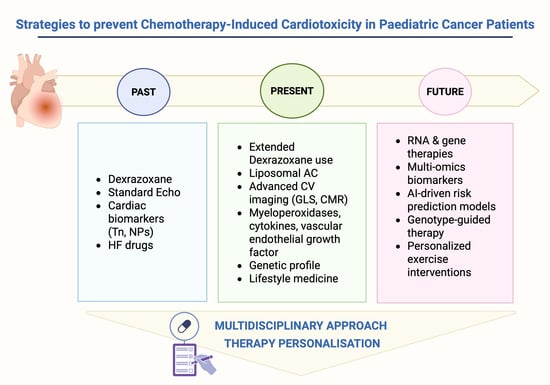
Emerging Strategies for the Prevention of Chemotherapy-Induced Cardiotoxicity in Paediatric Cancer Patients: Advances and Future Perspectives
Abstract
1. Introduction
2. Biomarkers for CIC Detection
2.1. Classical Biomarkers
2.2. Emerging Biomarkers
3. Emerging Therapeutic Approaches to Treat CIC
4. Ongoing Clinical Trials on Novel Strategies for Preventing or Reducing CIC
| Registration Name | Study Design | Inclusion Criteria | Age (Years) | Intervention(s)/Diagnostic Test(s) | Number of Enrolled Patients (Planned or Actual) | Primary Outcome(s) | Additional Outcome(s) | Involved Centres |
|---|---|---|---|---|---|---|---|---|
| NCT01671696 [63] | Observational Case–control Cross-sectional | CCSs having received ≥240 mg/m2 AC. Complete remission and off chemotherapy for ≥2 years | 9–35 | CMR Other: Echo, serological biomarkers of inflammation, myocyte injury, extracellular matrix remodelling, apoptosis, and BNP. Phenotype analysis of DNA/microRNA. | 80 | Changes in T1 Imapping-derived relaxation time and circumferential strain analysis | Changes in serological markers of extracellular matrix remodelling and tissue apoptosis; phenotype analysis of DNA/microRNA. | Connecticut Children’s Medical Centre, Hartford, Connecticut (USA) |
| NCT05781672 (SpeckleAnthra2) [64] | Interventional | CCSs included in the “SpeckleAnthra” Study. In remission of malignant disease. Discontinued chemotherapy for ≥6 years. | 11–27 | STE analysis. | 134 | 5-year evolution of LVGLS | LVEF. LV myocardial dysfunction; death secondary to toxic cardiomyopathy; Troponin T, NT-proBNP. | University Hospital, Montpellier (France) |
| NCT04262830 (CATCH-HF) [66] | Observational Cohort Prospective (3–5 years) | CCS previously treated with AC for cancer. Cancer diagnosis ≥ 2 years prior. | 13–39 | CMR Other: Accelerometer physical activity monitoring. | 150 | LVEF | - | Rady Children’s Hospital, San Diego, California (USA) |
| NCT04852965 [67] | Observational Cohort Cross-sectional | CCS having received at least 100 mg/m2 of AC. | ≥18 | 24 h Holter ECG; echo; CMR. Others: Serological biomarkers (troponin, NT-proBNP) and novel biomarkers (IL6, MPO, and sST2). | 103 | Cardiotoxicity (as defined by the BSE and BCOS guidelines) | Levels of hs-TnT and NT-proBNP. | Queen’s University, Belfast (United Kingdom) |
| NCT05023785 (HIMALAYAS) [73] | Interventional Randomised Open label |
≤39 years of age at the time of cancer diagnosis.
Received cancer treatment(s) with known cardiovascular risks. Be cancer-free at the time of enrolment. Stage B Heart Failure. | 18–45 | Supervised CORE (cardio-oncology rehabilitation) model, exercise therapy. | 336 | Cardiorespiratory fitness (cardiopulmonary exercise test, VO2peak) at 6-month follow-up |
Cardiorespiratory fitness (CPET, VO2peak, ventilatory and anaerobic thresholds, HR recovery).
LV systolic and diastolic function (LVEF, GLS). LV hypertrophy. Metabolic profile (lipid metabolism, insulin sensitivity, HOMA-IR). Health-related quality of life. | University Health Network, Toronto, Ontario, Canada |
| NCT04036032 [74] | Observational Prospective |
Long-term CCSs ≥ 9 years of age.
Exposed to AC chemotherapy. | 9–99 | Encourage work out 4 to 5 times a week for 3 months. Exercise support. | 65 | LV and RV volume and mass assessed by CMR | Cardiopulmonary parameters. Quality of life. MicroRNA expression. |
Connecticut Children’s Medical Center, Hartford, Connecticut (USA).
Nationwide Children’s Hospital, Columbus, Ohio (USA) |
| NCT05223413 (RESILIENCE) [79] | Interventional Phase II Randomised Double blind Sham-controlled Prospective Multinational |
≥18 years old at first lymphoma diagnosis. Scheduled to undergo ≥ 5 chemotherapy cycles including AC.
