Optimizing Biologic Treatment Selection in Chronic Rhinosinusitis with Nasal Polyps: A Network Meta-Analysis of Efficacy and Safety Across 22 RCTs
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
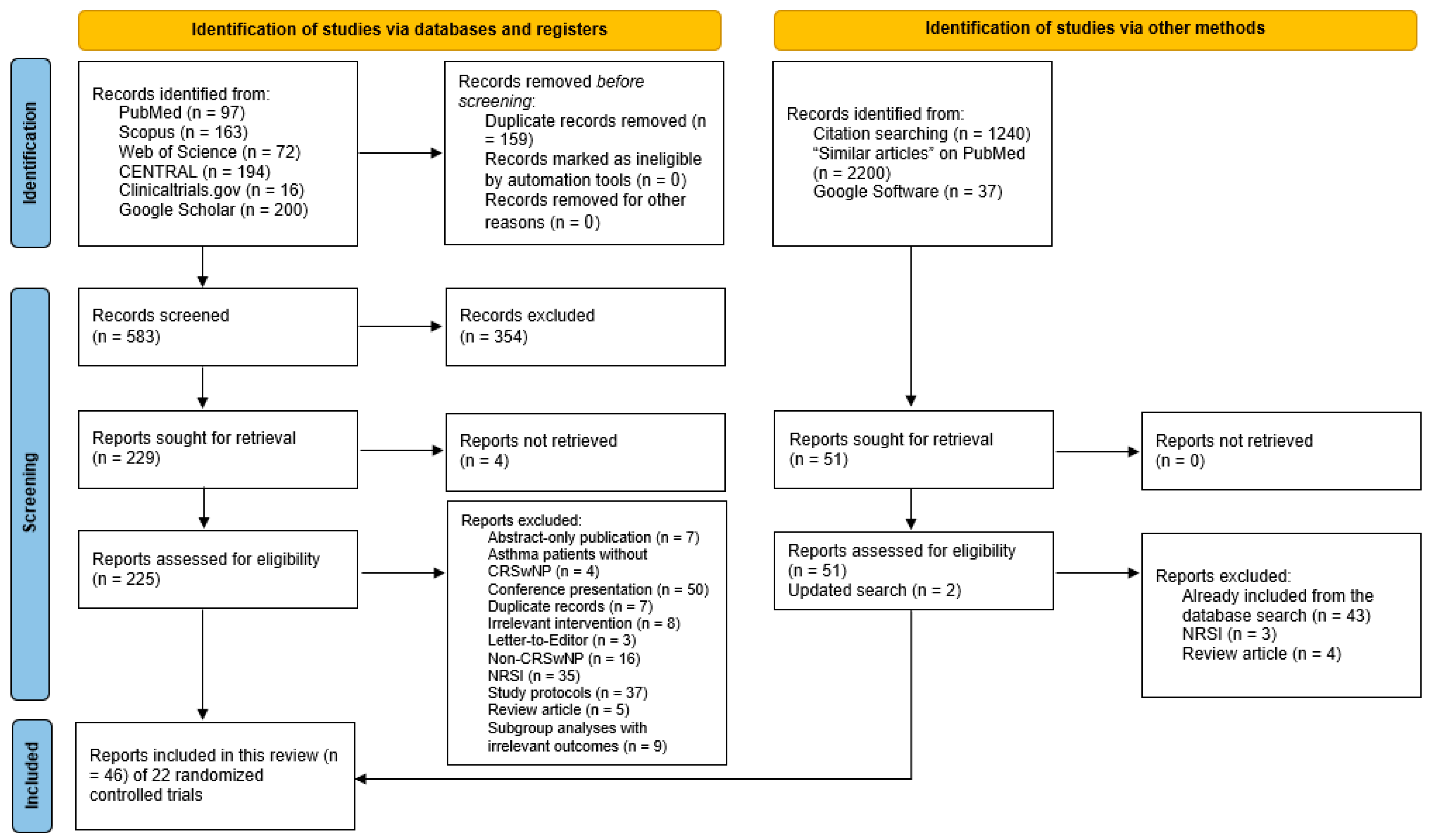
3.1. Literature Review
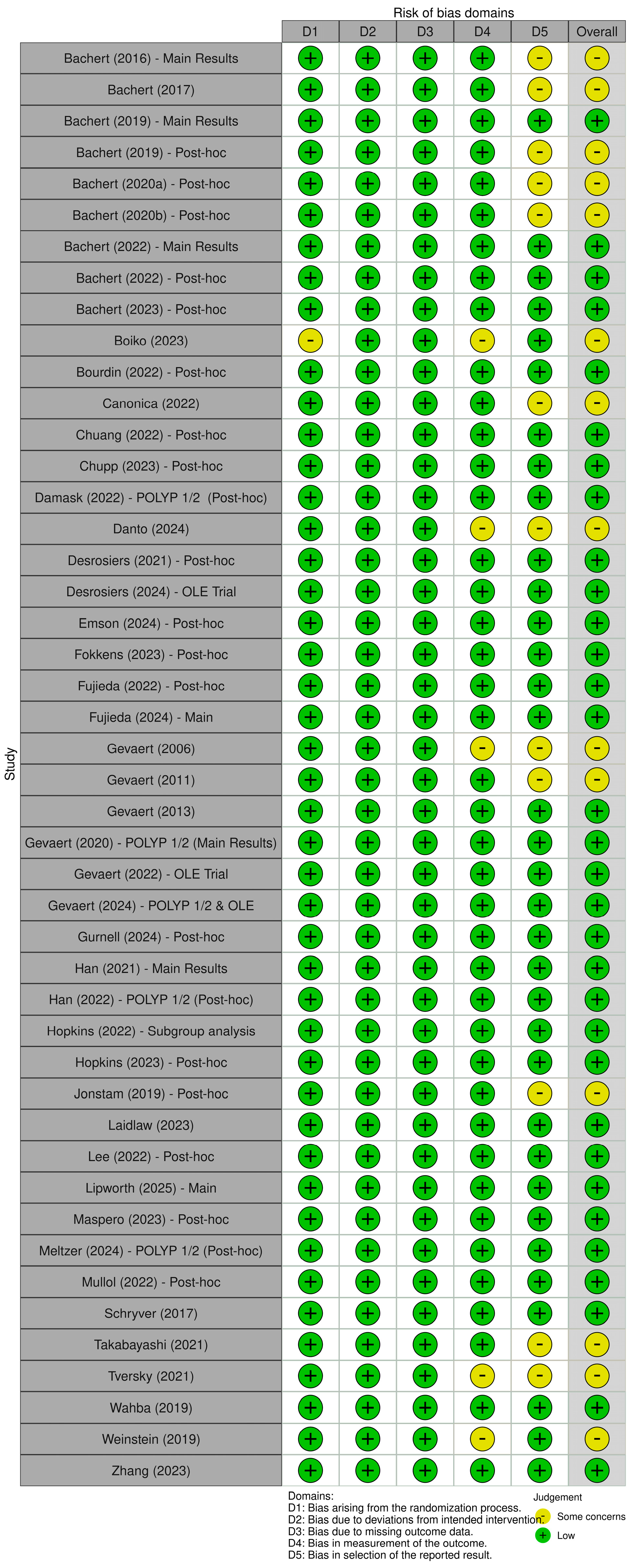
3.2. Risk of Bias Assessment
3.3. Characteristics of Included Trials
3.4. Efficacy Measures
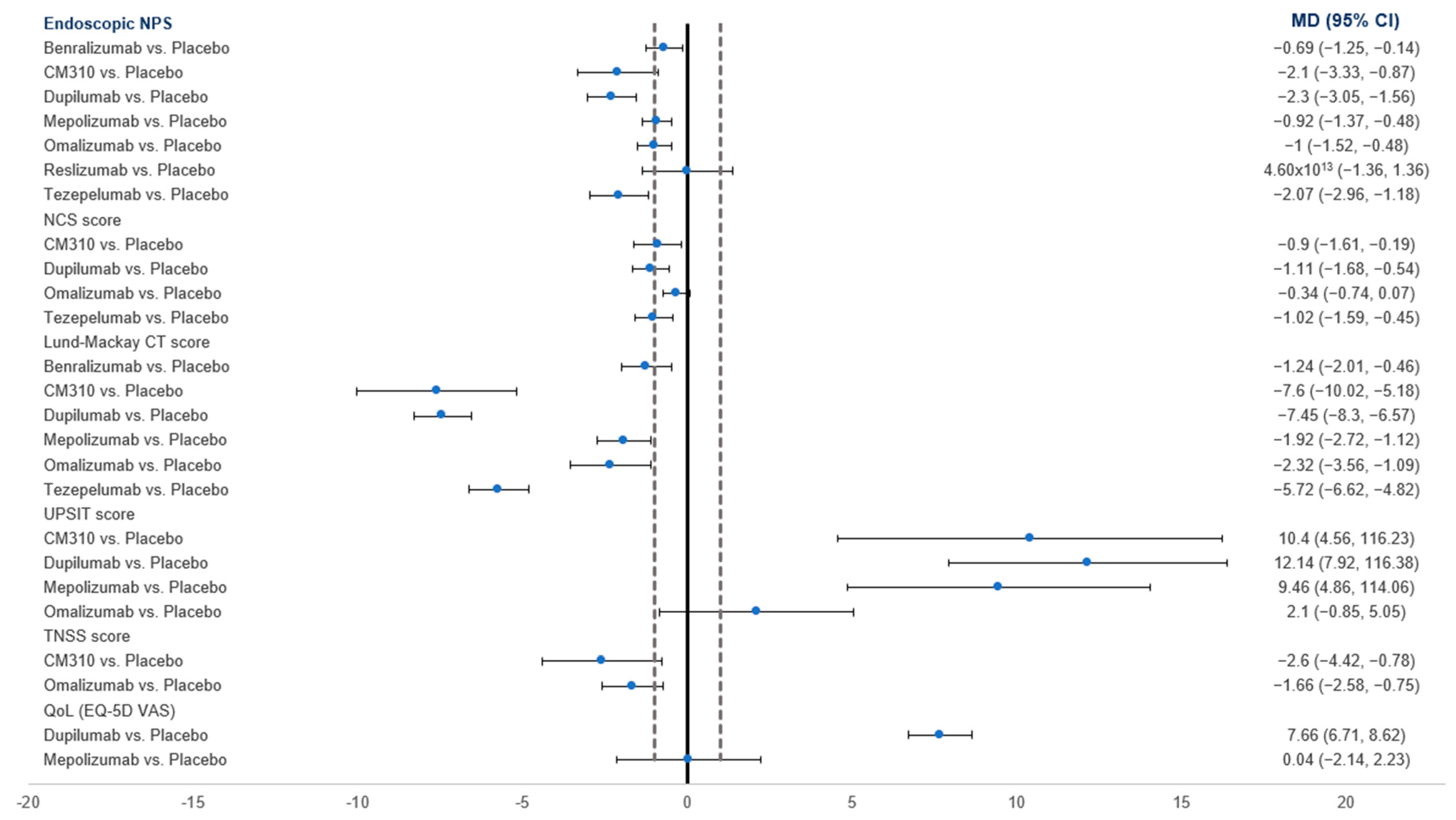
3.4.1. NPS
3.4.2. NCS
3.4.3. Lund–Mackay CT Score
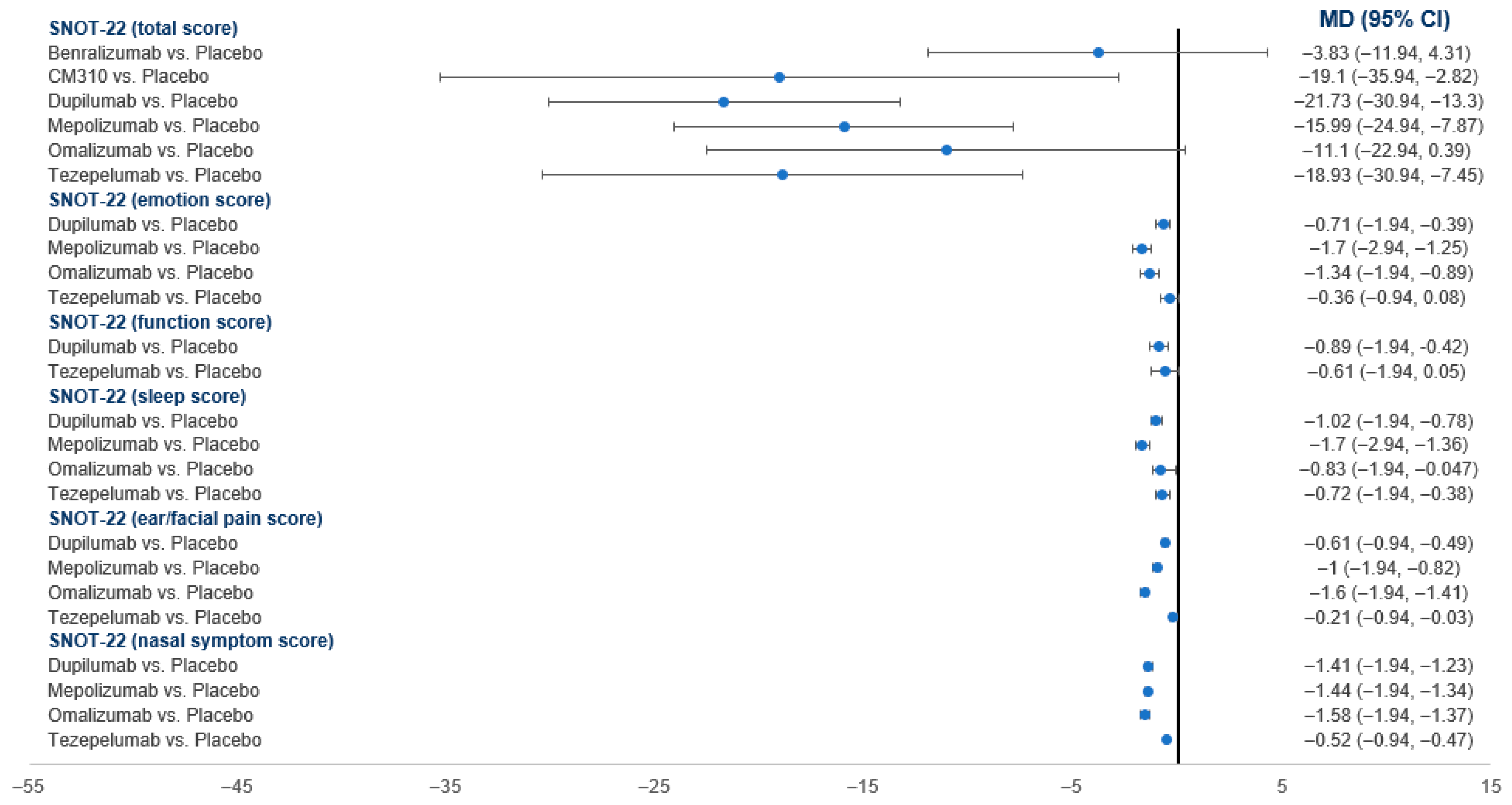
3.4.4. SNOT-22 Score
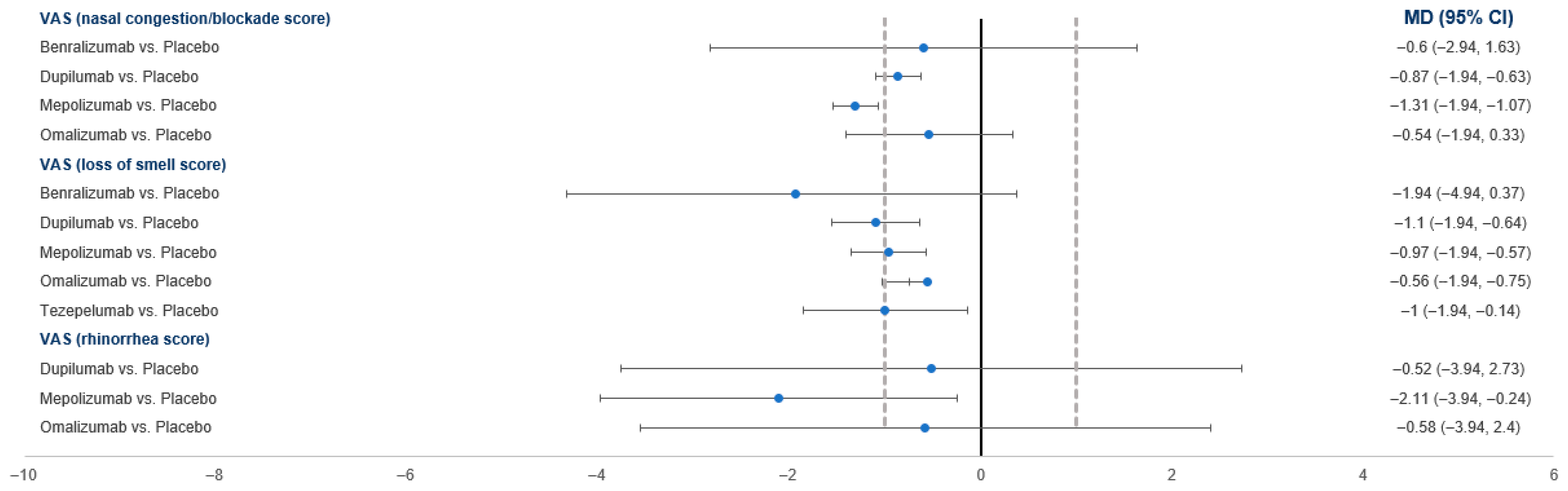
3.4.5. VAS Score
3.4.6. TNSS Score
3.4.7. UPSIT
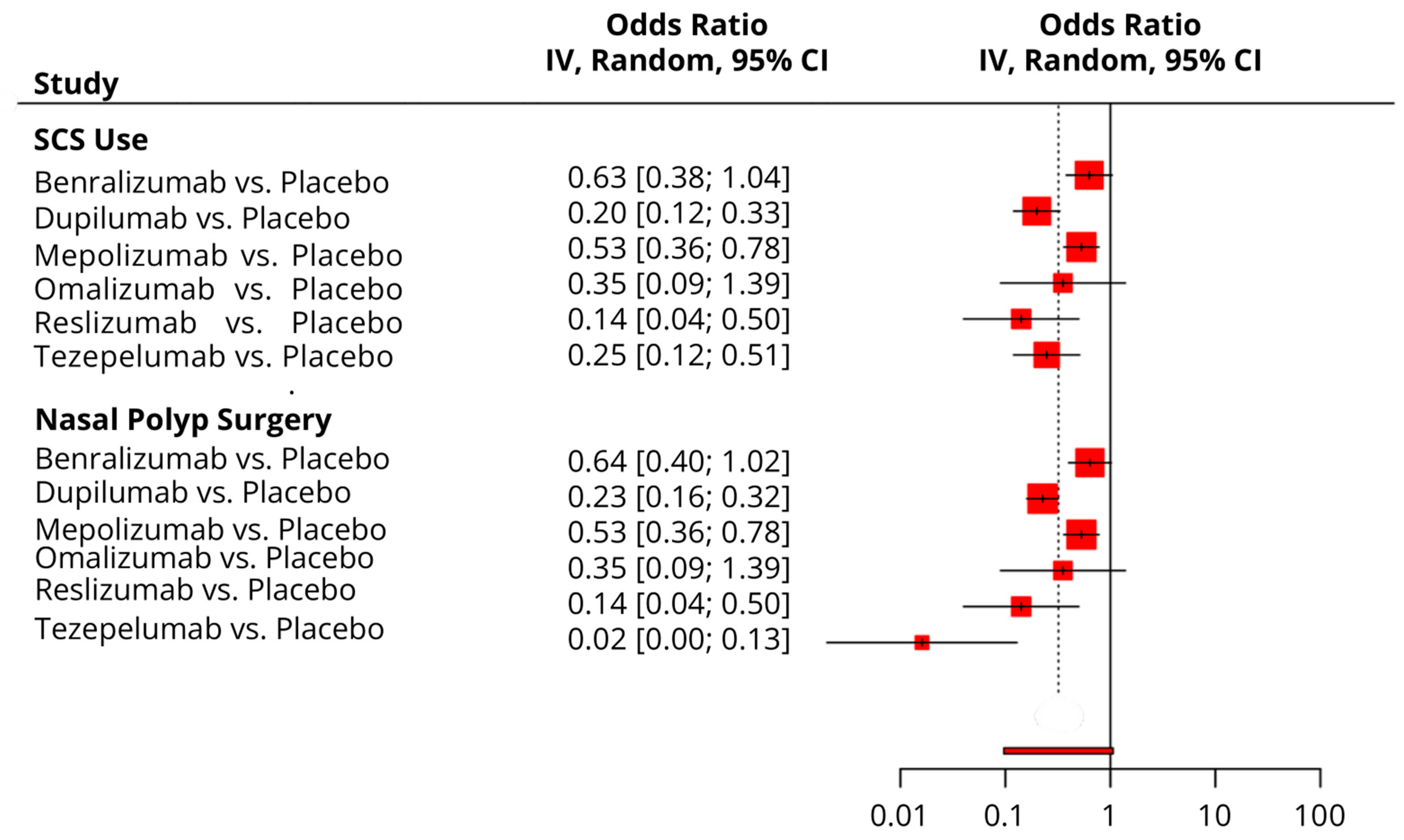
3.4.8. Time to First NP Surgery
3.4.9. Time to First SCS Use
3.5. Responder Analysis
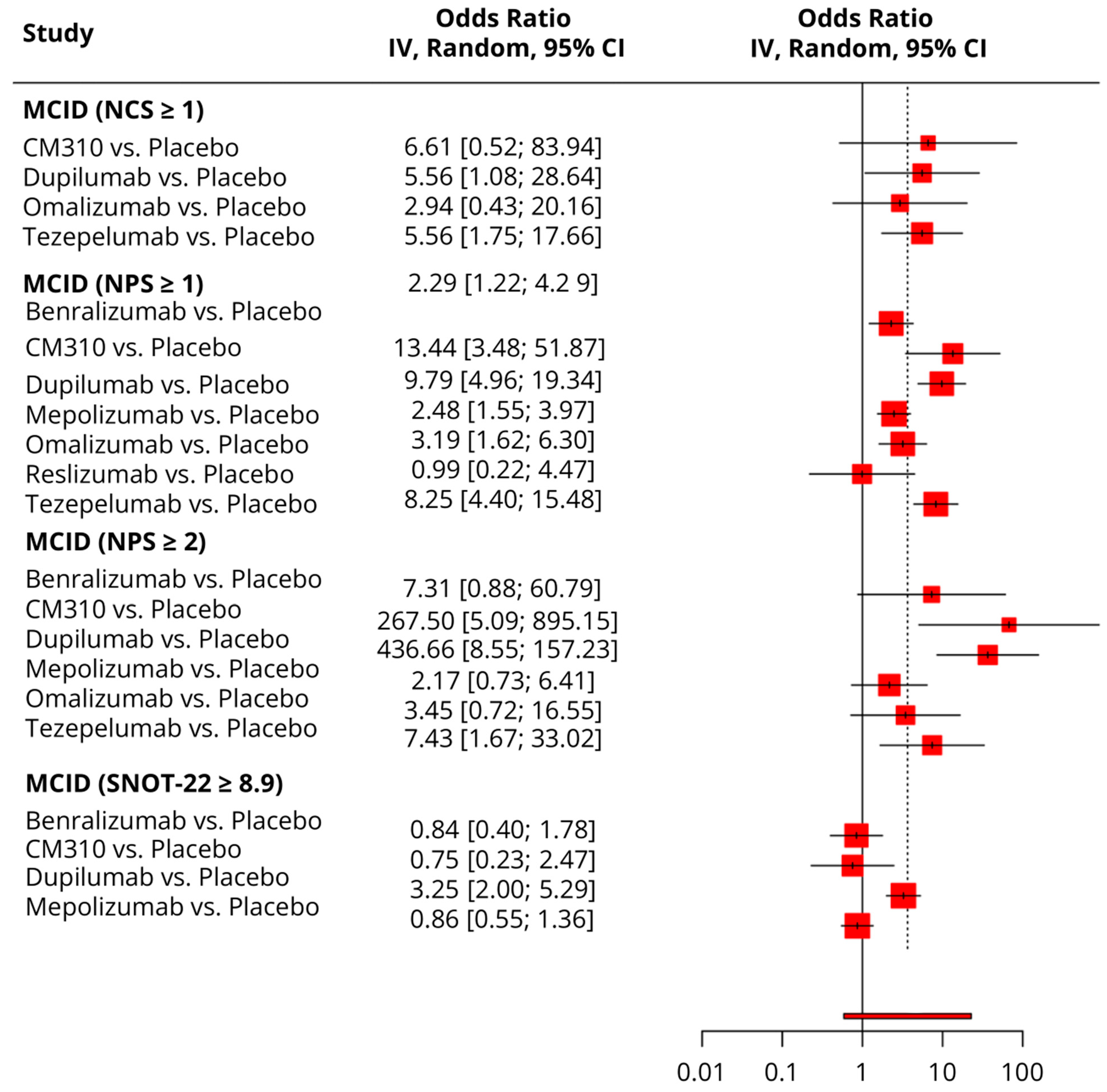
3.5.1. MCID (≥8.9 Points Reduction in SNOT-22)
3.5.2. MCID (≥1 Point Improvement in NCS)
3.5.3. MCDI (≥1 Point Improvement in NPS)
3.5.4. MCID (≥2 Points Improvement in NPS)
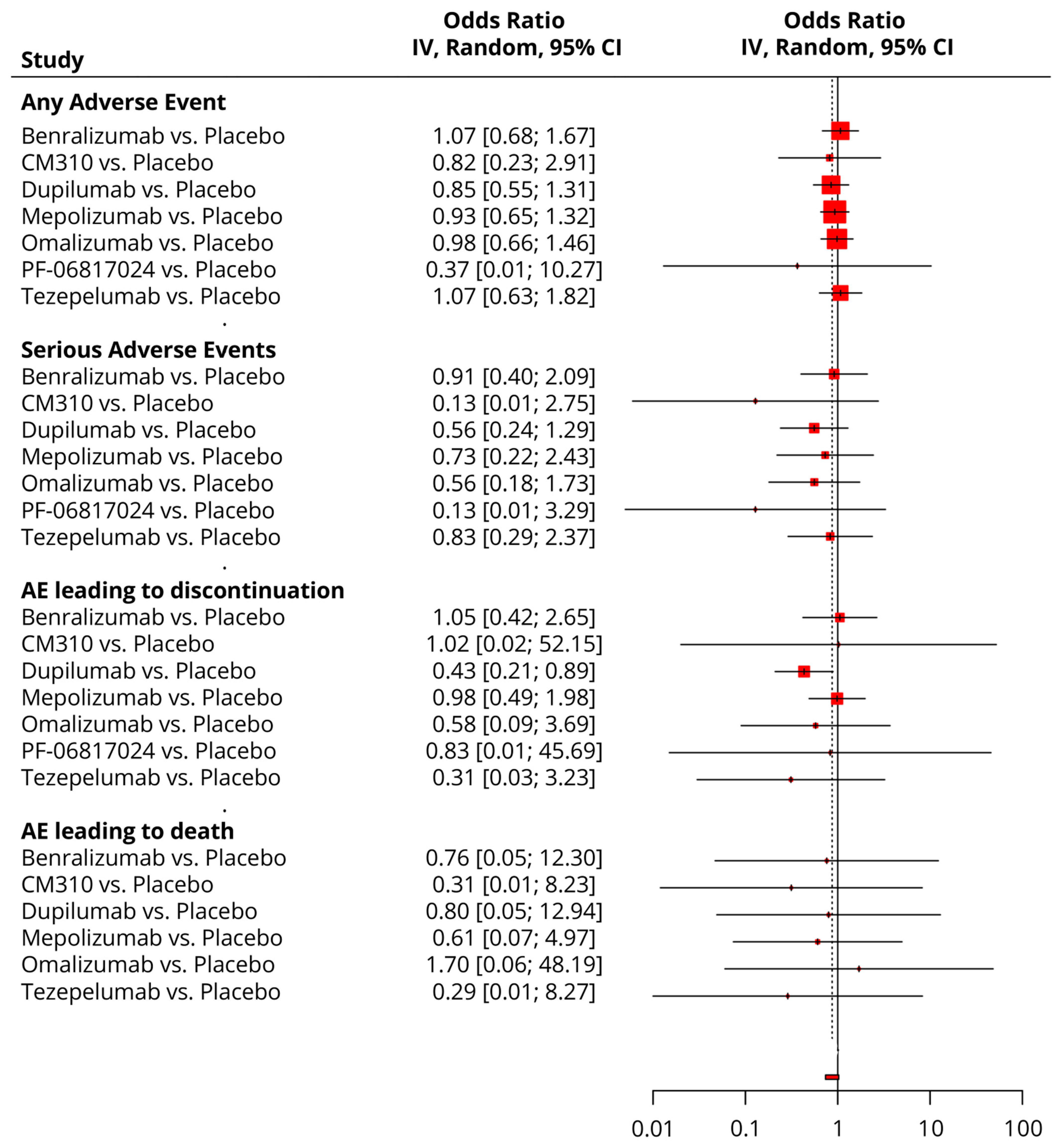
3.6. Safety Analysis
4. Discussion
4.1. Principal Findings
4.2. Comparison with Prior Reviews
4.3. Clinical Implications
4.4. Clinical Selection and Access (Practical Considerations)
4.5. Limitations
4.6. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| CRSwNP | Chronic rhinosinusitis with nasal polyps |
| CT | Computed tomography |
| ESS | Endoscopic sinus surgery |
| LOCF | Last observation carried forward |
| MCID | Minimal clinically important difference |
| MD | Mean difference |
| NCS | Nasal congestion score |
| NMA | Network meta-analysis |
| NP | Nasal polyp |
| NPS | Nasal polyp score |
| OR | Odds ratio |
| QoL | Quality of life |
| RCT | Randomized controlled trial |
| SAE | Serious adverse event |
| SC | Subcutaneous |
| SCS | Systemic corticosteroid |
| SNOT-22 | Sino-Nasal outcome test-22 |
| SUCRA | Surface under the cumulative ranking curve |
| TNSS | Total nasal symptom score |
| UPSIT | University of Pennsylvania smell identification test |
| VAS | Visual analog scale |
References
- Ma, L.; Shi, J.; Wang, K.; Sun, Y.; Xu, R. Clinical characteristics of patients with CRSwNP with intensely high eosinophil level. Laryngoscope Investig. Otolaryngol. 2022, 7, 316–324. [Google Scholar] [CrossRef]
- Rodriguez-Van Strahlen, C.; Arancibia, C.; Calvo-Henriquez, C.; Mullol, J.; Alobid, I. Systematic Review of Long Term Sinonasal Outcomes in CRSwNP after Endoscopic Sinus Surgery: A call for Unified and Standardized Criteria and Terms. Curr. Allergy Asthma Rep. 2024, 24, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Sella, G.C.P.; Tamashiro, E.; Sella, J.A.; Aragon, D.C.; de Mendonça, T.N.; de Paula Arruda, L.K.; Lima, W.T.A.; Valera, F.C.P. Asthma is the dominant factor for recurrence in chronic rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Schleimer, R.P.; Bleier, B.S. Mechanisms and pathogenesis of chronic rhinosinusitis. J. Allergy Clin. Immunol. 2022, 149, 1491–1503. [Google Scholar] [CrossRef]
