Development and Evaluation of 3D-Printed Losartan Potassium Tablets Using Semi-Solid Extrusion: The Effect of Geometry, Drug Loading and Superdisintegrant
Abstract
1. Introduction
- Identifying suitable polymer–solvent systems for printability;
- To evaluate the physicochemical properties of losartan potassium in the context of formulation design;
- Investigating the effects of tablet geometry, drug loading, and superdisintegrant concentration on drug release and disintegration, and;
- Applying kinetic modeling and spectroscopic analysis to understand release mechanisms and component compatibility;
- Develop improved or alternative formulations compared to existing commercial products considering quantitative and qualitative drug attributes.
2. Results and Discussion
2.1. Printability Assessment of the Polymer–Solvent–Excipient Mixtures
- Infill Density and Pattern: A 100% infill density and linear pattern were chosen to ensure mechanical integrity and prevent internal collapse, particularly during solvent loss. These settings also support reproducible drug release by minimizing porosity variation.
- Printing and Travel Speeds: A print speed of 15 mm/s was optimal for maintaining uniform deposition without filament discontinuity. Reduced initial layer speed (11 mm/s) was essential for proper anchoring of the base layer to the build plate.
- Cooling and Layer Adhesion: The fan was set to maximum (100%) to accelerate surface drying between layers, promoting strong interlayer bonding and dimensional stability.
2.2. Uniformity of Mass and Dimension of Printed Tablets
2.3. Drug Release
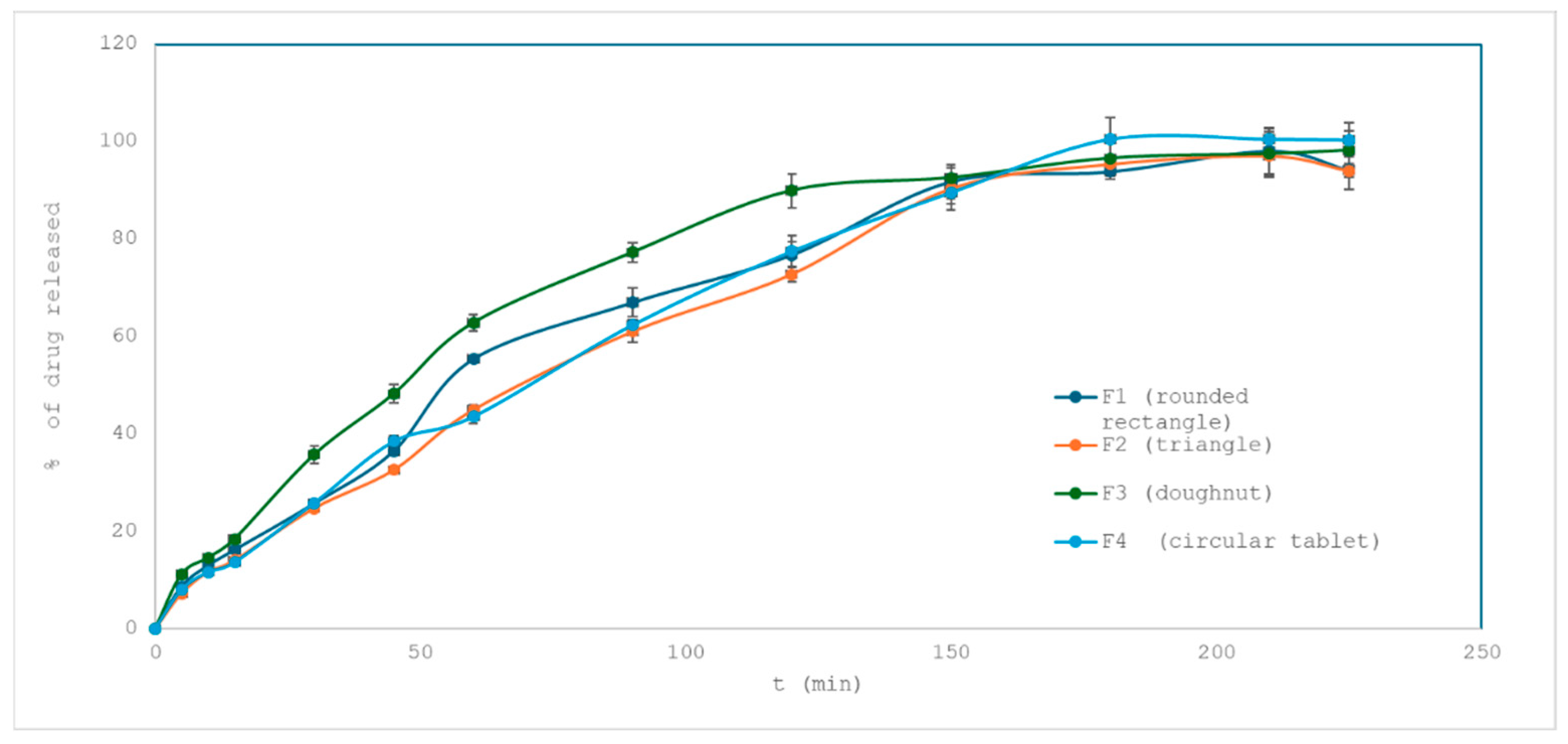
2.3.1. Influence of Tablet Shape on Drug Release
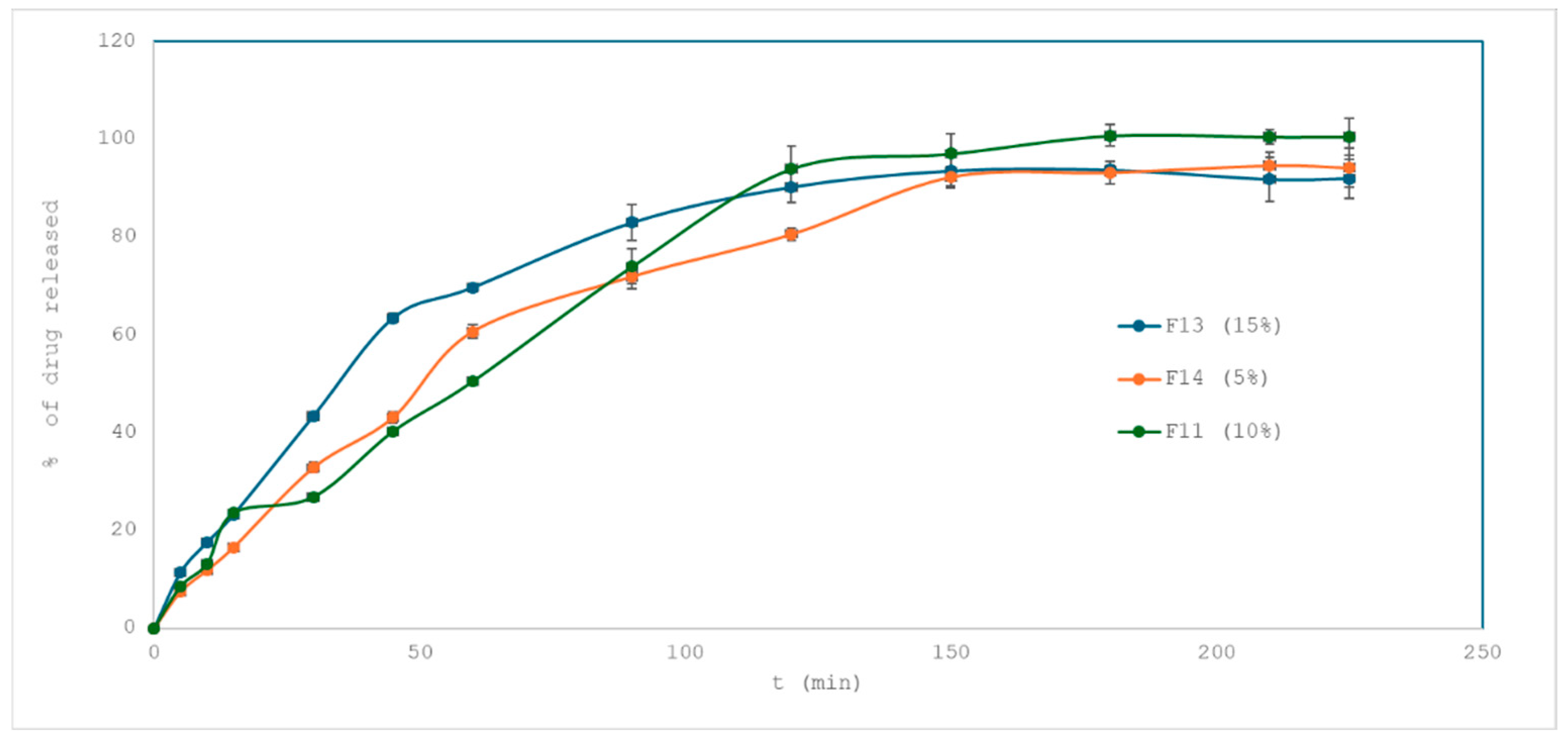
2.3.2. Effect of Superdisintegrant on Drug Release
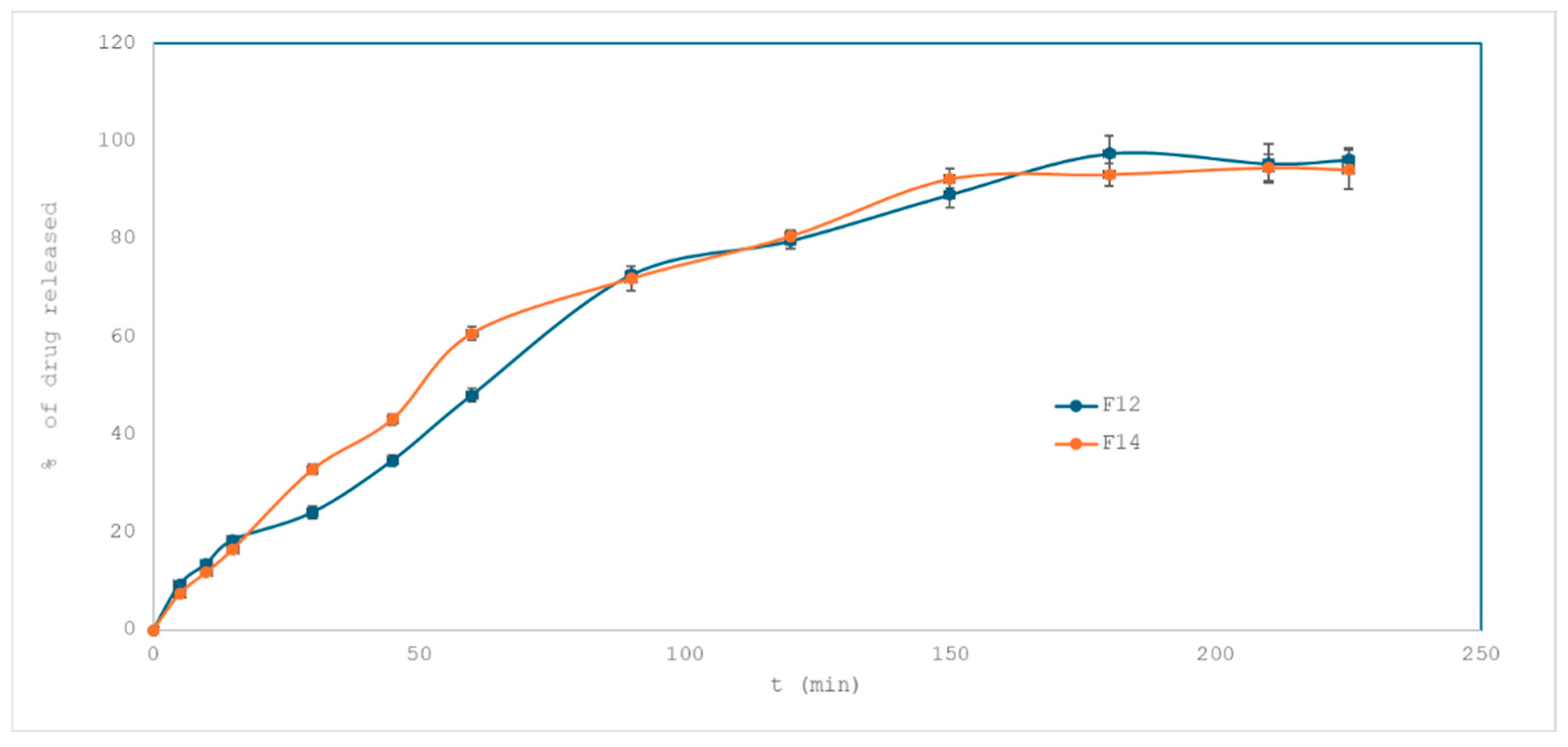
2.3.3. Effect of Drug Loading on Drug Release
- F13 vs. F11 (15% vs. 10%): f2 = 48.27 → profiles are not similar.
