Characterization of O-Glycosylation and N-Glycosylation in Bispecific Antibodies and Its Importance in Therapeutic Antibody Development
Abstract
1. Introduction
2. Results
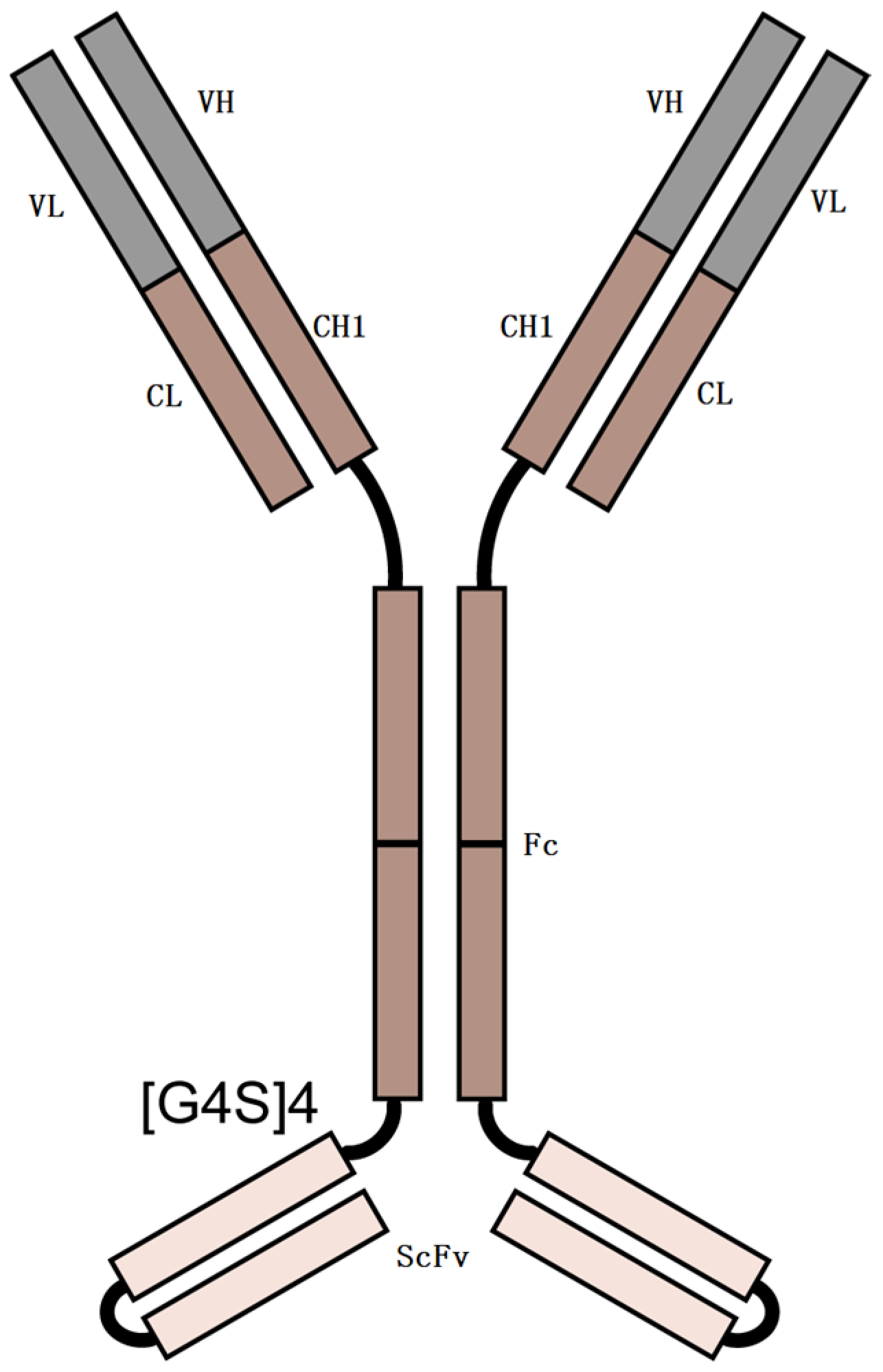
2.1. Characterization of N- and O-Glycosylations
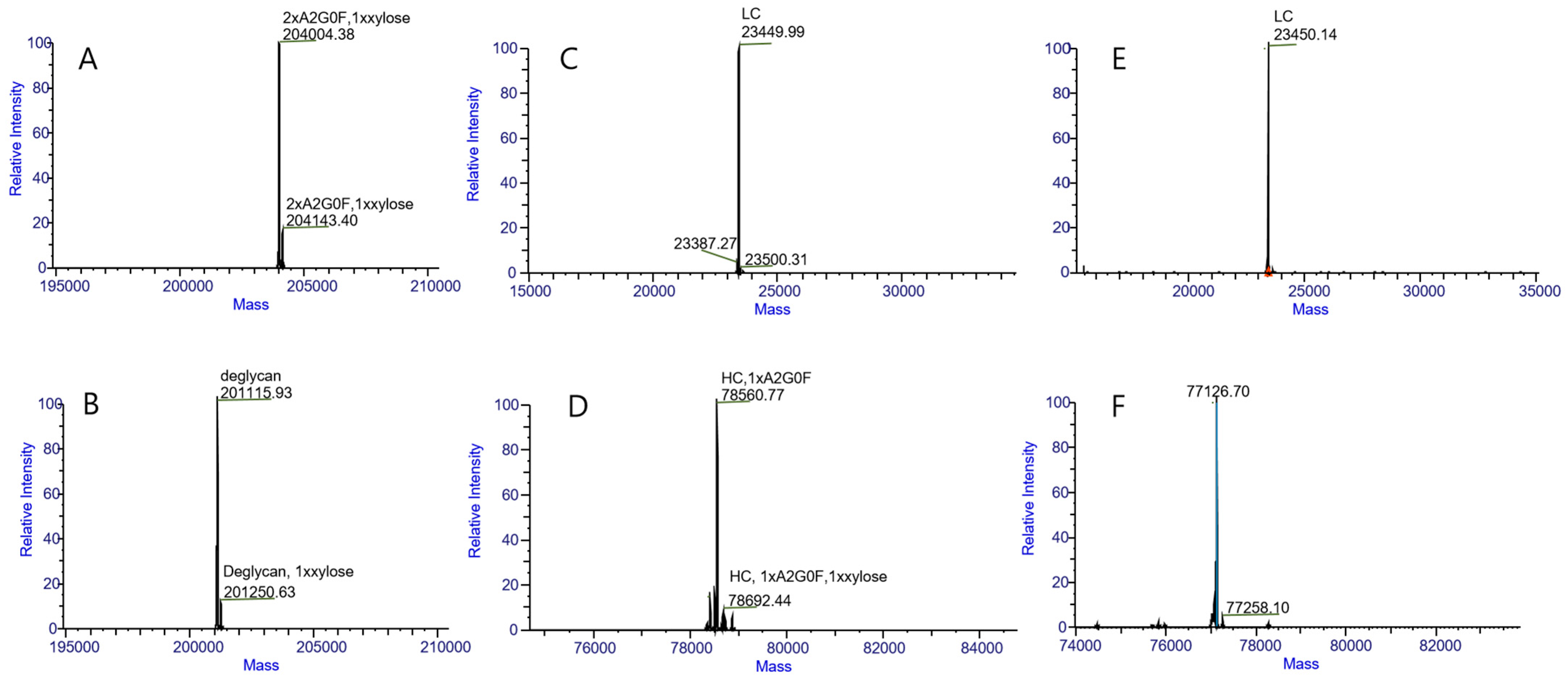
2.1.1. Intact Protein and HC-LC Analysis
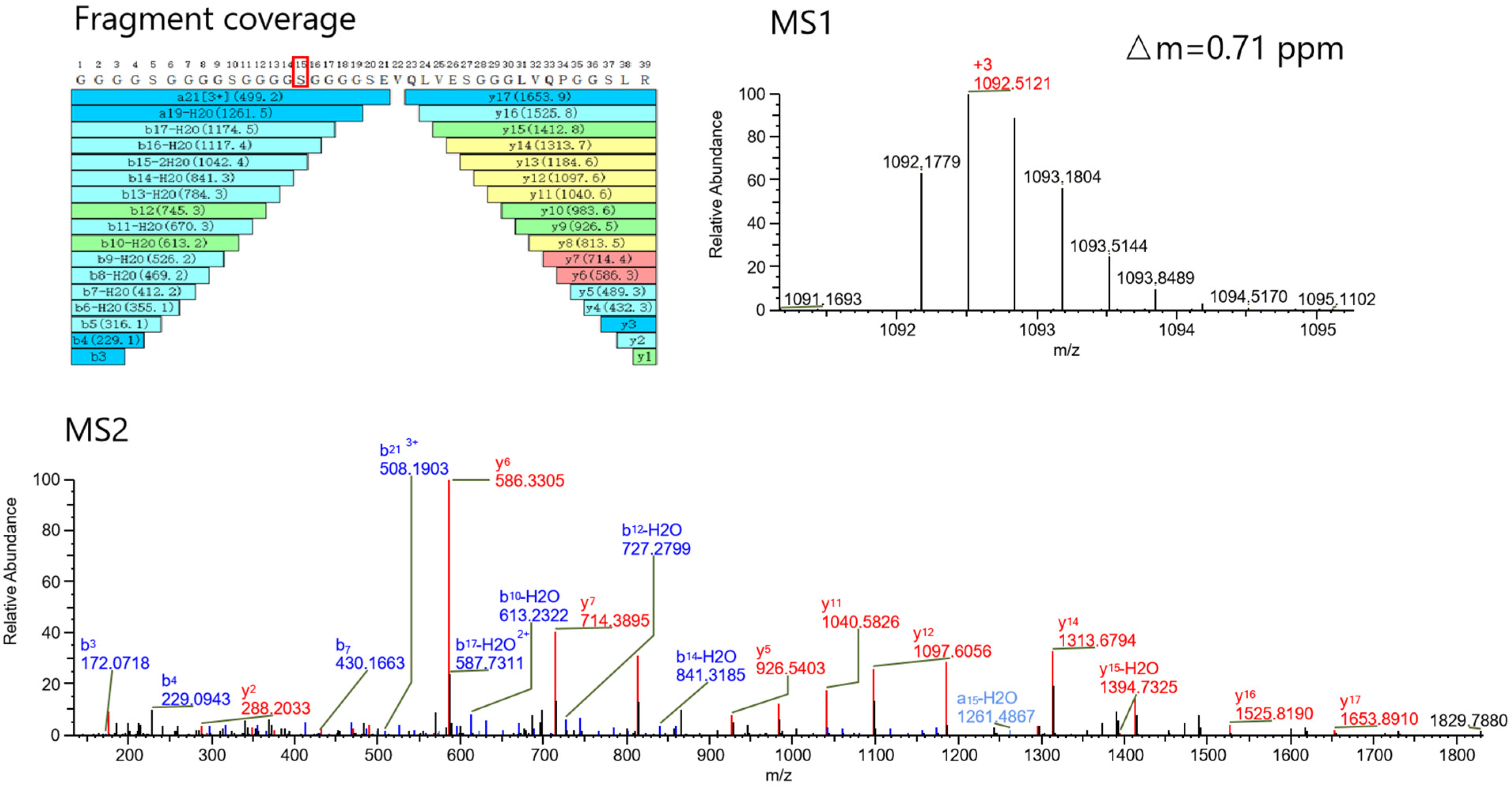
2.1.2. Peptide Mapping Analysis
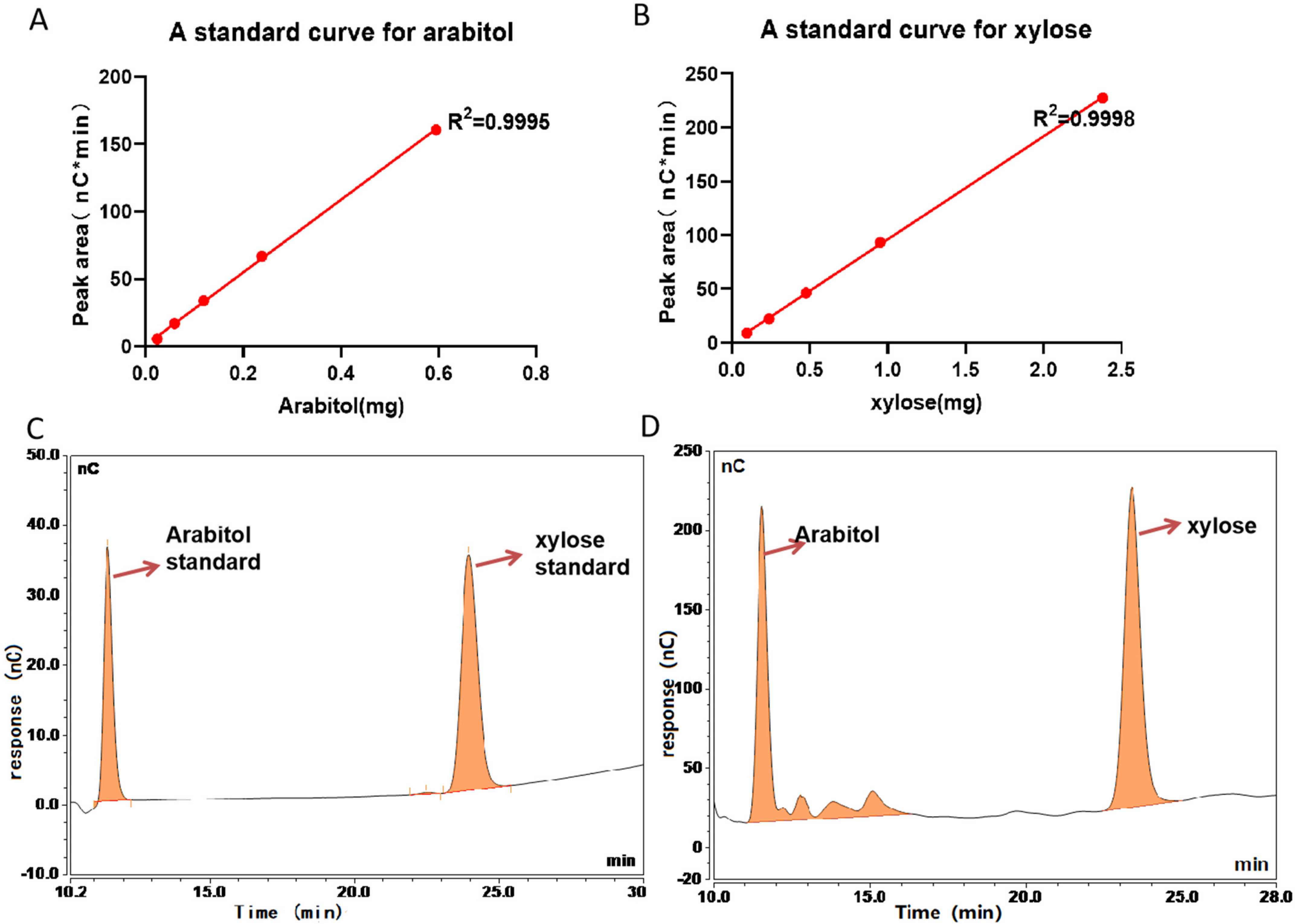
2.1.3. HPAEC-PAD Analysis of O-Glycosylation
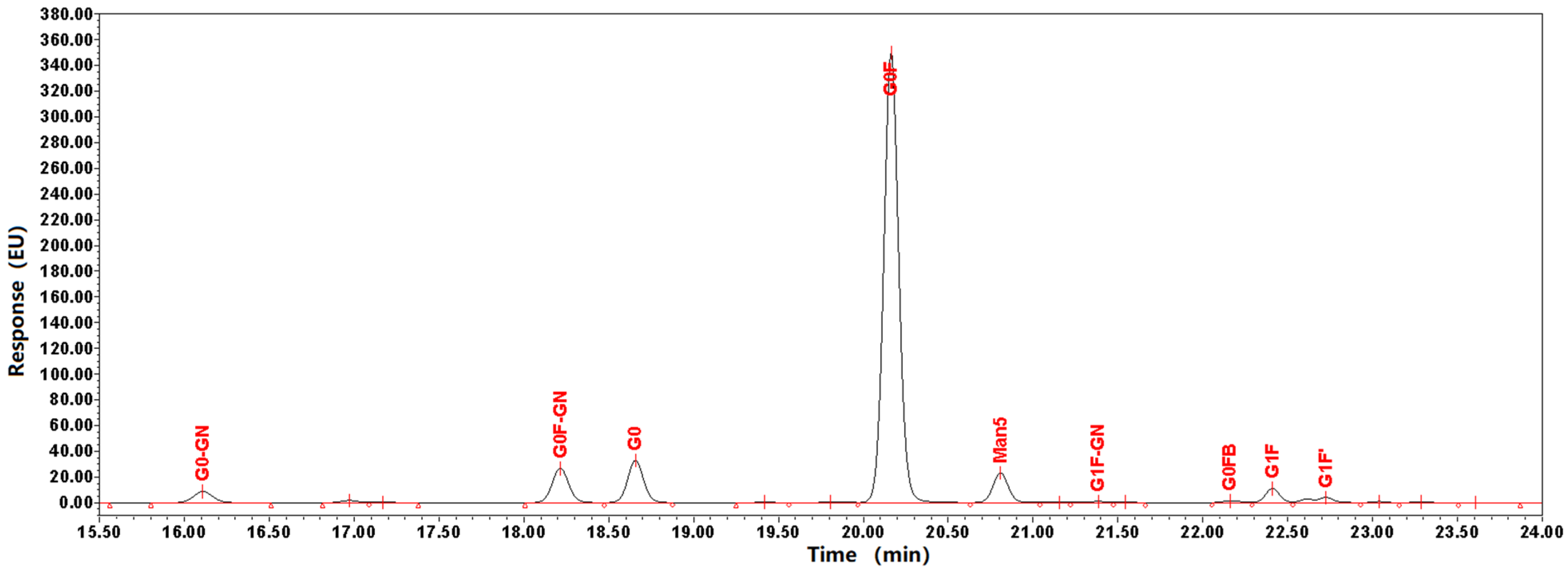
2.1.4. N-Glycosylation
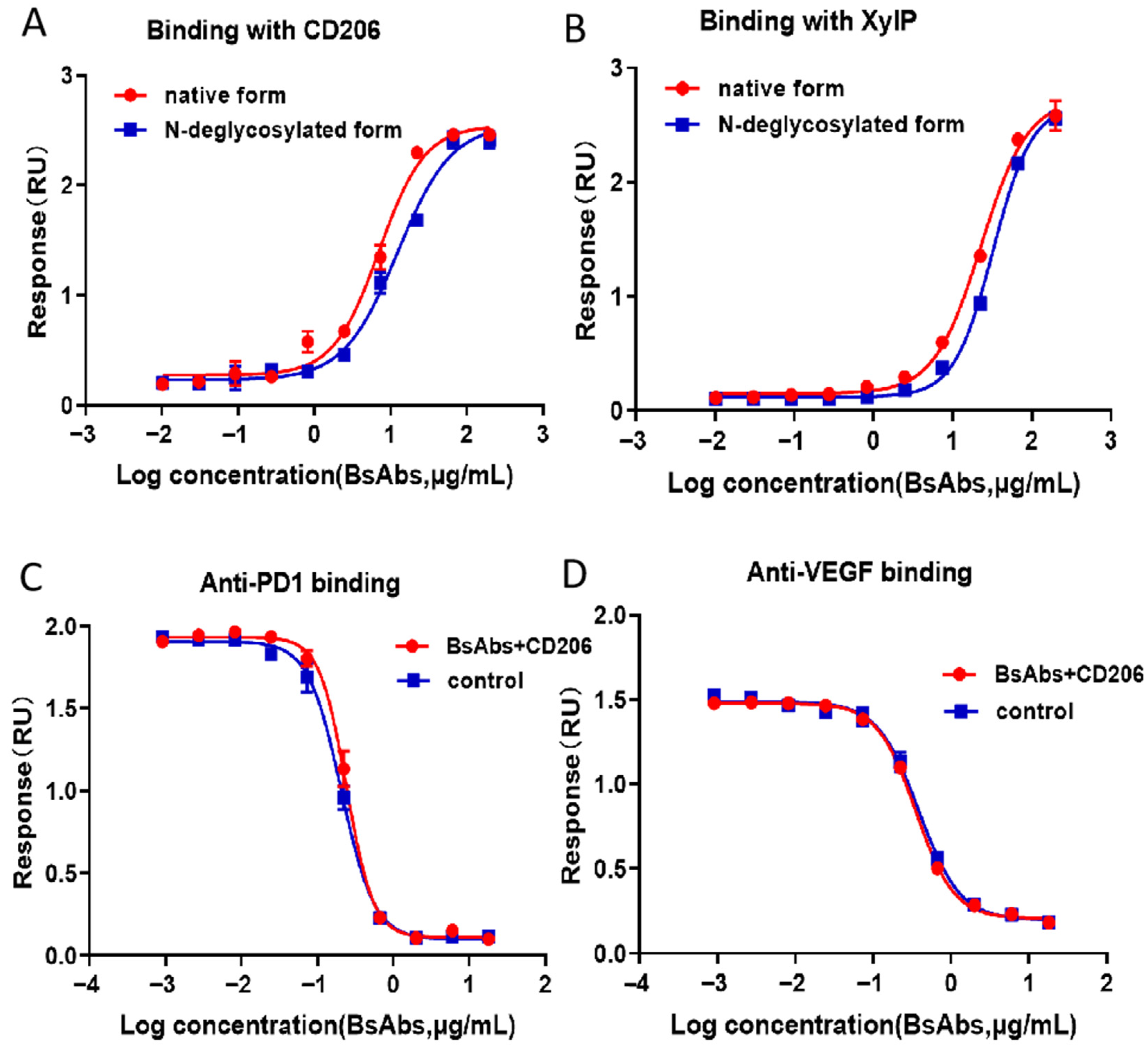
