Combined Effect of Size and Charge on the Interaction of Nanoparticles with Mucus-Mimicking Mucin Hydrogels
Abstract
1. Introduction
2. Results
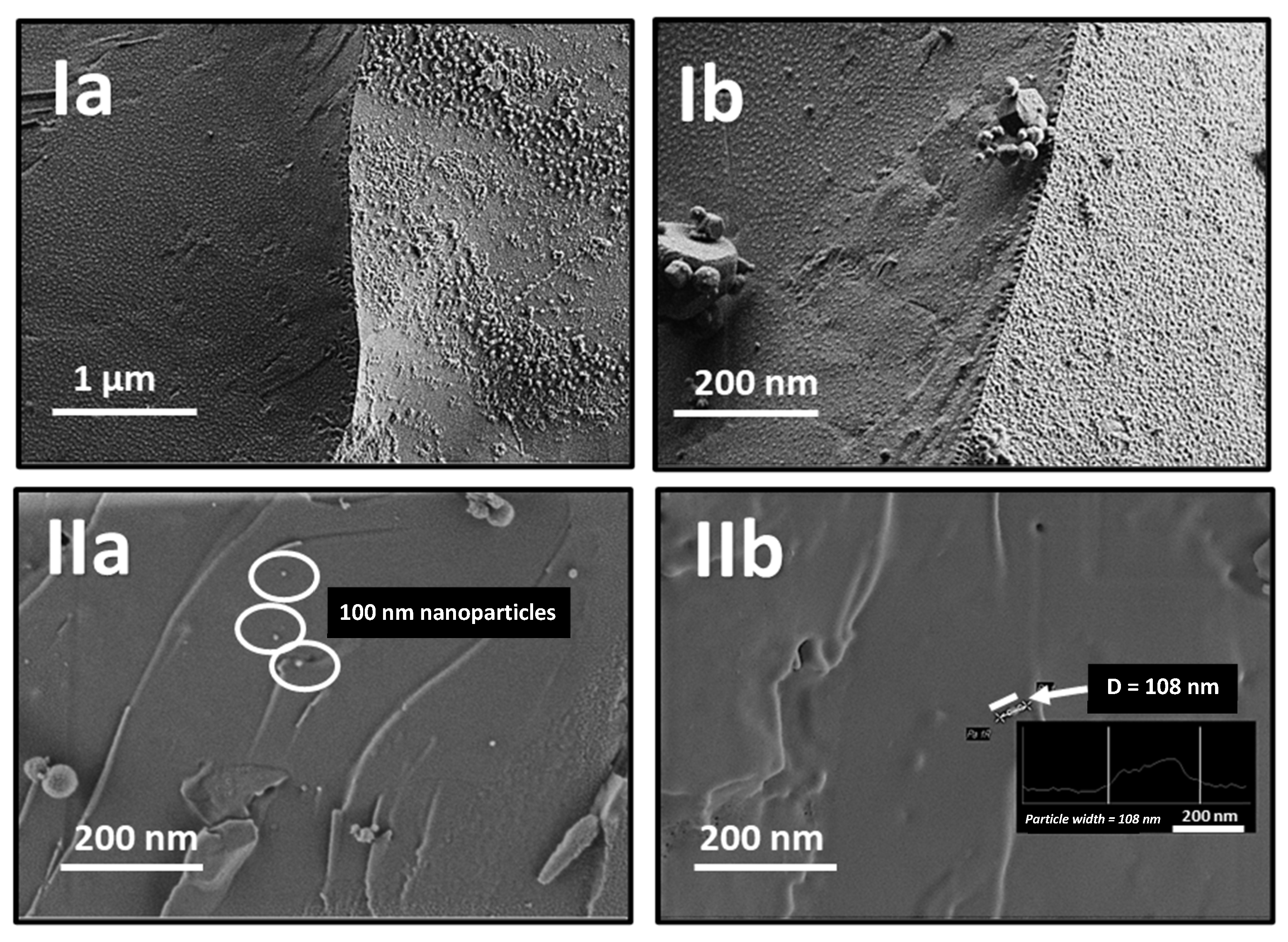
2.1. Synthesis and Characterization of Gold Nanoparticles
2.2. Particle Diffusion into Mucus-Mimicking Mucin-Based Hydrogels In Vitro
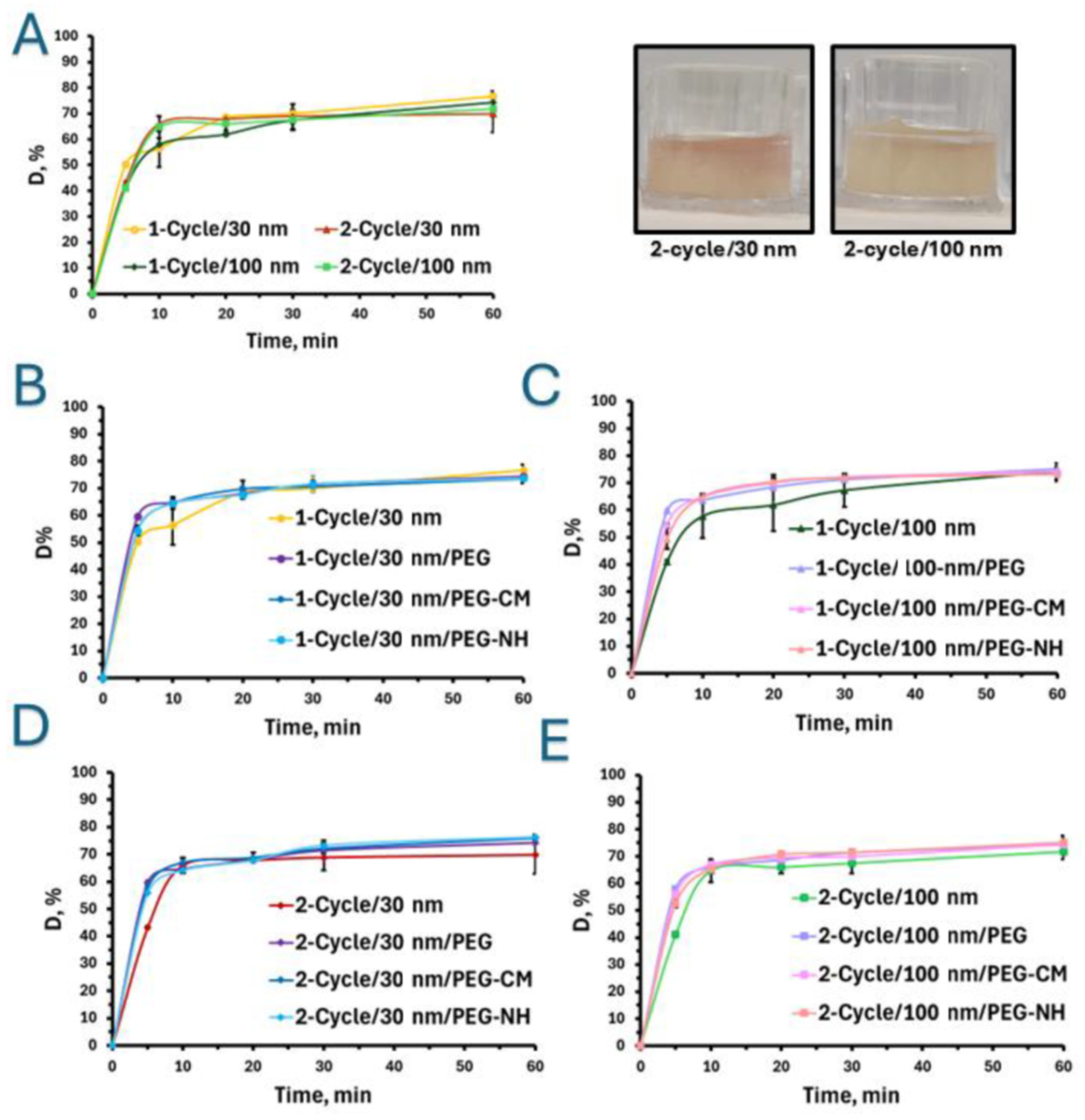
2.2.1. Effect of Nanoparticle Size
2.2.2. Effect of Particle Charge and Hydrogel Crosslinking Density
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Mucus-Mimicking Mucin-Based Hydrogels
4.3. Preparation of Gold Nanoparticles
4.4. Characterization of the Nanoparticles
4.5. In Vitro Particle Diffusion Studies on Mucus-Mimicking Hydrogels Top of Form
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McShane, A.; Bath, J.; Jaramillo, A.M.; Ridley, C.; Walsh, A.A.; Evans, C.M.; Thornton, D.J.; Ribbeck, K. Mucus. Curr. Biol. 2021, 31, R938. [Google Scholar] [CrossRef]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal mucus components and secretion mechanisms: What we do and do not know. Exp. Mol. Med. 2023, 55, 681–691. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, C.S.; St Leger, A.J.; Caspi, R.R. Mucosal immunology of the ocular surface. Mucosal Immunol. 2022, 15, 1143. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.A. Role of Cilia, Mucus, and Airway Surface Liquid in Mucociliary Dysfunction: Lessons from Mouse Models. J. Aerosol Med. Pulm. Drug Deliv. 2008, 21, 13–24. [Google Scholar] [CrossRef]
- Tomazic, P.V.; Darnhofer, B.; Birner-Gruenberger, R. Nasal mucus proteome and its involvement in allergic rhinitis. Expert Rev. Proteom. 2020, 17, 191. [Google Scholar] [CrossRef]
- Bej, R.; Stevens, C.A.; Nie, C.; Ludwig, K.; Degen, G.; Kerkhoff, Y.; Pigaleva, M.; Adler, J.M.; Bustos, N.A.; Page, T.M.; et al. Mucus-Inspired Self-Healing Hydrogels: A Protective Barrier for Cells against Viral Infection. Adv. Mater. 2024, 36, 2401745. [Google Scholar] [CrossRef]
- Biswas, M.; Nurunnabi, M.; Khatun, Z. Understanding Mucosal Physiology and Rationale of Formulation Design for Improved Mucosal Immunity. ACS Appl. Bio. Mater. 2024, 7, 5037. [Google Scholar] [CrossRef]
- Bustos, N.A.; Ribbeck, K.; Wagner, C.E. The role of mucosal barriers in disease progression and transmission. Adv. Drug Deliv. Rev. 2023, 200, 115008. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75. [Google Scholar] [CrossRef]