- F14 vs. F11 (5% vs. 10%): f2 = 59.33 → similar.
- F13 vs. F14 (15% vs. 5%): f2 = 53.53 → similar.
2.4. Disintegration
2.5. FT-IR Analysis
2.6. Process Development and Possible Scale-Up
3. Materials and Methods
3.1. Materials
3.1.1. Preparation and Evaluation of Polymer–Solvent Mixtures for Printability Assessment
3.1.2. Design of Printable Dosage Forms
3.1.3. Preparation of Drug-Loaded Formulations for SSE Printing
3.1.4. Three-dimensional Printing Procedure Using Semi-Solid Extrusion (SSE)
3.1.5. Characterization of Printed Tablets
Uniformity of Mass and Dimensions
Drug Release
- -
- Qₜ: amount of drug released at time t
- -
- Q0: initial amount of drug (usually zero)
- -
- k0: zero-order rate constant
- -
- Qₜ: amount of drug remaining at time t
- -
- Q0: initial amount of drug
- -
- k1: first-order rate constant
- -
- Qₜ: amount of drug released at time t
- -
- kH: Higuchi dissolution constant
- -
- t: time
- -
- Qₜ: amount of drug released at time t
- -
- Q∞: total amount of drug released at infinite time
- -
- kK: kinetic constant
- -
- n: release exponent (indicates mechanism of drug release)
- -
- Q0: initial amount of drug
- -
- Qₜ: amount of drug remaining at time t
- -
- kHC: Hixson–Crowell rate constant
- -
- t: time
Disintegration Testing
Fourier-Transform Infrared Spectroscopy (FT-IR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| SSE | Semi-solid extrusion |
| HPMC | Hydroxypropyl methylcellulose |
| FDM | Fused deposition modeling |
| PEG | Polyethylene glycol |
| CAD | Computer-aided design |
| FT-IR | Fourier-Transform Infrared |
| API | Active Pharmaceutical Ingredient |
References
- Aneesh, T.P.; Sekhar, S.; Jose, A.; Chandran, L.; Zachariah, S.M. Pharmacogenomics: The right drug to the right person. J. Clin. Med. Res. 2009, 1, 191–194. [Google Scholar] [CrossRef][Green Version]
- Roden, D.M.; Wilke, R.A.; Kroemer, H.K.; Stein, C.M. Pharmacogenomics The Genetics of Variable Drug Responses. Circulation 2011, 123, 1661–1670. [Google Scholar] [CrossRef]
- Jan, A.; Alanzi, A.R.; Mothana, R.; Kaimori, J.-Y.; Ali, S.S.; Muhammad, T.; Saeed, M.; Akbar, R.; Khan, M. Pharmacogenomic Study of Selected Genes Affecting Amlodipine Blood Pressure Response in Patients with Hypertension. Pharmacogenomics Pers. Med. 2024, 17, 473–486. [Google Scholar] [CrossRef]
- Srai, J.S.; Badman, C.; Krumme, M.; Futran, M.; Johnston, C. Future supply enabled by continuous opportunities and challenges. J. Pharm. Sci. 2015, 104, 840–849. [Google Scholar] [CrossRef]
- Mwema, F.M.; Akinlabi, E.T. Basics of Fused Deposition Modelling (FDM). Fused Depos. Model. 2020, 30, 1–15. [Google Scholar]
- ZipDose Technology | Spritam | Aprecia. Available online: https://www.aprecia.com/technology/zipdose (accessed on 28 April 2025).
- Sames, W.; Frederick, A.; Pannala, S.; Dehoff, R.R.; Babu, S.S. The metallurgy and processing science of metal additive manufacturing. Int. Mater. Rev. 2016, 61, 315–360. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef]
- Basit, A.W.; Gaisford, S. 3D Printing of Pharmaceuticals; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Liang, E.; Wang, Z.; Li, X.; Wang, S.; Han, X.; Chen, D.; Zheng, A. 3D printing technology based on versatile gelatin-carrageenan gel system for drug formulations. Pharmaceutics 2023, 15, 1218. [Google Scholar] [CrossRef]
