The Effects and Mechanisms of Ti-Fu-Kang Decoction in Alleviating Central Fatigue: Insights from Network Pharmacology and Metabolomics
Abstract
1. Introduction
2. Results
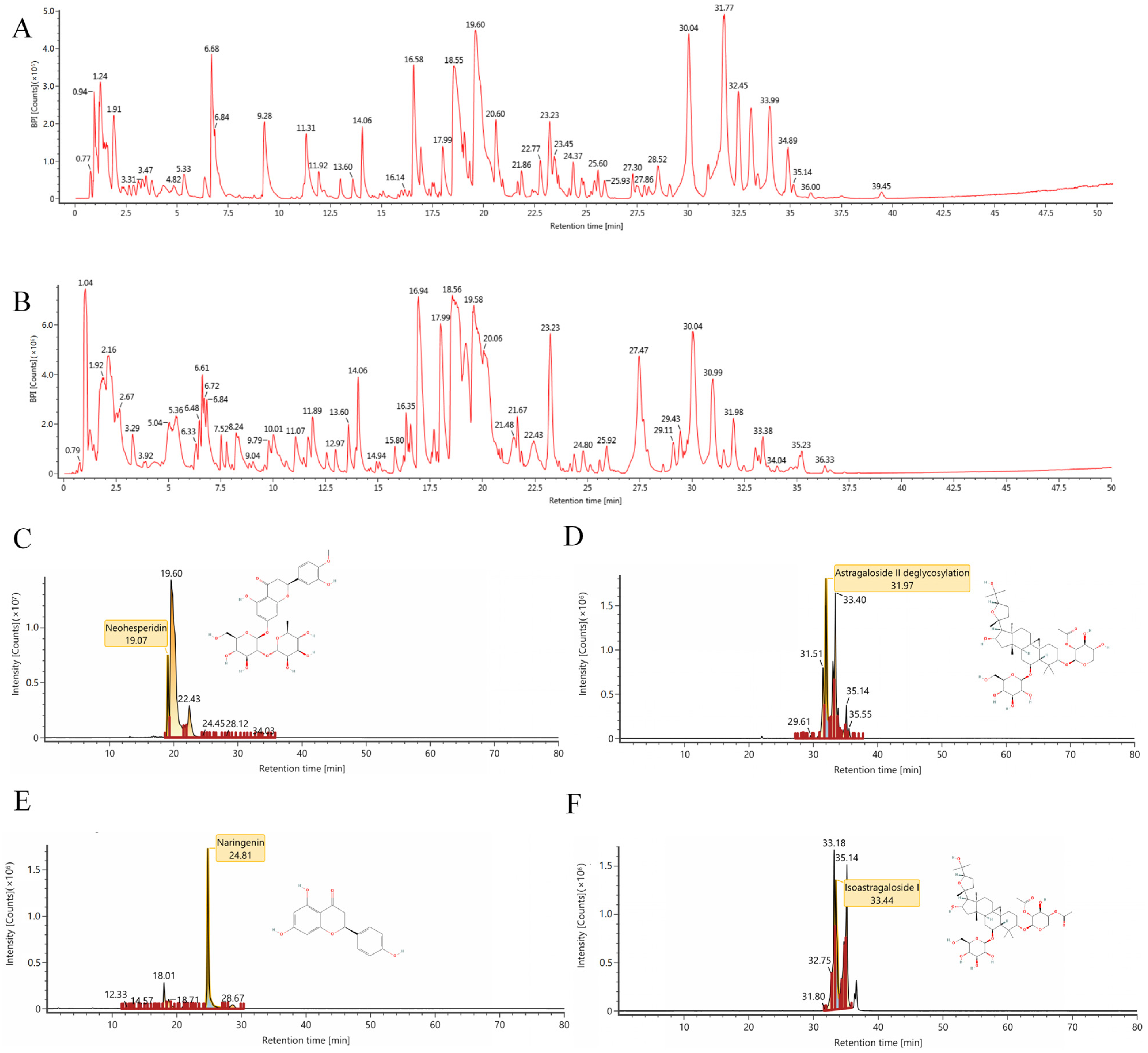
2.1. The Results of Ultra High-Performance Liquid Chromatography-Mass Spectrometry (UHPLC-MS/MS) Analysis
2.2. Network Pharmacology-Based Analysis
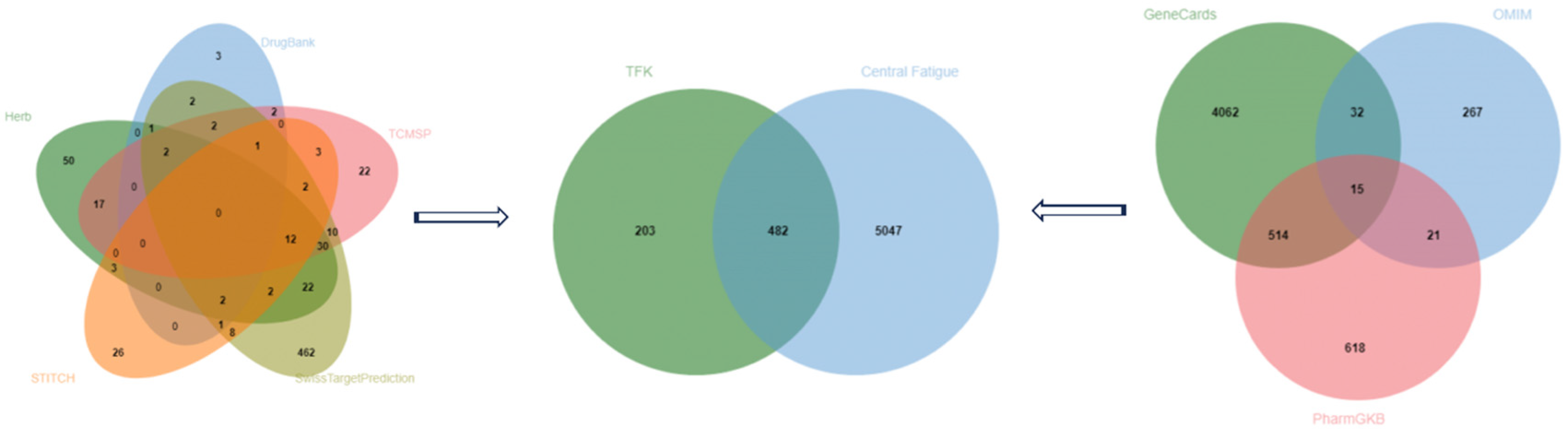
2.2.1. Potential Therapeutic Targets of TFK in the Treatment of CF
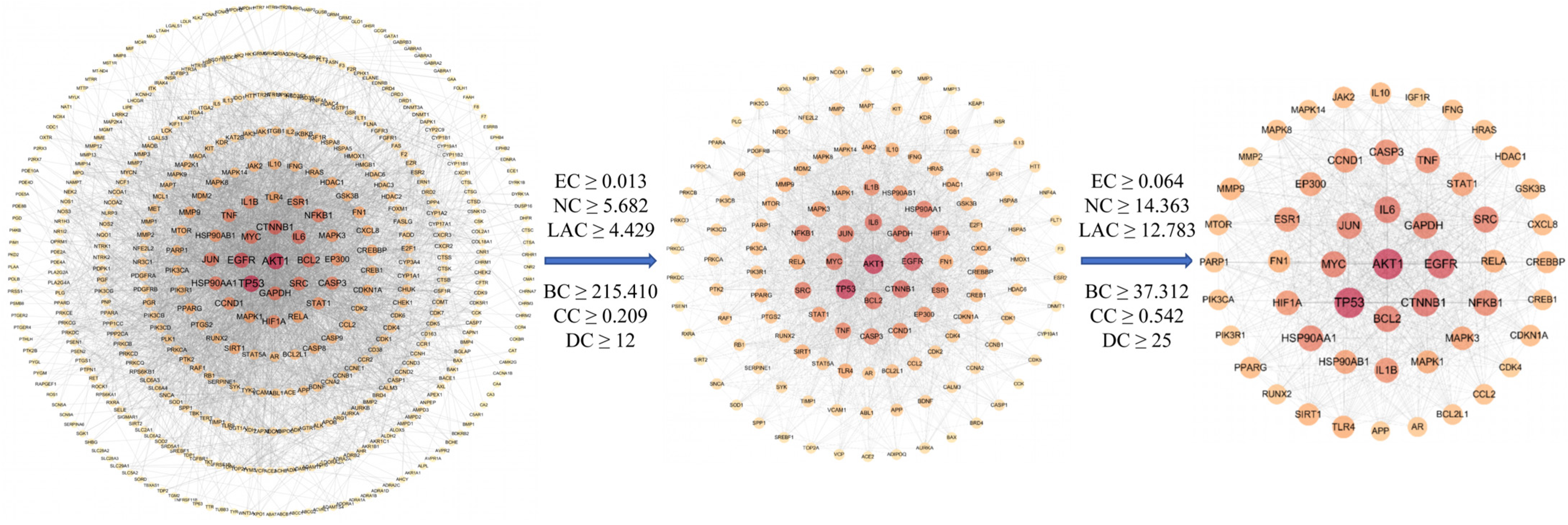
2.2.2. Protein–Protein Interaction (PPI) Network Construction and Topological Screening
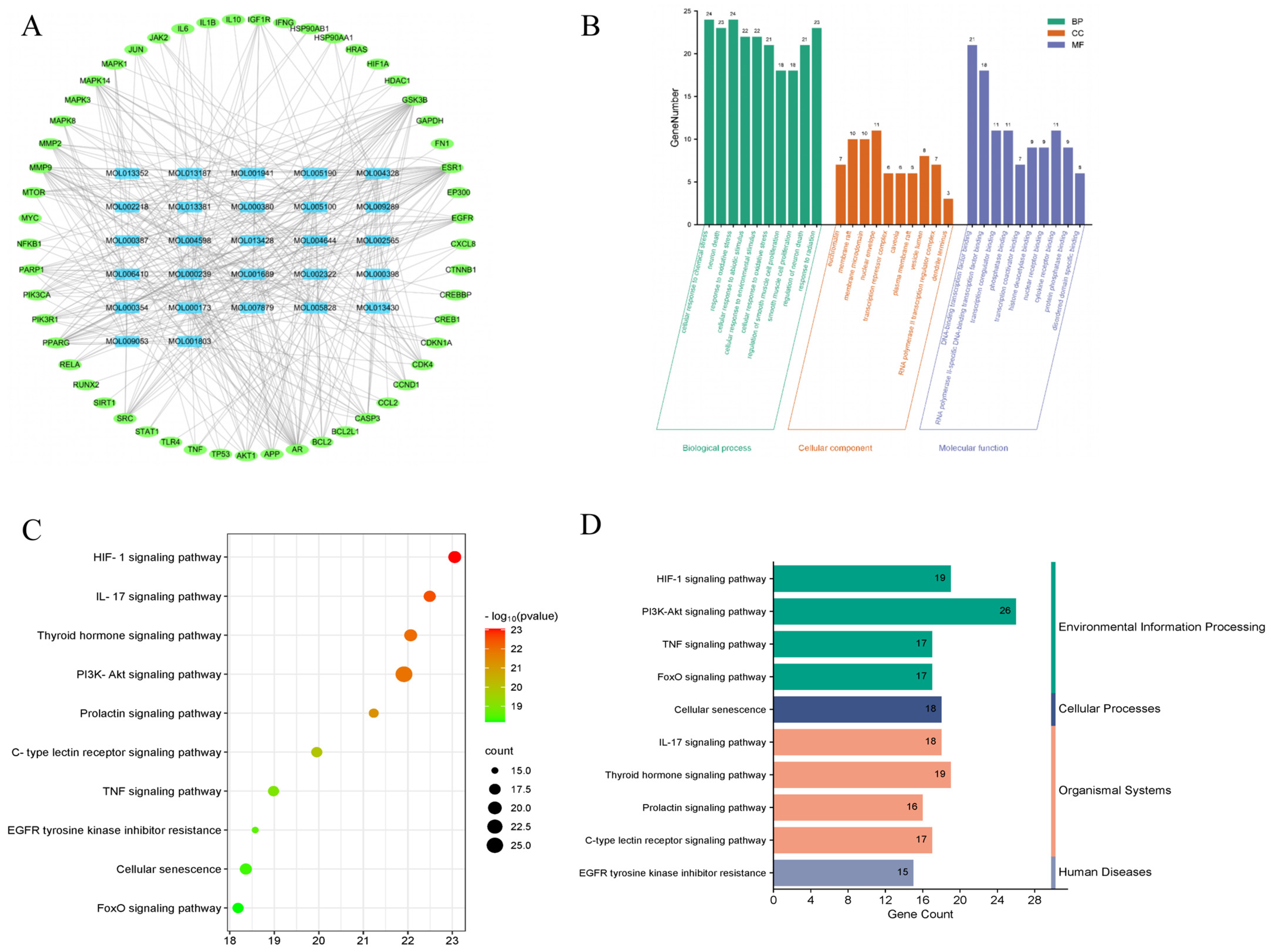
2.2.3. The Compound-Target Network
2.2.4. Gene Ontology (GO) Analysis of Hub Targets
2.2.5. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis of Hub Targets
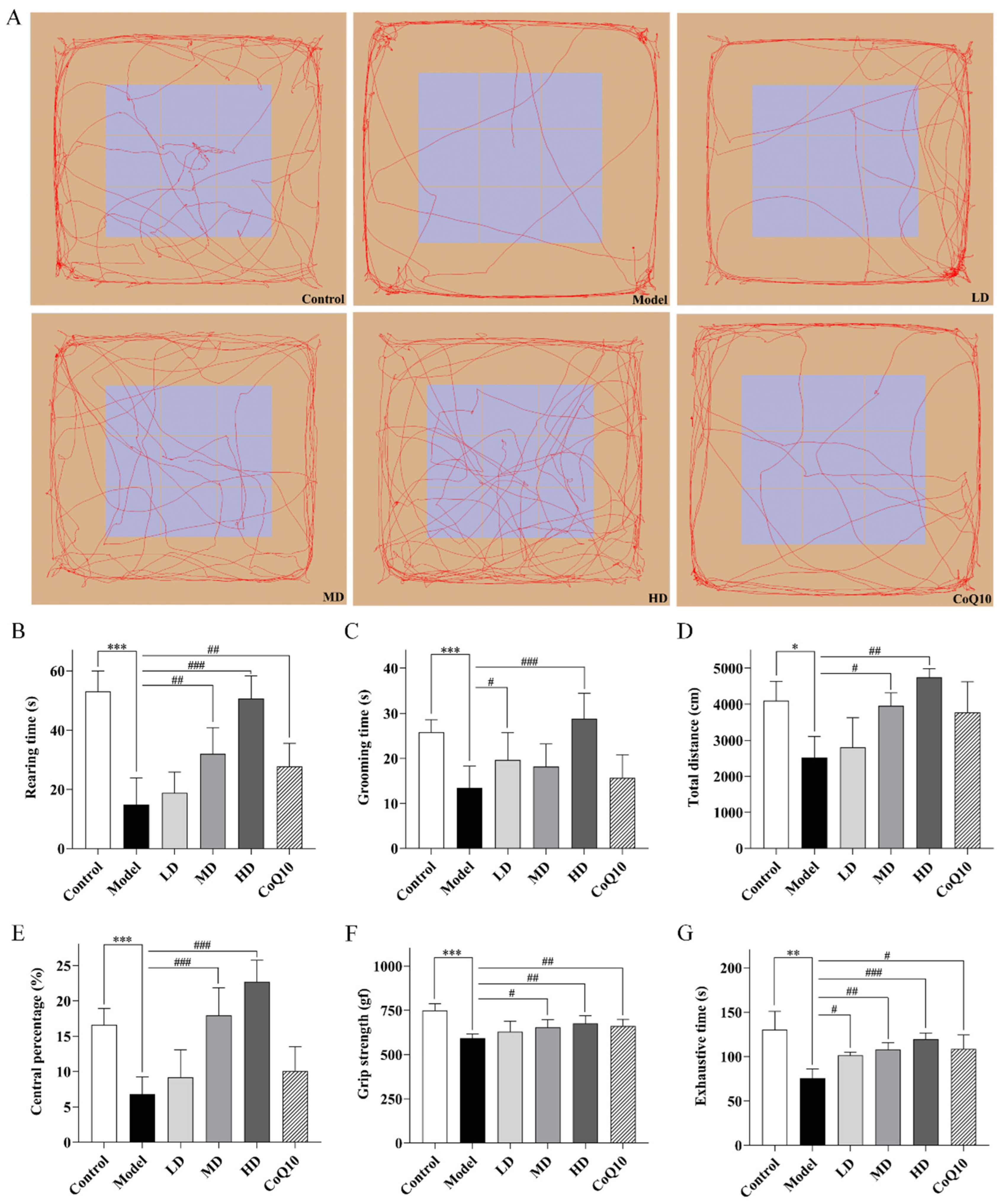
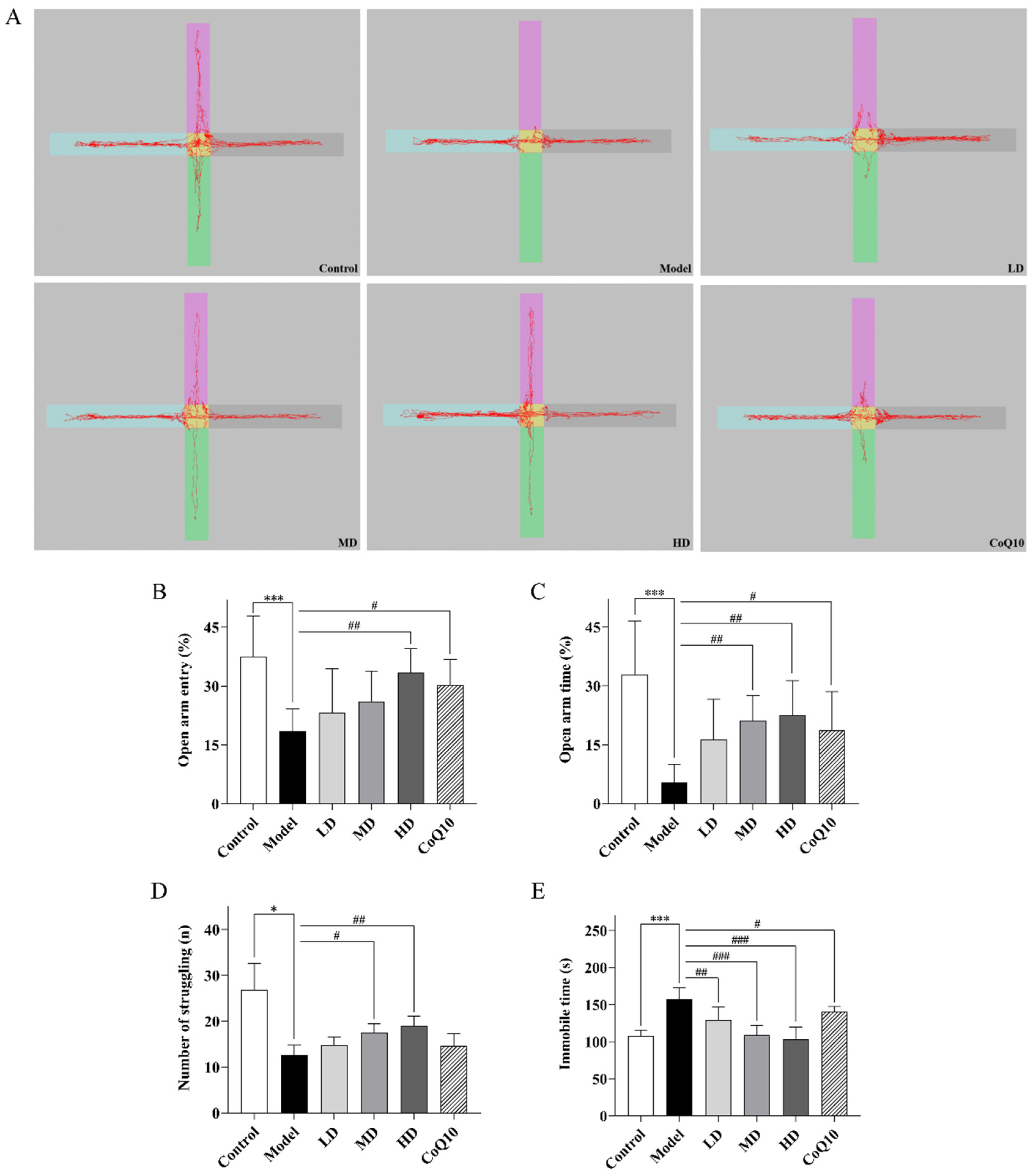
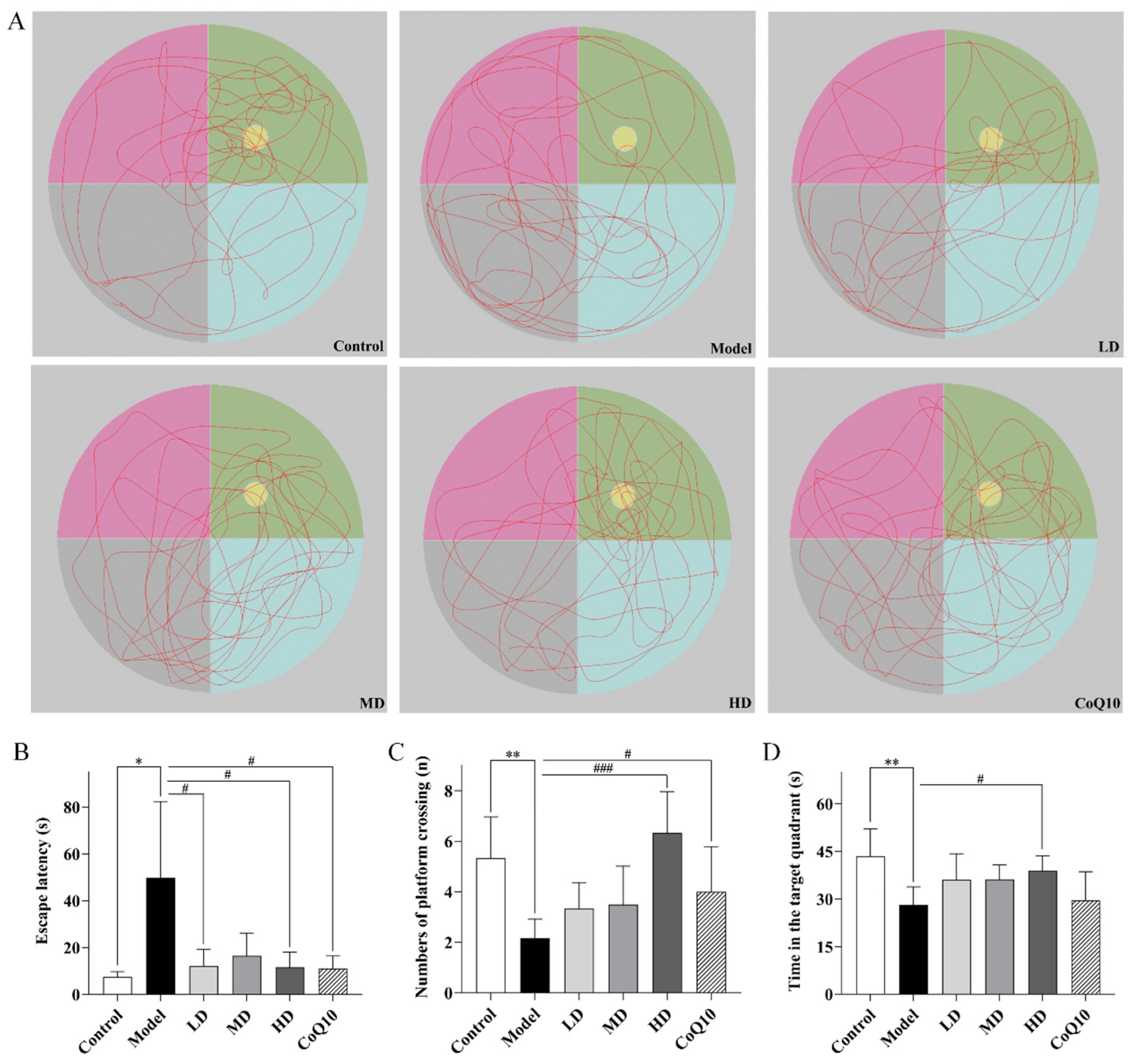
2.3. TFK Ameliorates Abnormal Behavioral Symptoms in Rats with Central Fatigue
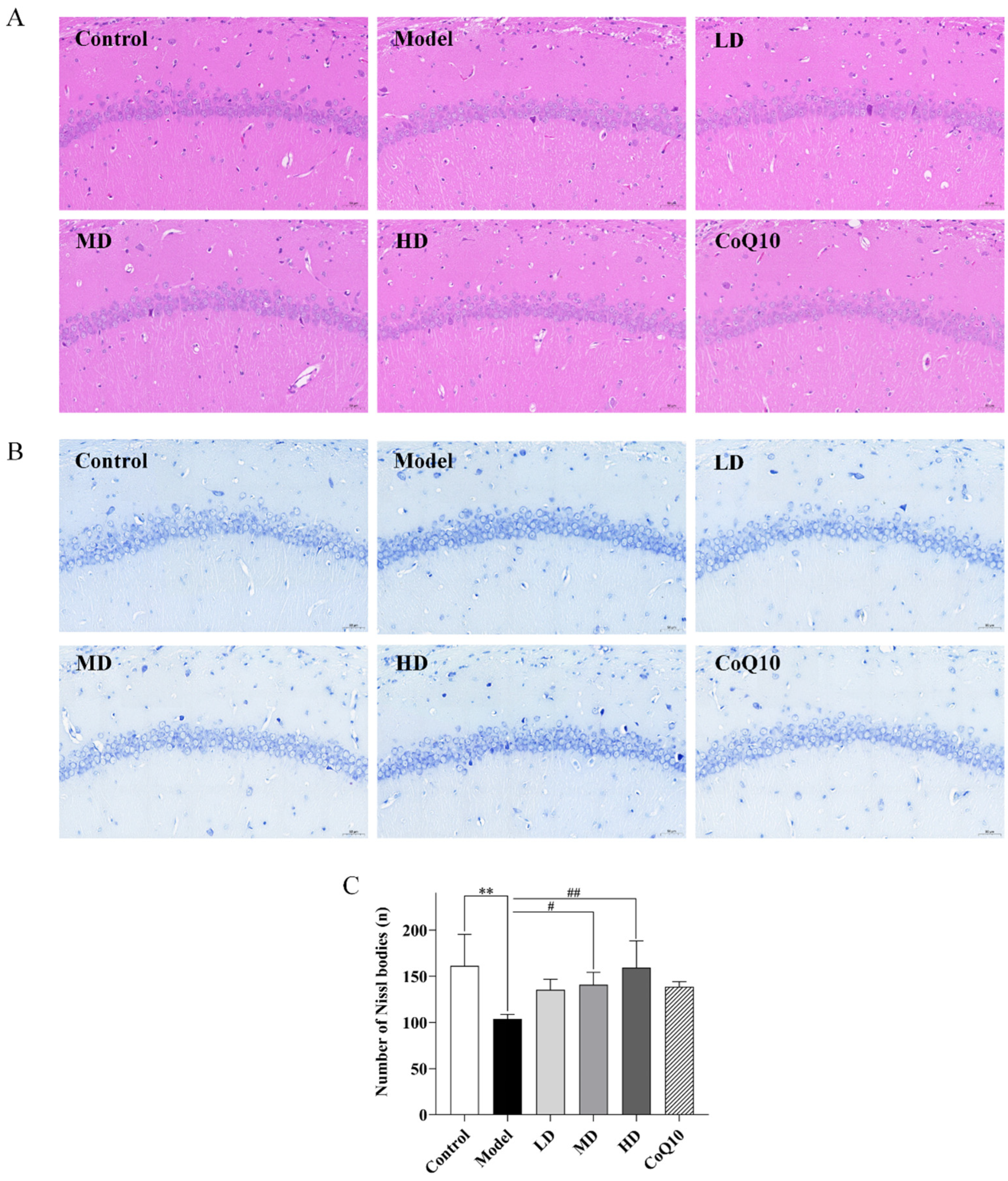
2.4. TFK Alleviates the Morphological Changes in the Hippocampal Tissues of Central Fatigue Rats
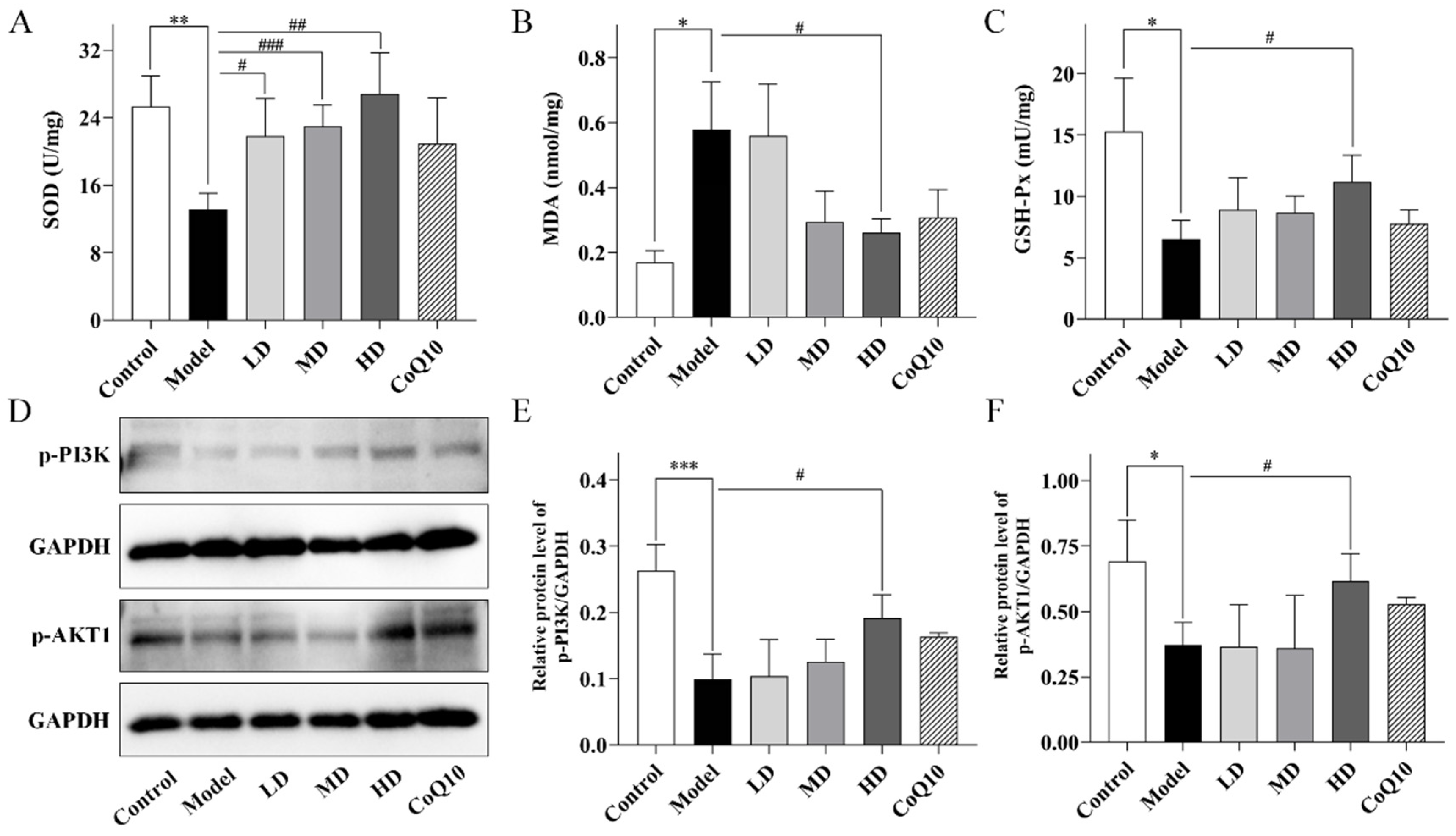
2.5. TFK Ameliorates Oxidative Stress Damage in the Hippocampal Tissues Involving PI3K-AKT Signaling Pathway
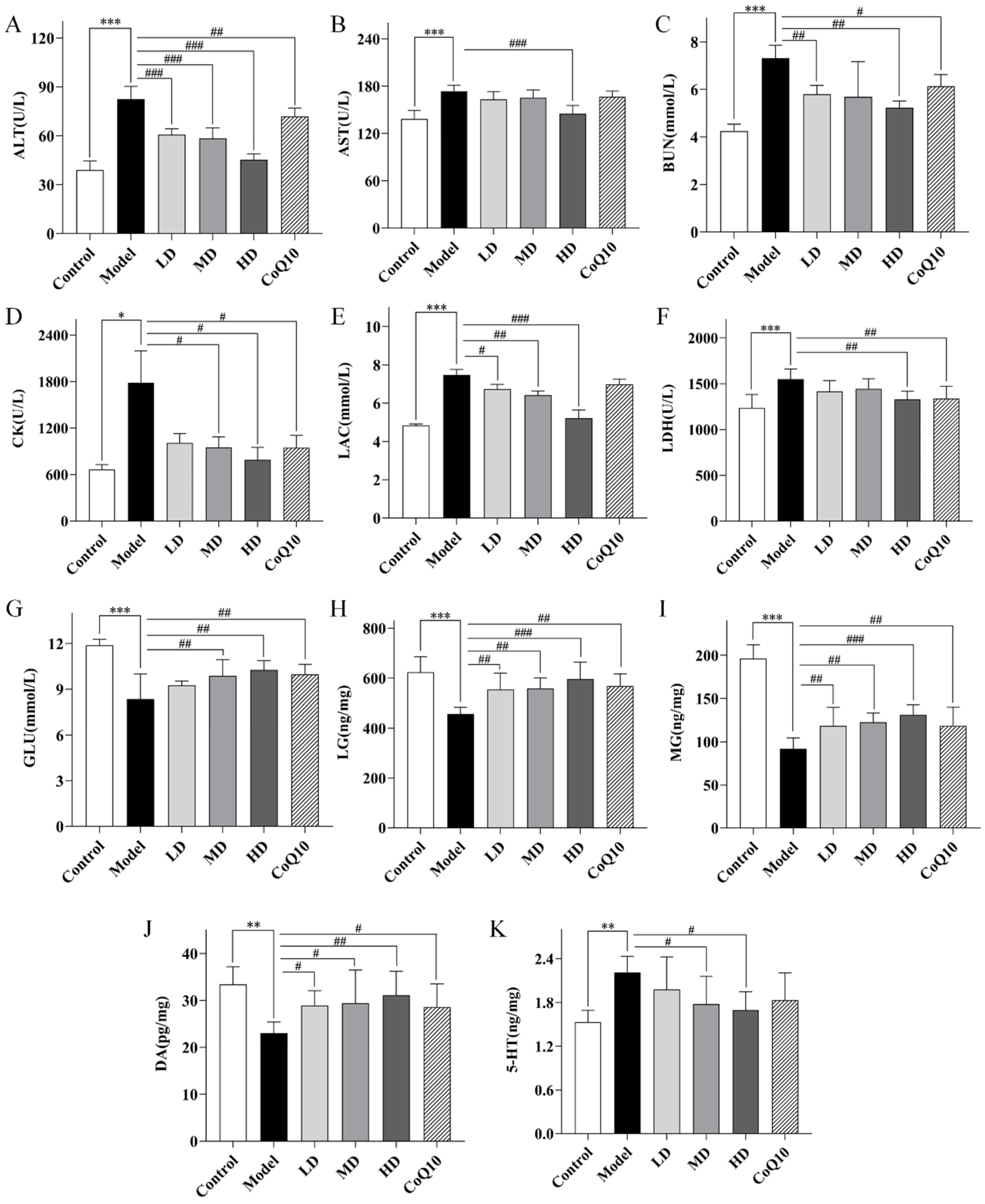
2.6. TFK Modulates Neurotransmitter Levels and Biochemical Metabolic Markers in Central Fatigue Rats
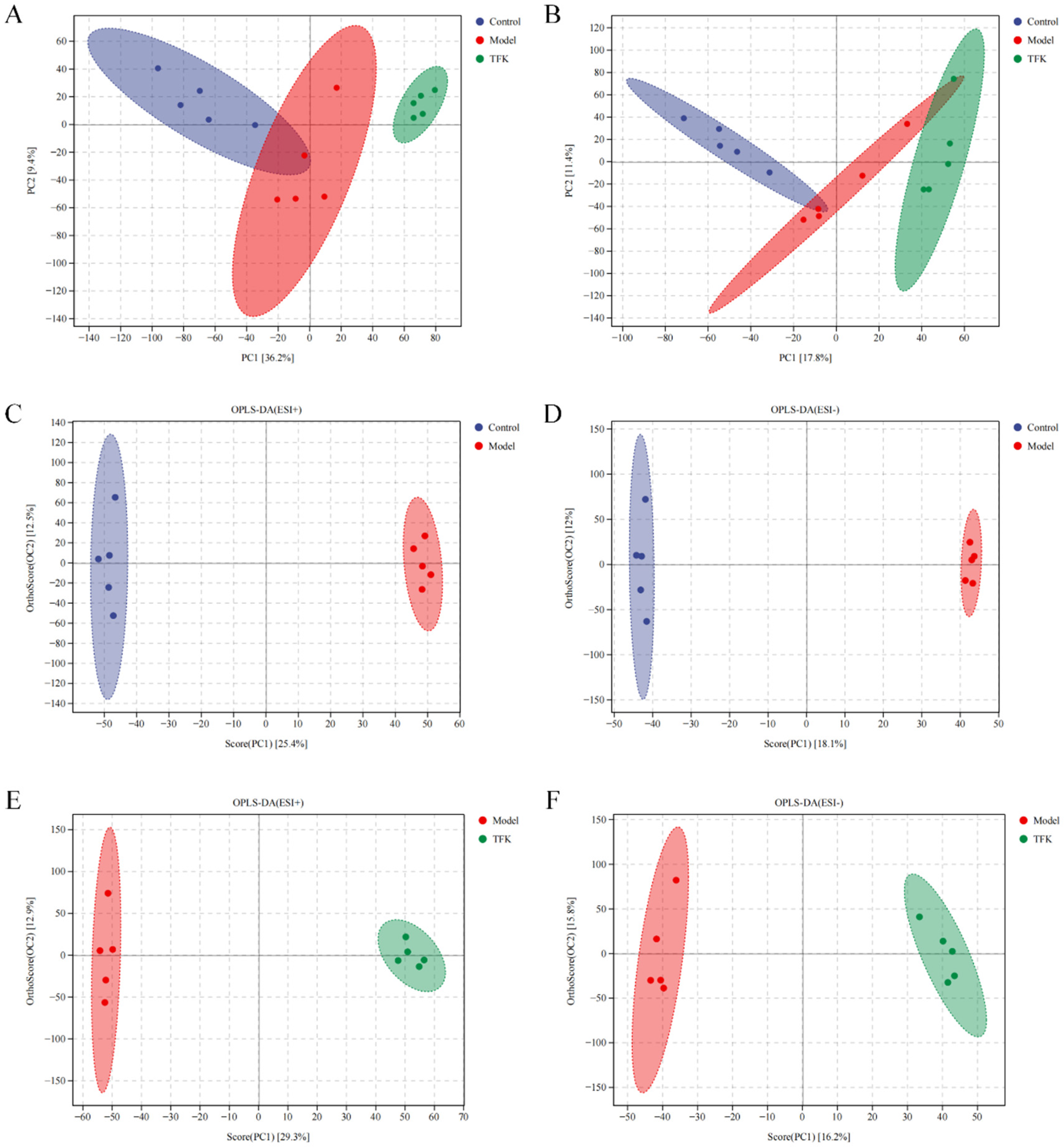
2.7. Untargeted Serum Metabolomics Analysis
3. Discussion
4. Materials and Methods
4.1. TFK Preparation and UHPLC-MS/MS Analysis
4.2. Network Pharmacology Analysis
4.2.1. Active Compounds and Targets of TFK
4.2.2. Disease Targets
4.2.3. Common Targets Screening
4.2.4. PPI Network
4.2.5. Compound-Target Network
4.2.6. GO and KEGG Enrichment Analyses
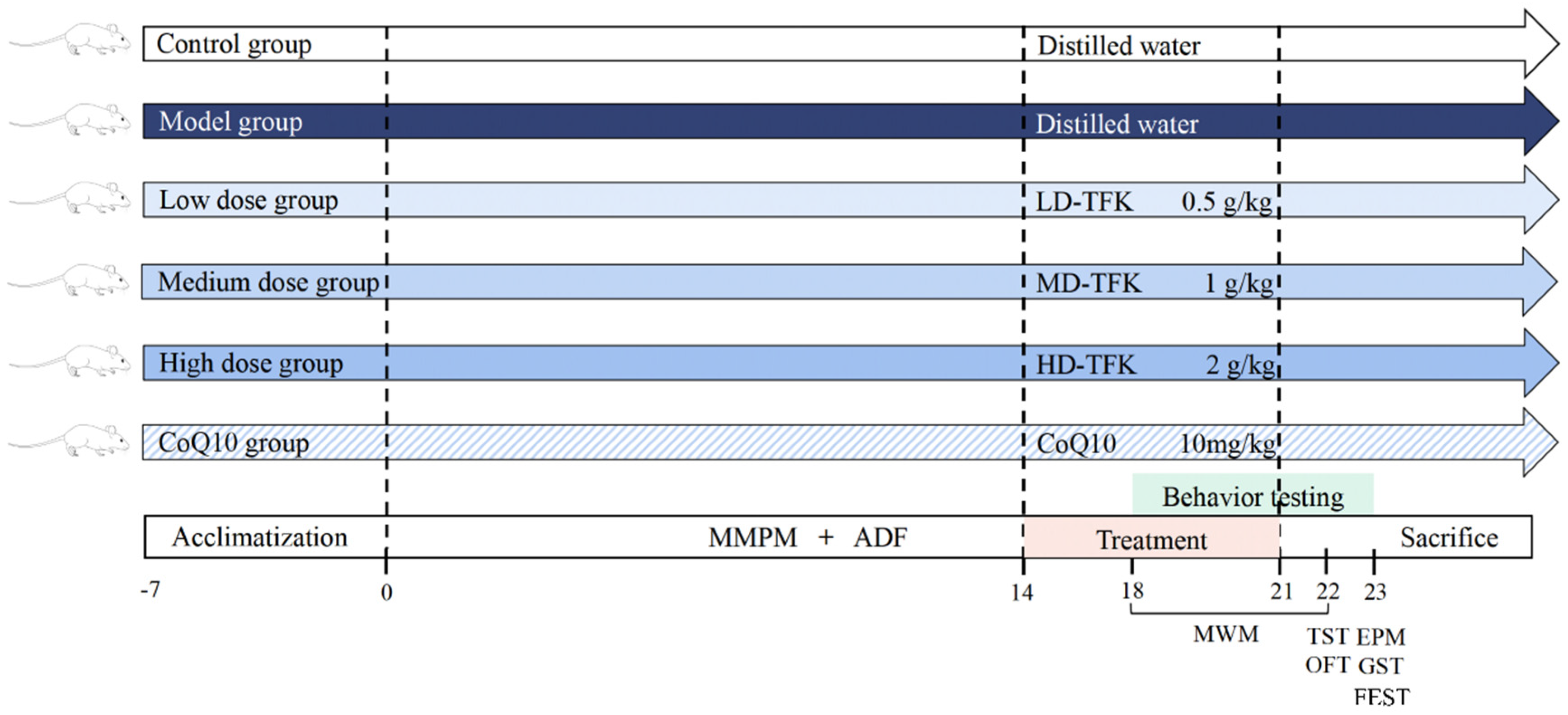
4.3. Animal Grouping and Model Establishment
4.4. Behavioral Testing
4.4.1. Tail Suspension Test
4.4.2. Morris Water Maze
4.4.3. Open Field Test
4.4.4. Elevated Plus Maze Test
4.4.5. Grip Strength Test
4.4.6. Forced Exhaustive Swimming Test
4.5. Histopathological Staining
4.6. Serum Biochemical Index Detection
4.7. ELISA
4.8. Measurement of SOD, MDA and GSH-Px
4.9. Western Blot
4.10. Serum Metabolomic Analysis
4.10.1. Preparation of Samples
4.10.2. Acquisition of LC-MS Data
4.10.3. Data Processing and Analysis
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Zou, Z.; Yang, J.; Li, X.; Zhu, B.; Zhang, H.; Sun, Y.; Zhang, Y.; Zhang, Z.-J.; Wang, W. Jianpi Jieyu Decoction, An Empirical Herbal Formula, Exerts Psychotropic Effects in Association with Modulation of Gut Microbial Diversity and GABA Activity. Front. Pharmacol. 2021, 12, 645638. [Google Scholar] [CrossRef]
