Cannabigerol Modulates Cannabinoid Receptor Type 2 Expression in the Spinal Dorsal Horn and Attenuates Neuropathic Pain Models
Abstract
1. Introduction
2. Results
2.1. Oral Cannabigerol Produces Central and Peripheral Antinociceptive Effects in Rodent Pain Models
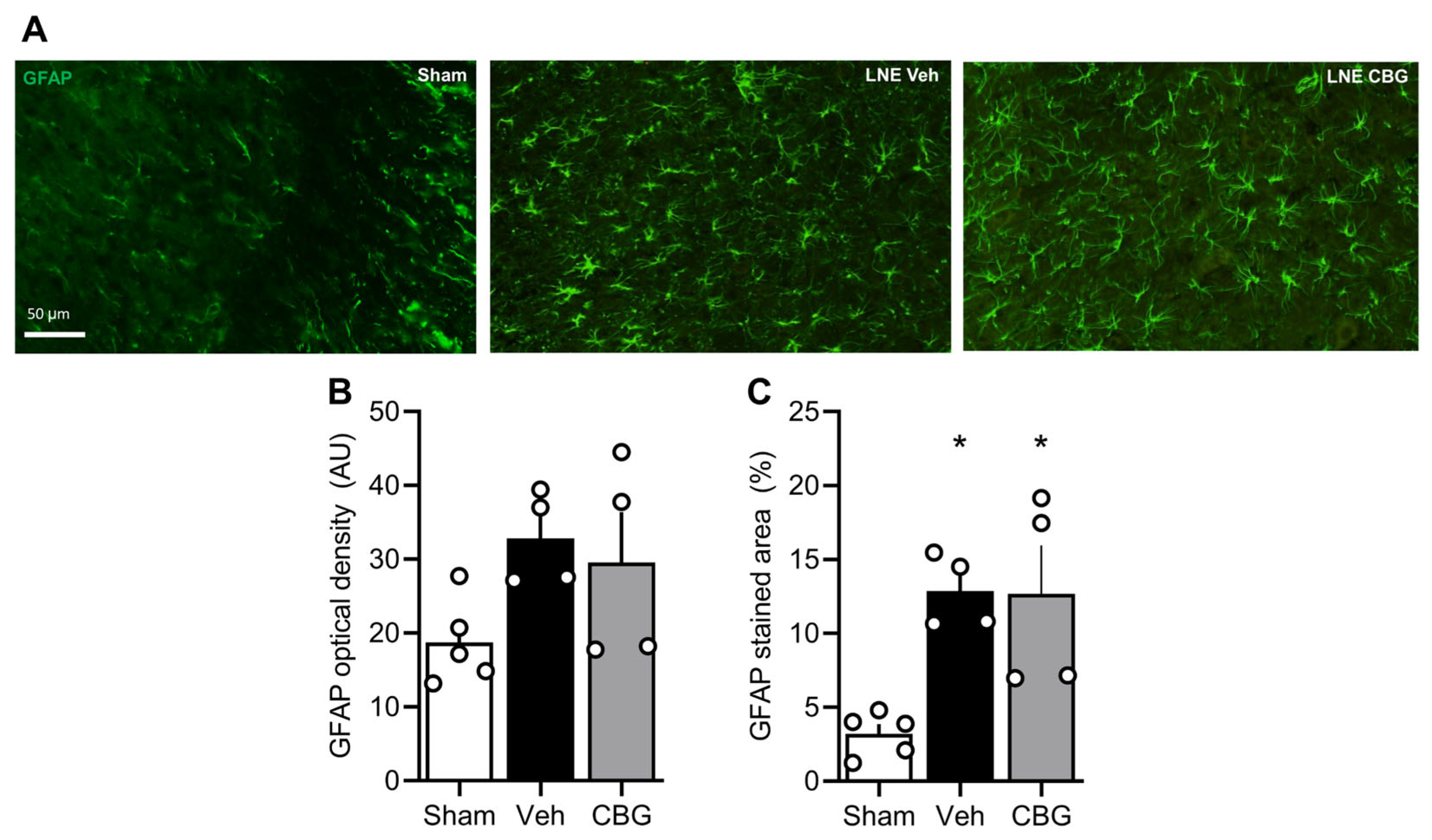
2.2. Cannabigerol Attenuates Microgliosis but Fails to Modulate Astrogliosis
2.3. Involvement of Cannabinoid 2 Receptors in CBG Effects
3. Discussion
4. Materials and Methods
4.1. Laboratory Animals
4.2. Drugs
4.3. Experimental Model
4.3.1. Formalin Test
4.3.2. Hot Plate Test
4.3.3. Chronic Nociception Model
4.3.4. Evaluation of Thermal and Mechanical Hypernociception in the SNL Model
4.4. Tissue Collection and Immunofluorescence
4.5. Image Acquisition and Quantification
4.6. Statistical Analysis
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of Chronic Pain in the UK: A Systematic Review and Meta-Analysis of Population Studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef]
- Jackson, T.; Thomas, S.; Stabile, V.; Shotwell, M.; Han, X.; McQueen, K. A Systematic Review and Meta-Analysis of the Global Burden of Chronic Pain Without Clear Etiology in Low- and Middle-Income Countries: Trends in Heterogeneous Data and a Proposal for New Assessment Methods. Anesth. Analg. 2016, 123, 739–748. [Google Scholar] [CrossRef]
- Steingrímsdóttir, Ó.A.; Landmark, T.; Macfarlane, G.J.; Nielsen, C.S. Defining Chronic Pain in Epidemiological Studies: A Systematic Review and Meta-Analysis. Pain 2017, 158, 2092–2107. [Google Scholar] [CrossRef]
- Santiago, B.V.M.; de Oliveira, A.B.G.; da Silva, G.M.R.; da Silva, M.D.F.; Bergamo, P.E.; Parise, M.; Villela, N.R. Prevalence of Chronic Pain in Brazil: A Systematic Review and Meta-Analysis. Clinics 2023, 78, 100209. [Google Scholar] [CrossRef] [PubMed]
- Udall, M.; Kudel, I.; Cappelleri, J.C.; Sadosky, A.; King-Concialdi, K.; Parsons, B.; Hlavacek, P.; Hopps, M.; Salomon, P.A.; DiBonaventura, M.D.; et al. Epidemiology of Physician-Diagnosed Neuropathic Pain in Brazil. J. Pain Res. 2019, 12, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Hylands-White, N.; Duarte, R.V.; Raphael, J.H. An Overview of Treatment Approaches for Chronic Pain Management. Rheumatol. Int. 2017, 37, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.P.; Brandow, A.M. Chronic Pain: Prevalence and Management. Hematol. Oncol. Clin. N. Am. 2022, 36, 1151–1165. [Google Scholar] [CrossRef]
- Pantoja-Ruiz, C.; Restrepo-Jimenez, P.; Castañeda-Cardona, C.; Ferreirós, A.; Rosselli, D. Cannabis and Pain: A Scoping Review. Braz. J. Anesthesiol. 2022, 72, 142–151. [Google Scholar] [CrossRef]
- Lessa, M.A.; Cavalcanti, I.L.; Figueiredo, N.V. Cannabinoid Derivatives and the Pharmacological Management of Pain. Rev. Dor 2016, 17, 47–51. [Google Scholar] [CrossRef]
- Rezende, B.; Alencar, A.K.; de Bem, G.F.; Fontes-Dantas, F.L.; Montes, G.C. Endocannabinoid System: Chemical Characteristics and Biological Activity. Pharmaceuticals 2023, 16, 148. [Google Scholar] [CrossRef]