- De Corso, E.; Bilò, M.B.; Matucci, A.; Seccia, V.; Braido, F.; Gelardi, M.; Heffler, E.; Latorre, M.; Malvezzi, L.; Pelaia, G. Personalized management of patients with chronic rhinosinusitis with nasal polyps in clinical practice: A multidisciplinary consensus statement. J. Pers. Med. 2022, 12, 846. [Google Scholar] [CrossRef]
- Scadding, G.K.; Scadding, G.W. Biologics for chronic rhinosinusitis with nasal polyps (CRSwNP). J. Allergy Clin. Immunol. 2022, 149, 895–897. [Google Scholar] [CrossRef]
- Oykhman, P.; Paramo, F.A.; Bousquet, J.; Kennedy, D.W.; Brignardello-Petersen, R.; Chu, D.K. Comparative efficacy and safety of monoclonal antibodies and aspirin desensitization for chronic rhinosinusitis with nasal polyposis: A systematic review and network meta-analysis. J. Allergy Clin. Immunol. 2022, 149, 1286–1295. [Google Scholar] [CrossRef]
- Fujieda, S.; Wang, C.; Yoshikawa, M.; Asako, M.; Suzaki, I.; Bachert, C.; Han, J.K.; Fuller, A.; Baylis, L.; Su, L. Mepolizumab in CRSwNP/ECRS and NP: The phase III randomised MERIT trial in Japan, China, and Russia. Rhinology 2024, 62, 576–589. [Google Scholar] [CrossRef]
- Lipworth, B.J.; Han, J.K.; Desrosiers, M.; Hopkins, C.; Lee, S.E.; Mullol, J.; Pfaar, O.; Li, T.; Chen, C.; Almqvist, G. Tezepelumab in adults with severe chronic rhinosinusitis with nasal polyps. N. Engl. J. Med. 2025, 392, 1178–1188. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Boiko, N.V.; Stagnieva, I.V.; Lodochkina, O.E.; Kurbatova, N.V. Experience with dupilumab in the treatment of chronic rhinosinusitis with nasal polyps. Vestn. Otorinolaringol. 2023, 88, 46–53. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef]
- Kim, J.H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019, 72, 558. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, P.; Calus, L.; Van Zele, T.; Blomme, K.; De Ruyck, N.; Bauters, W.; Hellings, P.; Brusselle, G.; De Bacquer, D.; van Cauwenberge, P.; et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J. Allergy Clin. Immunol. 2013, 131, 110–116.e111. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.F.; Katial, R.K.; Bardin, P.; Korn, S.; McDonald, M.; Garin, M.; Bateman, E.D.; Hoyte, F.C.L.; Germinaro, M. Effects of Reslizumab on Asthma Outcomes in a Subgroup of Eosinophilic Asthma Patients with Self-Reported Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2019, 7, 589–596.e583. [Google Scholar] [CrossRef]
- Gevaert, P.; Lang-Loidolt, D.; Lackner, A.; Stammberger, H.; Staudinger, H.; Van Zele, T.; Holtappels, G.; Tavernier, J.; van Cauwenberge, P.; Bachert, C. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J. Allergy Clin. Immunol. 2006, 118, 1133–1141. [Google Scholar] [CrossRef]
- Takabayashi, T.; Asaka, D.; Okamoto, Y.; Himi, T.; Haruna, S.; Yoshida, N.; Kondo, K.; Yoshikawa, M.; Sakuma, Y.; Shibata, K.; et al. A Phase II, Multicenter, Randomized, Placebo-Controlled Study of Benralizumab, a Humanized Anti-IL-5R Alpha Monoclonal Antibody, in Patients with Eosinophilic Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2021, 35, 861–870. [Google Scholar] [CrossRef]
- Wahba, A.A.; Abdelfattah, A.M. Anti-immunoglobulin E therapy: Is it a valid option for the management of chronic rhinosinusitis with nasal polyposis? Egypt. J. Otolaryngol. 2019, 35, 269–277. [Google Scholar] [CrossRef]
