The Effect of Liposomal DMU-212 on the Differentiation of Human Ovarian Granulosa Cells in a Primary 3D Culture Model
Abstract
1. Introduction
2. Results
2.1. Characterization of lipDMU-212
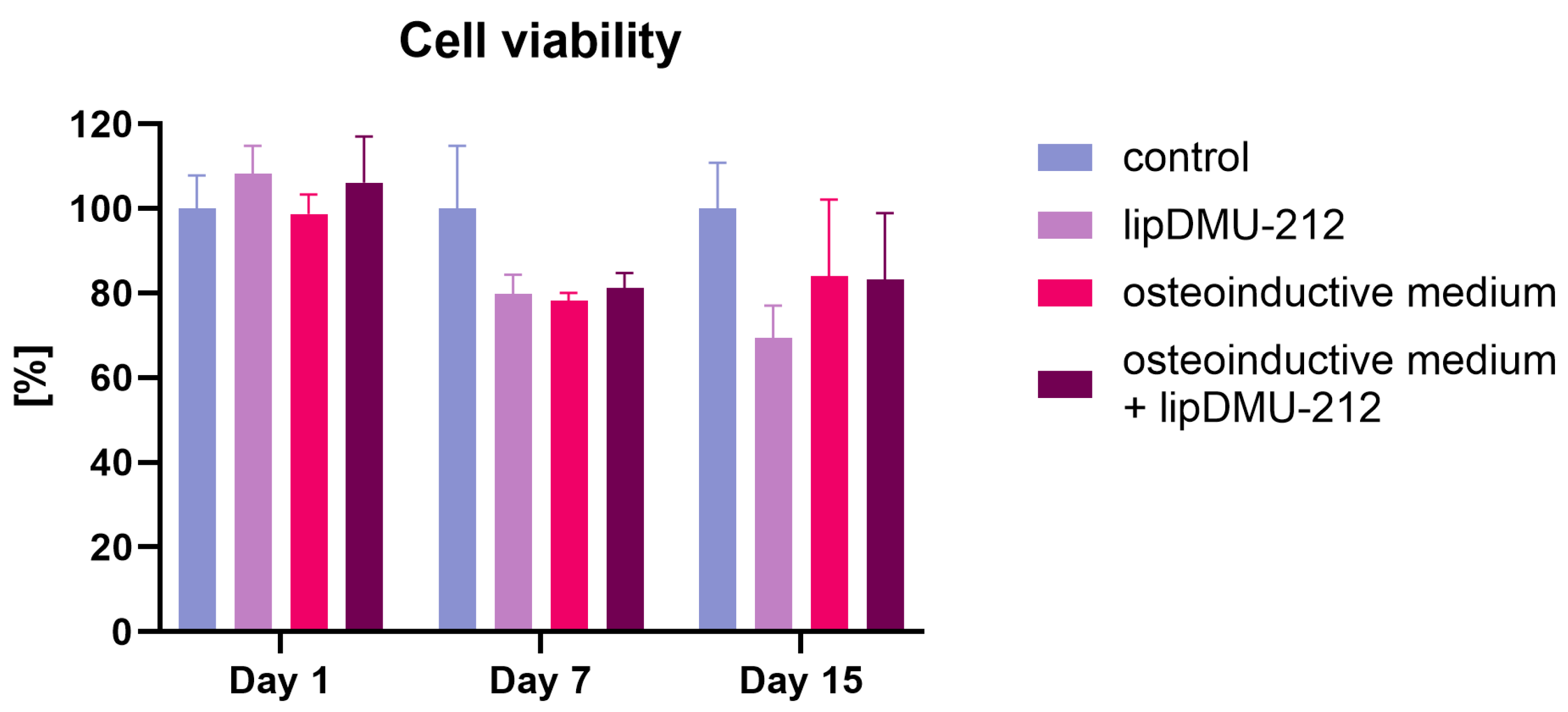
2.2. Cell Viability
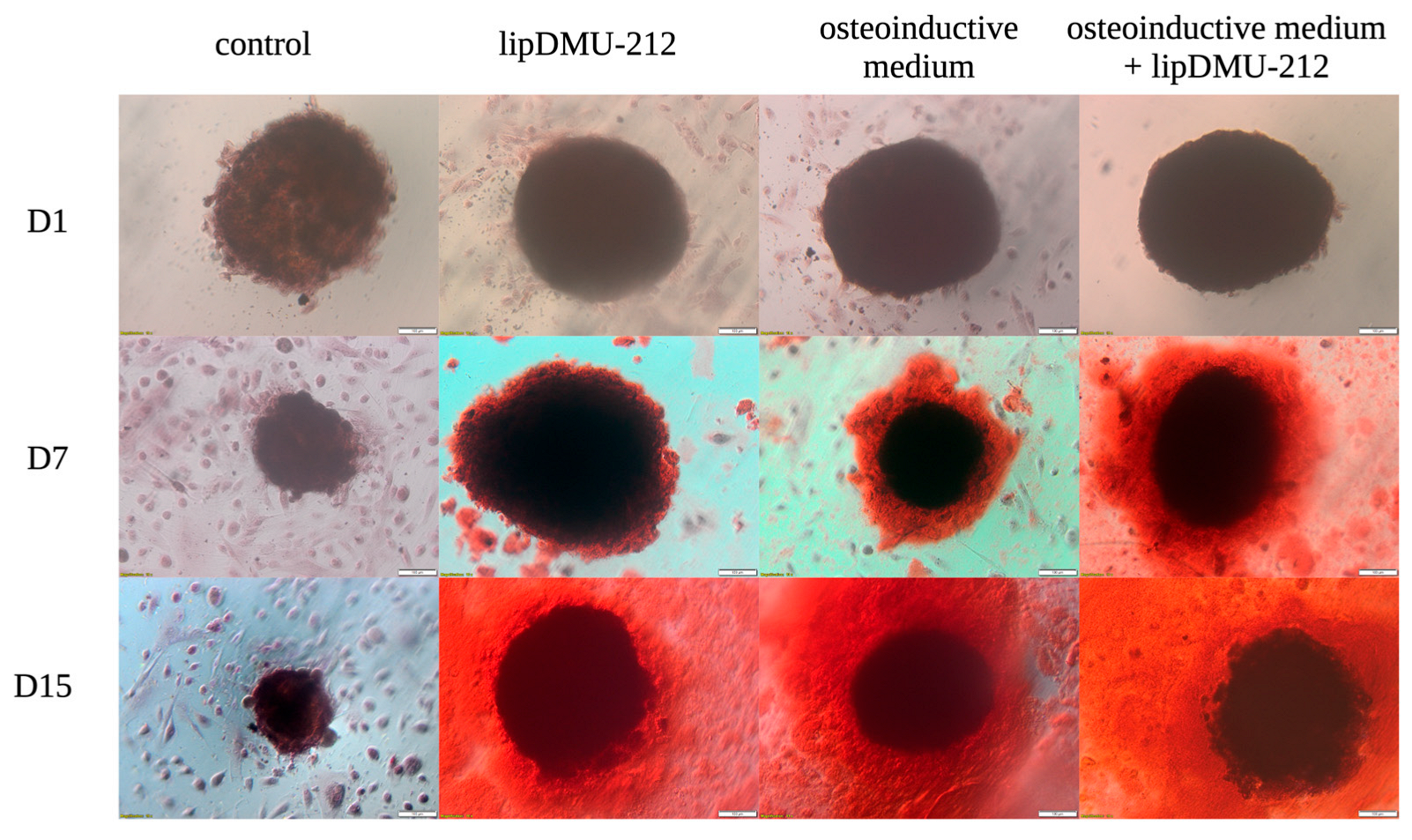
2.3. Alizarin Red Staining
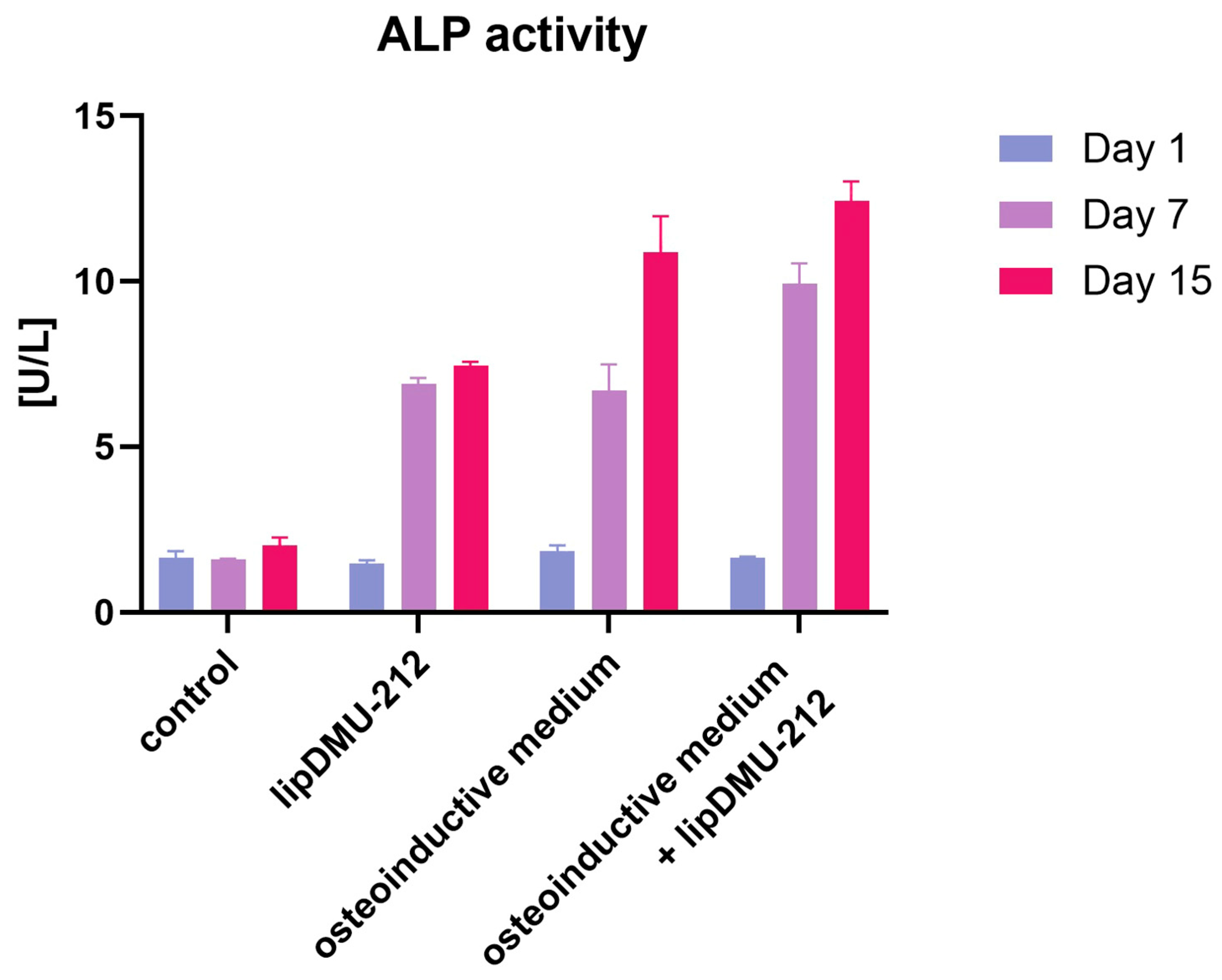
2.4. ALP Activity
2.5. RNA-Seq
2.6. Differential Modulation of the Wnt Pathway by lipDMU-212 and Osteoinductive Medium
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Liposome Formulation and Characterization
4.3. Source and Culture of Human Ovarian Granulosa Cells
4.4. Cell Viability Assay
4.5. Alizarin Red Staining
4.6. ALP Activity Assessment
4.7. RNA Isolation
4.8. Library Preparation and RNA Sequencing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, Y.; Hanai, J.I.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017, 25, 661–672. [Google Scholar] [CrossRef]
- Yu, B.; Huo, L.; Liu, Y.; Deng, P.; Szymanski, J.; Li, J.; Luo, X.; Hong, C.; Lin, J.; Wang, C.Y. PGC-1α Controls Skeletal Stem Cell Fate and Bone-Fat Balance in Osteoporosis and Skeletal Aging by Inducing TAZ. Cell Stem Cell 2018, 23, 193–209.e5, Erratum in: Cell Stem Cell 2018, 23, 615–623. https://doi.org/10.1016/j.stem.2018.09.001. [Google Scholar] [CrossRef]
- Barake, M.; Arabi, A.; Nakhoul, N.; El-Hajj Fuleihan, G.; El Ghandour, S.; Klibanski, A.; Tritos, N.A. Effects of Growth Hormone Therapy on Bone Density and Fracture Risk in Age-Related Osteoporosis in the Absence of Growth Hormone Deficiency: A Systematic Review and Meta-Analysis. Endocrine 2018, 59, 39–49. [Google Scholar] [CrossRef]
- Kossowska-Tomaszczuk, K.; De Geyter, C.; De Geyter, M.; Martin, I.; Holzgreve, W.; Scherberich, A.; Zhang, H. The multipotency of luteinizing granulosa cells collected from mature ovarian follicles. Stem Cells 2009, 27, 210–219. [Google Scholar] [CrossRef]
- Kossowska-Tomaszczuk, K.; De Geyter, C. Cells with stem cell characteristics in somatic compartments of the ovary. Biomed. Res. Int. 2013, 2013, 310859. [Google Scholar] [CrossRef]