- Panraksa, P.; Qi, S.; Udomsom, S.; Tipduangta, P.; Rachtanapun, P.; Jantanasakul-wong, K.; Jantrawut, P. Characterization of hydrophilic polymers as a syringe extrusion 3D printing material for orodispersible film. Polymers 2021, 13, 3454. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Rodríguez-González, D.; Alejandro Fernández, M.; Suñé-Pou, M.; Pérez-Lozano, P.; García-Montoya, E.; Aguilar, E. 3D printed gummies: Personalized drug dosage in a safe and appealing way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.Y.; Lin, X.; Xie, L.X.; Ivone, R.; Shen, J.; Yang, G.S. A tunable extruded 3D printing platform using thermo-sensitive pastes. Int. J. Pharm. 2020, 583, 119360. [Google Scholar] [CrossRef]
- Khaled, S.A.; Alexander, M.R.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int. J. Pharm. 2018, 538, 223–230. [Google Scholar] [CrossRef]
- Cheng, Y.; Qin, H.; Acevedo, N.C.; Jiang, X.; Shi, X. 3D printing of extended-release tablets of theophylline using hydroxypropyl methylcellulose (HPMC) hydrogels. Int. J. Pharm. 2020, 591, 119983. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef]
- Sjoholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef]
- Conceicao, J.; Farto-Vaamonde, X.; Goyanes, A.; Adeoye, O.; Concheiro, A.; Cabral-Marques, H.; Sousa Lobo, J.M.; Alvarez-Lorenzo, C. Hydroxypropyl-β-cyclodextrin-based fast dissolving carbamazepine printlets prepared by semisolid extrusion 3D printing. Carbohydr. Polym. 2019, 221, 55–62. [Google Scholar] [CrossRef]
- Cui, M.; Li, Y.; Wang, S.; Chai, Y.; Lou, J.; Chen, F.; Li, Q.; Pan, W.; Ding, P. Exploration and Preparation of a Dose-Flexible Regulation System for Levetiracetam Tablets via Novel Semi-Solid Extrusion Three-Dimensional Printing. J. Pharm. Sci. 2019, 108, 977–986. [Google Scholar] [CrossRef]
- Zhang, B.; Teoh, X.Y.; Yan, J.; Gleadall, A.; Belton, P.; Bibb, R.; Qi, S. Development of combi-pills using the coupling of semi-solid syringe extrusion 3D printing with fused deposition modelling. Int. J. Pharm. 2022, 625, 122140. [Google Scholar] [CrossRef]
- Kamlow, M.-A.; Vadodaria, S.; Gholamipour-Shirazi, A.; Spyropoulos, F.; Mills, T. 3D printing of edible hydrogels containing thiamine and their comparison to cast gels. Food Hydrocoll. 2021, 116, 106550. [Google Scholar] [CrossRef]
- Rodríguez-Maciñeiras, X.; Bendicho-Lavilla, C.; Rial, C.; Garba-Mohammed, K.; Wors-ley, A.; Díaz-Torres, E.; Orive-Martínez, C.; Orive-Mayor, Á.; Basit, A.W.; Alvarez-Lorenzo, C.; et al. Advancing medication compounding: Use of a pharmaceutical 3D printer to auto-fill minoxidil capsules for dispensing to patients in a community pharmacy. Int. J. Pharm. 2025, 671, 125251. [Google Scholar] [CrossRef] [PubMed]
- Cerveto, T.; Denis, L.; Stoops, M.; Lechanteur, A.; Jérôme, C.; Leenhardt, J.; Flynn, S.; Goyanes, A.; Mazet, R.; Annereau, M.; et al. promising role of semi-solid extrusion technology in custom drug formulation for pediatric medicine. Int. J. Bioprint. 2024, 10, 4063. [Google Scholar] [CrossRef]
- Zhou, L.; Meng, F.-B.; Li, Y.-C.; Shi, X.-D.; Yang, Y.-W.; Wang, M. Effect of peach gum polysaccharide on the rheological and 3D printing properties of gelatin-based functional gummy candy. Int. J. Biiol. Macromol. 2023, 253, 127186. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Optimization of semisolid extrusion (pressure-assisted microsyringe)-based 3D printing process for advanced drug delivery application. Ann. 3D Print. Med. 2021, 2, 100008. [Google Scholar] [CrossRef]