- Tversky, J.; Lane, A.P.; Azar, A. Benralizumab effect on severe chronic rhinosinusitis with nasal polyps (CRSwNP): A randomized double-blind placebo-controlled trial. Clin. Exp. Allergy 2021, 51, 836–844. [Google Scholar] [CrossRef]
- Gevaert, P.; Van Bruaene, N.; Cattaert, T.; Van Steen, K.; Van Zele, T.; Acke, F.; De Ruyck, N.; Blomme, K.; Sousa, A.R.; Marshall, R.P.; et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J. Allergy Clin. Immunol. 2011, 128, e981–e988. [Google Scholar] [CrossRef]
- Bachert, C.; Sousa, A.R.; Lund, V.J.; Scadding, G.K.; Gevaert, P.; Nasser, S.; Durham, S.R.; Cornet, M.E.; Kariyawasam, H.H.; Gilbert, J.; et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J. Allergy Clin. Immunol. 2017, 140, 1024–1031.e1014. [Google Scholar] [CrossRef]
- Danto, S.I.; Tsamandouras, N.; Reddy, P.; Gilbert, S.; Mancuso, J.; Page, K.; Peeva, E.; Vincent, M.S.; Beebe, J.S. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of PF-06817024 in Healthy Participants, Participants with Chronic Rhinosinusitis with Nasal Polyps, and Participants with Atopic Dermatitis: A Phase 1, Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Pharmacol. 2024, 64, 529–543. [Google Scholar] [CrossRef]
- De Schryver, E.; Derycke, L.; Calus, L.; Holtappels, G.; Hellings, P.W.; Van Zele, T.; Bachert, C.; Gevaert, P. The effect of systemic treatments on periostin expression reflects their interference with the eosinophilic inflammation in chronic rhinosinusitis with nasal polyps. Rhinology 2017, 55, 152–160. [Google Scholar] [CrossRef]
- Bachert, C.; Hellings, P.W.; Mullol, J.; Naclerio, R.M.; Chao, J.; Amin, N.; Grabher, A.; Swanson, B.N.; Hamilton, J.D.; Guillonneau, S.; et al. Dupilumab improves patient-reported outcomes in patients with chronic rhinosinusitis with nasal polyps and comorbid asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 2447–2449.e2442. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Peters, A.T.; Heffler, E.; Han, J.K.; Olze, H.; Pfaar, O.; Chuang, C.C.; Rout, R.; Attre, R.; Goga, L.; et al. Responder analysis to demonstrate the effect of targeting type 2 inflammatory mechanisms with dupilumab across objective and patient-reported endpoints for patients with severe chronic rhinosinusitis with nasal polyps in the SINUS-24 and SINUS-52 studies. Clin. Exp. Allergy 2022, 52, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Emson, C.; Han, J.K.; Hopkins, C.; Asimus, S.; Cann, J.A.; Chain, D.; Wu, Y.; Reddy, Y.; McCrae, C.; Cohen, D.; et al. Pharmacokinetics/pharmacodynamics of benralizumab in chronic rhinosinusitis with nasal polyps: Phase III, randomized, placebo-controlled OSTRO trial. Br. J. Clin. Pharmacol. 2024, 90, 1952–1963. [Google Scholar] [CrossRef]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, P.; Saenz, R.; Corren, J.; Han, J.K.; Mullol, J.; Lee, S.E.; Ow, R.A.; Zhao, R.; Howard, M.; Wong, K.; et al. Long-term efficacy and safety of omalizumab for nasal polyposis in an open-label extension study. J. Allergy Clin. Immunol. 2022, 149, 957–965.e953. [Google Scholar] [CrossRef]
