Challenges in the Treatment of HIV-Related Lymphomas Complicated by COVID-19: Case Study and Review of the Literature
Abstract
1. Introduction
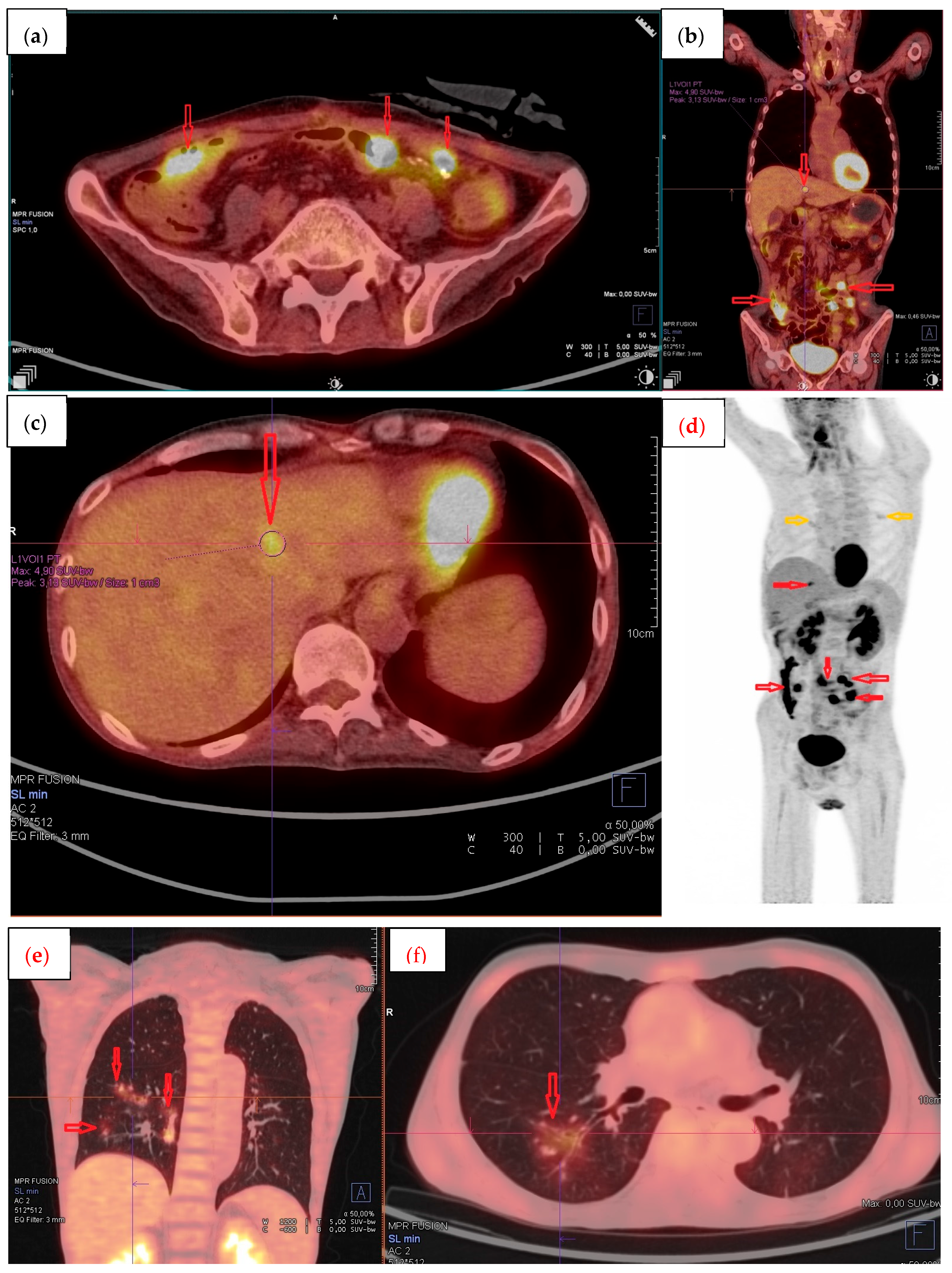
2. Case Report
3. Review of the Literature and Discussion
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO COVID-19 Dashboard. Geneva: World Health Organization. 2020. Available online: https://covid19.who.int/ (accessed on 21 April 2021).