2.2. ELISA-Based Analysis of the Effects of O-Glycosylation on BsAbs
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Sample Preparation
4.2.1. Pretreatment for Intact Protein, HC-LC Chain, N-Glycan and Peptide Mapping Analysis
4.2.2. Pretreatment for HPAEC-PAD Analysis
4.3. LC Conditions
4.3.1. LC Conditions for Intact Protein, HC LC Chain and Peptide Mapping Analysis
4.3.2. LC Conditions for N-Glycosylation
4.3.3. HPAEC-PAD Analysis
4.4. MS Parameters
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carter, P.J.; Rajpal, A. Designing antibodies as therapeutics. Cell 2022, 185, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C.; Carter, P.J. Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. Immunol. 2010, 10, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Brinkmann, U.; Reichert, J.M.; Kontermann, R.E. The present and future of bispecific antibodies for cancer therapy. Nat. Rev. Drug Discov. 2024, 23, 301–319. [Google Scholar] [CrossRef]
- Suurs, F.V.; Lub-de Hooge, M.N.; de Vries, E.G.E.; de Groot, D.J.A. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol. Ther. 2019, 201, 103–119. [Google Scholar] [CrossRef]
- Acheampong, D.O. Bispecific Antibody (bsAb) Construct Formats and their Application in Cancer Therapy. Protein Pept. Lett. 2019, 26, 479–493. [Google Scholar] [CrossRef]
- Yang, F.; Wen, W.; Qin, W. Bispecific Antibodies as a Development Platform for New Concepts and Treatment Strategies. Int. J. Mol. Sci. 2016, 18, 48. [Google Scholar] [CrossRef]
- Hashii, N.; Suzuki, J.; Hanamatsu, H.; Furukawa, J.I.; Ishii-Watabe, A. In-depth site-specific O-Glycosylation analysis of therapeutic Fc-fusion protein by electron-transfer/higher-energy collisional dissociation mass spectrometry. Biologicals 2019, 58, 35–43. [Google Scholar] [CrossRef]
- Gopal, A.K.; Tarantolo, S.R.; Bellam, N.; Green, D.J.; Griffin, M.; Feldman, T.; Mato, A.R.; Eisenfeld, A.J.; Stromatt, S.C.; Goy, A. Phase 1b study of otlertuzumab (TRU-016), an anti-CD37 monospecific ADAPTIR™ therapeutic protein, in combination with rituximab and bendamustine in relapsed indolent lymphoma patients. Investig. New Drugs 2014, 32, 1213–1225. [Google Scholar] [CrossRef]
- World Health Organization. International Nonproprietary Names for Pharmaceutical Substances. Available online: https://cdn.who.int/media/docs/default-source/international-nonproprietary-names-(inn)/pl131.pdf (accessed on 21 September 2025).