- Johnson, B.W.; Strand, N.H.; Raynak, J.C.; Jara, C.; Habtegiorgis, K.; Hand, B.A.; Hong, S.; Maloney, J.A. Cannabinoids in Chronic Pain Management: A Review of the History, Efficacy, Applications, and Risks. Biomedicines 2025, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Yarza-Sancho, M.D.; López, V. Cannabidiol (CBD): A Systematic Review of Clinical and Preclinical Evidence in the Treatment of Pain. Pharmaceuticals 2024, 17, 1438. [Google Scholar] [CrossRef] [PubMed]
- Moisset, X.; Bouhassira, D.; Avez Couturier, J.; Alchaar, H.; Conradi, S.; Delmotte, M.H.; Lanteri-Minet, M.; Lefaucheur, J.P.; Mick, G.; Piano, V.; et al. Pharmacological and Non-Pharmacological Treatments for Neuropathic Pain: Systematic Review and French Recommendations. Rev. Neurol. 2020, 176, 325–352. [Google Scholar] [CrossRef] [PubMed]
- Seevathee, K.; Kessomboon, P.; Manimmanakorn, N.; Luangphimai, S.; Thaneerat, T.; Wanaratna, K.; Plengphanich, S.; Thaenkham, T.; Sena, W. Efficacy and Safety of Transdermal Medical Cannabis (THC:CBD:CBN Formula) to Treat Painful Diabetic Peripheral Neuropathy of Lower Extremities. Med. Cannabis Cannabinoids 2025, 8, 1–14. [Google Scholar] [CrossRef]
- Bilbao, A.; Spanagel, R. Medical Cannabinoids: A Pharmacology-Based Systematic Review and Meta-Analysis for All Relevant Medical Indications. BMC Med. 2022, 20, 259. [Google Scholar] [CrossRef]
- Serpell, M.; Ratcliffe, S.; Hovorka, J.; Schofield, M.; Taylor, L.; Lauder, H.; Ehler, E. A Double-Blind, Randomized, Placebo-Controlled, Parallel Group Study of THC/CBD Spray in Peripheral Neuropathic Pain Treatment. Eur. J. Pain 2014, 18, 999–1012. [Google Scholar] [CrossRef]
- Rezende, B.; Marques, K.L.; de Carvalho, F.E.; Gonçalves, V.M.; de Oliveira, B.C.; Nascimento, G.G.; dos Santos, Y.B.; Antunes, F.; Barradas, P.C.; Fontes-Dantas, F.L.; et al. Cannabigerol Reduces Acute and Chronic Hypernociception in Animals Exposed to Prenatal Hypoxia-Ischemia. Sci. Pharm. 2024, 92, 53. [Google Scholar] [CrossRef]
- Diniz, L.P.; Almeida, J.C.; Tortelli, V.; Vargas Lopes, C.; Setti-Perdigão, P.; Stipursky, J.; Kahn, S.A.; Romão, L.F.; de Miranda, J.; Alves-Leon, S.V.; et al. Astrocyte-Induced Synaptogenesis Is Mediated by Transforming Growth Factor β Signaling through Modulation of D-Serine Levels in Cerebral Cortex Neurons. J. Biol. Chem. 2012, 287, 41432–41445. [Google Scholar] [CrossRef]
- Fernandes, G.G.; Costa, K.C.M.; Scomparin, D.S.; Freire, J.B.; Guimarães, F.S.; Campos, A.C. Genetic Ablation of the Inducible Form of Nitric Oxide in Male Mice Disrupts Immature Neuron Survival in the Adult Dentate Gyrus. Front. Immunol. 2021, 12, 782831. [Google Scholar] [CrossRef]
- Fontes-Dantas, F.L.; Fernandes, G.G.; Gutman, E.G.; De Lima, E.V.; Antonio, L.S.; Hammerle, M.B.; Mota-Araujo, H.P.; Colodeti, L.C.; Araújo, S.M.B.; Froz, G.M.; et al. SARS-CoV-2 Spike Protein Induces TLR4-Mediated Long-Term Cognitive Dysfunction Recapitulating Post-COVID-19 Syndrome in Mice. Cell Rep. 2023, 42, 112189. [Google Scholar] [CrossRef]