Pre-chemo LVEF > 40% on screening echo. ≥1 risk factor for developing cardiotoxicity | 18–99 | Device: RIPC Device: Simulated RIPC (Sham) | 608 | LVEF assessed by CMR | Incidence of AC cardiotoxicity events. Rate of tumour regression. Change in quality of life. Rate of heart failure hospitalisation. Other: Ability of T2 mapping to predict AC cardiotoxicity versus classical markers (LV strain, cardiac injury biomarkers); to validate a novel ultrafast CMR sequence. |
Fundación Centro Nacional de Investigaciones Cardiovasculares Carlos III, Madrid, Spain
and other European centres |
5. Impact of Exercise
6. Role of Genetic Testing
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armenian, S.H.; Armstrong, G.T.; Aune, G.; Chow, E.J.; Ehrhardt, M.J.; Ky, B.; Moslehi, J.; Mulrooney, D.; Nathan, P.C.; Ryan, T.D.; et al. Cardiovascular Disease in Survivors of Childhood Cancer: Insights into Epidemiology, Pathophysiology, and Prevention. J. Clin. Oncol. 2018, 36, 2135–2144. [Google Scholar] [CrossRef]
- Ryan, T.D.; Bates, J.E.; Kinahan, K.E.; Leger, K.J.; Mulrooney, D.A.; Narayan, H.K.; Ness, K.; Okwuosa, T.M.; Rainusso, N.C.; Steinberger, J.; et al. Cardiovascular Toxicity in Patients Treated for Childhood Cancer: A Scientific Statement from the American Heart Association. Circulation 2025, 151, e926–e943. [Google Scholar] [CrossRef] [PubMed]
- Tukenova, M.; Guibout, C.; Oberlin, O.; Doyon, F.; Mousannif, A.; Haddy, N.; Guerin, S.; Pacquement, H.; Aouba, A.; Hawkins, M.; et al. Role of Cancer Treatment in Long-Term Overall and Cardiovascular Mortality After Childhood Cancer. J. Clin. Oncol. 2010, 28, 1308–1315. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Dillenburg, R.F.; Nathan, P.; Mertens, L. Educational paper: Decreasing the burden of cardiovascular disease in childhood cancer survivors: An update for the pediatrician. Eur. J. Pediatr. 2013, 172, 1149–1160. [Google Scholar] [CrossRef]
- Leerink, J.M.; de Baat, E.C.; Feijen, E.A.M.; Bellersen, L.; van Dalen, E.C.; Grotenhuis, H.B.; Kapusta, L.; Kok, W.E.M.; Loonen, J.; van der Pal, H.; et al. Cardiac Disease in Childhood Cancer Survivors: Risk Prediction, Prevention, and Surveillance: JACC CardioOncology State-of-the-Art Review. JACC CardioOncol. 2020, 2, 363–378. [Google Scholar] [CrossRef]
- DeVine, A.; Landier, W.; Hudson, M.M.; Constine, L.S.; Bhatia, S.; Armenian, S.H.; Gramatges, M.M.; Chow, E.J.; Friedman, D.N.; Ehrhardt, M.J. The Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers: A Review. JAMA Oncol. 2025, 11, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Bertorello, N.; Luksch, R.; Bisogno, G.; Haupt, R.; Spallarossa, P.; Cenna, R.; Fagioli, F. Cardiotoxicity in children with cancer treated with anthracyclines: A position statement on dexrazoxane. Pediatr. Blood Cancer 2023, 70, e30515. [Google Scholar] [CrossRef] [PubMed]
- Loar, R.W.; Noel, C.V.; Tunuguntla, H.; Colquitt, J.L.; Pignatelli, R.H. State of the art review: Chemotherapy-induced cardiotoxicity in children. Congenit. Heart Dis. 2018, 13, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Amdani, S.; Lipshultz, E.R.; Lipshultz, S.E. Chemotherapy-induced cardiotoxicity in children. Expert Opin. Drug Metab. Toxicol. 2017, 13, 817–832. [Google Scholar] [CrossRef]
- Zaha, V.G.; Hayek, S.S.; Alexander, K.M.; Beckie, T.M.; Hundley, W.G.; Kondapalli, L. Future Perspectives of Cardiovascular Biomarker Utilization in Cancer Survivors: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e551–e563. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Miller, T.L.; Scully, R.E.; Lipsitz, S.R.; Rifai, N.; Silverman, L.B.; Colan, S.D.; Neuberg, D.S.; Dahlberg, S.E.; Henkel, J.M.; et al. Changes in Cardiac Biomarkers During Doxorubicin Treatment of Pediatric Patients with High-Risk Acute Lymphoblastic Leukemia: Associations with Long-Term Echocardiographic Outcomes. J. Clin. Oncol. 2012, 30, 1042–1049. [Google Scholar] [CrossRef]
- Michel, L.; Mincu, R.I.; Mrotzek, S.M.; Korste, S.; Neudorf, U.; Rassaf, T.; Totzeck, M. Cardiac biomarkers for the detection of cardiotoxicity in childhood cancer—A meta-analysis. ESC Heart Fail. 2020, 7, 423–433. [Google Scholar] [CrossRef]
- Leerink, J.M.; Feijen, E.A.M.; De Baat, E.C.; Merkx, R.; Van Der Pal, H.J.H.; Tissing, W.J.E.; Louwerens, M.; van den Heuvel-Eibrink, M.M.; Versluys, A.B.; van Dalen, E.C.; et al. A Biomarker-Based Diagnostic Model for Cardiac Dysfunction in Childhood Cancer Survivors. JACC CardioOncol. 2024, 6, 236–247. [Google Scholar] [CrossRef]
- Meo, L.; Savarese, M.; Munno, C.; Mirabelli, P.; Ragno, P.; Leone, O.; Alfieri, M. Circulating Biomarkers for Monitoring Chemotherapy-Induced Cardiotoxicity in Children. Pharmaceutics 2023, 15, 2712. [Google Scholar] [CrossRef]