- Zhang, B.; Teoh, X.Y.; Belton, P.; Gleadall, A.; Bibb, R.; Sheng, Q. The development of hypromellose based semisolid 3D printing inks for drug delivery. Transactions on Additive Manufacturing Meets Medicine Trans. AMMM 2021, 3, 541. [Google Scholar]
- Viidik, L.; Seera, D.; Antikainen, O.; Kogermann, K.; Heinämäki, J.; Laidmäe, I. 3D-printability of aqueous poly(ethylene oxide) gels. Eur. Polym. J. 2019, 120, 109206. [Google Scholar] [CrossRef]
- Jiang, Z.; Diggle, B.; Tan, M.L.; Viktorova, J.; Bennett, C.W.; Connal, L.A. Extrusion 3D printing of polymeric materials with advanced properties. Adv. Sci. 2020, 7, 2001379. [Google Scholar] [CrossRef]
- Hiremath, P.; Nuguru, K.; Agrahari, V. Handbook of Pharmaceutical Wet Granulation Chapter 8—Material Attributes and Their Impact on Wet Granulation Process Performance; Academic Press: Cambridge, MA, USA, 2019; pp. 263–315. ISBN 9780128104606. [Google Scholar] [CrossRef]
- Roche, A.; Sanchez-Ballester, N.M.; Aubert, A.; Rossi, J.-C.; Begu, S.; Soulairol, I. Preliminary Study on the Development of Caffeine Oral Solid Form 3D Printed by Semi-Solid Extrusion for Application in Neonates. AAPS PharmSciTech 2023, 24, 122. [Google Scholar] [CrossRef]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef]
- Siyawamwaya, M.; Toit, L.C.D.; Kumar, P.; Choonara, Y.E.; Kondiah, P.P.D.; Pillay, V. 3D printed, controlled release, tritherapeutic tablet matrix for advanced anti-HIV-1 drug delivery. Eur. J. Pharm. Biopharm. 2019, 138, 99–110. [Google Scholar] [CrossRef]
- Aina, M.; Baillon, F.; Sescousse, R.; Sanchez-Ballester, N.M.; Begu, S.; Soulairol, I.; Sauceau, M. From conception to consumption: Applications of semi-solid extrusion 3D printing in oral drug delivery. Int. J. Pharm. 2025, 674, 125436. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Robles Martínez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Yang, X.L.; Huang, W.D.; Liu, J.; Wang, Y.G.; Xu, H. Electrospun biphasic drug release polyvinylpyrrolidone/ethyl cellulose core–shell nanofibers. Int. J. Pharm. 2010, 384, 215–221. [Google Scholar] [CrossRef]
- Devi, D.; Ghosh, A.; Mandal, U.K. Sustained release matrix tablet of 100 mg losartan potassium: Formulation development and in vitro characterization. Braz. J. Pharm. Sci. 2022, 58, e20079. [Google Scholar] [CrossRef]
- Vohra, D.D.; Pagi, K.S.; Rajesh, K.S. Losartan potassium loaded sustained release matrix tablets: Influence of various hydrophilic and hydrophobic polymers on drug release behaviour. J. Pharm. Bioallied Sci. 2012, 4 (Suppl. S1), S79–S80. [Google Scholar] [CrossRef]
- Fatima, H.; Mohammed, S.; Begum, A. Effect of Croscormellose Sodium in Sustained Release Layer of Valsartan in Bilayer Tablet with Clopidogrel as Immediate Drug Release and Valsartan as Sustained Drug Release. J. Drug Deliv. Ther. 2023, 13, 35–50. [Google Scholar] [CrossRef]
- Stanojević, G.; Medarević, D.; Adamov, I.; Pešić, N.; Kovačević, J.; Ibrić, S. Tailoring Atomoxetine Release Rate from DLP 3D-Printed Tablets Using Artificial Neural Networks: Influence of Tablet Thickness and Drug Loading. Molecules 2021, 26, 111. [Google Scholar] [CrossRef]
- Franca, C.A.; Etcheverry, S.B.; Diez, R.P.; Williams, P.A.M. Irbesartan: FTIR and Raman spectra. Density functional study on vibrational and NMR spectra. J. Raman Spectrosc. 2006, 40, 1296–1300. [Google Scholar] [CrossRef]
