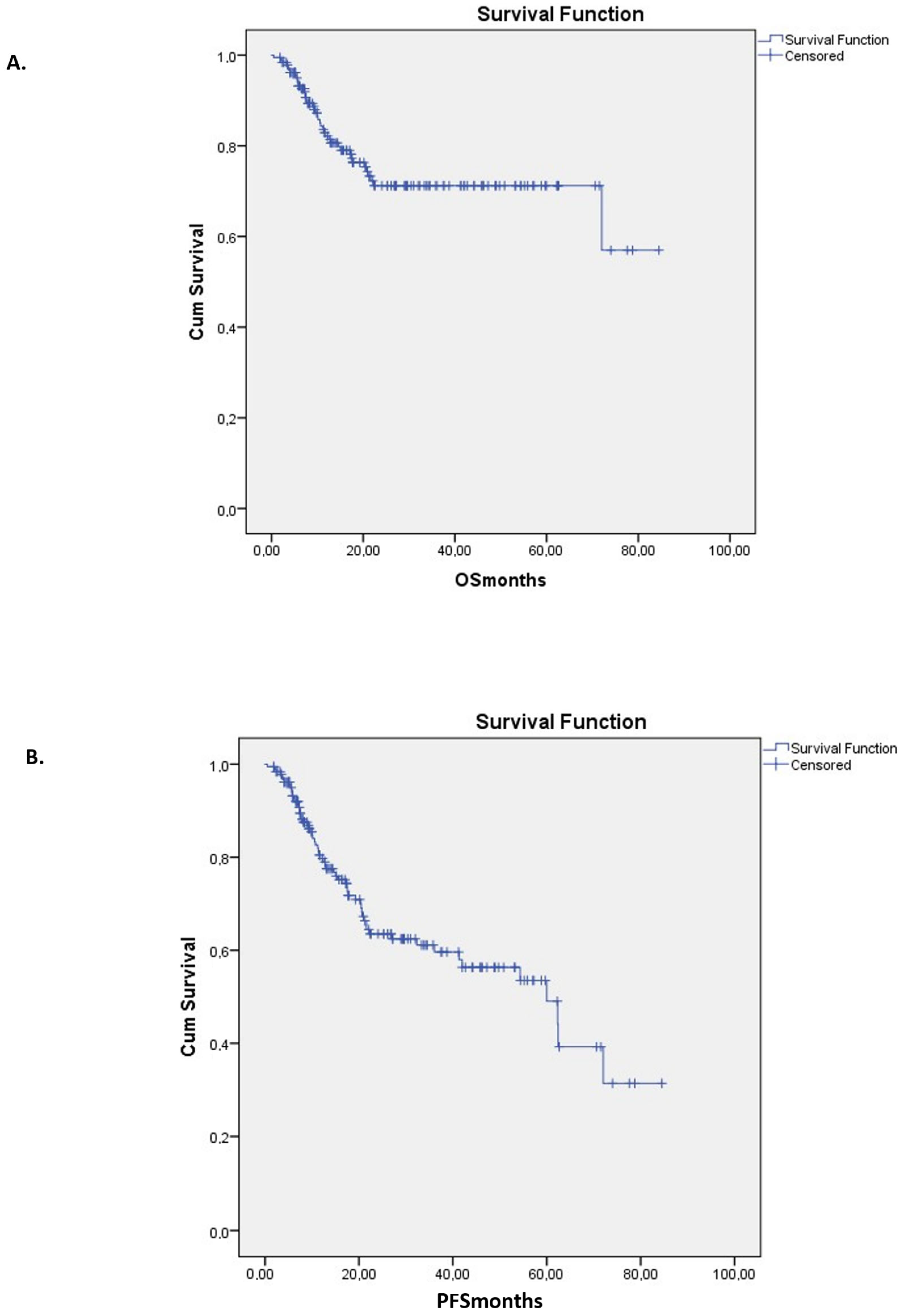
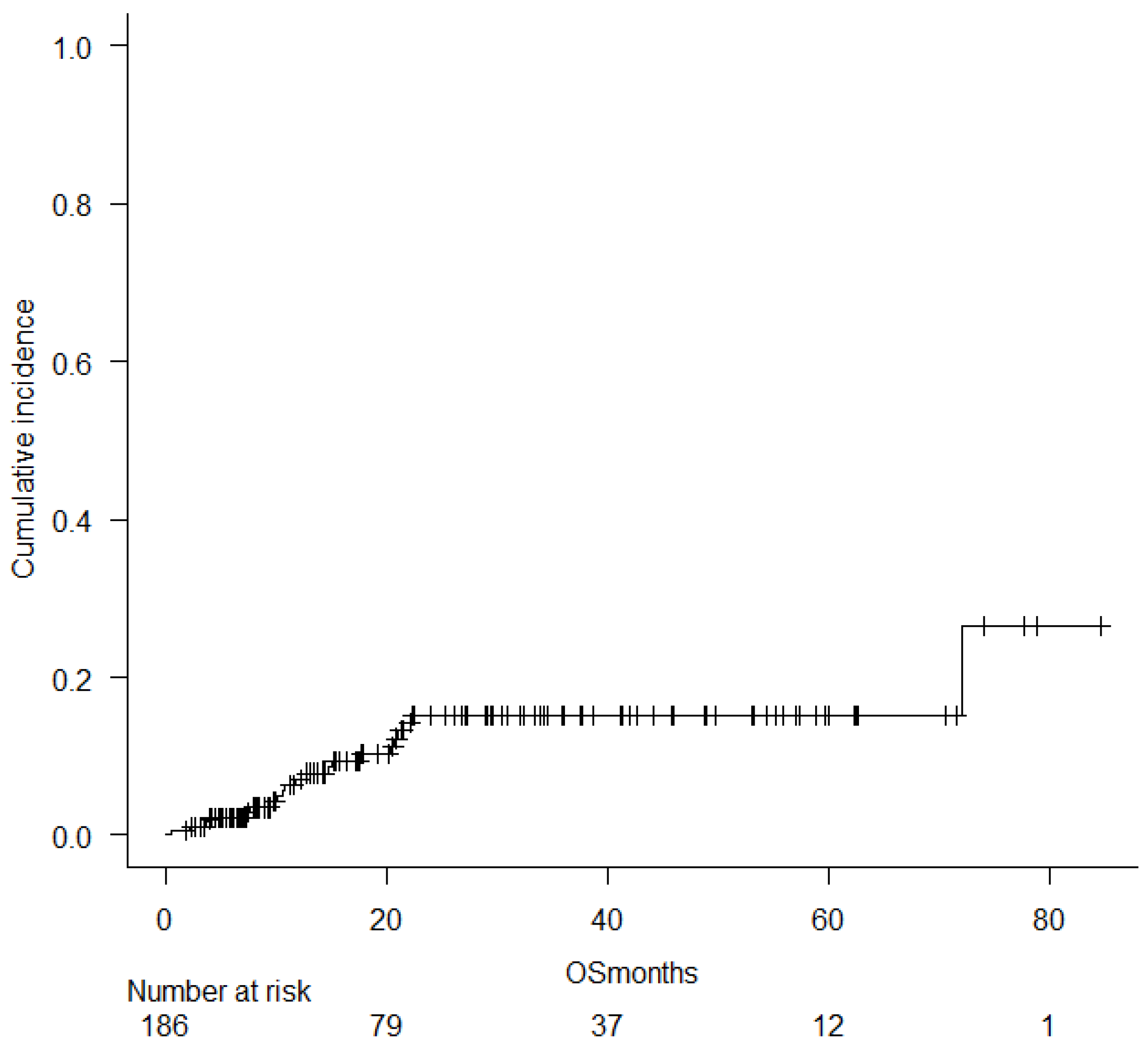
2.4. Relapse, Survival, and Treatment-Related Mortality
2.5. Multivariate Analyses
3. Discussion
4. Materials and Methods
4.1. Study Design and Patient Population
4.2. Conditioning Regimen
4.3. GVHD Prophylaxis
4.4. Chimerism
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pasic, I.; Moya, T.A.; Remberger, M.; Chen, C.; Gerbitz, A.; Kim, D.D.H.; Kumar, R.; Lam, W.; Law, A.D.; Lipton, J.H.; et al. Treosulfan- Versus Busulfan-based Conditioning in Allogeneic Hematopoietic Cell Transplantation for Myelodysplastic Syndrome: A Single-center Retrospective Propensity Score-matched Cohort Study. Transplant. Cell Ther. 2024, 30, e1–e681.e11. [Google Scholar] [CrossRef]
- Sakellari, I. Hematopoietic stem cell transplantation: Historical perspectives. In New Insights in Hematopoietic Cell Transplantation; Research Signpost: Pontiac, MI, USA, 2010. [Google Scholar]
- Haskoloğlu, Ş.; Köstel Bal, S.; İslamoğlu, C.; Altun, D.; Kendirli, T.; Doğu, E.F.; İkIncioğulları, A. Outcome of treosulfan-based reduced-toxicity conditioning regimens for HSCT in high-risk patients with primary immune deficiencies. Pediatr. Transplant. 2018, 22, e13266. [Google Scholar] [CrossRef]
- Shimoni, A.; Radici, V.; Nagler, A. Conditioning. The EBMT Handbook: Hematopoietic Cell Transplantation and Cellular Therapies; Springer International Publishing: Cham, Switzerland, 2024; pp. 125–134. [Google Scholar]
- Bernardi, C.; Morisset, S.; Pradier, A.; Mamez, A.-C.; Giannotti, F.; Morin, S.; Levrat, S.M.; Roth-Guepin, G.; D’AVeni, M.; Kicki, C.; et al. Fludarabine and Treosulfan Conditioning Is Feasible and Leads to High OS and Low NRM. Blood 2024, 144 (Suppl. S1), 7301. [Google Scholar] [CrossRef]
- O’Hagan Henderson, S.; Frietsch, J.J.; Hilgendorf, I.; Hochhaus, A.; Köhne, C.H.; Casper, J. Combination of treosulfan, fludarabine and cytarabine as conditioning in patients with acute myeloid leukemia, myelodysplastic syndrome and myeloproliferative neoplasms. J. Cancer Res. Clin. Oncol. 2022, 148, 2599–2609. [Google Scholar] [CrossRef]