- Ehrhardt, M.J.; Leerink, J.M.; Mulder, R.L.; Mavinkurve-Groothuis, A.; Kok, W.; Nohria, A.; Nathan, P.C.; Merkx, R.; de Baat, E.; Asogwa, O.A.; et al. Systematic review and updated recommendations for cardiomyopathy surveillance for survivors of childhood, adolescent, and young adult cancer from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2023, 24, e108–e120. [Google Scholar] [CrossRef]
- Ehrhardt, M.J.; Liu, Q.; Mulrooney, D.A.; Rhea, I.B.; Dixon, S.B.; Lucas, J.T.; Sapkota, Y.; Shelton, K.; Ness, K.K.; Srivastava, D.K.; et al. Improved Cardiomyopathy Risk Prediction Using Global Longitudinal Strain and N-Terminal-Pro-B-Type Natriuretic Peptide in Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy. J. Clin. Oncol. 2024, 42, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Leger, K.J.; Leonard, D.; Nielson, D.; De Lemos, J.A.; Mammen, P.P.A.; Winick, N.J. Circulating microRNAs: Potential Markers of Cardiotoxicity in Children and Young Adults Treated with Anthracycline Chemotherapy. J. Am. Heart Assoc. 2017, 6, e004653. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, X.; Li, S.; Liu, Y.; Cui, Y.; Deng, X. Advances in Biomarkers for Detecting Early Cancer Treatment-Related Cardiac Dysfunction. Front. Cardiovasc. Med. 2021, 8, 753313. [Google Scholar] [CrossRef] [PubMed]
- Nettersheim, F.S.; Schlüter, J.D.; Kreuzberg, W.; Mehrkens, D.; Grimm, S.; Nemade, H. Myeloperoxidase is a critical mediator of anthracycline-induced cardiomyopathy. Basic Res. Cardiol. 2023, 118, 36. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, H.; Liu, J.; Xu, M.; Ma, L. GDF-15 is a potential candidate biomarker for an elevated risk of cardiotoxicity in breast cancer patients receiving neoadjuvant dual anti-HER2 therapy. Front. Pharmacol. 2024, 15, 1396133. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, U.; Di Sarro, E.; Tricarico, L.; Di Lisi, D.; Armentaro, G.; Miceli, S.; Fioretti, F.; Deidda, M.; Correale, M.; Novo, G.; et al. Cardiovascular Biomarkers in Cardio-Oncology: Antineoplastic Drug Cardiotoxicity and Beyond. Biomolecules 2024, 14, 199. [Google Scholar] [CrossRef]
- Ky, B.; Putt, M.; Sawaya, H.; French, B.; Januzzi, J.L.; Sebag, I.A.; Plana, J.C.; Cohen, V.; Banchs, J.; Carver, J.R.; et al. Early Increases in Multiple Biomarkers Predict Subsequent Cardiotoxicity in Patients with Breast Cancer Treated with Doxorubicin, Taxanes, and Trastuzumab. J. Am. Coll. Cardiol. 2014, 63, 809–816. [Google Scholar] [CrossRef]
- Kuang, Z.; Kong, M.; Yan, N.; Ma, X.; Wu, M.; Li, J. Precision Cardio-oncology: Update on Omics-Based Diagnostic Methods. Curr. Treat. Options Oncol. 2024, 25, 679–701. [Google Scholar] [CrossRef]
- Kronenberger, D.W.; Zimmers, T.A.; Ralston, R.K.; Runco, D.V. Circulating Growth Differentiation Factor 15 (GDF15) in Paediatric Disease: A Systematic Review. J. Cachexia Sarcopenia Muscle 2025, 16, e13712. [Google Scholar] [CrossRef] [PubMed]
- Runco, D.V.; DiMeglio, L.A.; Vanderpool, C.P.; Han, Y.; Daggy, J.; Kelley, M.M.; Mikesell, R.; Zimmers, T.A. Growth differentiation factor 15 (GDF15) elevation in children with newly diagnosed cancer. Front. Oncol. 2023, 13, 1295228. [Google Scholar] [CrossRef]
- Cartas-Espinel, I.; Telechea-Fernández, M.; Manterola Delgado, C.; Ávila Barrera, A.; Saavedra Cuevas, N.; Riffo-Campos, A.L. Novel molecular biomarkers of cancer therapy-induced cardiotoxicity in adult population: A scoping review. ESC Heart Fail. 2022, 9, 1651–1665. [Google Scholar] [CrossRef] [PubMed]
- Boen, H.M.; Cherubin, M.; Franssen, C.; Gevaert, A.B.; Witvrouwen, I.; Bosman, M.; Guns, P.J.; Heidbuchel, H.; Loeys, B.; Alaerts, M.; et al. Circulating MicroRNA as Biomarkers of Anthracycline-Induced Cardiotoxicity. JACC CardioOncol. 2024, 6, 183–199. [Google Scholar] [CrossRef]
- Joolharzadeh, P.; Rodriguez, M.; Zaghlol, R.; Pedersen, L.N.; Jimenez, J.; Bergom, C.; Mitchell, J.D. Recent Advances in Serum Biomarkers for Risk Stratification and Patient Management in Cardio-Oncology. Curr. Cardiol. Rep. 2023, 25, 133–146. [Google Scholar] [CrossRef]
- Van Den Berg, P.F.; Aboumsallem, J.P.; Screever, E.M.; Shi, C.; De Wit, S.; Bracun, V.; Yousif, L.I.; Geerlings, L.; Wang, D.; Ho, J.E.; et al. Fibrotic Marker Galectin-3 Identifies Males at Risk of Developing Cancer and Heart Failure. JACC CardioOncol. 2023, 5, 445–453. [Google Scholar] [CrossRef]
- Poudel, S.; Shrestha, H.; Pan, Y.; Li, Q.; Li, K.; Im, C.; Dixon, S.B.; Ehrhardt, M.J.; Mulrooney, D.A.; Zhou, S.; et al. Serum Proteins Predict Treatment-Related Cardiomyopathy Among Survivors of Childhood Cancer. JACC CardioOncol. 2025, 7, 56–67. [Google Scholar] [CrossRef]