- Akinosho, H.; Hawkins, S.; Wicker, L. Hydroxypropyl methylcellulose substituent analysis and rheological properties. Carbohydr. Polym. 2013, 98, 276–281. [Google Scholar] [CrossRef]
- Bashir, S.; Zafar, N.; Lebaz, N.; Mahmood, A.; Elaissari, A. Hydroxypropyl methylcellulose-based hydrogel copolymeric for controlled delivery of galantamine hydrobromide in Dementia. Processes 2020, 8, 1350. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022; Volume 11, pp. 3260–3262. [Google Scholar]
- Lastra, O.C.; Lemus, I.G.; Sánchez, H.J.; Pérez, R.F. Development and validation of an UV derivative spectrophotometric determination of Losartan potassium in tablets. J. Pharm. Biomed. Anal. 2003, 33, 175–180. [Google Scholar] [CrossRef]
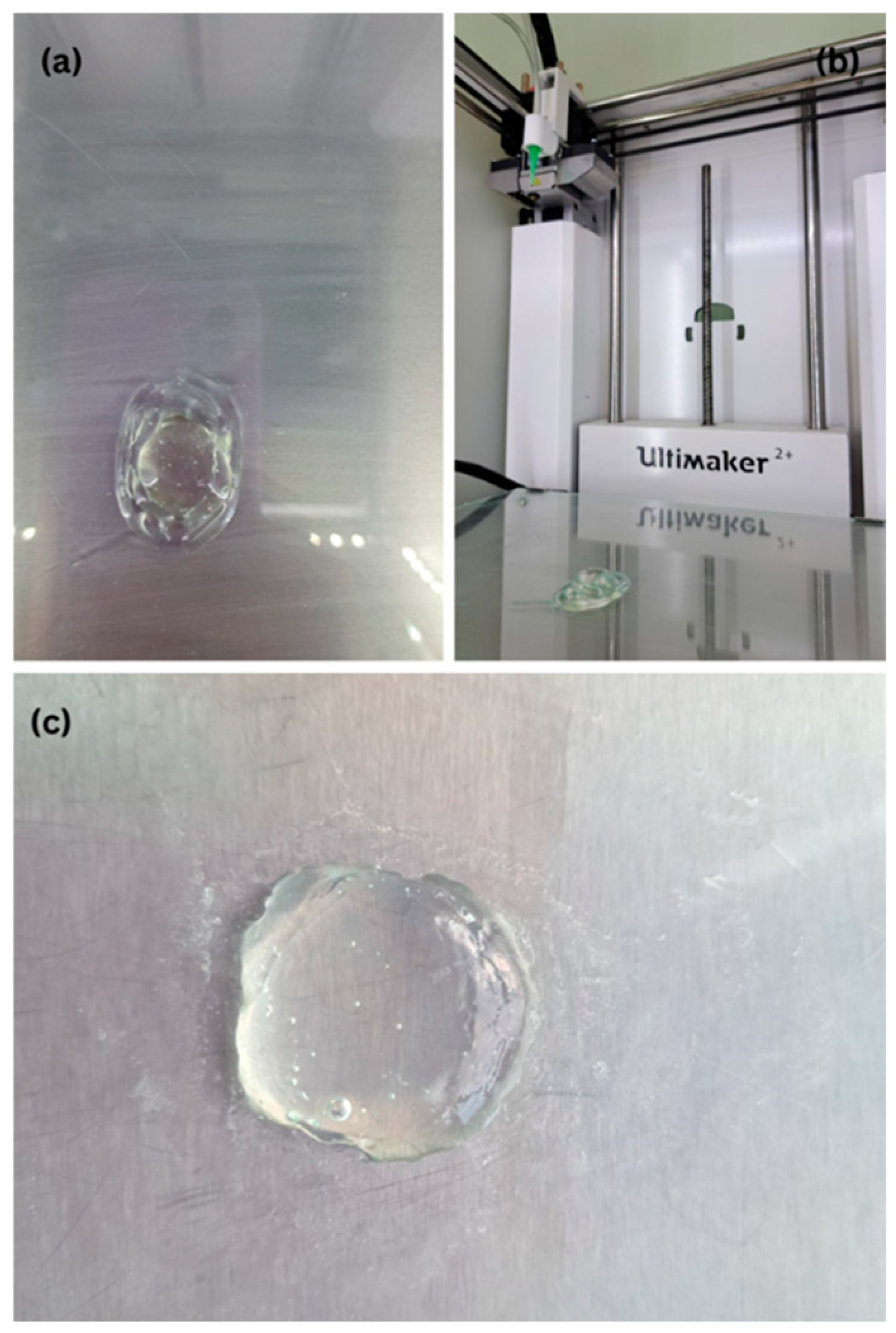

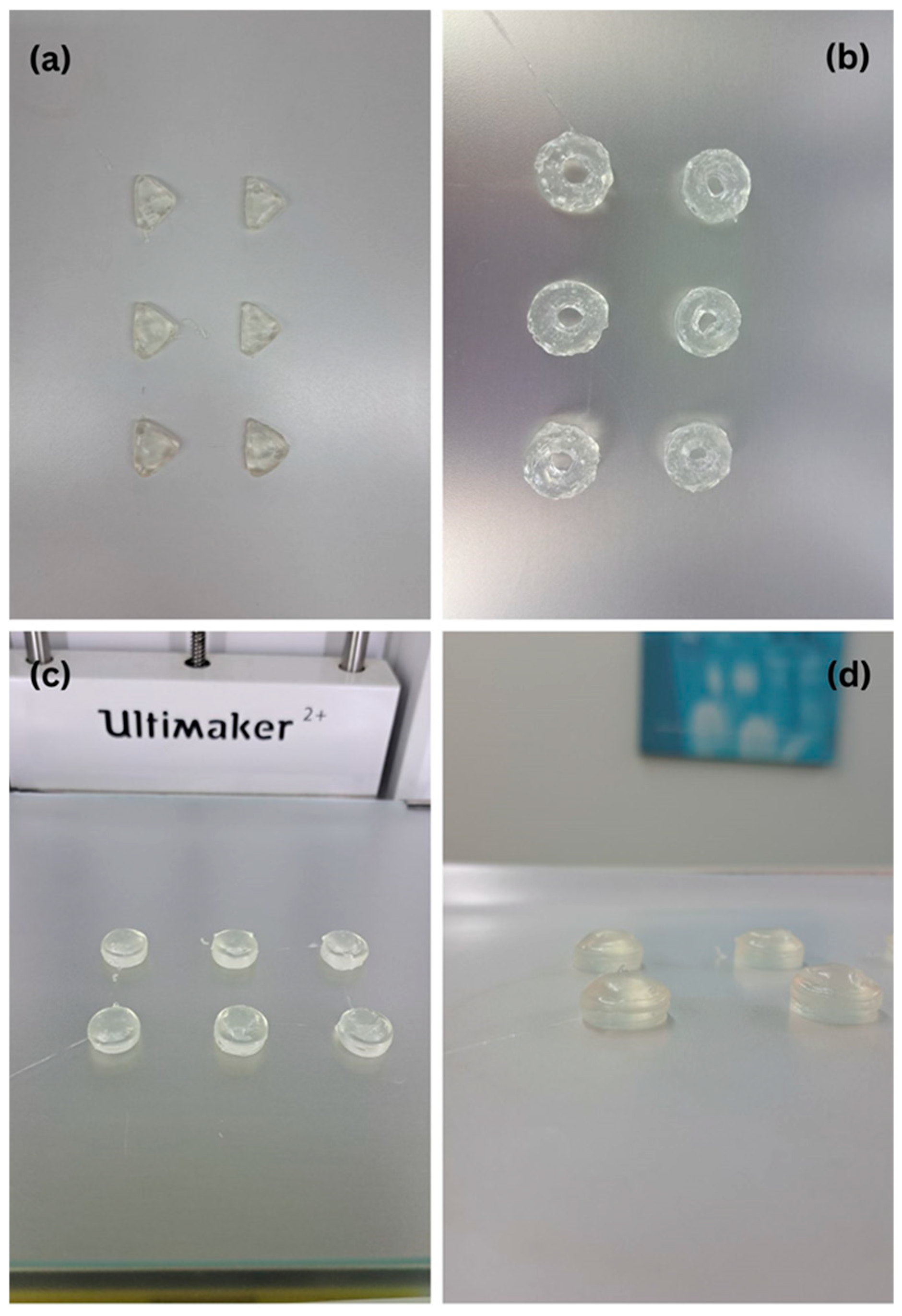
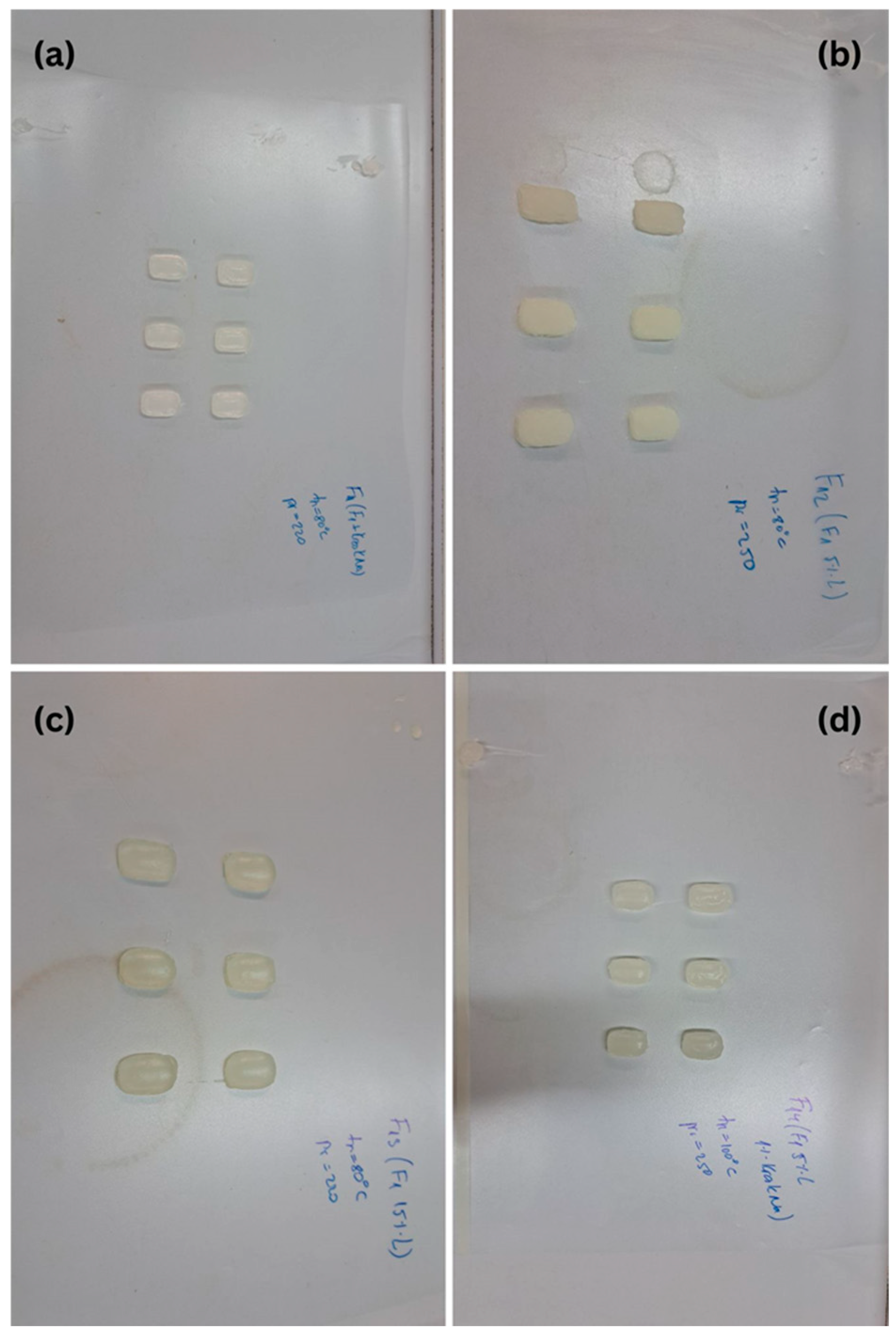




| Polymer/Croscarmellose Sodium/Solvent | Concentration (% w/w) | Observations |
|---|---|---|
| Benecel K35M + Croscarmellose sodium/Water | 1% + 1% | Exhibited excessively low viscosity. |
| Benecel K35M + Croscarmellose sodium/Water | 2% + 2% | Optimal viscosity; high presence of bubbles (disappear with settling). |
| Benecel K35M + Croscarmellose sodium/Water | 2% + 4% | Fewer bubbles present; increased viscosity (printed with flow rate 280%); shape spreading during print (poor plastic properties). |
| HPMC 615 + Croscarmellose sodium/Water | 10% + 1% | High bubble presence; optimal viscosity; shape spreading and poor adhesion between layers. |
| HPMC 615/Water | 12% | High viscosity and bubble presence; unsuitable for printing. |
| HPMC 615/Ethanol–water (50:50 v/v) | 12% | Inhomogeneous; mixing difficulties. |
| HPMC 615 + Croscarmellose sodium/Ethanol–water (50:50 v/v) | 10% + 1% | Inhomogeneous; mixing difficulties. |
| HPMC 4500 + Croscarmellose sodium/Ethanol–water (9:1 v/v) | 5% + 1% | Slight mixing difficulties; suitable viscosity and flow; successful harder form. |
| Excipient | Role | F1 | F2 | F3 | F4 | F11 | F12 | F13 | F14 |
|---|---|---|---|---|---|---|---|---|---|
| HPMC 4500 | binder | x | x | x | x | x | x | x | x |
| PEG 6000 | binder | x | x | x | x | x | x | x | x |
| SiO2 | plasticizer | x | x | x | x | x | x | x | x |
| Croscarmellose sodium | disintegrant | - | - | - | - | x | x | x | x |
| Ethanol–water (9:1 v/v) | solvent | x | x | x | x | x | x | x | x |
| Tablet No | Length (mm) | Width (mm) | Height (mm) |
|---|---|---|---|
| 1 | 18.50 | 10.00 | 4.00 |
| 2 | 19.00 | 10.50 | 3.50 |
| 3 | 19.50 | 11.00 | 4.00 |
| 4 | 19.00 | 9.50 | 4.00 |
| 5 | 20.00 | 11.50 | 4.00 |
| 6 | 19.50 | 12.00 | 4.00 |
| 7 | 18.50 | 11.50 | 3.50 |
| 8 | 19.00 | 11.00 | 4.00 |
| 9 | 20.00 | 11.50 | 4.00 |
| 10 | 19.00 | 12.00 | 4.00 |
| 11 | 18.50 | 11.00 | 3.50 |
| 12 | 19.50 | 11.50 | 4.00 |
| Average | 19.17 | 11.08 | 3.87 |
| SD | 0.54 | 0.76 | 0.22 |
| Formulation | Average Mass (g) | SD (g) | CV (%) |
|---|---|---|---|
| F1 | 0.425 | 0.026 | 6.12 |
| F2 | 0.398 | 0.047 | 11.81 |
| F3 | 0.489 | 0.063 | 12.89 |
| F4 | 0.513 | 0.051 | 9.94 |
| F11 | 0.573 | 0.060 | 10.47 |
| F12 | 0.623 | 0.107 | 17.17 |
| F13 | 0.821 | 0.116 | 14.13 |
| F14 | 0.709 | 0.070 | 9.87 |
| Model | Rounded Rectangle | Triangle | Doughnut | Round Tablet |
|---|---|---|---|---|