- De Witte, T.; Bowen, D.; Robin, M.; Mamez, A.-C.; Giannotti, F.; Morin, S.; Levrat, S.M.; Roth-Guepin, G.; D’AVeni, M.; Kicki, C.; et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: Recommendations from an international expert panel. Blood 2017, 129, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Berning, P.; Kolloch, L.; Reicherts, C.; Call, S.; Marx, J.; Floeth, M.; Esseling, E.; Ronnacker, J.; Albring, J.; Schliemann, C.; et al. Comparable outcomes for TBI-based versus treosulfan based conditioning prior to allogeneic hematopoietic stem cell transplantation in AML and MDS patients. Bone Marrow Transplant. 2024, 59, 1097–1106. [Google Scholar] [CrossRef]
- Deeg, H.J.; Stevens, E.A.; Salit, R.B.; Ermoian, R.P.; Fang, M.; Gyurkocza, B.; Sorror, M.L.; Fatobene, G.; Baumgart, J.; Burroughs, L.M.; et al. Transplant Conditioning with Treosulfan/Fludarabine with or without Total Body Irradiation: A Randomized Phase II Trial in Patients with Myelodysplastic Syndrome and Acute Myeloid Leukemia. Biol. Blood Marrow Transplant. 2018, 24, 956–963. [Google Scholar] [CrossRef]
- Gagelmann, N.; Schuh, C.; Flossdorf, S.; Kunadt, D.; Stelljes, M.; Blau, I.W.; Brecht, A.; Bethge, W.; Schroeder, T.; Wulf, G.; et al. Impact of busulfan versus treosulfan dose intensity in myelofibrosis undergoing hematopoietic cell transplantation. Am. J. Hematol. 2024, 99, 1540–1549. [Google Scholar] [CrossRef]
- Robin, M.; Iacobelli, S.; Koster, L.; Passweg, J.; Avenoso, D.; Wilson, K.M.O.; Salmenniemi, U.; Dreger, P.; Borne, P.v.D.; Snowden, J.A.; et al. Treosulfan compared to busulfan in allogeneic haematopoietic stem cell transplantation for myelofibrosis: A registry-based study from the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant. 2024, 59, 928–935. [Google Scholar] [CrossRef]
- Finke, J.; Schmoor, C.; Stelljes, M.; Burchert, A.; Dreger, P.; Hegenbart, U.; Wagner-Drouet, E.-M.; Bornhäuser, M.; Sohlbach, K.; Schub, N.; et al. Thiotepa–fludarabine–treosulfan conditioning for 2nd allogeneic HCT from an alternative unrelated donor for patients with AML: A prospective multicenter phase II trial. Bone Marrow Transplant. 2022, 57, 1664. [Google Scholar] [CrossRef]
- Shimoni, A.; Robin, M.; Iacobelli, S.; Beelen, D.; Mufti, G.J.; Ciceri, F.; Bethge, W.; Volin, L.; Blaise, D.; Ganser, A.; et al. Allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome using treosulfan based compared to other reduced-intensity or myeloablative conditioning regimens. A report of the chronic malignancies working party of the EBMT. Br. J. Haematol. 