- Damask, C.; Chen, M.; Holweg, C.T.J.; Yoo, B.; Millette, L.A.; Franzese, C. Defining the Efficacy of Omalizumab in Nasal Polyposis: A POLYP 1 and POLYP 2 Subgroup Analysis. Am. J. Rhinol. Allergy 2022, 36, 135–141. [Google Scholar] [CrossRef]
- Chuang, C.C.; Guillemin, I.; Bachert, C.; Lee, S.E.; Hellings, P.W.; Fokkens, W.J.; Duverger, N.; Fan, C.; Daizadeh, N.; Amin, N.; et al. Dupilumab in CRSwNP: Responder Analysis Using Clinically Meaningful Efficacy Outcome Thresholds. Laryngoscope 2022, 132, 259–264. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Mullol, J.; Ko, J.; Saenz, R.; Steinke, J.W.; Millette, L.A.; Gevaert, P. Omalizumab improves sleep and health status for patients with chronic rhinosinusitis with nasal polyps: An analysis of randomized clinical trials. Int. Forum Allergy Rhinol. 2024, 14, 1163–1172. [Google Scholar] [CrossRef]
- Gevaert, P.; Mullol, J.; Saenz, R.; Ko, J.; Steinke, J.W.; Millette, L.A.; Meltzer, E.O. Omalizumab improves sinonasal outcomes in patients with chronic rhinosinusitis with nasal polyps regardless of allergic status. Ann. Allergy Asthma Immunol. 2024, 132, 355–362.e351. [Google Scholar] [CrossRef]
- Hopkins, C.; Buchheit, K.M.; Heffler, E.; Cohen, N.A.; Olze, H.; Khan, A.H.; Msihid, J.; Siddiqui, S.; Nash, S.; Jacob-Nara, J.A.; et al. Improvement in Health-Related Quality of Life with Dupilumab in Patients with Moderate-to-Severe Asthma with Comorbid Chronic Rhinosinusitis with/without Nasal Polyps: An Analysis of the QUEST Study. J. Asthma Allergy 2022, 15, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Bourdin, A.; Virchow, J.C.; Papi, A.; Lugogo, N.L.; Bardin, P.; Antila, M.; Halpin, D.M.G.; Daizadeh, N.; Djandji, M.; Ortiz, B.; et al. Dupilumab efficacy in subgroups of type 2 asthma with high-dose inhaled corticosteroids at baseline. Respir. Med. 2022, 202, 106938. [Google Scholar] [CrossRef] [PubMed]
- Maspero, J.F.; Shafazand, S.; Cole, J.; Pavord, I.D.; Busse, W.W.; Msihid, J.; Gall, R.; Soler, X.; Radwan, A.; Khan, A.H.; et al. Dupilumab efficacy in high sleep disturbance management among patients with type 2 asthma. Respir. Med. 2023, 218, 107344. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, M.; Mannent, L.P.; Amin, N.; Canonica, G.W.; Hellings, P.W.; Gevaert, P.; Mullol, J.; Lee, S.E.; Fujieda, S.; Han, J.K.; et al. Dupilumab reduces systemic corticosteroid use and sinonasal surgery rate in CRSwNP. Rhinology 2021, 59, 301–311. [Google Scholar] [CrossRef]
- Mullol, J.; Bachert, C.; Amin, N.; Desrosiers, M.; Hellings, P.W.; Han, J.K.; Jankowski, R.; Vodicka, J.; Gevaert, P.; Daizadeh, N.; et al. Olfactory Outcomes with Dupilumab in Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2022, 10, 1086–1095.e1085. [Google Scholar] [CrossRef]
- Fujieda, S.; Matsune, S.; Takeno, S.; Ohta, N.; Asako, M.; Bachert, C.; Inoue, T.; Takahashi, Y.; Fujita, H.; Deniz, Y.; et al. Dupilumab efficacy in chronic rhinosinusitis with nasal polyps from SINUS-52 is unaffected by eosinophilic status. Allergy 2022, 77, 186–196. [Google Scholar] [CrossRef]