- Bakshani, C.R.; Morales-Garcia, A.L.; Althaus, M.; Wilcox, M.D.; Pearson, J.P.; Bythell, J.C.; Burgess, J.G. Evolutionary conservation of the antimicrobial function of mucus: A first defence against infection. npj Biofilms Microbiomes 2018, 4, 14. [Google Scholar] [CrossRef]
- Randall, T.D.; Mebius, R.E. The development and function of mucosal lymphoid tissues: A balancing act with micro-organisms. Mucosal Immunol. 2014, 7, 455. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered defense: How mucus and tight junctions seal the intestinal barrier. J. Mol. Med. 2017, 95, 927. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.-Y.; Wirtz, D.; Hanes, J. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 2009, 61, 86. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.H.; Hasnain, S.Z. Mucus and Mucins: The Underappreciated Host Defence System. Front. Cell. Infect. Microbiol. 2022, 12, 856962. [Google Scholar] [CrossRef]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 236–252. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162. [Google Scholar] [CrossRef]
- Wang, C.-M.; Fernez, M.T.; Woolston, B.M.; Carrier, R.L.; Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2023, 200, 555. [Google Scholar]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Johansson, M.E.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712. [Google Scholar] [CrossRef]
- Bruschi, M.L.; de Souza Ferreira, S.B.; Bassi da Silva, J. Mucoadhesive and mucus-penetrating polymers for drug delivery. In Nanotechnology for Oral Drug Delivery; Academic Press: Cambridge, MA, USA, 2020; pp. 77–141. [Google Scholar]
- Ways, T.M.M.; Filippov, S.K.; Maji, S.; Glassner, M.; Cegłowski, M.; Hoogenboom, R.; King, S.; Lau, W.M.; Khutoryanskiy, V.V. Mucus-penetrating nanoparticles based on chitosan grafted with various non-ionic polymers: Synthesis, structural characterisation and diffusion studies. J. Colloid Interface Sci. 2022, 626, 251. [Google Scholar] [CrossRef]
- Wang, C.-M.; Fernez, M.T.; Woolston, B.M.; Carrier, R.L. Native gastrointestinal mucus: Critical features and techniques for studying interactions with drugs, drug carriers, and bacteria. Adv. Drug Deliv. Rev. 2023, 200, 114966. [Google Scholar] [CrossRef]