- Lee, H.; Choi, S. Factors Affecting Fatigue among Nurses during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 11380. [Google Scholar]
- Ishii, A.; Tanaka, M.; Yoshikawa, T.; Watanabe, Y. Evidence for unconscious regulation of performance in fatigue. Sci. Rep. 2017, 7, 16103. [Google Scholar] [CrossRef]
- Barrios, L.; Amon, R.; Oldrati, P.; Hilty, M.; Holz, C.; Lutterotti, A. Cognitive fatigability assessment test (cFAST): Development of a new instrument to assess cognitive fatigability and pilot study on its association to perceived fatigue in multiple sclerosis. Digit. Health 2022, 8, 20552076221117740. [Google Scholar]
- Kośnik, A.; Wójcicki, M. Fatigue in chronic liver disease patients: Prevalence, pathophysiology, and management. Gastroenterol. Rev./Przegląd Gastroenterol. 2022, 17, 21–27. [Google Scholar] [CrossRef]
- Zaehle, T. Frontal Transcranial Direct Current Stimulation as a Potential Treatment of Parkinson’s Disease-Related Fatigue. Brain Sci. 2021, 11, 467. [Google Scholar]
- Yang, M.; Tao, L.; Zhao, C.-C.; Wang, Z.-L.; Yu, Z.-J.; Zhou, W.; Wen, Y.-L.; Li, L.-F.; Tian, Y.; Sheng, J. Antifatigue Effect of Panax Notoginseng Leaves Fermented with Microorganisms: In-vitro and In-vivo Evaluation. Front. Nutr. 2022, 9, 824525. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Kim, D.-Y.; Lee, J.-S.; Hwang, S.-J.; Kim, G.-H.; Hyun, S.-H.; Son, C.-G. Korean Red Ginseng Ameliorates Fatigue via Modulation of 5-HT and Corticosterone in a Sleep-Deprived Mouse Model. Nutrients 2021, 13, 3121. [Google Scholar] [CrossRef]
- Kwak, J.-J.; Yook, J.S.; Ha, M.-S. Potential Biomarkers of Peripheral and Central Fatigue in High-Intensity Trained Athletes at High-Temperature: A Pilot Study with Momordica charantia (Bitter Melon). J. Immunol. Res. 2020, 2020, 4768390. [Google Scholar]
- Qazi, T. Fatigue in inflammatory bowel disease: A problematic ailment. Curr. Opin. Gastroenterol. 2020, 36, 284–294. [Google Scholar] [CrossRef]
- Yamashita, M. Potential Role of Neuroactive Tryptophan Metabolites in Central Fatigue: Establishment of the Fatigue Circuit. Int. J. Tryptophan Res. 2020, 13, 1178646920936279. [Google Scholar] [CrossRef] [PubMed]
- Liang, R. Human Clinical Trial Investigating the Efficacy of Chinese Herbal Formula ‘Tifukang’ in Alleviating Exercise-Induced Fatigue. China J. Basic Med. Tradit. Chin. Med. 1998, 10, 41–42. [Google Scholar]
- Li, F.; Yang, W.; Ji, S.; Feng, L.; Gao, D.; Liang, R.; Lv, D.; Liu, X.; Wang, T.; Kang, C.; et al. Experimental Study on the Function of the Chinese Medicine Recipe “TiFuKang” in the Regulation of the Gene Expression of ET-1 and cNOS in Cerebrum. Chin. J. Sports Med. 2001, 2, 116–120. [Google Scholar]