- Wen, D.; Foley, S.F.; Hronowski, X.L.; Gu, S.; Meier, W. Discovery and investigation of O-xylosylation in engineered proteins containing a (GGGGS)n linker. Anal. Chem. 2013, 85, 4805–4812. [Google Scholar] [CrossRef]
- Lis, H.; Sharon, N. Protein glycosylation. Structural and functional aspects. Eur. J. Biochem. 1993, 218, 1–27. [Google Scholar] [CrossRef]
- Beck, A.; Wagner-Rousset, E.; Bussat, M.C.; Lokteff, M.; Klinguer-Hamour, C.; Haeuw, J.F.; Goetsch, L.; Wurch, T.; Van Dorsselaer, A.; Corvaïa, N. Trends in glycosylation, glycoanalysis and glycoengineering of therapeutic antibodies and Fc-fusion proteins. Curr. Pharm. Biotechnol. 2008, 9, 482–501. [Google Scholar] [CrossRef]
- Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009, 8, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H. Characterization of glycosylation in monoclonal antibodies and its importance in therapeutic antibody development. Crit. Rev. Biotechnol. 2021, 41, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Joshi, S.; Guttman, A.; Rathore, A.S. N-Glycosylation of monoclonal antibody therapeutics: A comprehensive review on significance and characterization. Anal. Chim. Acta 2022, 1209, 339828. [Google Scholar] [CrossRef] [PubMed]
- Kohda, D. Structural Basis of Protein Asn-Glycosylation by Oligosaccharyltransferases. Adv. Exp. Med. Biol. 2018, 1104, 171–199. [Google Scholar] [CrossRef]
- Quast, I.; Peschke, B.; Lünemann, J.D. Regulation of antibody effector functions through IgG Fc N-glycosylation. Cell. Mol. Life Sci. 2017, 74, 837–847. [Google Scholar] [CrossRef]
- Iida, S.; Misaka, H.; Inoue, M.; Shibata, M.; Nakano, R.; Yamane-Ohnuki, N.; Wakitani, M.; Yano, K.; Shitara, K.; Satoh, M. Nonfucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to FcgammaRIIIa. Clin. Cancer Res. 2006, 12, 2879–2887. [Google Scholar] [CrossRef]
- Raju, T.S.; Briggs, J.B.; Borge, S.M.; Jones, A.J. Species-specific variation in glycosylation of IgG: Evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 2000, 10, 477–486. [Google Scholar] [CrossRef]
- Hodoniczky, J.; Zheng, Y.Z.; James, D.C. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol. Prog. 2005, 21, 1644–1652. [Google Scholar] [CrossRef]
- Thomann, M.; Schlothauer, T.; Dashivets, T.; Malik, S.; Avenal, C.; Bulau, P.; Rüger, P.; Reusch, D. In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PLoS ONE 2015, 10, e0134949. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lou, Y.; Zhang, Y.; Liu, S.; Li, J.; Tao, J. Sialylated immunoglobulin G: A promising diagnostic and therapeutic strategy for autoimmune diseases. Theranostics 2021, 11, 5430–5446. [Google Scholar] [CrossRef] [PubMed]
- Melo-Braga, M.N.; Carvalho, M.B.; Ferreira, M.C.E.; Lavinder, J.; Abbasi, A.; Palmisano, G.; Thaysen-Andersen, M.; Sajadi, M.M.; Ippolito, G.C.; Felicori, L.F. Unveiling the multifaceted landscape of N-glycosylation in antibody variable domains: Insights and implications. Int. J. Biol. Macromol. 2024, 257 Pt 1, 128362. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Handelman, G.; Liu, N.; Xie, D.; Yoon, S. At-line N-linked glycan profiling for monoclonal antibodies with advanced sample preparation and high-performance liquid chromatography. J. Biosci. Bioeng. 2020, 130, 327–333. [Google Scholar] [CrossRef]
- Cook, M.C.; Kaldas, S.J.; Muradia, G.; Rosu-Myles, M.; Kunkel, J.P. Comparison of orthogonal chromatographic and lectin-affinity microarray methods for glycan profiling of a therapeutic monoclonal antibody. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 997, 162–178. [Google Scholar] [CrossRef]
- Sroka-Bartnicka, A.; Karlsson, I.; Ndreu, L.; Quaranta, A.; Pijnappel, M.; Thorsén, G. Particle-based N-linked glycan analysis of selected proteins from biological samples using nonglycosylated binders. J. Pharm. Biomed. Anal. 2017, 132, 125–132. [Google Scholar] [CrossRef]