- Valibeknejad, M.; Abdoli, S.M.; Alizadeh, R.; Mihăilă, S.M.; Raoof, A. Insights into transport in mucus barrier: Exploring particle penetration through the intestinal mucus layer. J. Drug. Deliv. Sci. Technol. 2023, 86, 104752. [Google Scholar] [CrossRef]
- Vanukuru, S.; Al-Obaidi, H.; Khutoryanskiy, V.V. Transmucosal Drug Delivery: Main Physiological Features and Modern Approaches. In Fundamentals of Pharmaceutical Nanoscience; Springer Nature: Cham, Switzerland, 2024; pp. 213–239. [Google Scholar]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.-Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158. [Google Scholar] [CrossRef]
- Chater, P.I.; Wilcox, M.D.; Pearson, J.P. Efficacy and safety concerns over the use of mucus modulating agents for drug delivery using nanoscale systems. Adv. Drug Deliv. Rev. 2018, 124, 184. [Google Scholar] [CrossRef]
- Bandi, S.P.; Bhatnagar, S.; Venuganti, V.V.K. Advanced materials for drug delivery across mucosal barriers. Acta Biomater. 2021, 119, 13. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Li, S.; Han, B. Nanomedicines for intranasal delivery: Understanding the nano-bio interactions at the nasal mucus-mucosal barrier. Expert Opin. Drug Deliv. 2024, 21, 553. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Fanse, S.; Burgess, D.J. Mucoadhesive drug delivery systems: A promising non-invasive approach to bioavailability enhancement. Part I: Biophysical considerations. Expert Opin. Drug Deliv. 2023, 20, 395. [Google Scholar] [CrossRef] [PubMed]
- Khutoryanskiy, V.V. Beyond PEGylation: Alternative surface-modification of nanoparticles with mucus-inert biomaterials. Adv. Drug Deliv. Rev. 2018, 124, 140. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Shan, W.; Huang, Y. Developments of mucus penetrating nanoparticles. Asian J. Pharm. Sci. 2015, 10, 275. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, D.; Qin, X.; Zhang, D.; Zhang, P. Mucoadhesive-to-Mucopenetrating Nanoparticles for Mucosal Drug Delivery: A Mini Review. Int. J. Nanomed. 2025, 20, 2241. [Google Scholar] [CrossRef]
- Bandi, S.P.; Kumbhar, Y.S.; Venuganti, V.V.K. Effect of particle size and surface charge of nanoparticles in penetration through intestinal mucus barrier. J. Nanopart. Res. 2020, 22, 62. [Google Scholar] [CrossRef]
- Kočevar-Nared, J.; Kristl, J.; Šmid-Korbar, J. Comparative rheological investigation of crude gastric mucin and natural gastric mucus. Biomaterials 1997, 18, 677. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; das Neves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 39, 2030. [Google Scholar] [CrossRef]