2021, 195, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, L.; Ruggeri, A.; Lupo Stanghellini, M.T.; Mastaglio, S.; Messina, C.; Giglio, F.; Lorusso, A.; Perini, T.; Piemontese, S.; Marcatti, M.; et al. Treosulfan-Based Conditioning Regimen Prior to Allogeneic Stem Cell Transplantation: Long-Term Results From a Phase 2 Clinical Trial. Front. Oncol. 2021, 11, 731478. [Google Scholar] [CrossRef] [PubMed]
- Beelen, D.W.; Trenschel, R.; Stelljes, M.; Groth, C.; Masszi, T.; Reményi, P.; Wagner-Drouet, E.-M.; Hauptrock, B.; Dreger, P.; Luft, T.; et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): A randomised, non-inferiority, phase 3 trial. Lancet Haematol. 2020, 7, e28–e39. [Google Scholar] [CrossRef]
- Wedge, E.; Sengeløv, H.; Hansen, J.W.; Andersen, N.S.; Schjødt, I.; Petersen, S.L.; Kornblit, B.; Grønbæk, K.; Friis, L.S. Improved Outcomes after Allogenic Hematopoietic Stem Cell Transplantation with Fludarabine/Treosulfan for Patients with Myelodysplastic Syndromes. Biol. Blood Marrow Transplant. 2020, 26, 1091–1098. [Google Scholar] [CrossRef]
- Braitsch, K.; Schwarz, A.; Koch, K.; Hubbuch, M.; Menzel, H.; Keller, U.; Götze, K.S.; Bassermann, F.; Herhaus, P.; Verbeek, M. Conditioning with fludarabine and treosulfan compared to FLAMSA-RIC in allogeneic stem cell transplantation for myeloid malignancies: A retrospective single-center analysis. Ann. Hematol. 2022, 101, 1311. [Google Scholar] [CrossRef]
- Sakellari, I.; Gavriilaki, E.; Mallouri, D.; Batsis, I.; Varelas, C.; Tagara, S.; Bousiou, Z.; Papathanasiou, M.; Vardi, A.; Papalexandri, A.; et al. Survival Advantage of Treosulfan Plus Fludarabine Before Allogeneic Hematopoietic Cell Transplantation for Older or Comorbid Patients with Myeloid Malignancies. Transplant. Cell Ther. 2021, 27, e1–e916.e6. [Google Scholar] [CrossRef] [PubMed]
- Nagler, A.; Labopin, M.; Beelen, D.; Ciceri, F.; Volin, L.; Shimoni, A.; Foá, R.; Milpied, N.; Peccatori, J.; Polge, E.; et al. Long-term outcome after a treosulfan-based conditioning regimen for patients with acute myeloid leukemia: A report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer 2017, 123, 2671–2679. [Google Scholar] [CrossRef]