- Oki, Y.; Ono, H.; Motohashi, T.; Sugiura, N.; Nobusue, H.; Kano, K. Dedifferentiated follicular granulosa cells derived from pig ovary can transdifferentiate into osteoblasts. Biochem. J. 2012, 447, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Dzafic, E.; Stimpfel, M.; Virant-Klun, I. Plasticity of granulosa cells: On the crossroad of stemness and transdifferentiation potential. J. Assist. Reprod. Genet. 2013, 30, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, F.; Basini, G.; Tirelli, M.; Cavalli, V.; Bussolati, S.; Tamanini, C. Angiogenic activity of porcine granulosa cells co-cultured with endothelial cells in a microcarrier-based three-dimensional fibrin gel. J. Physiol. Pharmacol. 2003, 54, 361–370. [Google Scholar] [PubMed]
- Jozkowiak, M.; Hutchings, G.; Jankowski, M.; Kulcenty, K.; Mozdziak, P.; Kempisty, B.; Spaczynski, R.Z.; Piotrowska-Kempisty, H. The Stemness of Human Ovarian Granulosa Cells and the Role of Resveratrol in the Differentiation of MSCs—A Review Based on Cellular and Molecular Knowledge. Cells 2020, 9, 1418. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Posa, F.; De Maria, S.; Ravagnan, G.; Ballini, A.; Porro, C.; Trotta, T.; Grano, M.; Muzio, L.L.; Mori, G. Polydatin, natural precursor of resveratrol, promotes osteogenic differentiation of mesenchymal stem cells. Int. J. Med. Sci. 2018, 15, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Baur, A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, Y.; Ye, X.; Zeng, M.; Zhao, Q.; Keefe, D.L.; Liu, L. Resveratrol protects against age-associated infertility in mice. Hum. Reprod. 2013, 28, 707–717. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Luo, L.-L.; Xu, J.-J.; Zhuang, X.-L.; Kong, X.-X.; Fu, Y.-C. Effects of plant polyphenols on ovarian follicular reserve in aging rats. Biochem. Cell Biol. 2010, 88, 737–745. [Google Scholar] [CrossRef]