- Khera, R.; Asnani, A.H.; Krive, J.; Addison, D.; Zhu, H.; Vasbinder, A.; Fleming, M.R.; Arnaout, R.; Razavi, P.; Okwuosa, T.M.; et al. Artificial Intelligence to Enhance Precision Medicine in Cardio-Oncology: A Scientific Statement from the American Heart Association. Circ. Genom. Precis. Med. 2025, 18, e000097. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Xiao, R.; Deng, A.; Liu, W. Safety Evaluation of the Combination with Dexrazoxane and Anthracyclines: A Disproportionality Analysis Based on the Food and Drug Administration Adverse Event Reporting System Database. Pharmaceuticals 2024, 17, 1739. [Google Scholar] [CrossRef]
- Shaddy, R.E.; Boucek, M.M.; Hsu, D.T.; Boucek, R.J.; Canter, C.E.; Mahony, L.; Ross, R.D.; Pahl, E.; Blume, E.D.; Dodd, D.A.; et al. Carvedilol for children and adolescents with heart failure: A randomized controlled trial. JAMA 2007, 298, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, F.; Dupuis, L.L.; Alexander, S.; Gupta, A.; Mertens, L.; Nathan, P.C. Cardioprotection and Second Malignant Neoplasms Associated with Dexrazoxane in Children Receiving Anthracycline Chemotherapy: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2016, 108, djv357. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Karnik, R.; Sambatakos, P.; Franco, V.I.; Ross, S.W.; Miller, T.L. Anthracycline-related cardiotoxicity in childhood cancer survivors. Curr. Opin. Cardiol. 2014, 29, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Franco, V.I.; Lipshultz, S.E. Cardiac complications in childhood cancer survivors treated with anthracyclines. Cardiol. Young 2015, 25 (Suppl. S2), 107–116. [Google Scholar] [CrossRef]
- Iarussi, D.; Indolfi, P.; Casale, F.; Martino, V.; Di Tullio, M.T.; Calabrò, R. Anthracycline-induced cardiotoxicity in children with cancer: Strategies for prevention and management. Paediatr. Drugs 2005, 7, 67–76. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Rifai, N.; Dalton, V.M.; Levy, D.E.; Silverman, L.B.; Lipsitz, S.R.; Colan, S.D.; Asselin, B.L.; Barr, R.D.; Clavell, L.A.; et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N. Engl. J. Med. 2004, 351, 145–153. [Google Scholar] [CrossRef]
- Toro, C.; Felmingham, B.; Jessop, S.; Celermajer, D.S.; Kotecha, R.S.; Govender, D.; Terese Hanna, D.M.; O’Connor, M.; Manudhane, R.; Ayer, J.; et al. Cardio-Oncology Recommendations for Pediatric Oncology Patients. JACC Adv. 2022, 1, 100155. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, E.C.; van der Pal, H.J.H.; Kremer, L.C.M. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst. Rev. 2016, 3, CD005008. [Google Scholar] [CrossRef]
- Berrak, S.G.; Ewer, M.S.; Jaffe, N.; Pearson, P.; Ried, H.; Zietz, H.A.; Benjamin, R.S. Doxorubicin cardiotoxicity in children: Reduced incidence of cardiac dysfunction associated with continuous-infusion schedules. Oncol. Rep. 2001, 8, 611–614. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Miller, T.L.; Lipsitz, S.R.; Neuberg, D.S.; Dahlberg, S.E.; Colan, S.D.; Silverman, L.B.; Henkel, J.M.; Franco, V.I.; Cushman, L.L.; et al. Continuous Versus Bolus Infusion of Doxorubicin in Children with ALL: Long-term Cardiac Outcomes. Pediatrics 2012, 130, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- De Baat, E.C.; Van Dalen, E.C.; Mulder, R.L.; Hudson, M.M.; Ehrhardt, M.J.; Engels, F.K.; Feijen, E.A.M.; Grotenhuis, H.B.; Leerink, J.M.; Kapusta, L.; et al. Primary cardioprotection with dexrazoxane in patients with childhood cancer who are expected to receive anthracyclines: Recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Child Adolesc. Health 2022, 6, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Creutzig, U.; Zimmermann, M.; Bourquin, J.-P.; Dworzak, M.N.; Fleischhack, G.; Graf, N.; Klingebiel, T.; Kremens, B.; Lehrnbecher, T.; von Neuhoff, C.; et al. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: Results from Study AML-BFM 2004. Blood 2013, 122, 37–43. [Google Scholar] [CrossRef]
- Kaspers, G.J.L.; Zimmermann, M.; Reinhardt, D.; Gibson, B.E.S.; Tamminga, R.Y.J.; Aleinikova, O.; Armendariz, H.; Dworzak, M.; Ha, S.Y.; Hasle, H.; et al. Improved outcome in pediatric relapsed acute myeloid leukemia: Results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 599–607. [Google Scholar] [CrossRef]
- Rose-Felker, K.; Border, W.L.; Hong, B.J.; Chow, E.J. Cardio-oncology Related to Heart Failure: Pediatric Considerations for Cardiac Dysfunction. Heart Fail. Clin. 2017, 13, 311–325. [Google Scholar] [CrossRef]