- Guo, S. Mechanism Study on the Effect of Body Fukang Plus and Minus Formula on the Cognitive Function of Rats with Psychological Fatigue Model. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2016. [Google Scholar]
- Xu, Y.; Lian, Y.; Li, J.; Zhang, Y.; Liu, Y.; Wang, X.; Ma, J.; Li, F. KangPiLao decoction modulates cognitive and emotional disorders in rats with central fatigue through the GABA/Glu pathway. Front. Pharmacol. 2022, 13, 939169. [Google Scholar] [CrossRef]
- Wei, X.; Xin, J.; Chen, W.; Wang, J.; Lv, Y.; Wei, Y.; Li, Z.; Ding, Q.; Shen, Y.; Xu, X.; et al. Astragalus polysaccharide ameliorated complex factor-induced chronic fatigue syndrome by modulating the gut microbiota and metabolites in mice. Biomed. Pharmacother. 2023, 163, 114862. [Google Scholar] [CrossRef]
- Li, L.; Hou, X.; Xu, R.; Liu, C.; Tu, M. Research review on the pharmacological effects of astragaloside IV. Clin. Pharmacol. 2016, 31, 17–36. [Google Scholar]
- Jiang, P.; Ji, X.; Xia, J.; Xu, M.; Hao, F.; Tong, H.; Jiao, L. Structure and potential anti-fatigue mechanism of polysaccharides from Bupleurum chinense DC. Carbohydr. Polym. 2023, 306, 120608. [Google Scholar] [CrossRef]
- Zuo, X.; Shi, X.; Zhang, X.; Chen, Z.; Yang, Z.; Pan, X.; Lai, R.; Zhao, Z. Postoperative Ileus with the Topical Application of Tongfu Decoction Based on Network Pharmacology and Experimental Validation. Evid.-Based Complement. Altern. Med. 2022, 2022, 2347419. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Zhang, X.; Zhang, S.; Jiang, Y.; Yu, Z.; Xie, T.; Chen, Y.; Chen, L.; Li, J. Mechanism of Yangxin Tongmai Decoction in the Treatment of Coronary Heart Disease with Blood Stasis Syndrome Based on Network Pharmacology and Molecular Docking. Evid.-Based Complement. Altern. Med. 2022, 2022, 4692217. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Y.; Chen, Q.; Zheng, W.; Wang, J.; Zeng, H.; Wu, Y.; Cao, L.; Chen, Z. Metabolomics Analysis of Laparoscopic Surgery Combined with Wuda Granule to Promote Rapid Recovery of Patients with Colorectal Cancer Using UPLC/Q-TOF-MS/MS. Evid.-Based Complement. Altern. Med. 2020, 2020, 5068268. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, A.; Wu, F.; Wang, X. UPLC-G2Si-HDMS Untargeted Metabolomics for Identification of Yunnan Baiyao’s Metabolic Target in Promoting Blood Circulation and Removing Blood Stasis. Molecules 2022, 27, 3208. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Tsai, A.C.; Huang, A.C.W.; Yu, Y.H.; Kuo, C.-S.; Hsu, C.-C.; Lim, Y.S.; Shyu, B.C. A Wireless Magnetic Resonance Device for Optogenetic Applications in an Animal Model. Sensors 2020, 20, 5869. [Google Scholar] [CrossRef] [PubMed]