- Hao, J.; Tuck, A.R.; Sjödin, M.O.D.; Lindberg, J.; Sand, A.; Niklasson, B.; Argyraki, M.; Hovatta, O.; Damdimopoulou, P. Resveratrol supports and alpha-naphthoflavone disrupts growth of human ovarian follicles in an in vitro tissue culture model. Toxicol. Appl. Pharmacol. 2018, 338, 73–82. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Sale, S.; Verschoyle, R.D.; Boocock, D.; Jones, D.J.L.; Wilsher, N.; Ruparelia, K.C.; Potter, G.A.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Br. J. Cancer 2004, 90, 736–744. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Lee, J.K.; Choi, O.; Kim, C.Y.; Jang, J.-H.; Hwang, B.Y.; Hong, Y.-S. Biosynthesis of methylated resveratrol analogs through the construction of an artificial biosynthetic pathway in E. coli. BMC Biotechnol. 2014, 14, 67. [Google Scholar] [CrossRef]
- Nowicki, A.; Wawrzyniak, D.; Czajkowski, M.; Józkowiak, M.; Pawlak, M.; Wierzchowski, M.; Rolle, K.; Skupin-Mrugalska, P.; Piotrowska-Kempisty, H. Enhanced biological activity of liposomal methylated resveratrol analog 3′-hydroxy-3,4,5,4′-tetramethoxystilbene (DMU-214) in 3D patient-derived ovarian cancer model. Drug Deliv. 2022, 29, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Józkowiak, M.; Kobylarek, D.; Bryja, A.; Piotrowska-Kempisty, H.; Celichowski, P.; Kocherova, I.; Dyszkiewicz-Konwińska, M.; Bukowska, D.; Brązert, M.; Ożegowska, K.; et al. Steroidogenic activity of liposomal methylated resveratrol analog 3,4,5,4′-tetramethoxystilbene (DMU-212) in human luteinized granulosa cells in a primary three-dimensional in vitro model. Endocrine 2023, 82, 681–694. [Google Scholar] [CrossRef]
- Bernar, A.; Gebetsberger, J.V.; Bauer, M.; Streif, W.; Schirmer, M. Optimization of the Alizarin Red S Assay by Enhancing Mineralization of Osteoblasts. Int. J. Mol. Sci. 2022, 24, 723. [Google Scholar] [CrossRef] [PubMed]
- Kolter, M.; Wittmann, M.; Köll-Weber, M.; Süss, R. The suitability of liposomes for the delivery of hydrophobic drugs—A case study with curcumin. Eur. J. Pharm. Biopharm. 2019, 140, 20–28. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Olimpio, R.M.C.; de Oliveira, M.; De Sibio, M.T.; Moretto, F.C.F.; Deprá, I.C.; Mathias, L.S.; Gonçalves, B.M.; Rodrigues, B.M.; Tilli, H.P.; Coscrato, V.E.; et al. Cell viability assessed in a reproducible model of human osteoblasts derived from human adipose-derived stem cells. PLoS ONE 2018, 13, e0194847. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.C.; Hou, S.M.; Chen, R.J.; Peng, H.W.; Hsieh, C.F.; Kuo, M.L.; Yen, M.L. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Miner. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.E.; Yang, Z.; Zhang, H.; Yao, G.; Liu, J.; Wei, Q.; Ma, B. Resveratrol Promotes Osteogenic Differentiation of Canine Bone Marrow Mesenchymal Stem Cells Through Wnt/Beta-Catenin Signaling Pathway. Cell. Reprogram. 2018, 20, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Carboni, G.A.; Guemes, M.; Bailey, S.; Anaya, E.; Corselli, M.; Peault, B.; Krum, S.A. GATA4 regulates estrogen receptor-alpha-mediated osteoblast transcription. Mol. Endocrinol. 2011, 25, 1126–1136. [Google Scholar] [CrossRef]
- Güemes, M.; Garcia, A.J.; Rigueur, D.; Runke, S.; Wang, W.; Zhao, G.; Mayorga, V.H.; Atti, E.; Tetradis, S.; Péault, B.; et al. GATA4 is essential for bone mineralization via ERα and TGFβ/BMP pathways. J. Bone Miner. Res. 2014, 29, 2676–2687. [Google Scholar] [CrossRef]
- Brązert, M.; Kranc, W.; Celichowski, P.; Ożegowska, K.; Budna-Tukan, J.; Jeseta, M.; Pawelczyk, L.; Bruska, M.; Zabel, M.; Nowicki, M.; et al. Novel markers of human ovarian granulosa cell differentiation toward osteoblast lineage: A microarray approach. Mol. Med. Rep. 2019, 20, 4403–4414. [Google Scholar] [CrossRef]
- Maruelli, S.; Besio, R.; Rousseau, J.; Garibaldi, N.; Amiaud, J.; Brulin, B.; Layrolle, P.; Escriou, V.; Rossi, A.; Trichet, V.; et al. Osteoblasts mineralization and collagen matrix are conserved upon specific Col1a2 silencing. Matrix Biol. Plus. 2020, 31, 100028. [Google Scholar] [CrossRef]
- Izu, Y.; Ezura, Y.; Koch, M.; Birk, D.E.; Noda, M. Collagens VI and XII form complexes mediating osteoblast interactions during osteogenesis. Cell Tissue Res. 2016, 364, 623–635. [Google Scholar] [CrossRef]