- Das, B. Pharmacotherapy for Cancer Treatment-Related Cardiac Dysfunction and Heart Failure in Childhood Cancer Survivors. Paediatr. Drugs 2023, 25, 695–707. [Google Scholar] [CrossRef]
- Simela, C.; Walker, J.M.; Ghosh, A.K.; Chen, D.H. SGLT2 inhibitors for prevention and management of cancer treatment-related cardiovascular toxicity: A review of potential mechanisms and clinical insights. Cardio-Oncol. Lond. Engl. 2025, 11, 15. [Google Scholar] [CrossRef]
- Guo, Z.; Javaheri, A. Empagliflozin to Prevent Doxorubicin Cardiotoxicity. JACC CardioOncol. 2025, 7, 185–187. [Google Scholar] [CrossRef]
- Novo, G.; Madaudo, C.; Cannatà, A.; Ameri, P.; Di Lisi, D.; Bromage, D.I.; Galassi, A.R.; Minotti, G.; Lyon, A.R. Effects of sodium-glucose cotransporter 2 inhibitors in patients with cancer and Diabetes mellitus: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2025, 11, 343–352. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Reinal, I.; Ontoria-Oviedo, I.; Selva, M.; Casini, M.; Peiró-Molina, E.; Fambuena-Santos, C.; Climent, A.M.; Balaguer, J.; Cañete, A.; Mora, J.; et al. Modeling Cardiotoxicity in Pediatric Oncology Patients Using Patient-Specific iPSC-Derived Cardiomyocytes Reveals Downregulation of Cardioprotective microRNAs. Antioxidants 2023, 12, 1378. [Google Scholar] [CrossRef]
- Sapp, V.; Aguirre, A.; Mainkar, G.; Ding, J.; Adler, E.; Liao, R.; Sharma, S.; Jain, M. Genome-wide CRISPR/Cas9 screening in human iPS derived cardiomyocytes uncovers novel mediators of doxorubicin cardiotoxicity. Sci. Rep. 2021, 11, 13866. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, M.; Liu, Y.; Manhas, A.; Zhao, S.R.; Zhang, M.; Caudal, A.; Nishiga, M.; Thomas, D.; Zhang, A.; et al. CRISPRi/a screens in human iPSC-cardiomyocytes identify glycolytic activation as a druggable target for doxorubicin-induced cardiotoxicity. Cell Stem Cell 2024, 31, 1760–1776.e9. [Google Scholar] [CrossRef]
- Hutchins, E.; Yang, E.H.; Stein-Merlob, A.F. Inflammation in Chemotherapy-Induced Cardiotoxicity. Curr. Cardiol. Rep. 2024, 26, 1329–1340. [Google Scholar] [CrossRef]
- Rurik, J.G.; Tombácz, I.; Yadegari, A.; Méndez Fernández, P.O.; Shewale, S.V.; Li, L.; Kimura, T.; Soliman, O.Y.; Papp, T.E.; Tam, Y.K.; et al. CAR T cells produced in vivo to treat cardiac injury. Science 2022, 375, 91–96. [Google Scholar] [CrossRef]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; de Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Han, X.; Bai, X.; Yu, J.; Ma, Y.; Chen, W.; Zhang, D.; Li, Z. Revolutionizing cancer treatment: Enhancing CAR-T cell therapy with CRISPR/Cas9 gene editing technology. Front. Immunol. 2024, 15, 1354825. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, F.; Sun, B.; Liu, Y.; Tang, H.; Luo, J.; Chen, H.; Luo, Z. Intestinal Microbiome Associated with Immune-Related Adverse Events for Patients Treated with Anti-PD-1 Inhibitors, a Real-World Study. Front. Immunol. 2021, 12, 756872. [Google Scholar] [CrossRef]
- Liu, X.; Lu, B.; Tang, H.; Jia, X.; Zhou, Q.; Zeng, Y.; Zeng, Y.; Gao, X.; Chen, M.; Xu, Y.; et al. Gut microbiome metabolites, molecular mimicry, and species-level variation drive long-term efficacy and adverse event outcomes in lung cancer survivors. EBioMedicine 2024, 109, 105427. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Defining Late Onset Occult Asymptomatic Cardiotoxicity in Childhood Cancer Survivors Exposed to Anthracycline Therapy; Report No.: NCT01671696; National Library of Medicine (US): Bethesda, MD, USA, 2012. Available online: https://clinicaltrials.gov/study/NCT01671696 (accessed on 1 August 2025).
- ClinicalTrials.gov. Longitudinal Analysis of Myocardial Function by Speckle Tracking Echocardiography and Prediction of Delayed Toxic Cardiomyopathy Associated with Anthracycline Therapy in Children (SpeckleAnthra2); Report No.: NCT05781672; National Library of Medicine (US): Bethesda, MD, USA, 2023. Available online: https://clinicaltrials.gov/study/NCT05781672 (accessed on 1 August 2025).
- Amedro, P.; Vincenti, M.; Abassi, H.; Lanot, N.; De La Villeon, G.; Guillaumont, S.; Gamon, L.; Mura, T.; Lopez-Perrin, K.; Haouy, S.; et al. Use of speckle tracking echocardiography to detect late anthracycline-induced cardiotoxicity in childhood cancer: A prospective controlled cross-sectional study. Int. J. Cardiol. 2022, 354, 75–83. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Cardiotoxicity Assessment Through Comprehensive Heart Imaging to Predict Heart Failure (CATCH-HF); Identifier NCT04262830; National Library of Medicine (US): Bethesda, MD, USA, 2019. Available online: https://clinicaltrials.gov/study/NCT04262830 (accessed on 1 August 2025).