| Zero-order | 0.9165 | 0.9437 | 0.8632 | 0.9517 |
| First-order | 0.7886 | 0.8191 | 0.7335 | 0.8313 |
| Higuchi | 0.9649 | 0.9563 | 0.9641 | 0.9602 |
| Korsmeyer–Peppas | 0.9747 | 0.9809 | 0.9641 | 0.9863 |
| Hixson–Crowell | 0.8309 | 0.8623 | 0.7742 | 0.8733 |
| Formulation | Geometry | Drug Loading (%) | Superdisintegrant | Conc. (%) | Disintegration Time (min) |
|---|---|---|---|---|---|
| F1 | Rounded Rectangle | 5 | None | 0 | 55 |
| F2 | Triangle | 5 | None | 0 | 55 |
| F3 | Doughnut | 5 | None | 0 | 55 |
| F4 | Round | 5 | None | 0 | 60 |
| F11 | Rounded Rectangle | 10 | Croscarmellose Sodium | 1 | 25 |
| F12 | Rounded Rectangle | 5 | Croscarmellose Sodium | 3 | 50 |
| F13 | Rounded Rectangle | 15 | Croscarmellose Sodium | 1 | 40 |
| F14 | Rounded Rectangle | 5 | Croscarmellose Sodium | 1 | 50 |
| Quality Target Product Profile (QTPP) | Shape |
|---|---|
| Dosage Form | 3D SSE printed tablet |
| Route of Administration | oral |
| Appearance | Solid, tablet with smooth layers rounded rectangle, triangle, doughnut or circle shape |
| Dimensions | Rounded rectangle 20 × 12 × 4 mm Triangle 22 × 15 × 5 mm Doughnut 20 × 20 × 5 mm (outer ⌀) 10 mm (inner ⌀) Circle 15 × 15 × 5 mm |
| Dissolution profile | L1: 20% in 1 h L2: 50% in 2 h L3: >80% in 3 h |
| Mechanical Strength | No cracking or shape collapse post-printing/drying |
| Critical Material Attributes (CMAs) | Impact on CQAs | Control Strategy |
|---|---|---|
| API solubility in hydroalcoholic vehicle | Affects drug content uniformity and release rate | Fixed solvent ratio (ethanol:water), temperature-controlled dissolution |
| PEG 6000 content (15% w/w) | Affects plasticity, flowability, and printability | Optimized based on prior screening |
| HPMC viscosity (4500 cps) | Impacts gel formation, print fidelity, and layer adhesion | Pre-characterized polymer |
| SiO2 (1% w/w) | Improves thixotropy and prevents phase separation | Fixed concentration |
| Critical Process Parameters (CPPs) | Impact on CQAs | Control Strategy |
|---|---|---|
| Mixing temperature (~35 °C) | Solubilization of API, blend uniformity | Maintained ± 2 °C using hotplate/stirrer |
| Stirring duration and speed | API dispersion, bubble formation | Time and speed controlled mixing |
| Gelation/resting time (10 min at 22 °C) | Air removal, shape formation | Constant across batches |
| Syringe filling method | Uniform loading, air bubble prevention | Manual, standardised |
| Extrusion speed/pressure | Layer quality, dimensional accuracy | Pre-set in G-code, verified before runs |
| Nozzle diameter (e.g., 0.84 mm) | Affects resolution and flow | Constant |
| Drying conditions (e.g., 40 °C/4 h) | Mechanical integrity, residual moisture | Fixed drying protocol validated for shape retention |
| Critical Quality Attributes (CQAs) | Specification/Target | Test Method |
|---|---|---|
| Dosage unit mass | 500 ± 5 mg | Mass variation by individually weighing 12 units per formulation |
| Drug content | 90–110% of label claim | UV/Vis spectrophotometry |
| Dimensional accuracy | CV < 3% for height and diameter | Digital caliper measurement |
| Disintegration time | <60 min | USP <701> |
| Dissolution profile | L1: 20% in 1 h L2: 50% in 2 h L3: >80% in 3 h | USP Apparatus I (basket) |
| Mechanical integrity | No cracking or delamination | Visual assessment |