- Zhang, Y.; Stefanovic, B. LARP6 Meets Collagen mRNA: Specific Regulation of Type I Collagen Expression. Int. J. Mol. Sci. 2016, 17, 419. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Xie, L.; Pi, X. Low-Density Lipoprotein Receptor-Related Protein-1 Signaling in Angiogenesis. Front. Cardiovasc. Med. 2017, 4, 34. [Google Scholar] [CrossRef]
- Park, S.; Lee, M.S.; Gwak, J.; Choi, T.I.; Lee, Y.; Ju, B.G.; Kim, C.H.; Oh, S. CCAAT/enhancer-binding protein-β functions as a negative regulator of Wnt/β-catenin signaling through activation of AXIN1 gene expression. Cell Death Dis. 2018, 9, 1023. [Google Scholar] [CrossRef]
- Rubinfeld, B.; Albert, I.; Porfiri, E.; Fiol, C.; Munemitsu, S.; Polakis, P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science 1996, 272, 1023–1026. [Google Scholar] [CrossRef]
- Shah, K.; Kazi, J.U. Phosphorylation-Dependent Regulation of WNT/Beta-Catenin Signaling. Front. Oncol. 2022, 12, 858782. [Google Scholar] [CrossRef]
- Vega, O.A.; Lucero, C.M.J.; Araya, H.F.; Jerez, S.; Tapia, J.C.; Antonelli, M.; Salazar-Onfray, F.; Las Heras, F.; Thaler, R.; Riester, S.M.; et al. Wnt/β-Catenin Signaling Activates Expression of the Bone-Related Transcription Factor RUNX2 in Select Human Osteosarcoma Cell Types. J. Cell Biochem. 2017, 118, 3662–3674. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.P. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Doro, D.; Liu, A.; Grigoriadis, A.E.; Liu, K.J. The Osteogenic Potential of the Neural Crest Lineage May Contribute to Craniosynostosis. Mol. Syndromol. 2019, 10, 48–57. [Google Scholar] [CrossRef]
- Muriel, J.M.; O′Neill, A.; Kerr, J.P.; Kleinhans-Welte, E.; Lovering, R.M.; Bloch, R.J. Keratin 18 is an integral part of the intermediate filament network in murine skeletal muscle. Am. J. Physiol. Cell Physiol. 2020, 318, C215–C224. [Google Scholar] [CrossRef] [PubMed]