- ClinicalTrials.gov. Late Anthracycline Induced Cardiotoxicity—Childhood Cancer Survivors; Identifier NCT04852965; National Library of Medicine (US): Bethesda, MD, USA, 2021. Available online: https://clinicaltrials.gov/study/NCT04852965 (accessed on 1 August 2025).
- Götte, M.; Söntgerath, R.; Gauß, G.; Wiskemann, J.; Buždon, M.; Kesting, S. A National Implementation Approach for Exercise as Usual Care in Pediatric and Adolescent Oncology: Network ActiveOncoKids. Pediatr. Exerc. Sci. 2022, 34, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Wurz, A.; McLaughlin, E.; Lategan, C.; Chamorro Viña, C.; Grimshaw, S.L.; Hamari, L.; Götte, M.; Kesting, S.; Rossi, F.; van der Torre, P.; et al. The international Pediatric Oncology Exercise Guidelines (iPOEG). Transl. Behav. Med. 2021, 11, 1915–1922. [Google Scholar] [CrossRef]
- Kesting, S.; Giordano, U.; Weil, J.; McMahon, C.J.; Albert, D.C.; Berger, C. Association of European Paediatric and Congenital Cardiology practical recommendations for surveillance and prevention of cardiac disease in childhood cancer survivors: The importance of physical activity and lifestyle changes from the Association of European Paediatric and Congenital Cardiology Working Group Sports Cardiology, Physical Activity and Prevention, Working Group Adult Congenital Heart Disease, Working Group Imaging and Working Group Heart Failure. Cardiol. Young 2024, 34, 250–261. [Google Scholar] [CrossRef]
- Braam, K.I.; van der Torre, P.; Takken, T.; Veening, M.A.; van Dulmen-den Broeder, E.; Kaspers, G.J.L. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst. Rev. 2016, 3, CD008796. [Google Scholar] [CrossRef]
- Bourdon, A.; Grandy, S.A.; Keats, M.R. Aerobic exercise and cardiopulmonary fitness in childhood cancer survivors treated with a cardiotoxic agent: A meta-analysis. Support Care Cancer 2018, 26, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Harmonized Interventions to Maintain Health via Appropriate Risk Factor Modification and Lifestyle Changes in Pediatric, Adolescent and Young Adult Cancer Survivors Study (HIMALAYAS); Identifier NCT05023785; National Library of Medicine (US): Bethesda, MD, USA, 2024. Available online: https://clinicaltrials.gov/study/NCT05023785 (accessed on 1 August 2025).
- ClinicalTrials.gov. Role of Aerobic Exercise to Modulate Cardiotoxicity in Long-Term Cancer Survivors Exposed to Anthracycline Therapy; Identifier NCT04036032; National Library of Medicine (US): Bethesda, MD, USA, 2019. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04036032 (accessed on 1 August 2025).
- Brown, M.C.; Araújo-Soares, V.; Skinner, R.; Glaser, A.W.; Sarwar, N.; Saxton, J.M.; Montague, K.; Hall, J.; Burns, O.; Sharp, L. Using qualitative and co-design methods to inform the development of an intervention to support and improve physical activity in childhood cancer survivors: A study protocol for BEing Active after ChildhOod caNcer (BEACON). BMJ Open 2020, 10, e041073. [Google Scholar] [CrossRef]
- Galán-Arriola, C.; Villena-Gutiérrez, R.; Higuero-Verdejo, M.I.; Díaz-Rengifo, I.A.; Pizarro, G.; López, G.J.; Molina-Iracheta, A.; Pérez-Martínez, C.; García, R.D.; González-Calle, D.; et al. Remote ischaemic preconditioning ameliorates anthracycline-induced cardiotoxicity and preserves mitochondrial integrity. Cardiovasc. Res. 2021, 117, 1132–1143. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Kharbanda, R.K.; Møller, U.K.; Ramlall, M.; Aarøe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): A single-blind randomised controlled trial. Lancet Lond. Engl. 2019, 394, 1415–1424. [Google Scholar] [CrossRef]
- Cheung, Y.-F.; Li, V.W.-Y.; So, E.K.-F.; Cheng, F.W.-T.; Yau, J.P.-W.; Chiu, S.-Y.; Yau, J.P.; Chiu, S.Y.; Wong, W.H.; Cheuk, D.K. Remote Ischemic Conditioning in Pediatric Cancer Patients Receiving Anthracycline Chemotherapy: A Sham-Controlled Single-Blind Randomized Trial. JACC CardioOncol. 2023, 5, 332–342. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. REmote iSchemic Conditioning in Lymphoma PatIents REceiving ANthraCyclinEs (RESILIENCE); Identifier NCT05223413; National Library of Medicine (US): Bethesda, MD, USA, 2022. Available online: https://clinicaltrials.gov/study/NCT05223413 (accessed on 1 August 2025).