- Arcani, R.; Colle, J.; Cauchois, R.; Koubi, M.; Jarrot, P.A.; Jean, R.; Boyer, A.; Lachamp, J.; Tichadou, A.; Couderc, A.L.; et al. Clinical characteristics and outcomes of patients with haematologic malignancies and COVID-19 suggest that prolonged SARS-CoV-2 carriage is an important issue. Ann. Hematol. 2021, 100, 2799–2803. [Google Scholar] [CrossRef]
- Martinez, J.C.; Sica, R.A.; Stockerl-Goldstein, K.; Rubinstein, S.M. COVID-19 in Patients with Hematologic Malignancies: Outcomes and Options for Treatments. Acta Haematol. 2022, 145, 244–256. [Google Scholar] [CrossRef]
- Goldman, J.D.; Robinson, P.C.; Uldrick, T.S.; Ljungman, P. COVID-19 in immunocompromised populations: Implications for prognosis and repurposing of immunotherapies. J. Immunother. Cancer 2021, 9, e002630. [Google Scholar] [CrossRef] [PubMed]
- Pula, B.; Pruszczyk, K.; Pietrusza, E.; Morawska, M.; Piszczek, W.; Kalicinska, E.; Szeremet, A.; Tryc-Szponder, J.; Wasik-Szczepanek, E.; Drozd-Sokolowska, J.; et al. Outcome of SARS-CoV-2-Infected Polish Patients with Chronic Lymphocytic Leukemia. Cancers 2022, 14, 558. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.; Curley, H.M.; Varnai, C.; Arnold, R.; Lee, L.Y.W.; Campton, N.A.; Cook, G.; Purshouse, K.; Aries, J.; Innes, A.; et al. Key findings from the UKCCMP cohort of 877 patients with haematological malignancy and COVID-19: Disease control as an important factor relative to recent chemotherapy or anti-CD20 therapy. Br. J. Haematol. 2022, 196, 892–901. [Google Scholar] [CrossRef]
- Lahmer, T.; Salmanton-Garcia, J.; Marchesi, F.; El-Ashwah, S.; Nucci, M.; Besson, C.; Itri, F.; Jaksic, O.; Colovic, N.; Weinbergerova, B.; et al. Need for ICU and outcome of critically ill patients with COVID-19 and haematological malignancies: Results from the EPICOVIDEHA survey. Infection 2024, 52, 1125–1141. [Google Scholar] [CrossRef]
- Hus, I.; Szymczyk, A.; Manko, J.; Drozd-Sokolowska, J. COVID-19 in Adult Patients with Hematological Malignancies-Lessons Learned after Three Years of Pandemic. Biology 2023, 12, 545. [Google Scholar] [CrossRef]
- Baang, J.H.; Smith, C.; Mirabelli, C.; Valesano, A.L.; Manthei, D.M.; Bachman, M.A.; Wobus, C.E.; Adams, M.; Washer, L.; Martin, E.T.; et al. Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 Replication in an Immunocompromised Patient. J. Infect. Dis. 2021, 223, 23–27. [Google Scholar] [CrossRef]
- Ueda, Y.; Asakura, S.; Wada, S.; Saito, T.; Yano, T. Prolonged COVID-19 in an Immunocompromised Patient Treated with Obinutuzumab and Bendamustine for Follicular Lymphoma. Intern. Med. 2022, 61, 2523–2526. [Google Scholar] [CrossRef]
- Maan, I.; Paraskevopoulou, S.M.; Cwynarski, K.; Shrestha, M.; Waters, L.; Miller, R.; Ahmed, N. Prolonged SARS-CoV-2 shedding in a person living with advanced HIV and diffuse large B-cell lymphoma: A case report. Infect. Dis. 2022, 54, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, H.; Recoing, A.; Bridier-Nahmias, A.; Rahi, M.; Lambert, C.; Martres, P.; Lucet, J.C.; Rioux, C.; Bouzid, D.; Lebourgeois, S.; et al. Long-Term Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infectiousness Among Three Immunocompromised Patients: From Prolonged Viral Shedding to SARS-CoV-2 Superinfection. J. Infect. Dis. 2021, 223, 1522–1527. [Google Scholar] [CrossRef]
- Meiring, S.; Tempia, S.; Bhiman, J.N.; Buys, A.; Kleynhans, J.; Makhasi, M.; McMorrow, M.; Moyes, J.; Quan, V.; Walaza, S.; et al. Prolonged Shedding of Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at High Viral Loads Among Hospitalized Immunocompromised Persons Living With Human Immunodeficiency Virus (HIV), South Africa. Clin. Infect. Dis. 2022, 75, e144–e156. [Google Scholar] [CrossRef]
- Szwebel, T.A.; Veyer, D.; Robillard, N.; Eshagh, D.; Canoui, E.; Bruneau, T.; Contejean, A.; Azoulay, C.; Serrano, T.; Hueso, T.; et al. Usefulness of Plasma SARS-CoV-2 RNA Quantification by Droplet-based Digital PCR to Monitor Treatment Against COVID-19 in a B-cell Lymphoma Patient. Stem Cell Rev. Rep. 2021, 17, 296–299. [Google Scholar] [CrossRef]