- Dompe, C.; Kranc, W.; Jopek, K.; Kowalska, K.; Ciesiółka, S.; Chermuła, B.; Bryja, A.; Jankowski, M.; Perek, J.; Józkowiak, M.; et al. Muscle cell morphogenesis, structure, development and differentiation processes are significantly regulated during human ovarian granulosa cells in vitro cultivation. J. Clin. Med. 2020, 9, 2006. [Google Scholar] [CrossRef]
- Gogola, J.; Hoffmann, M.; Ptak, A. Persistent endocrine-disrupting chemicals found in human follicular fluid stimulate IGF1 secretion by adult ovarian granulosa cell tumor spheroids and thereby increase proliferation of non-cancer ovarian granulosa cells. Toxicol. Vitr. 2020, 65, 104769. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jόzkowiak, M.; Wawrzyniak, D.; Kawczyńska, A.; Skupin-Mrugalska, P.; Czajkowski, M.; Mozdziak, P.; Podralska, M.; Żywicki, M.; Kempisty, B.; Spaczyński, R.Z.; et al. The Effect of Liposomal DMU-212 on the Differentiation of Human Ovarian Granulosa Cells in a Primary 3D Culture Model. Pharmaceuticals 2025, 18, 1460. https://doi.org/10.3390/ph18101460
Jόzkowiak M, Wawrzyniak D, Kawczyńska A, Skupin-Mrugalska P, Czajkowski M, Mozdziak P, Podralska M, Żywicki M, Kempisty B, Spaczyński RZ, et al. The Effect of Liposomal DMU-212 on the Differentiation of Human Ovarian Granulosa Cells in a Primary 3D Culture Model. Pharmaceuticals. 2025; 18(10):1460. https://doi.org/10.3390/ph18101460
Chicago/Turabian StyleJόzkowiak, Małgorzata, Dariusz Wawrzyniak, Alicja Kawczyńska, Paulina Skupin-Mrugalska, Mikołaj Czajkowski, Paul Mozdziak, Marta Podralska, Marek Żywicki, Bartosz Kempisty, Robert Z. Spaczyński, and et al. 2025. "The Effect of Liposomal DMU-212 on the Differentiation of Human Ovarian Granulosa Cells in a Primary 3D Culture Model" Pharmaceuticals 18, no. 10: 1460. https://doi.org/10.3390/ph18101460
APA StyleJόzkowiak, M., Wawrzyniak, D., Kawczyńska, A., Skupin-Mrugalska, P., Czajkowski, M., Mozdziak, P., Podralska, M., Żywicki, M., Kempisty, B., Spaczyński, R. Z., & Piotrowska-Kempisty, H. (2025). The Effect of Liposomal DMU-212 on the Differentiation of Human Ovarian Granulosa Cells in a Primary 3D Culture Model. Pharmaceuticals, 18(10), 1460. https://doi.org/10.3390/ph18101460