- Misorin, A.K.; Chernyshova, D.O.; Karbyshev, M.S. State-of-the-Art Approaches to Heterologous Expression of Bispecific Antibodies Targeting Solid Tumors. Biochemistry 2023, 88, 1215–1231. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Y.; Zhang, Z.; Song, L.; Wang, P.; Chai, W. Effect and limitation of excess ammonium on the release of O-glycans in reducing forms from glycoproteins under mild alkaline conditions for glycomic and functional analysis. Anal. Chem. 2010, 82, 9534–9542. [Google Scholar] [CrossRef]
- Turyan, I.; Hronowski, X.; Sosic, Z.; Lyubarskaya, Y. Comparison of two approaches for quantitative O-linked glycan analysis used in characterization of recombinant proteins. Anal. Biochem. 2014, 446, 28–36. [Google Scholar] [CrossRef]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef]
- Stadheim, T.A.; Li, H.; Kett, W.; Burnina, I.N.; Gerngross, T.U. Use of high-performance anion exchange chromatography with pulsed amperometric detection for O-glycan determination in yeast. Nat. Protoc. 2008, 3, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Merry, A.H.; Neville, D.C.; Royle, L.; Matthews, B.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M. Recovery of intact 2-aminobenzamide-labeled O-glycans released from glycoproteins by hydrazinolysis. Anal. Biochem. 2002, 304, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Melmer, M.; Stangler, T.; Premstaller, A.; Lindner, W. Comparison of hydrophilic-interaction, reversed-phase and porous graphitic carbon chromatography for glycan analysis. J. Chromatogr. A 2011, 1218, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Dell, A.; Haslam, S.M.; Tissot, B.; Canis, K.; Azadi, P.; Bäckström, M.; Costello, C.E.; Hansson, G.C.; Hiki, Y.; et al. Comparison of methods for profiling O-glycosylation: Human Proteome Organisation Human Disease Glycomics/Proteome Initiative multi-institutional study of IgA1. Mol. Cell Proteom. 2010, 9, 719–727. [Google Scholar] [CrossRef]
- Robbe, C.; Michalski, J.C.; Capon, C. Structural determination of O-glycans by tandem mass spectrometry. Methods Mol. Biol. 2006, 347, 109–123. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Wu, J.; Huang, Y.; Zhao, X.; Nguyen, J.B.; Rosconi, M.P.; Pyles, E.A.; Qiu, H.; Li, N. High-sensitivity and high-resolution therapeutic antibody charge variant and impurity characterization by microfluidic native capillary electrophoresis-mass spectrometry. J. Pharm. Biomed. Anal. 2023, 223, 115147. [Google Scholar] [CrossRef]
- Loh, S.H.; Park, J.Y.; Cho, E.H.; Nah, S.Y.; Kang, Y.S. Animal lectins: Potential receptors for ginseng polysaccharides. J. Ginseng Res. 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. Lectins: Production and practical applications. Appl. Microbiol. Biotechnol. 2011, 89, 45–55. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Chellapandian, H.; Ramasamy, P.; Jeyachandran, S. Back2Basics: Animal lectins: An insight into a highly versatile recognition protein. J. Proteins Proteom. 2023, 14, 43–59. [Google Scholar] [CrossRef]
- East, L.; Isacke, C.M. The mannose receptor family. Biochim. Biophys. Acta 2002, 1572, 364–386. [Google Scholar] [CrossRef]
- Taylor, P.R.; Gordon, S.; Martinez-Pomares, L. The mannose receptor: Linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005, 26, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Leteux, C.; Chai, W.; Loveless, R.W.; Yuen, C.T.; Uhlin-Hansen, L.; Combarnous, Y.; Jankovic, M.; Maric, S.C.; Misulovin, Z.; Nussenzweig, M.C.; et al. The cysteine-rich domain of the macrophage mannose receptor is a multispecific lectin that recognizes chondroitin sulfates A and B and sulfated oligosaccharides of blood group Lewis(a) and Lewis(x) types in addition to the sulfated N-glycans of lutropin. J. Exp. Med. 2000, 191, 1117–1126. [Google Scholar] [CrossRef]
- Taylor, P.R.; Zamze, S.; Stillion, R.J.; Wong, S.Y.; Gordon, S.; Martinez-Pomares, L. Development of a specific system for targeting protein to metallophilic macrophages. Proc. Natl. Acad. Sci. USA 2004, 101, 1963–1968. [Google Scholar] [CrossRef]