- Venus, M. Interactions of International Pilots’ Stress, Fatigue, Symptoms of Depression, Anxiety, Common Mental Disorders and Wellbeing. Int. J. Aviat. Aeronaut. Aerosp. 2022, 9, 4. [Google Scholar] [CrossRef]
- Liu, X.-J.; Wang, Y.-Z.; Wei, F.-X.; Lv, M.; Qu, P.; Chen, S.-J.; Li, S.-Y.; Qin, X. The synergistic anti-depression effects of different efficacy groups of Xiaoyaosan as demonstrated by the integration of network pharmacology and serum metabolomics. J. Pharm. Biomed. Anal. 2021, 197, 113949. [Google Scholar] [CrossRef]
- Ayache, S.S.; Chalah, M.A. Cognitive fatigability in the healthy brain: Neurophysiological substrates and the use of tDCS. Clin. Neurophysiol. 2021, 132, 1714–1715. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, R.-T.; Liu, Y.; Li, F. The Effect of Anti-fatigue Decoction on the Behaviors and Serological Indicators in a Central Fatigue Rat Model. J. Vis. Exp. 2024, 206, e66481. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Jiang, Y.; Zhang, Y.; Zhang, Y.; Wang, B.; Lu, H.; Su, H.; Liao, W.; Liu, L.; et al. Shenling Baizhu San alleviates central fatigue through SIRT1-PGC-1α-Mediated mitochondrial biogenesis. J. Ethnopharmacol. 2024, 339, 119110. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Yu, Q.; Lan, B.; Shi, Q.; Li, R.; Jiao, Z.; Zhang, W.; Li, F. Replicating human characteristics: A promising animal model of central fatigue. Brain Res. Bull. 2024, 212, 110951. [Google Scholar] [CrossRef]
- Seguro, C.K.; Beckler, M.D.; Kesselman, M.M. Targeting the NOD-, LRR- and Pyrin Domain-Containing Protein 3 (NLRP3) Inflammasome in Psoriasis and Fatigue. Cureus 2022, 14, e24704. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Z.; Wei, L.; Zhao, H.-F.; Huang, B.-K.; Rahman, K.; Qin, L.-P. The effect of Eleutheroside E on behavioral alterations in murine sleep deprivation stress model. Eur. J. Pharmacol. 2011, 658, 150–155. [Google Scholar] [CrossRef]
- Owen, A.M.; Patel, S.P.; Smith, J.D.; Balasuriya, B.K.; Mori, S.F.; Hawk, G.S.; Stromberg, A.J.; Kuriyama, N.; Kaneki, M.; Rabchevsky, A.G.; et al. Chronic muscle weakness and mitochondrial dysfunction in the absence of sustained atrophy in a preclinical sepsis model. eLife 2019, 8, e49920. [Google Scholar] [CrossRef]
- Arsenopoulos, K.V.; Triantafillou, E.; Gelasakis, A.I.; Papadopoulos, E. Deltamethrin Application on Pre-Weaned Calves Improves Feed Consumption, Stress and Fatigue Status under Heat Stress Conditions. Pathogens 2022, 11, 85. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, M.; Li, H.; Xu, R.; Liu, J. miR-130b regulates PTEN to activate the PI3K/Akt signaling pathway and attenuate oxidative stress-induced injury in diabetic encephalopathy. Int. J. Mol. Med. 2021, 48, 141. [Google Scholar]