- Lee, S.E.; Hopkins, C.; Mullol, J.; Msihid, J.; Guillemin, I.; Amin, N.; Mannent, L.P.; Li, Y.; Siddiqui, S.; Chuang, C.C.; et al. Dupilumab improves health related quality of life: Results from the phase 3 SINUS studies. Allergy 2022, 77, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.Y.; Gevaert, P.; Heffler, E.; Hopkins, C.; Tversky, J.R.; Barker, P.; Cohen, D.; Emson, C.; et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2022, 149, 1309–1317.e1312. [Google Scholar] [CrossRef]
- Bachert, C.; Laidlaw, T.M.; Cho, S.H.; Mullol, J.; Swanson, B.N.; Naimi, S.; Classe, M.; Harel, S.; Jagerschmidt, A.; Laws, E.; et al. Effect of Dupilumab on Type 2 Biomarkers in Chronic Rhinosinusitis with Nasal Polyps: SINUS-52 Study Results. Ann. Otol. Rhinol. Laryngol. 2023, 132, 1649–1661. [Google Scholar] [CrossRef]
- Gurnell, M.; Radwan, A.; Bachert, C.; Lugogo, N.; Cho, S.H.; Nash, S.; Zhang, H.; Khan, A.H.; Jacob-Nara, J.A.; Rowe, P.J.; et al. Dupilumab Reduces Asthma Disease Burden and Recurrent SCS Use in Patients with CRSwNP and Coexisting Asthma. J. Asthma Allergy 2024, 17, 1–8. [Google Scholar] [CrossRef]
- Saruhan, S.; Savran, A.; Yıldız, M.; Şener, M. Reconstruction of proximal pole scaphoid non-union with avascular necrosis using proximal hamate: A four-case series. Hand Surg Rehabil. 2021, 40, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Han, J.K.; Lund, V.J.; Bachert, C.; Fokkens, W.J.; Diamant, Z.; Mullol, J.; Sousa, A.R.; Smith, S.G.; Yang, S.; et al. Evaluating treatment response to mepolizumab in patients with severe CRSwNP. Rhinology 2023, 61, 108–117. [Google Scholar] [CrossRef]
- Chupp, G.; Alobid, I.; Lugogo, N.L.; Kariyawasam, H.H.; Bourdin, A.; Chaker, A.M.; Smith, S.G.; Sousa, A.R.; Mayer, B.; Chan, R.H.; et al. Mepolizumab Reduces Systemic Corticosteroid Use in Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2023, 11, 3504–3512.e3502. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, M.; Diamant, Z.; Castelnuovo, P.; Hellings, P.W.; Han, J.K.; Peters, A.T.; Silver, J.; Smith, S.G.; Fuller, A.; Sousa, A.R.; et al. Sustained efficacy of mepolizumab in patients with severe chronic rhinosinusitis with nasal polyps: SYNAPSE 24-week treatment-free follow-up. Int. Forum Allergy Rhinol. 2024, 14, 18–31. [Google Scholar] [CrossRef]
- Bachert, C.; Mannent, L.; Naclerio, R.M.; Mullol, J.; Ferguson, B.J.; Gevaert, P.; Hellings, P.; Jiao, L.; Wang, L.; Evans, R.R.; et al. Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients with Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. JAMA 2016, 315, 469–479. [Google Scholar] [CrossRef]
- Jonstam, K.; Swanson, B.N.; Mannent, L.P.; Cardell, L.O.; Tian, N.; Wang, Y.; Zhang, D.; Fan, C.; Holtappels, G.; Hamilton, J.D.; et al. Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy 2019, 74, 743–752. [Google Scholar] [CrossRef]
- Bachert, C.; Zinreich, S.J.; Hellings, P.W.; Mullol, J.; Hamilos, D.L.; Gevaert, P.; Naclerio, R.M.; Amin, N.; Joish, V.N.; Fan, C.; et al. Dupilumab reduces opacification across all sinuses and related symptoms in patients with CRSwNP. Rhinology 2020, 58, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Hellings, P.W.; Mullol, J.; Hamilos, D.L.; Gevaert, P.; Naclerio, R.M.; Joish, V.N.; Chao, J.; Mannent, L.P.; Amin, N.; et al. Dupilumab improves health-related quality of life in patients with chronic rhinosinusitis with nasal polyposis. Allergy 2020, 75, 148–157. [Google Scholar] [CrossRef]