- Kuczborska, K.; Buda, P.; Ksiazyk, J.B. Different Course of SARS-CoV-2 Infection in Two Adolescents With Other Immunosuppressive Factors. Cureus 2022, 14, e22710. [Google Scholar] [CrossRef]
- Mardani, M.; Sharifi-Razavi, A.; Sheibani, S.; Baziboroun, M. Outcome of a HIV Positive Patient Infected with COVID-19 After an Autologous Bone Marrow Transplantation: A Case Report. Curr. HIV Res. 2021, 19, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Padurariu-Covit, M.D.; Andreescu, M.; Niculet, E.; Plesea-Condratovici, A.; Arbune, M. Managing HIV-Associated Hodgkin Lymphoma During the COVID-19 Pandemic: Case Report and Literature Review. Viruses 2025, 17, 404. [Google Scholar] [CrossRef] [PubMed]
- Lundell, L.R.; Dent, J.; Bennett, J.R.; Blum, A.L.; Armstrong, D.; Galmiche, J.P.; Johnson, F.; Hongo, M.; Richter, J.E.; Spechler, S.J.; et al. Endoscopic assessment of oesophagitis: Clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999, 45, 172–180. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, A.L.; Lymphoma, G.; Eastern Cooperative Oncology, G.; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [Google Scholar] [CrossRef]
- Freifeld, A. NCCN Best Practices Guidance: Management of COVID-19 Infection in Patients with Cancer (March 2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf (accessed on 23 September 2025).
- European Society for Medical Oncology. Cancer Patient Management During the COVID-19 Pandemic; European Society for Medical Oncology: Lugano, Switzerland, 2020. [Google Scholar]
- Cesaro, S.; Ljungman, P.; Mikulska, M.; Hirsch, H.H.; Navarro, D.; Cordonnier, C.; Mehra, V.; Styczynski, J.; Marchesi, F.; Pinana, J.L.; et al. Post-pandemic recommendations for the management of COVID-19 in patients with haematological malignancies or undergoing cellular therapy, from the European Conference on Infections in Leukaemia (ECIL-10). Leukemia 2025, 39, 2061–2071. [Google Scholar] [CrossRef]
- Hubel, K.; Bower, M.; Aurer, I.; Bastos-Oreiro, M.; Besson, C.; Brunnberg, U.; Cattaneo, C.; Collins, S.; Cwynarski, K.; Pria, A.D.; et al. Human immunodeficiency virus-associated Lymphomas: EHA-ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Hemasphere 2024, 8, e150. [Google Scholar] [CrossRef]
- Kimby, E. Tolerability and safety of rituximab (MabThera). Cancer Treat. Rev. 2005, 31, 456–473. [Google Scholar] [CrossRef]
- Dulery, R.; Lamure, S.; Delord, M.; Di Blasi, R.; Chauchet, A.; Hueso, T.; Rossi, C.; Drenou, B.; Deau Fischer, B.; Soussain, C.; et al. Prolonged in-hospital stay and higher mortality after COVID-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am. J. Hematol. 2021, 96, 934–944. [Google Scholar] [CrossRef]
- Avouac, J.; Drumez, E.; Hachulla, E.; Seror, R.; Georgin-Lavialle, S.; El Mahou, S.; Pertuiset, E.; Pham, T.; Marotte, H.; Servettaz, A.; et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: A cohort study. Lancet Rheumatol. 2021, 3, e419–e426. [Google Scholar] [CrossRef] [PubMed]
- Visco, C.; Marcheselli, L.; Mina, R.; Sassone, M.; Guidetti, A.; Penna, D.; Cattaneo, C.; Bonuomo, V.; Busca, A.; Ferreri, A.J.M.; et al. A prognostic model for patients with lymphoma and COVID-19: A multicentre cohort study. Blood Adv. 2022, 6, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martin-Quiros, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Bolkun, L.; Pula, B.; Kolkowska-Lesniak, A.; Morawska, M.; Cichocka, E.; Charlinski, G.; Garus, B.; Giebel, S.; Piszcz, J.; Drozd-Sokolowska, J.; et al. Molnupiravir is effective in patients with haematological malignancies. Int. J. Cancer 2023, 153, 1251–1256. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Adriano, A.; Berhane, S.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; Beese, S.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2020, 8, CD013705. [Google Scholar] [CrossRef]