- Zeng, X.; Yu, J.; Liu, P.; Liu, Y.; Zeng, T.; Li, B. Asiaticoside alleviates cardiomyocyte apoptosis and oxidative stress in myocardial ischemia/reperfusion injury via activating the PI3K-AKT-GSK3β pathway in vivo and in vitro. Ann. Transl. Med. 2022, 10, 69. [Google Scholar]
- Zhao, S.; Chi, A.; Wan, B.; Liang, J. Differential Metabolites and Metabolic Pathways Involved in Aerobic Exercise Improvement of Chronic Fatigue Symptoms in Adolescents Based on Gas Chromatography-Mass Spectrometry. Int. J. Environ. Res. Public Health 2022, 19, 2377. [Google Scholar]
- Liu, K.; Du, Y.; Li, H.; Lin, X. Identification of super-enhancer-associated transcription factors regulating glucose metabolism in poorly differentiated thyroid carcinoma. Genet. Mol. Biol. 2022, 45, e20210370. [Google Scholar]
- Yen, C.-C.; Liang, Y.-K.; Cheng, C.-P.; Hsu, M.-C.; Wu, Y.-T. Oral Bioavailability Enhancement and Anti-Fatigue Assessment of the Andrographolide Loaded Solid Dispersion. Int. J. Mol. Sci. 2020, 21, 2506. [Google Scholar] [CrossRef]
- Rama, S.; Manjabhat, S.N. Protective effect of shrimp carotenoids against ammonia stress in common carp, Cyprinus carpio. Ecotoxicol. Environ. Saf. 2014, 107, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Lin, W.; Zou, J.; Mei, J.; Wen, Y.; Lu, L.; Guo, R. Development and Validation of a Novel Nomogram for Predicting Vessels that Encapsulate Tumor Cluster in Hepatocellular Carcinoma. Cancer Control. 2022, 29, 10732748221102820. [Google Scholar] [CrossRef] [PubMed]
- Saunoriene, L.; Siauciunaite, V.; Vainoras, A.; Bertasiute, V.; Navickas, Z.; Ragulskis, M. The characterization of the transit through the anaerobic threshold based on relationships between RR and QRS cardiac intervals. PLoS ONE 2019, 14, e0216938. [Google Scholar] [CrossRef] [PubMed]
- Juan, S.; Lee, J.-H.; Won, S.-J.; Oh, S.; Ha, M.-S. Effect of Saengmaeksan on Fatigue, Liver Function, and Immunity Combined with High-Intensity Training. J. Immunol. Res. 2023, 2023, 3269293. [Google Scholar] [CrossRef]
- Webster, R.G.; Cossins, J.; Lashley, D.; Maxwell, S.; Liu, W.W.; Wickens, J.R.; Martinez-Martinez, P.; de Baets, M.; Beeson, D. A mouse model of the slow channel myasthenic syndrome: Neuromuscular physiology and effects of ephedrine treatment. Exp. Neurol. 2013, 248, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Kan, R.L.; Xu, G.X.; Shu, K.T.; Lai, F.H.; Kranz, G.; Kranz, G.S. Effects of non-invasive brain stimulation in multiple sclerosis: Systematic review and meta-analysis. Ther. Adv. Chronic Dis. 2022, 13, 20406223211069198. [Google Scholar] [CrossRef]