- Cai, S.; Xu, S.; Zhao, Y.; Zhang, L. Efficacy and Safety of Biologics for Chronic Rhinosinusitis with Nasal Polyps: A Meta-Analysis of Real-World Evidence. Allergy 2025, 80, 1256–1270. [Google Scholar] [CrossRef]
- Iqbal, I.Z.; Kao, S.S.T.; Ooi, E.H. The role of biologics in chronic rhinosinusitis: A systematic review. Int. Forum Allergy Rhinol. 2020, 10, 165–174. [Google Scholar] [CrossRef]
- Kim, C.; Han, J.; Wu, T.; Bachert, C.; Fokkens, W.; Hellings, P.; Hopkins, C.; Lee, S.; Mullol, J.; Lee, J.T. Role of biologics in chronic rhinosinusitis with nasal polyposis: State of the art review. Otolaryngol.-Head Neck Surg. 2021, 164, 57–66. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Zhang, C.; Shi, L.; Zhang, Q.; Song, X.; Wang, D.; Hu, L.; Yu, H.; Sun, X. Comparative short-term efficacy of endoscopic sinus surgery and biological therapies in chronic rhinosinusitis with nasal polyps: A network meta-analysis. Clin. Transl. Allergy 2023, 13, e12269. [Google Scholar] [CrossRef] [PubMed]
- Otten, J.; van der Lans, R.; de Corso, E.; Dziadziulia, K.; Hilvering, B.; Weersink, E.; Bonini, M.; Hagemann, J.; Thaitrakool, W.; Montuori, C. Evaluation of switching or simultaneous use of biologic treatment in patients with severe chronic rhinosinusitis with nasal polyps and severe asthma. Considerations in clinical decision making. Expert Rev. Clin. Immunol. 2023, 19, 1041–1049. [Google Scholar] [CrossRef]
- Fokkens, W.; Trigg, A.; Lee, S.E.; Chan, R.H.; Diamant, Z.; Hopkins, C.; Howarth, P.; Lund, V.; Mayer, B.; Sousa, A.R.; et al. Mepolizumab improvements in health-related quality of life and disease symptoms in a patient population with very severe chronic rhinosinusitis with nasal polyps: Psychometric and efficacy analyses from the SYNAPSE study. J. Patient-Rep. Outcomes 2023, 7, 4. [Google Scholar] [CrossRef]
- Canonica, G.W.; Harrison, T.W.; Chanez, P.; Menzella, F.; Louis, R.; Cosio, B.G.; Lugogo, N.L.; Mohan, A.; Burden, A.; Garcia Gil, E. Benralizumab improves symptoms of patients with severe, eosinophilic asthma with a diagnosis of nasal polyposis. Allergy 2022, 77, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, B.; Shen, S.; Song, X.; Jiang, Y.; Shi, L.; Zhao, C.; Yang, Y.; Jiang, L.; Li, J.; et al. Efficacy and safety of CM310 in severe eosinophilic chronic rhinosinusitis with nasal polyps (CROWNS-1): A multicentre, randomised, double-blind, placebo-controlled phase 2 clinical trial. EClinicalMedicine 2023, 61, 102076. [Google Scholar] [CrossRef]
- Laidlaw, T.M.; Menzies-Gow, A.; Caveney, S.; Han, J.K.; Martin, N.; Israel, E.; Lee, J.K.; Llanos, J.P.; Martin, N.; Megally, A.; et al. Tezepelumab Efficacy in Patients with Severe, Uncontrolled Asthma with Comorbid Nasal Polyps in NAVIGATOR. J. Asthma Allergy 2023, 16, 915–932. [Google Scholar] [CrossRef]