- Sakellari, I.; Mallouri, D.; Gavriilaki, E.; Batsis, I.; Kaliou, M.; Constantinou, V.; Papalexandri, A.; Lalayanni, C.; Vadikolia, C.; Athanasiadou, A.; et al. Survival Advantage and Comparable Toxicity in Reduced-Toxicity Treosulfan-Based versus Reduced-Intensity Busulfan-Based Conditioning Regimen in Myelodysplastic Syndrome and Acute Myeloid Leukemia Patients after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 445–451. [Google Scholar] [CrossRef]
- Beelen, D.W.; Iacobelli, S.; Koster, L.; Eikema, D.-J.; van Biezen, A.; Stölzel, F.; Ciceri, F.; Bethge, W.; Dreger, P.; Wagner-Drouet, E.-M.; et al. Fludarabine-treosulfan versus fludarabine-melphalan or busulfan-cyclophosphamide conditioning in older AML or MDS patients—A clinical trial to registry data comparison. Bone Marrow Transplant. 2024, 59, 670. [Google Scholar] [CrossRef]
- Gran, C.; Wang, J.; Nahi, H.; Koster, L.; Gahrton, G.; Einsele, H.; Niittyvoupio, R.; Edinger, M.; Beelen, D.; Ciceri, F.; et al. Treosulfan conditioning for allogeneic transplantation in multiple myeloma—Improved overall survival in first line haematopoietic stem cell transplantation—A large retrospective study by the Chronic Malignancies Working Party of the EBMT. Br. J. Haematol. 2020, 189, e213–e217. [Google Scholar] [CrossRef]
- Styczynski, J.; Van Der Velden, W.; Fox, C.P.; Engelhard, D.; Camara, R.; Cordonnier, C.; Ljungman, P. Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica 2016, 101, 803. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Hakki, M.; Boeckh, M. Cytomegalovirus in Hematopoietic Stem Cell Transplant Recipients. Hematol. Oncol. Clin. N. Am. 2011, 25, 151. [Google Scholar] [CrossRef] [PubMed]
- Przepiorka, D.; Weisdorf, D.; Martin, P.; Klingemann, H.G.; Beatty, P.; Hows, J.; Thomas, E.D. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995, 15, 825–828. [Google Scholar]
- Flowers, M.E.D.; Kansu, E.; Sullivan, K.M. Pathophysiology and treatment of graft-versus-host disease. Hematol. Oncol. Clin. N. Am. 1999, 13, 1091–1112. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Category | n (%) |
|---|---|---|
| Age at transplant | Median (range), years | 59 (18–70) |
| Underlying disease | De novo AML | 80 (43.0) |
| Secondary AML | 30 (16.1) | |
| Myelodysplastic syndrome (MDS) | 46 (24.7) | |
| Myelofibrosis (MF) | 30 (16.1) | |
| Disease status at transplant | First CR (CR1) | 89 (47.8) |
| Second CR (CR2) | 62 (33.3) | |
| Refractory/relapsed | 35 (18.8) | |
| Donor | Matched unrelated | 90 (48.4) |
| Mismatched unrelated | 23 (12.4) | |
| HLA-matched sibling | 59 (31.7) | |