- das Neves, J.; Sverdlov Arzi, R.; Sosnik, A. Molecular and cellular cues governing nanomaterial–mucosae interactions: From nanomedicine to nanotoxicology. Chem. Soc. Rev. 2020, 49, 5058. [Google Scholar] [CrossRef]
- Lock, J.Y.; Carlson, T.L.; Carrier, R.L. Mucus models to evaluate the diffusion of drugs and particles. Adv. Drug Deliv. Rev. 2018, 124, 34. [Google Scholar] [CrossRef]
- Imperiale, J.C.; Nejamkin, P.; del Sole, M.J.; ELanusse, C.; Sosnik, A. Novel protease inhibitor-loaded Nanoparticle-in-Microparticle Delivery System leads to a dramatic improvement of the oral pharmacokinetics in dogs. Biomaterials 2015, 37, 383. [Google Scholar] [CrossRef]
- Arzi, R.S.; Sosnik, A.; Cohen, N. A Microscopically Motivated Model for Particle Penetration into Swollen Biological Networks. Polymers 2020, 12, 1912. [Google Scholar] [CrossRef]
- Arzi, R.S.; Davidovich-Pinhas, M.; Cohen, N.; Sosnik, A. An experimental and theoretical approach to understand the interaction between particles and mucosal tissues. Acta Biomater. 2023, 158, 449. [Google Scholar] [CrossRef]
- Porfiryeva, N.N.; Zlotver, I.; Davidovich-Pinhas, M.; Sosnik, A. Mucus-Mimicking Mucin-Based Hydrogels by Tandem Chemical and Physical Crosslinking. Macromol. Biosci. 2024, 24, 2400028. [Google Scholar] [CrossRef]
- Gupta, R.; Rai, B. Effect of Size and Surface Charge of Gold Nanoparticles on their Skin Permeability: A Molecular Dynamics Study. Sci. Rep. 2017, 7, 45292. [Google Scholar] [CrossRef]
- Memon, A.; Channa, I.; Shaikh, A.; Ahmad, J.; Soomro, A.; Giwa, A.; Baig, Z.; Mahdi, W.; Alshehri, S. Citrate-Capped AuNP Fabrication, Characterization and Comparison with Commercially Produced Nanoparticles. Crystals 2022, 12, 1747. [Google Scholar] [CrossRef]
- Porfiryeva, N.N.; Moustafine, R.I.; Khutoryanskiy, V.V. PEGylated Systems in Pharmaceutics. Polym. Sci. Ser. C 2020, 62, 62–74. [Google Scholar] [CrossRef]
- Zuber, A.; Purdey, M.; Schartner, E.; Forbes, C.; van der Hoek, B.; Giles, D.; Abell, A.; Monro, T.; Ebendorff-Heidepriem, H. Detection of gold nanoparticles with different sizes using absorption and fluorescence based method. Sens. Actuators B Chem. 2016, 227, 117. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Moradi Khaniabadi, P.; Jameel, M.S.; Oladzadabbasabadi, N.; Mohammed, S.A.; Abdullah, R.S.; Mehrdel, B. Monodisperse Gold Nanoparticles: A Review on Synthesis and Their Application in Modern Medicine. Int. J. Mol. Sci. 2022, 23, 7400. [Google Scholar] [CrossRef] [PubMed]