- Hong, H.; Liao, S.; Chen, F.; Yang, Q.; Wang, D.Y. Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy 2020, 75, 2794–2804. [Google Scholar] [CrossRef]
- Kariyawasam, H.H.; James, L.K.; Gane, S.B. Dupilumab: Clinical efficacy of blocking IL-4/IL-13 signalling in chronic rhinosinusitis with nasal polyps. Drug Des. Dev. Ther. 2020, 14, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Marcotte, G.V.; MacGlashan Jr, D.; Togias, A.; Saini, S. Omalizumab-induced reductions in mast cell FcεRI expression and function. J. Allergy Clin. Immunol. 2004, 114, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Kobayashi, Y.; Yasuba, H. Role of eosinophilic chronic rhinosinusitis in switching to benralizumab treatment in mepolizumab responders. Int. J. Clin. Pharmacol. Ther. 2020, 58, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Diver, S.; Khalfaoui, L.; Emson, C.; Wenzel, S.E.; Menzies-Gow, A.; Wechsler, M.E.; Johnston, J.; Molfino, N.; Parnes, J.R.; Megally, A.; et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 1299–1312. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Y.; He, Y.; Yan, X.; Yu, J.; Song, Q.; Zhang, L.; Dong, B.; Xu, G.; Wang, C.; et al. Stapokibart (CM310) targets IL-4Rα for the treatment of type 2 inflammation. iScience 2024, 27, 110721. [Google Scholar] [CrossRef]
- Agache, I.; Song, Y.; Alonso-Coello, P.; Vogel, Y.; Rocha, C.; Solà, I.; Santero, M.; Akdis, C.A.; Akdis, M.; Canonica, G.W. Efficacy and safety of treatment with biologicals for severe chronic rhinosinusitis with nasal polyps: A systematic review for the EAACI guidelines. Allergy 2021, 76, 2337–2353. [Google Scholar] [CrossRef]
- Papacharalampous, G.X.; Constantinidis, J.; Fotiadis, G.; Zhang, N.; Bachert, C.; Katotomichelakis, M. Chronic rhinosinusitis with nasal polyps (CRSwNP) treated with omalizumab, dupilumab, or mepolizumab: A systematic review of the current knowledge towards an attempt to compare agents’ efficacy. Int. Forum Allergy Rhinol. 2024, 14, 96–109. [Google Scholar] [CrossRef]
- Laidlaw, T.M.; Buchheit, K.M. Biologics in chronic rhinosinusitis with nasal polyposis. Ann. Allergy Asthma Immunol. 2020, 124, 326–332. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, N.; Zhang, L.; Bachert, C. Biologics for the treatment of chronic rhinosinusitis with nasal polyps-state of the art. World Allergy Organ. J. 2019, 12, 100050. [Google Scholar] [CrossRef]
- Kariyawasam, H.H.; Langan, D.; Rimmer, J. Chronic rhinosinusitis with nasal polyps and biologics: A call for better data standardisation and presentation in clinical studies. Ther. Clin. Risk Manag. 2025, 21, 27–34. [Google Scholar] [CrossRef] [PubMed]
| Trial Name | Author (YOP) | Country | YOI | Population | Allocation | Sample | |||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Total | |||||
| BREATHE Phase III Trials (post hoc) NCT01287039/NCT01285323 | Weinstein (2019) [16] | Trial 1: 128 centers Trial 2: 104 centers (Asia, Australia, North America, South America, South Africa, and Europe) | Trial 1 (Apr 2011–Mar 2014) Trial 2 (Mar 2011–Apr 2014) | Self-reported CRSwNP (with or without Aspirin sensitivity) | Reslizumab (3 mg/kg, IV) every 4 wks for 52 wks | Placebo | 78 | 72 | 150 |