- Krasowski, M.D.; Harrison, N.L. General anaesthetic actions on ligand-gated ion channels. Cell. Mol. Life Sci. 1999, 55, 1278–1303. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Large neutral amino acids: Dietary effects on brain neurochemistry and function. Amino Acids 2013, 45, 419–430. [Google Scholar]
- Zhang, W.; Zhang, W.; Dai, N.; Han, C.; Wu, F.; Wang, X.; Tan, L.; Li, J.; Li, F.; Ren, Q. A Rat Model of Central Fatigue Using a Modified Multiple Platform Method. J. Vis. Exp. 2018, 138, 57362. [Google Scholar]
- Sur, B.; Lee, B. Myricetin Inhibited Fear and Anxiety-Like Behaviors by HPA Axis Regulation and Activation of the BDNF-ERK Signaling Pathway in Posttraumatic Stress Disorder Rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 8320256. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, P.; Rueda-Robles, A.; Sánchez-Rodríguez, L.; Blanca-Herrera, R.M.; Quirantes-Piné, R.M.; Borrás-Linares, I.; Segura-Carretero, A.; Lozano-Sánchez, J. Analysis and Screening of Commercialized Protein Supplements for Sports Practice. Foods 2022, 11, 3500. [Google Scholar] [CrossRef]
- Nielsen, C.U.; Frølund, S.; Abdulhadi, S.; Sari, H.; Langthaler, L.; Nøhr, M.K.; A Kall, M.; Brodin, B.; Holm, R. Sertraline inhibits the transport of PAT1 substrates in vivo and in vitro. Br. J. Pharmacol. 2013, 170, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Yu, Q.Q.; Zhang, Z.H.; Lan, B.J.; Shi, Q.H.; Li, F. Intervention mechanism of Ti-Fu-Kang in hippocampal oxidative stress damage in rats with central fatigue liver stagnation and spleen deficiency syndrome by regulation of mitophagy. China J. Chin. Mater. Medica 2025, 40, 2316–2323. [Google Scholar]
- Panteleev, J.; Gao, H.; Jia, L. Recent applications of machine learning in medicinal chemistry. Bioorganic Med. Chem. Lett. 2018, 28, 2807–2815. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Spinelli, D.; Geronikaki, A.; Kartsev, V.; Hakobyan, E.K.; Petrou, A.; Paronikyan, R.G.; Nazaryan, I.M.; Akopyan, H.H.; Hovakimyan, A.A. Synthesis and Neurotropic Activity of New Heterocyclic Systems: Pyridofuro[3,2-d]pyrrolo[1,2-a]pyrimidines, Pyridofuro[3,2-d]pyrido[1,2-a]pyrimidines and Pyridofuro[3′,2′:4,5]pyrimido[1,2-a]azepines. Molecules 2021, 26, 3320. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Fang, S.; Dong, L.; Liu, L.; Guo, J.; Zhao, L.; Zhang, J.; Bu, D.; Liu, X.; Huo, P.; Cao, W.; et al. HERB: A high-throughput experiment- and reference-guided database of traditional Chinese medicine. Nucleic Acids Res. 2021, 49, D1197–D1206. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Manuel, A.M.; Dai, Y.; Freeman, L.A.; Jia, P.; Zhao, Z. Dense module searching for gene networks associated with multiple sclerosis. BMC Med Genom. 2020, 13 (Suppl. 5), 48. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Szklarczyk, D.; Franceschini, A.; Campillos, M.; von Mering, C.; Jensen, L.J.; Beyer, A.; Bork, P. STITCH 2: An interaction network database for small molecules and proteins. Nucleic Acids Res. 2010, 38, D552–D556. [Google Scholar] [CrossRef]