- Cheng, T.; Xu, Z.; Ma, X. The Role of Astrocytes in Neuropathic Pain. Front. Mol. Neurosci. 2022, 15, 1007889. [Google Scholar] [CrossRef]
- Smith, P.A. BDNF in Neuropathic Pain; the Culprit That Cannot Be Apprehended. Neuroscience 2024, 543, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and Brain Pharmacokinetic Profile of Cannabidiol (CBD), Cannabidivarine (CBDV), Δ9-Tetrahydrocannabivarin (THCV) and Cannabigerol (CBG) in Rats and Mice Following Oral and Intraperitoneal Administration and CBD Action on Obsessive-Compulsive Behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef]
- Lacerda, M.; Carona, A.; Castanheira, S.; Falcão, A.; Bicker, J.; Fortuna, A. Pharmacokinetics of Non-Psychotropic Phytocannabinoids. Pharmaceutics 2025, 17, 236. [Google Scholar] [CrossRef] [PubMed]
- Nachnani, R.; Sepulveda, D.E.; Booth, J.L.; Zhou, S.; Graziane, N.M.; Raup-Konsavage, W.M.; Vrana, K.E. Chronic Cannabigerol as an Effective Therapeutic for Cisplatin-Induced Neuropathic Pain. Pharmaceuticals 2023, 16, 1442. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, D.E.; Morris, D.P.; Raup-Konsavage, W.M.; Sun, D.; Vrana, K.E.; Graziane, N.M. Cannabigerol (CBG) Attenuates Mechanical Hypersensitivity Elicited by Chemotherapy-Induced Peripheral Neuropathy. Eur. J. Pain 2022, 26, 1950–1966. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Malhi, N.K.; Huang, J.; Li, Q.; Zhou, Z.; Wang, R.; Peng, J.; Yin, T.; Wang, H. Cannabigerol (CBG): A Comprehensive Review of Its Molecular Mechanisms and Therapeutic Potential. Molecules 2024, 29, 5471. [Google Scholar] [CrossRef]
- Geng, S.-J.; Liao, F.-F.; Dang, W.-H.; Ding, X.; Liu, X.-D.; Cai, J.; Han, J.-S.; Wan, Y.; Xing, G.-G. Contribution of the Spinal Cord BDNF to the Development of Neuropathic Pain by Activation of the NR2B-Containing NMDA Receptors in Rats with Spinal Nerve Ligation. Exp. Neurol. 2010, 222, 256–266. [Google Scholar] [CrossRef]
- Zhao, L.; Levine, E.S. BDNF-Endocannabinoid Interactions at Neocortical Inhibitory Synapses Require Phospholipase C Signaling. J. Neurophysiol. 2014, 111, 1008–1015. [Google Scholar] [CrossRef]
- Gangarossa, G.; Perez, S.; Dembitskaya, Y.; Prokin, I.; Berry, H.; Venance, L. BDNF Controls Bidirectional Endocannabinoid Plasticity at Corticostriatal Synapses. Cereb. Cortex 2020, 30, 197–214. [Google Scholar] [CrossRef]