| Surface quality | Smooth, no visible layering defects | Visual inspection |
| Mixture Code | Polymer/Superdisintegrant/Solvent | Concentration (%w/w) | Quantity (g) |
|---|---|---|---|
| M1 | Benecel K35M + Croscarmellose sodium/Water | 1% + 1% | 0.2 + 0.2 |
| M2 | Benecel K35M + Croscarmellose sodium/Water | 2% + 2% | 0.4 + 0.4 |
| M3 | Benecel K35M + Croscarmellose sodium/Water | 2% + 4% | 0.4 + 0.8 |
| M4 | HPMC 615 + Croscarmellose sodium/Water | 10% + 1% | 2.0 + 0.2 |
| M5 | HPMC 615/Water | 12% | 2.4 |
| M6 | HPMC 615/Ethanol–water (50:50 v/v) | 12% | 2.4 |
| M7 | HPMC 615 + Croscarmellose sodium/Ethanol–water (50:50 v/v) | 10% + 1% | 2.0 + 0.2 |
| M8 | HPMC 4500 + Croscarmellose sodium/Ethanol–water (9:1 v/v) | 5% + 1% | 1.0 + 0.2 |
| Shape | Dimensions | Volume (mm3) | Surface Area (mm2) | SA:V Ratio (mm−1) |
|---|---|---|---|---|
| Rounded Rectangle | 20 × 12 × 4 mm | 946.3 | 736.0 | 0.778 |
| Triangle | 22 × 15 × 5 mm | 825.0 | 648.1 | 0.786 |
| Doughnut | 20 × 20 × 5 mm (outer ⌀) 10 mm (inner ⌀) | 1178.1 | 942.5 | 0.8 |
| Circle | 15 × 15 × 5 mm | 883.6 | 589.0 | 0.667 |
| Component | F1 | F2 | F3 | F4 | F11 | F12 | F13 | F14 |
|---|---|---|---|---|---|---|---|---|
| Losartan potassium | 10 | 10 | 10 | 10 | 10 | 5 | 15 | 5 |
| HPMC 4500 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| PEG 6000 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| SiO2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Croscarmellose sodium | - | - | - | - | 1 | 3 | 1 | 1 |
| Ethanol–water (9:1 v/v) | 69 | 69 | 69 | 69 | 68 | 71 | 63 | 73 |
| Tablet shape | RR | T | D | C | RR | RR | RR | RR |
| Parameter | Value |
|---|---|
| Layer Height | 1.2 mm |
| Top/Bottom Thickness | 1.2 mm |
| Top/Bottom Layers | 1/1 |
| Infill Density | 100% |
| Infill Pattern | Lines |
| Infill Layer Thickness | 1.2 mm |
| Print Speed | 15.0 mm/s |
| Travel Speed | 80.0 mm/s |
| Initial Layer Speed | 11.0 mm/s |
| Fan Speed | 100% |
| Build Plate Adhesion | None |
| Adaptive Layers | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojinović, A.; Medarević, Đ.; Stanojević, G.; Mirković, D.; Mugoša, S.; Adamov, I.; Ibrić, S. Development and Evaluation of 3D-Printed Losartan Potassium Tablets Using Semi-Solid Extrusion: The Effect of Geometry, Drug Loading and Superdisintegrant. Pharmaceuticals 2025, 18, 1504. https://doi.org/10.3390/ph18101504
Vojinović A, Medarević Đ, Stanojević G, Mirković D, Mugoša S, Adamov I, Ibrić S. Development and Evaluation of 3D-Printed Losartan Potassium Tablets Using Semi-Solid Extrusion: The Effect of Geometry, Drug Loading and Superdisintegrant. Pharmaceuticals. 2025; 18(10):1504. https://doi.org/10.3390/ph18101504
Chicago/Turabian StyleVojinović, Aleksandra, Đorđe Medarević, Gordana Stanojević, Dušica Mirković, Snežana Mugoša, Ivana Adamov, and Svetlana Ibrić. 2025. "Development and Evaluation of 3D-Printed Losartan Potassium Tablets Using Semi-Solid Extrusion: The Effect of Geometry, Drug Loading and Superdisintegrant" Pharmaceuticals 18, no. 10: 1504. https://doi.org/10.3390/ph18101504
APA StyleVojinović, A., Medarević, Đ., Stanojević, G., Mirković, D., Mugoša, S., Adamov, I., & Ibrić, S. (2025). Development and Evaluation of 3D-Printed Losartan Potassium Tablets Using Semi-Solid Extrusion: The Effect of Geometry, Drug Loading and Superdisintegrant. Pharmaceuticals, 18(10), 1504. https://doi.org/10.3390/ph18101504