- Binnicker, M.J. Can Testing Predict SARS-CoV-2 Infectivity? The Potential for Certain Methods To Be Surrogates for Replication-Competent Virus. J. Clin. Microbiol. 2021, 59, e0046921. [Google Scholar] [CrossRef]
| Reference | Age (at Diagnosis of SARS- CoV-2 Infection) | Type of Lymphoma | Anti-Lymphoma Regimen | Last Administration of Anti-Lymphoma Treatment | HIV Infection Management | Concomitant Diseases, Risk Factors of Severe COVID-19 | COVID-19 Vaccination | COVID-19 Severity | SARS-CoV-2 Shedding (Days) | COVID-19 Treatment | Hematologic Response at Last Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maan I, et al., 2022 [11] | 28 | DLBCL | R-CHOP (5 cycles), IT MTX (1 cycle) | Ongoing during SARS-CoV-2 infection | ART temporary interrupted during 5th cycle of CIT | None | NA | Asymptomatic | 164 | Not required | Remission |
| Szwebel TA et al., 2020 [14] | NA | B-cell lymphoma | CTx + auto-HSCT, 2 lines CIT + rituximab, 1 line CIT with obinutuzumab + ibrutinib | 2 months prior SARS-CoV-2 infection (obinutuzumab and ibrutinib) | ART, undetectable viral load | Lymphopenia, hypogammaglobulinemia | NA | Severe | 66 | Corticoids, lopinavir/ritonavir, tocilizumab, convalescent plasma | VGPR |
| Kuczborska K, et al., 2022 [15] | 17 | Primary CNS B-cell lymphoma | CTx + XRT | Ongoing XRT | No ART before COVID-19 | Cachexia, drug-induced nephropathy | No | Mild | NA | Remdesivir | NA |
| Mardani M, et al., 2021 [16] | 61 | HL | CTx + CRT + auto-HSCT | Auto-HSCT 2 months prior SARS-CoV-2 infection | ART, undetectable viral load | Underweight | NA | Mild | NA | Atazanavir | NA |
| Arbune M, et al., 2025 [17] | 57 | HL | NA | NA | darunavir/cobicistat/emtricitabine/tenofovir alafenamide | Hypoalbuminemia | No | Severe | NA | Remdesivir | NA |
| Day | Test | Result |
|---|---|---|
| 1 | RT-qPCR | +, Ct: 27 (gene N), 20 (gene RdRP) |
| 9 | RAT | + |
| 14 | RAT | + |
| 18 | RAT | + |
| 21 | RAT | + |
| 23 | RAT | + |
| 25 | RAT | + |
| 29 | RAT | + |
| 36 | RT-qPCR, RAT | +, Ct: 21 (gene N1), 21 (gene N2) |
| 43 | RAT | + |
| 50 | RT-qPCR, RAT | +, Ct: 17 (gene N1), 19 (gene N2) |
| 57 | RAT | + |
| 65 | RT-qPCR, RAT | +, Ct: 11 (gene N1), 11 (gene N2) |
| 71 | RAT | + |
| 77 | RAT | + |
| 78 | RT-qPCR | +, Ct 11 (gene ORF1), 10 (gene ORF8), 10 (gene N) |
| 85 | RAT | + |
| 92 | RT-qPCR, RAT | +, Ct 13 (gene ORF1), 14 (gene ORF8), 16 (gene N) |
| 99 | RAT | + |
| 106 | RAT | + |
| 113 | RAT | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siewiorek, K.; Jasiński, M.; Izdebski, B.; Przybylski, M.; Kobylecka, M.; Mączewska, J.; Jamroziak, K.; Drozd-Sokołowska, J. Challenges in the Treatment of HIV-Related Lymphomas Complicated by COVID-19: Case Study and Review of the Literature. Pharmaceuticals 2025, 18, 1461. https://doi.org/10.3390/ph18101461
Siewiorek K, Jasiński M, Izdebski B, Przybylski M, Kobylecka M, Mączewska J, Jamroziak K, Drozd-Sokołowska J. Challenges in the Treatment of HIV-Related Lymphomas Complicated by COVID-19: Case Study and Review of the Literature. Pharmaceuticals. 2025; 18(10):1461. https://doi.org/10.3390/ph18101461
Chicago/Turabian StyleSiewiorek, Kinga, Marcin Jasiński, Błażej Izdebski, Maciej Przybylski, Małgorzata Kobylecka, Joanna Mączewska, Krzysztof Jamroziak, and Joanna Drozd-Sokołowska. 2025. "Challenges in the Treatment of HIV-Related Lymphomas Complicated by COVID-19: Case Study and Review of the Literature" Pharmaceuticals 18, no. 10: 1461. https://doi.org/10.3390/ph18101461
APA StyleSiewiorek, K., Jasiński, M., Izdebski, B., Przybylski, M., Kobylecka, M., Mączewska, J., Jamroziak, K., & Drozd-Sokołowska, J. (2025). Challenges in the Treatment of HIV-Related Lymphomas Complicated by COVID-19: Case Study and Review of the Literature. Pharmaceuticals, 18(10), 1461. https://doi.org/10.3390/ph18101461