- Moreno-Arciniegas, A.; García, A.; Kelm, M.; D’Amore, F.; da Silva, M.G.; Sánchez-González, J.; Sánchez, P.L.; López-Fernández, T.; Córdoba, R.; Asteggiano, R.; et al. Rationale and design of RESILIENCE: A prospective randomized clinical trial evaluating remote ischaemic conditioning for the prevention of anthracycline cardiotoxicity. Eur. J. Heart Fail. 2024, 26, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Alvarez, J.A.; Scully, R.E. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart Br. Card. Soc. 2008, 94, 525–533. [Google Scholar] [CrossRef]
- Scott, J.M.; Nilsen, T.S.; Gupta, D.; Jones, L.W. Exercise Therapy and Cardiovascular Toxicity in Cancer. Circulation 2018, 137, 1176–1191. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Lipsitz, S.R.; Sallan, S.E.; Dalton, V.M.; Mone, S.M.; Gelber, R.D.; Colan, S.D. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2005, 23, 2629–2636. [Google Scholar] [CrossRef]
- Brown, T.M.; Pack, Q.R.; Aberegg, E.; Brewer, L.C.; Ford, Y.R.; Forman, D.E.; Gathright, E.C.; Khadanga, S.; Ozemek, C.; Ryan, T. Core Components of Cardiac Rehabilitation Programs: 2024 Update: A Scientific Statement from the American Heart Association and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2024, 150, e328–e347. [Google Scholar] [CrossRef]
- Morales, J.S.; Valenzuela, P.L.; Rincón-Castanedo, C.; Takken, T.; Fiuza-Luces, C.; Santos-Lozano, A. Exercise training in childhood cancer: A systematic review and meta-analysis of randomized controlled trials. Cancer Treat. Rev. 2018, 70, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.S.; Padilla, J.R.; Valenzuela, P.L.; Santana-Sosa, E.; Rincón-Castanedo, C.; Santos-Lozano, A.; Lucia, A. Inhospital Exercise Training in Children with Cancer: Does It Work for All? Front. Pediatr. 2018, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- van Kalsbeek, R.J.; Mulder, R.L.; Haupt, R.; Muraca, M.; Hjorth, L.; Follin, C.; Kepak, T.; Kepakova, K.; Uyttebroeck, A.; Mangelschots, M.; et al. The PanCareFollowUp Care Intervention: A European harmonised approach to person-centred guideline-based survivorship care after childhood, adolescent and young adult cancer. Eur. J. Cancer 2022, 162, 34–44. [Google Scholar] [CrossRef]
- Visscher, H.; Ross, C.J.D.; Rassekh, S.R.; Sandor, G.S.S.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; van der Pal, H.J.; Rogers, P.C.; Rieder, M.J.; et al. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr. Blood Cancer 2013, 60, 1375–1381. [Google Scholar] [CrossRef]
- Visscher, H.; Ross, C.J.D.; Rassekh, S.R.; Barhdadi, A.; Dubé, M.-P.; Al-Saloos, H.; Sandor, G.S.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J. Clin. Oncol. 2012, 30, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Atkins, C.D. Single nucleotide polymorphisms and anthracycline cardiotoxicity in children: Potential implications for adult oncology. J. Clin. Oncol. 2012, 30, 3563–3564. [Google Scholar] [CrossRef] [PubMed]
- Orgil, B.-O.; Bajpai, A.K.; Alberson, N.; Lander, M.; Enkhzul, B.; Martinez, H.R.; Towbin, J.A.; Lu, L.; Purevjav, E. Unraveling the genetic blueprint of doxorubicin-induced cardiotoxicity through systems genetics approaches. Cardio-Oncol. Lond. Engl. 2025, 11, 53. [Google Scholar] [CrossRef]
- Wilcox, N.S.; Rotz, S.J.; Mullen, M.; Song, E.J.; Ky Hamilton, B.; Moslehi, J.; Armenian, S.H.; Wu, J.C.; Rhee, J.W.; Ky, B. Sex-Specific Cardiovascular Risks of Cancer and Its Therapies. Circ. Res. 2022, 130, 632–651. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Trivieri, M.G.; Khaper, N.; Liu, P.P.; Backx, P.H. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J. Mol. Med. Berl. Ger. 2006, 84, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C.; Schmidt, P.J. Iron homeostasis. Annu. Rev. Physiol. 2007, 69, 69–85. [Google Scholar] [CrossRef]
- Wang, X.; Singh, P.; Cejas, R.B.; Zhou, L.; Sharafeldin, N.; Trainor, P.J.; Landier, W.; Cheng, C.; Hageman, L.; Wang, F.; et al. DNA Damage Response and Repair Genes and Anthracycline-Induced Cardiomyopathy in Childhood Cancer Survivors: A Report from the Children’s Oncology Group and the Childhood Cancer Survivor Study. Circ. Genom. Precis. Med. 2025, 18, e004813. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Kim, Y.; Restrepo-Cordoba, M.A.; Lunde, I.G.; Wakimoto, H.; Smith, A.M.; Toepfer, C.N.; Getz, K.; Gorham, J.; Patel, P.; et al. Genetic Variants Associated with Cancer Therapy-Induced Cardiomyopathy. Circulation 2019, 140, 31–41. [Google Scholar] [CrossRef]
- Bennati, E.; Capponi, G.; Favilli, S.; Girolami, F.; Gozzini, A.; Spaziani, G.; Passantino, S.; Tamburini, A.; Tondo, A.; Olivotto, I. Role of Genetic Testing for Cardiomyopathies in Pediatric Patients with Left Ventricular Dysfunction Secondary to Chemotherapy. Circ. Genom. Precis. Med. 2024, 17, e004353. [Google Scholar] [CrossRef]
- Armenian, S.H.; Bhatia, S. Chronic health conditions in childhood cancer survivors: Is it all treatment-related--or do genetics play a role? J. Gen. Intern. Med. 2009, 24 (Suppl. S2), 395–400. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S. Role of genetic susceptibility in development of treatment-related adverse outcomes in cancer survivors. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2048–2067. [Google Scholar] [CrossRef] [PubMed]