- Kaberova, Z.; Karpushkin, E.; Nevoralová, M.; Vetrík, M.; Šlouf, M.; Dušková-Smrčková, M. Microscopic Structure of Swollen Hydrogels by Scanning Electron and Light Microscopies: Artifacts and Reality. Polymers 2020, 12, 578. [Google Scholar] [CrossRef] [PubMed]
- Meziu, E.; Koch, M.; Fleddermann, J.; Schwarzkopf, K.; Schneider, M.; Kraegeloh, A. Visualization of the structure of native human pulmonary mucus. Int. J. Pharm. 2021, 597, 120238. [Google Scholar] [CrossRef]
- Hill, D.B.; Long, R.F.; Kissner, W.J.; Atieh, E.; Garbarine, I.C.; Markovetz, M.R.; Fontana, N.C.; Christy, M.; Habibpour, M.; Tarran, R.; et al. Pathological mucus and impaired mucus clearance in cystic fibrosis patients result from increased concentration, not altered pH. Eur. Respir. J. 2018, 52, 1801297. [Google Scholar] [CrossRef]
- Borah, R.; Ninakanti, R.; Bals, S.; Verbruggen, S.W. Plasmon resonance of gold and silver nanoparticle arrays in the Kretschmann (attenuated total reflectance) vs. direct incidence configuration. Sci. Rep. 2022, 12, 15738. [Google Scholar] [CrossRef]
- Semwal, V.; Jensen, O.R.; Bang, O.; Janting, J. Investigation of Performance Parameters of Spherical Gold Nanoparticles in Localized Surface Plasmon Resonance Biosensing. Micromachines 2023, 14, 1717. [Google Scholar] [CrossRef]
- Fan, J.; Cheng, Y.; Sun, M. Functionalized Gold Nanoparticles: Synthesis, Properties and Biomedical Applications. Chem. Rec. 2020, 20, 1474. [Google Scholar] [CrossRef]
- Barbero, N.; Coletti, M.; Catalano, F.; Visentin, S. Exploring gold nanoparticles interaction with mucins: A spectroscopic-based study. Int. J. Pharm. 2018, 535, 438. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ma, Y.; Chen, X.; Li, M.; Ma, X.; Cheng, G.; Xue, C.; Zuo, Y.Y.; Sun, B. Mucus Penetration of Surface-Engineered Nanoparticles in Various pH Microenvironments. ACS Nano 2023, 17, 2813. [Google Scholar] [CrossRef] [PubMed]
- Zabetakis, K.; Ghann, W.E.; Kumar, S.; Daniel, M.-C. Effect of high gold salt concentrations on the size and polydispersity of gold nanoparticles prepared by an extended Turkevich–Frens method. Gold Bull. 2012, 45, 203. [Google Scholar] [CrossRef]