| Haploidentical family | 12 (6.5) |
| Variable | Exp (B) | p Value | Confidence Interval (CI) |
|---|---|---|---|
| Disease Relapse (Yes/No) | 0.147 | 0.000 | 0.121–0.452 |
| aGVHD | 0.360 | 0.001 | 0.252–0.563 |
| Disease Phase | 1.155 | 0.144 | 0.921–1.457 |
| Independent Predictor | Hazard Ratio | p Value | Confidence Interval (CI) |
|---|---|---|---|
| aGVHD | 0.2694 | 0.0031 | 0.1898–0.4892 |
| Disease | 0.9902 | 0.7400 | 0.8901–1.8933 |
| Donor | 1.2400 | 0.5100 | 0.7831–2.1894 |
| Lines | 0.8351 | 0.4600 | 0.5678–1.9832 |
| Phase | 1.1440 | 0.6700 | 0.7832–3.2312 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavriilaki, E.; Dolgyras, P.; Konstantinidis, I.; Mallouri, D.; Salvaras, G.; Demosthenous, C.; Batsis, I.; Vardi, A.; Papadopoulos, I.; Tsokkou, S.; et al. Treosulfan-Based Conditioning Regimens for Allogeneic Hematopoietic Cell Transplantation in Acute Myeloid Leukemia and Other Myeloid Malignancies. Pharmaceuticals 2025, 18, 1631. https://doi.org/10.3390/ph18111631
Gavriilaki E, Dolgyras P, Konstantinidis I, Mallouri D, Salvaras G, Demosthenous C, Batsis I, Vardi A, Papadopoulos I, Tsokkou S, et al. Treosulfan-Based Conditioning Regimens for Allogeneic Hematopoietic Cell Transplantation in Acute Myeloid Leukemia and Other Myeloid Malignancies. Pharmaceuticals. 2025; 18(11):1631. https://doi.org/10.3390/ph18111631
Chicago/Turabian StyleGavriilaki, Eleni, Panagiotis Dolgyras, Ioannis Konstantinidis, Despina Mallouri, Grigorios Salvaras, Christos Demosthenous, Ioannis Batsis, Anna Vardi, Ioannis Papadopoulos, Sophia Tsokkou, and et al. 2025. "Treosulfan-Based Conditioning Regimens for Allogeneic Hematopoietic Cell Transplantation in Acute Myeloid Leukemia and Other Myeloid Malignancies" Pharmaceuticals 18, no. 11: 1631. https://doi.org/10.3390/ph18111631
APA StyleGavriilaki, E., Dolgyras, P., Konstantinidis, I., Mallouri, D., Salvaras, G., Demosthenous, C., Batsis, I., Vardi, A., Papadopoulos, I., Tsokkou, S., Bousiou, Z., Karavalakis, G., Varelas, C., Panteliadou, A., Spyridis, N., Syrigou, A., Marvaki, A., Papathanasiou, M., Papalexandri, A., ... Sakellari, I. (2025). Treosulfan-Based Conditioning Regimens for Allogeneic Hematopoietic Cell Transplantation in Acute Myeloid Leukemia and Other Myeloid Malignancies. Pharmaceuticals, 18(11), 1631. https://doi.org/10.3390/ph18111631