- Sinan, K.I.; Zengin, G.; Zheleva-Dimitrova, D.; Etienne, O.K.; Mahomoodally, M.F.; Bouyahya, A.; Lobine, D.; Chiavaroli, A.; Ferrante, C.; Menghini, L.; et al. Qualitative Phytochemical Fingerprint and Network Pharmacology Investigation of Achyranthes aspera Linn. Extracts. Molecules 2020, 25, 1973. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Scott, A.F.; Hamosh, A. OMIM.org: Leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. 2019, 47, D1038–D1043. [Google Scholar] [CrossRef] [PubMed]
- Whirl-Carrillo, M.; Huddart, R.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Whaley, R.; Klein, T.E. An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2021, 110, 563–572. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Tsai, I.-C.; Hsu, C.-W.; Chang, C.-H.; Tseng, P.-T.; Chang, K.-V. Effectiveness of Coenzyme Q10 Supplementation for Reducing Fatigue: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2022, 13, 883251. [Google Scholar] [CrossRef]
- Li, Y. Experimental Methodology of Traditional Chinese Medicine Pharmacology; Shanghai Science and Technology Press: Shanghai, China, 2016; Volume 119. [Google Scholar]
- Lindberg, F.A.; Nordenankar, K.; Fredriksson, R. SLC38A10 Knockout Mice Display a Decreased Body Weight and an Increased Risk-Taking Behavior in the Open Field Test. Front. Behav. Neurosci. 2022, 16, 840987. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.R.; Simpson, S.R.; Sherman, E.R.; Raby-Smith, W.; Azvine, K.; Arribas, M.; Zou, J.; Deiana, S.; Hengerer, B.; Cahill, E.N. Reactivity to conditioned threat cues is distinct from exploratory drive in the elevated plus maze. Eur. J. Neurosci. 2023, 57, 54–63. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, R.; Hua, H.; Qian, H.; Du, P. Deciphering the potential role of Maca compounds prescription influencing gut microbiota in the management of exercise-induced fatigue by integrative genomic analysis. Front. Nutr. 2022, 9, 1004174. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, Y.; Gao, J.; Ding, X.; Gao, S. The anti-fatigue effect of 20(R)-ginsenoside Rg3 in mice by intranasally administration. Biol. Pharm. Bull. 2008, 31, 2024–2027. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.; Bagnall, A.M.; Sowden, A.J.; Cornell, J.E.; Mulrow, C.D.; Ramírez, G. Interventions for the treatment and management of chronic fatigue syndrome: A systematic review. Jama 2001, 286, 1360–1368. [Google Scholar] [CrossRef]