- António, M.; Lima, T.; Vitorino, R.; Daniel-da-Silva, A.L. Interaction of Colloidal Gold Nanoparticles with Urine and Saliva Biofluids: An Exploratory Study. Nanomaterials 2022, 12, 4434. [Google Scholar] [CrossRef]
- Wang, W.; Ding, X.; Xu, Q.; Wang, J.; Wang, L.; Lou, X. Zeta-potential data reliability of gold nanoparticle biomolecular conjugates and its application in sensitive quantification of surface absorbed protein. Colloids Surf. B Biointerfaces 2016, 148, 541. [Google Scholar] [CrossRef]
- Omping, J.; Unabia, R.; Reazo, R.L.; Lapening, M.; Lumod, R.; Ruda, A.; Rivera, R.B.; Sayson, N.L.; Latayada, F.; Capangpangan, R.; et al. Facile Synthesis of PEGylated Gold Nanoparticles for Enhanced Colorimetric Detection of Histamine. ACS Omega 2024, 9, 14269. [Google Scholar] [CrossRef]
- Wang, W.; Wei, Q.-Q.; Wang, J.; Wang, B.-C.; Zhang, S.; Yuan, Z. Role of thiol-containing polyethylene glycol (thiol-PEG) in the modification process of gold nanoparticles (AuNPs): Stabilizer or coagulant? J. Colloid Interface Sci. 2023, 404, 223. [Google Scholar] [CrossRef]
- Schuster, B.S.; Suk, J.S.; Woodworth, G.F.; Hanes, J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials 2013, 34, 3439–3446. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.-Y.; Hida, K.; Cone, R.; Hanes, J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 598. [Google Scholar] [CrossRef]
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. A 2009, 90A, 720. [Google Scholar] [CrossRef]
- Jory, M.; Donnarumma, D.; Blanc, C.; Bellouma, K.; Fort, A.; Vachier, I.; Casanellas, L.; Bourdin, A.; Massiera, G. Mucus from human bronchial epithelial cultures: Rheology and adhesion across length scales. Interface Focus 2022, 12, 20220028. [Google Scholar] [CrossRef]
- Crater, J.S.; Carrier, R.L. Barrier Properties of Gastrointestinal Mucus to Nanoparticle Transport. Macromol. Biosci. 2010, 10, 1473. [Google Scholar] [CrossRef]
- Chavva, S.R.; San Juan, A.M.T.; Jaitpal, S.; Vu, N.N.; Mabbott, S. Efficient production of uniform gold nanoparticles via a streamlined low-cost, semi-automated, open-source platform. Nanoscale 2024, 16, 9944. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Dobrynin, D.; Zlotver, I.; Polishchuk, I.; Kauffmann, Y.; Suharenko, S.; Koifman, R.; Kuhrts, L.; Katsman, A.; Sosnik, A.; Pokroy, B. Controlled Synthesis of Bimetallic Gold-Silver Nanostars: Atomic Insights and Predictive Formation Model. Small 2025, 21, 2410152. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.; Pati, P.; Vikesland, P.J. Room temperature seed mediated growth of gold nanoparticles: Mechanistic investigations and life cycle assesment. Environ. Sci. Nano 2015, 2, 440. [Google Scholar] [CrossRef]
- Tsvirkun, D.; Ben-Nun, Y.; Merquiol, E.; Zlotver, I.; Meir, K.; Weiss-Sadan, T.; Matok, I.; Popovtzer, R.; Blum, G. CT Imaging of Enzymatic Activity in Cancer Using Covalent Probes Reveal a Size-Dependent Pattern. J. Am. Chem. Soc. 2018, 140, 12010. [Google Scholar] [CrossRef] [PubMed]

| Nanoparticle | Surface Modification | Z-Average ± S.D. (nm) | Dh by Intensity ± S.D. (nm) | Dh Size by Number ± S.D. (nm) | PDI ± S.D. | Z-Potential ± S.D. (mV) |
|---|---|---|---|---|---|---|
| 30 nm | Citrate | 26 ± 6 | 27 ± 5 | 12 ± 1 | 0.15 ± 0.05 | −18 ± 3 |
| PEG | 36 ± 3 | 42 ± 5 | 20 ± 4 | 0.24 ± 0.01 | −3 ± 1 | |
| PEG-CM | 34 ± 4 | 40 ± 1 | 18 ± 3 | 0.22 ± 0.01 | −20 ± 2 | |
| PEG-NH | 43 ± 5 | 54 ±5 | 19 ± 2 | 0.32 ±0.02 | +9 ± 1 | |
| 100 nm | Citrate | 96 ± 5 | 120 ± 2 | 63 ± 1 | 0.21 ± 0.01 | −17 ± 1 |
| PEG | 113 ± 2 | 110 ± 4 | 77 ± 2 | 0.20 ± 0.03 | −4 ± 1 | |
| PEG-CM | 111 ± 4 | 108 ± 2 | 70 ± 2 | 0.19 ± 0.01 | −16 ± 1 | |
| PEG-NH | 120 ± 5 | 129 ± 3 | 73 ± 2 | 0.18 ± 0.04 | +17 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porfiryeva, N.N.; Zlotver, I.; Sosnik, A. Combined Effect of Size and Charge on the Interaction of Nanoparticles with Mucus-Mimicking Mucin Hydrogels. Pharmaceuticals 2025, 18, 1498. https://doi.org/10.3390/ph18101498
Porfiryeva NN, Zlotver I, Sosnik A. Combined Effect of Size and Charge on the Interaction of Nanoparticles with Mucus-Mimicking Mucin Hydrogels. Pharmaceuticals. 2025; 18(10):1498. https://doi.org/10.3390/ph18101498
Chicago/Turabian StylePorfiryeva, Natalia N., Ivan Zlotver, and Alejandro Sosnik. 2025. "Combined Effect of Size and Charge on the Interaction of Nanoparticles with Mucus-Mimicking Mucin Hydrogels" Pharmaceuticals 18, no. 10: 1498. https://doi.org/10.3390/ph18101498
APA StylePorfiryeva, N. N., Zlotver, I., & Sosnik, A. (2025). Combined Effect of Size and Charge on the Interaction of Nanoparticles with Mucus-Mimicking Mucin Hydrogels. Pharmaceuticals, 18(10), 1498. https://doi.org/10.3390/ph18101498