- De Chiara, V.; Angelucci, F.; Rossi, S.; Musella, A.; Cavasinni, F.; Cantarella, C.; Mataluni, G.; Sacchetti, L.; Napolitano, F.; Castelli, M.; et al. Brain-Derived Neurotrophic Factor Controls Cannabinoid CB1 Receptor Function in the Striatum. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 8127–8137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yeh, M.L.-W.; Levine, E.S. Role for Endogenous BDNF in Endocannabinoid-Mediated Long-Term Depression at Neocortical Inhibitory Synapses. eNeuro 2015, 2, e0029-14.2015. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.S.; Park, H.; Kim, S.K. Neural Plasticity in the Brain during Neuropathic Pain. Biomedicines 2021, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Kuner, T.; Kuner, R. Synaptic Plasticity in Pathological Pain. Trends Neurosci. 2014, 37, 343–355. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jun, J.; Wang, J.; Bittar, A.; Chung, K.; Chung, J.M. Induction of Long-Term Potentiation and Long-Term Depression Is Cell-Type Specific in the Spinal Cord. Pain 2015, 156, 618–625. [Google Scholar] [CrossRef]
- Randić, M.; Jiang, M.C.; Cerne, R. Long-Term Potentiation and Long-Term Depression of Primary Afferent Neurotransmission in the Rat Spinal Cord. J. Neurosci. Off. J. Soc. Neurosci. 1993, 13, 5228–5241. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; E, S.; Zhang, Z.; Liang, Y. Neuroplasticity in the Transition from Acute to Chronic Pain. Neurother. J. Am. Soc. Exp. Neurother. 2024, 21, e00464. [Google Scholar] [CrossRef]
- Cascio, M.G.; Gauson, L.A.; Stevenson, L.A.; Ross, R.A.; Pertwee, R.G. Evidence That the Plant Cannabinoid Cannabigerol Is a Highly Potent Alpha2-Adrenoceptor Agonist and Moderately Potent 5HT1A Receptor Antagonist. Br. J. Pharmacol. 2010, 159, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; Sánchez de Medina, V.; Rivas-Santisteban, R.; Sánchez-Carnerero Callado, C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol Action at Cannabinoid CB(1) and CB(2) Receptors and at CB(1)-CB(2) Heteroreceptor Complexes. Front. Pharmacol. 2018, 9, 632. [Google Scholar] [CrossRef]
- Pollastro, F.; Taglialatela-Scafati, O.; Allarà, M.; Muñoz, E.; Di Marzo, V.; De Petrocellis, L.; Appendino, G. Bioactive Prenylogous Cannabinoid from Fiber Hemp (Cannabis Sativa). J. Nat. Prod. 2011, 74, 2019–2022. [Google Scholar] [CrossRef]
- Rosenthaler, S.; Pöhn, B.; Kolmanz, C.; Nguyen Huu, C.; Krewenka, C.; Huber, A.; Kranner, B.; Rausch, W.-D.; Moldzio, R. Differences in Receptor Binding Affinity of Several Phytocannabinoids Do Not Explain Their Effects on Neural Cell Cultures. Neurotoxicol. Teratol. 2014, 46, 49–56. [Google Scholar] [CrossRef]
- Zhang, J.; Hoffert, C.; Vu, H.K.; Groblewski, T.; Ahmad, S.; O’Donnell, D. Induction of CB2 Receptor Expression in the Rat Spinal Cord of Neuropathic but Not Inflammatory Chronic Pain Models. Eur. J. Neurosci. 2003, 17, 2750–2754. [Google Scholar] [CrossRef]
- Grabon, W.; Ruiz, A.; Gasmi, N.; Degletagne, C.; Georges, B.; Belmeguenai, A.; Bodennec, J.; Rheims, S.; Marcy, G.; Bezin, L. CB2 Expression in Mouse Brain: From Mapping to Regulation in Microglia under Inflammatory Conditions. J. Neuroinflam. 2024, 21, 206. [Google Scholar] [CrossRef]
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the Cannabinoid CB2 Receptor in Microglial Cells in Response to Inflammatory Stimuli. J. Neurochem. 2005, 95, 437–445. [Google Scholar] [CrossRef]