- Fazzini, L.; Campana, N.; Cossu, S.; Deidda, M.; Madaudo, C.; Quagliariello, V.; Maurea, N.; Di Lisi, D.; Novo, G.; Zito, C.; et al. Genetic Background in Patients with Cancer Therapy-Induced Cardiomyopathy. J. Clin. Med. 2025, 14, 1286. [Google Scholar] [CrossRef]
- Kondapalli, L.; Overholser, L.; Lenneman, C. Cardiac Care of Childhood Cancer Survivors: Time to Act Instead of React. J. Am. Coll. Cardiol. 2024, 83, 839–842. [Google Scholar] [CrossRef]
- Sayed, A.; Abdelfattah, O.M.; Munir, M.; Shazly, O.; Awad, A.K.; Ghaith, H.S.; Moustafa, K.; Gerew, M.; Guha, A.; Barac, A.; et al. Long-term effectiveness of empiric cardio-protection in patients receiving cardiotoxic chemotherapies: A systematic review & bayesian network meta-analysis. Eur. J. Cancer 2022, 169, 82–92. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Ong, G.; Fazelzad, R.; Amir, E.; Lee, D.S.; Thavendiranathan, P.; Tomlinson, G. Interventions for preventing cardiomyopathy due to anthracyclines: A Bayesian network meta-analysis. Ann. Oncol. 2017, 28, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Bogle, C.; Colan, S.D.; Miyamoto, S.D.; Choudhry, S.; Baez-Hernandez, N.; Brickler, M.M.; Feingold, B.; Lal, A.K.; Lee, T.M.; Canter, C.E.; et al. Treatment Strategies for Cardiomyopathy in Children: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 174–195. [Google Scholar] [CrossRef] [PubMed]
| Emerging Biomarker | Biomarker Mechanism of Action | Therapeutic Target |
|---|---|---|
| Circulating miRNAs | Regulation of apoptosis, angiogenesis, cardiac contractility | Epigenetic modulation therapies targeting signalling pathways influencing cell survival, angiogenesis, and cardiac remodelling |
| MPO | Oxidative stress, extracardiac matrix degradation | Anti-MPO to reduce oxidative damage and fibrosis |
| hs-TnI | Cardiomyocyte injury | Cardioprotective agents |
| GDF-15 | Myocardial strain, inflammation | Anti-inflammatory therapies; modulation of stress–response pathways to limit myocardial remodelling |
| ST-2 | Myocardial strain, fibrosis | Anti-fibrotic/anti remodelling agents |
| PlGF | Angiogenesis | Angiogenesis modulators |
| Gal3 | Cardiac fibrosis and remodelling | Inhibitors of Gal-3, antifibrotic strategies |
| Category | Examples | Mechanism/Rationale |
|---|---|---|
| Approved primary cardioprotective drugs | Dexrazoxane | Reduces free radicals and DNA damage in cardiomyocytes. |
| Conventional Drugs | Beta-blockers, ACE inhibitors, ARBs | Support heart function and reduce strain on the heart. |
| Repurposed Drugs | SGLT2 inhibitors | Lower inflammation and improve heart energy metabolism. |
| Molecular Targets | NOX2 inhibitors, Ferroptosis blockers | Block oxidative and inflammatory damage to heart tissue. |
| RNA-Based Therapies | siRNA, mRNA (SIRT1, VEGF) | Adjust gene activity to support heart cell survival. |
| Stem Cell Therapies | MSCs, iPSC-derived patches | Promote repair and reduce heart scarring. |
| Gene Editing | CRISPR/Cas9 (Top2β, SOD2) | Switch off harmful pathways linked to heart damage. |
| Exosome/Mito Therapies | MSC exosomes, MitoQ, SS-31 | Deliver protective signals and boost mitochondria. |
| Gut Microbiota Modulation | Probiotics, Prebiotics | Reduce systemic inflammation |
| Protein Homeostasis | Proteasome modulators | Maintain healthy proteins and prevent heart cell stress. |
| microRNA Therapies | miR-146a, miR-21 | Fine-tune repair and reduce fibrosis and cardiomyocytes’ death. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozza, A.; Di Candia, A.; Zanella, L.; Stefors, E.J.; Bennati, E.; Somigli, C.; Poli, E.; Fournier, E.; Joye, R.; Mura, R.; et al. Emerging Strategies for the Prevention of Chemotherapy-Induced Cardiotoxicity in Paediatric Cancer Patients: Advances and Future Perspectives. Pharmaceuticals 2025, 18, 1604. https://doi.org/10.3390/ph18111604
Pozza A, Di Candia A, Zanella L, Stefors EJ, Bennati E, Somigli C, Poli E, Fournier E, Joye R, Mura R, et al. Emerging Strategies for the Prevention of Chemotherapy-Induced Cardiotoxicity in Paediatric Cancer Patients: Advances and Future Perspectives. Pharmaceuticals. 2025; 18(11):1604. https://doi.org/10.3390/ph18111604
Chicago/Turabian StylePozza, Alice, Angela Di Candia, Luca Zanella, Emil Joly Stefors, Elena Bennati, Camilla Somigli, Elena Poli, Emmanuelle Fournier, Raphael Joye, Rossella Mura, and et al. 2025. "Emerging Strategies for the Prevention of Chemotherapy-Induced Cardiotoxicity in Paediatric Cancer Patients: Advances and Future Perspectives" Pharmaceuticals 18, no. 11: 1604. https://doi.org/10.3390/ph18111604
APA StylePozza, A., Di Candia, A., Zanella, L., Stefors, E. J., Bennati, E., Somigli, C., Poli, E., Fournier, E., Joye, R., Mura, R., Fagioli, F., & Bertorello, N. (2025). Emerging Strategies for the Prevention of Chemotherapy-Induced Cardiotoxicity in Paediatric Cancer Patients: Advances and Future Perspectives. Pharmaceuticals, 18(11), 1604. https://doi.org/10.3390/ph18111604