| Intact Protein | HC-LC Analysis | Peptide Mapping | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mobile phase | A: FA/water (1:1000, v/v); B: FA/acetonitrile (1:1000, v/v) | ||||||||
| Flow rate (mL/min) | 0.3 | 0.3 | 0.2 | ||||||
| column temperature (°C) | 60 | ||||||||
| wavelength for UV | 280 nm | ||||||||
| injection amount | 2 µg | 2 µg | 25 µg | ||||||
| gradient | Time (min) | A% | B% | Time (min) | A% | B% | Time (min) | A% | B% |
| 0 | 80 | 20 | 0 | 75 | 25 | 0 | 98 | 2 | |
| 15 | 68 | 32 | 15 | 60 | 40 | 5 | 98 | 2 | |
| 18 | 10 | 90 | 18 | 10 | 90 | 120 | 65 | 35 | |
| 20 | 10 | 90 | 20 | 10 | 90 | 128 | 0 | 100 | |
| 20.1 | 80 | 20 | 20.1 | 75 | 25 | 128.1 | 98 | 2 | |
| 25 | 80 | 20 | 25 | 75 | 25 | 135 | 98 | 2 | |
| N-Glycosylation | ||||
|---|---|---|---|---|
| Mobile phase | A: 50 mmol/L ammonium formate solution (pH 4.5); B: 1.0 N Sodium Hydroxide and Acetonitrile | |||
| column temperature (°C) | 40 | |||
| Wavelength for Fluorescence | excitation at 330 nm and emission at 420 nm | |||
| injection volume | 1 µL | |||
| gradient | Time (min) | Flow rate (mL/min) | A% | B% |
| 0 | 0.4 | 22 | 78 | |
| 10 | 0.4 | 22 | 78 | |
| 34.8 | 0.4 | 47 | 53 | |
| 36 | 0.25 | 80 | 20 | |
| 39 | 0.25 | 80 | 20 | |
| 40 | 0.25 | 22 | 78 | |
| 45 | 0.25 | 22 | 78 | |
| 45.1 | 0.4 | 22 | 78 | |
| 50 | 0.4 | 22 | 78 | |
| O-Glycosylation | ||||
|---|---|---|---|---|
| Mobile phase | A: 50 mM Sodium Acetate; B: 1.0 N Sodium Hydroxide and 50 mM Sodium Acetate | |||
| column temperature (°C) | 40 | |||
| Detection | Pulsed Amperometric detection, gold working electrode, pH/Ag/AgCl reference | |||
| injection volume | 50 µL | |||
| gradient | Time (min) | Flow rate (mL/min) | A% | B% |
| 0 | 0.4 | 80 | 20 | |
| 30 | 0.4 | 80 | 20 | |
| 30.1 | 0.4 | 35 | 65 | |
| 57 | 0.4 | 35 | 65 | |
| 57.1 | 0.4 | 80 | 20 | |
| 70 | 0.4 | 80 | 20 | |
| Intact Analysis | HC-LC Analysis | Peptide Mapping | |
|---|---|---|---|
| Spray voltage (kV) | 3.6 | 3.6 | 3.6 |
| sheath gas (arb) | 35 | 35 | 35 |
| aux gas (arb) | 10 | 10 | 10 |
| gas heater temperature (°C) | 350 | 350 | 350 |
| capillary temperature (°C) | 320 | 320 | 320 |
| m/z for MS1 | 1000–4000 | 2000–8000 | 200–2000 |
| isolation window (Da) | - | - | 2 |
| First mass for MS2 | - | - | 110 |
| AGC (%) for MS1 | 500 | 300 | 300 |
| Resolution for MS1 | 17,500 | 17,500 | 70,000 |
| maximum injection time (ms) for MS1 | 200 | 100 | 100 |
| AGC (%) for MS2 | - | - | 100 |
| Resolution for MS2 | - | - | 17,500 |
| maximum injection time (ms) for MS2 | - | - | 50 |
| (N)CE (%) | - | - | 27 |
| top N | - | - | 5 |
| microscan | 5 | 5 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, M.; Guo, L.; Long, Z.; Ni, Y.; Yang, Y.; Du, J.; Li, M.; Zhang, J.; Tang, T.; Yu, C.; et al. Characterization of O-Glycosylation and N-Glycosylation in Bispecific Antibodies and Its Importance in Therapeutic Antibody Development. Pharmaceuticals 2025, 18, 1538. https://doi.org/10.3390/ph18101538
Duan M, Guo L, Long Z, Ni Y, Yang Y, Du J, Li M, Zhang J, Tang T, Yu C, et al. Characterization of O-Glycosylation and N-Glycosylation in Bispecific Antibodies and Its Importance in Therapeutic Antibody Development. Pharmaceuticals. 2025; 18(10):1538. https://doi.org/10.3390/ph18101538
Chicago/Turabian StyleDuan, Maoqin, Luyun Guo, Zhen Long, Yongbo Ni, Yalan Yang, Jialiang Du, Meng Li, Jialing Zhang, Tao Tang, Chuanfei Yu, and et al. 2025. "Characterization of O-Glycosylation and N-Glycosylation in Bispecific Antibodies and Its Importance in Therapeutic Antibody Development" Pharmaceuticals 18, no. 10: 1538. https://doi.org/10.3390/ph18101538
APA StyleDuan, M., Guo, L., Long, Z., Ni, Y., Yang, Y., Du, J., Li, M., Zhang, J., Tang, T., Yu, C., & Wang, L. (2025). Characterization of O-Glycosylation and N-Glycosylation in Bispecific Antibodies and Its Importance in Therapeutic Antibody Development. Pharmaceuticals, 18(10), 1538. https://doi.org/10.3390/ph18101538