- Alhouayek, M.; Muccioli, G.G. COX-2-Derived Endocannabinoid Metabolites as Novel Inflammatory Mediators. Trends Pharmacol. Sci. 2014, 35, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.; Cahill, C.M. TNF-Alpha and Neuropathic Pain—A Review. J. Neuroinflam. 2010, 7, 27. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Zhou, L.-J.; Hu, N.-W.; Xu, J.-T.; Wu, C.-Y.; Zhang, T.; Li, Y.-Y.; Liu, X.-G. Tumor Necrosis Factor-Alpha Induces Long-Term Potentiation of C-Fiber Evoked Field Potentials in Spinal Dorsal Horn in Rats with Nerve Injury: The Role of NF-Kappa B, JNK and P38 MAPK. Neuropharmacology 2007, 52, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Fleisher-Berkovich, S.; Ventura, Y.; Amoyal, M.; Dahan, A.; Feinshtein, V.; Alfahel, L.; Israelson, A.; Bernstein, N.; Gorelick, J.; Ben-Shabat, S. Therapeutic Potential of Phytocannabinoid Cannabigerol for Multiple Sclerosis: Modulation of Microglial Activation In Vitro and In Vivo. Biomolecules 2023, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and Analgesia: The Dual Effect of Nitric Oxide in the Nociceptive System. Nitric Oxide Biol. Chem. 2011, 25, 243–254. [Google Scholar] [CrossRef]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V.; et al. Beneficial Effect of the Non-Psychotropic Plant Cannabinoid Cannabigerol on Experimental Inflammatory Bowel Disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef]
- Hervera, A.; Negrete, R.; Leánez, S.; Martín-Campos, J.M.; Pol, O. The Spinal Cord Expression of Neuronal and Inducible Nitric Oxide Synthases and Their Contribution in the Maintenance of Neuropathic Pain in Mice. PLoS ONE 2010, 5, e14321. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.C.; Monte, F.B.D.M.; Eduardo Dematte, B.; Montagnoli, T.L.; Montes, G.C.; da Silva, J.S.; Mendez-Otero, R.; Trachez, M.M.; Sudo, R.T.; Zapata-Sudo, G. Antinociceptive Effect of Lodenafil Carbonate in Rodent Models of Inflammatory Pain and Spinal Nerve Ligation-Induced Neuropathic Pain. J. Pain Res. 2021, 14, 857–866. [Google Scholar] [CrossRef]
- Maia, J.R.L.C.B.; Machado, L.K.A.; Fernandes, G.G.; Vitorino, L.C.; Antônio, L.S.; Araújo, S.M.B.; Colodeti, L.C.; Fontes-Dantas, F.L.; Zeidler, J.D.; Saraiva, G.N.; et al. Mitotherapy Prevents Peripheral Neuropathy Induced by Oxaliplatin in Mice. Neuropharmacology 2024, 245, 109828. [Google Scholar] [CrossRef]
- Melato, J.; Goldoni, F.C.; Benvenutti, L.; Corrêa, T.P.; Remor, A.P.; Varela, K.G.; Stoeberl, L.C.; Fernandes, G.G.; de Lima Rasga, G.; Passos, G.F.; et al. Omega-3-Enriched Fish Oil Reduces the Chemotherapy-Induced Peripheral Neuropathy in Mice. Neuropharmacology 2025, 271, 110384. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, R.; Li, X.; Zou, H.; Yu, W.; Liang, Z.; Li, L.; Chen, L.; Zhou, L.; Chen, Z. Astrocytes Constitute the Major TNF-α-Producing Cell Population in the Infarct Cortex in dMCAO Rats Receiving Intravenous MSC Infusion. Biomed. Pharmacother. 2021, 142, 111971. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, J.K.; Suh, H.W. Antinociceptive Profiles of Aspirin and Acetaminophen in Formalin, Substance P and Glutamate Pain Models. Brain Res. 2001, 921, 233–239. [Google Scholar] [CrossRef]