- Demurtas, A.; Pescina, S.; Nicoli, S.; Santi, P.; de Araujo, D.R.; Padula, C. Validation of a HPLC-UV method for the quantification of budesonide in skin layers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1164, 122512. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, M.; Song, J.; Shi, Y.; Qin, X.; Gao, Z.; Lv, Y.; Du, G. The cardioprotective effects of the new crystal form of puerarin in isoproterenol-induced myocardial ischemia rats based on metabolomics. Sci. Rep. 2020, 10, 17787. [Google Scholar] [CrossRef] [PubMed]
- Pohlert, T. PMCMR: Calculate Pairwise Multiple Comparisons of Mean Rank Sums, Version 4.0; Giessen, Germany, 2015. Available online: https://www.rdocumentation.org/packages/PMCMR/versions/4.0 (accessed on 20 April 2024).
- Kumar, V.; Torben, W.; Mansfield, J.; Alvarez, X.; Stouwe, C.V.; Li, J.; Byrareddy, S.N.; Didier, P.J.; Pahar, B.; Molina, P.E.; et al. Cannabinoid Attenuation of Intestinal Inflammation in Chronic SIV-Infected Rhesus Macaques Involves T Cell Modulation and Differential Expression of Micro-RNAs and Pro-inflammatory Genes. Front. Immunol. 2019, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- López-Ibáñez, J.; Pazos, F.; Chagoyen, M. MBROLE 2.0-functional enrichment of chemical compounds. Nucleic Acids Res. 2016, 44, W201–W204. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, Z.; Yu, Q.; Shi, Q.; Lan, B.; Liu, Y.; Zhang, W.; Li, F. The Effects and Mechanisms of Ti-Fu-Kang Decoction in Alleviating Central Fatigue: Insights from Network Pharmacology and Metabolomics. Pharmaceuticals 2025, 18, 1545. https://doi.org/10.3390/ph18101545
Zhang Y, Zhang Z, Yu Q, Shi Q, Lan B, Liu Y, Zhang W, Li F. The Effects and Mechanisms of Ti-Fu-Kang Decoction in Alleviating Central Fatigue: Insights from Network Pharmacology and Metabolomics. Pharmaceuticals. 2025; 18(10):1545. https://doi.org/10.3390/ph18101545
Chicago/Turabian StyleZhang, Yifei, Zehan Zhang, Qingqian Yu, Qinghuan Shi, Bijuan Lan, Yan Liu, Weiyue Zhang, and Feng Li. 2025. "The Effects and Mechanisms of Ti-Fu-Kang Decoction in Alleviating Central Fatigue: Insights from Network Pharmacology and Metabolomics" Pharmaceuticals 18, no. 10: 1545. https://doi.org/10.3390/ph18101545
APA StyleZhang, Y., Zhang, Z., Yu, Q., Shi, Q., Lan, B., Liu, Y., Zhang, W., & Li, F. (2025). The Effects and Mechanisms of Ti-Fu-Kang Decoction in Alleviating Central Fatigue: Insights from Network Pharmacology and Metabolomics. Pharmaceuticals, 18(10), 1545. https://doi.org/10.3390/ph18101545