- Łuszczki, J.J. Dose-Response Relationship Analysis of Pregabalin Doses and Their Antinociceptive Effects in Hot-Plate Test in Mice. Pharmacol. Rep. 2010, 62, 942–948. [Google Scholar] [CrossRef]
- Ho Kim, S.; Mo Chung, J. An Experimental Model for Peripheral Neuropathy Produced by Segmental Spinal Nerve Ligation in the Rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef]
- Lolignier, S.; Amsalem, M.; Maingret, F.; Padilla, F.; Gabriac, M.; Chapuy, E.; Eschalier, A.; Delmas, P.; Busserolles, J. Nav1.9 Channel Contributes to Mechanical and Heat Pain Hypersensitivity Induced by Subacute and Chronic Inflammation. PLoS ONE 2011, 6, e23083. [Google Scholar] [CrossRef]
- Santos-Nogueira, E.; Redondo Castro, E.; Mancuso, R.; Navarro, X. Randall-Selitto Test: A New Approach for the Detection of Neuropathic Pain after Spinal Cord Injury. J. Neurotrauma 2012, 29, 898–904. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezende, B.; Fernandes, G.G.; de Simas Gonçalves, V.M.; Nascimento, G.G.; Marques, K.L.; de Oliveira, B.C.C.A.; dos Santos, Y.B.; de Andrade, M.E.B.; Calumbi, K.S.; Maia, E.P.; et al. Cannabigerol Modulates Cannabinoid Receptor Type 2 Expression in the Spinal Dorsal Horn and Attenuates Neuropathic Pain Models. Pharmaceuticals 2025, 18, 1508. https://doi.org/10.3390/ph18101508
Rezende B, Fernandes GG, de Simas Gonçalves VM, Nascimento GG, Marques KL, de Oliveira BCCA, dos Santos YB, de Andrade MEB, Calumbi KS, Maia EP, et al. Cannabigerol Modulates Cannabinoid Receptor Type 2 Expression in the Spinal Dorsal Horn and Attenuates Neuropathic Pain Models. Pharmaceuticals. 2025; 18(10):1508. https://doi.org/10.3390/ph18101508
Chicago/Turabian StyleRezende, Bismarck, Gabriel Gripp Fernandes, Vitória Macario de Simas Gonçalves, Gabriela Guedes Nascimento, Kethely Lima Marques, Barbara Conceição Costa Azeredo de Oliveira, Yure Bazilio dos Santos, Maria Eduarda Barros de Andrade, Karine Simões Calumbi, Eduardo Perdigão Maia, and et al. 2025. "Cannabigerol Modulates Cannabinoid Receptor Type 2 Expression in the Spinal Dorsal Horn and Attenuates Neuropathic Pain Models" Pharmaceuticals 18, no. 10: 1508. https://doi.org/10.3390/ph18101508
APA StyleRezende, B., Fernandes, G. G., de Simas Gonçalves, V. M., Nascimento, G. G., Marques, K. L., de Oliveira, B. C. C. A., dos Santos, Y. B., de Andrade, M. E. B., Calumbi, K. S., Maia, E. P., Trefilio, L. M., Antunes, F., Fontes-Dantas, F. L., & Montes, G. C. (2025). Cannabigerol Modulates Cannabinoid Receptor Type 2 Expression in the Spinal Dorsal Horn and Attenuates Neuropathic Pain Models. Pharmaceuticals, 18(10), 1508. https://doi.org/10.3390/ph18101508