| Phase IIIb Trial NCT03170271 | Canonica (2022) [58] | 221 clinical research centers | Jul 2017–Sept 2019 | CRSwNP of any severity | Benralizumab (30 mg every 8 wks; first 3 doses every 4 wks) | Placebo | 96 | 57 | 153 |
| CROWNS-1 Phase II Trial NCT04805398 | Zhang (2023) [59] | 19 hospitals in China | Apr 2021–Mar 2022 | CRSwNP patients had received SCS treatment within 2 years prior to the run-in period, or have contraindicated or intolerant to SCS treatment or have undergone nasal polyp surgery 6 months before the run-in period, to have an NPS of at least 5 points (at least 2 points for each nostril), and to have moderate or severe nasal congestion with a weekly average nasal congestion score (NCS) of 2 or 3 points and any other symptoms such as loss of smell or rhinorrhea. | CM310 (subcutaneous 300 mg; every 2 wks) for 16 wks | Placebo | 28 | 28 | 56 |
| NAVIGATORY Phase III Trial (subgroup) NCT03347279 | Laidlaw (2023) [60] | 297 sites in 18 countries | Nov 2017–Sep 2020 | CRSwNP of any severity | Tezepelumab (subcutaneous 210 mg every 4 wks) for 52 wks | Placebo | 62 | 56 | 118 |
| Phase I Trial | Gevaert (2006) [17] | 2 centers | - | CRSwNP [massive bilateral NP grade 3–4 or recurrent NP after surgery] | Reslizumab (single IV infusion at 1 mg/kg) | Placebo | 8 | 8 | 24 |
| Reslizumab (single IV infusion at 3 mg/kg) | Placebo | 8 | |||||||
| - | Gevaert (2013) [18] | Belgium | Jan 2007–Oct 2008 | CRSwNP [according to European Position Paper on Rhinosinusitis and Nasal Polyps Guidelines] | Omalizumab (4–8 subcutaneous doses; once every 2 wks) for 16 wks | Placebo | 15 | 8 | 23 |
| Phase II Trial NCT02772419 | Takabayashi (2021) [18] | Multicenter, Japan | May 2016–May 2017 | Eosinophilic CRSwNP [bilateral NPS of 3 with a score ≥ 1 in each nostril] | Benralizumab (single dose 30 mg) | Placebo | 22 | 11 | 56 |
| Benralizumab (3 doses of 30 mg; once every 4 weeks) | 23 | ||||||||
| - | Wahba (2019) [19] | Egypt | Jan 2015–May 2018 | CRSwNP [according to the criteria defined by the rhinosinusitis Task Force] | Omalizumab (0.016 mg/kg/IgE (IU/mL)); once every 2 wks | Conventional therapy (ABs + corticosteroid) | 43 | 43 | 86 |
| NCT03450083 | Tversky (2021) [20] | USA | Jan 2018–Dec 2019 | Severe CRSwNP (average bilateral NPS ≥ 5), eosinophil count of 300/Ml or greater, refractory symptoms despite prior surgical or endoscopic NP removal, and at least one OCS course over the previous 12 months | Benralizumab (30 mg SC) every 4 wks for 20 wks | Placebo | 12 | 12 | 24 |
| - | Boiko (2023) [11] | Russia | Jan 2019–Nov 2022 | CRSwNP [with the need for SCS over the past 2 years and deteriorating QoL with reduction in olfaction] | Dupilumab (300 mg SC every 2 wks for 24 wks) | Reslizumab (3 mg/kh IV once every 4 wks for 24 wks) | 10 | 9 | 19 |
| CRT110178 | Gevaert (2011) [21] | Belgium | - | Severe CRSwNP (grade 3–4 or recurrent after surgery) refractory to CS | Mepolizumab (750 mg, 2 IV injections 28 days apart) | Placebo | 20 | 10 | 30 |
| NCT01362244 | Bachert (2017) [22] | 6 centers (Belgium, The Netherlands, and UK) | May 2009–Dec 2014 | Severe, recurrent, bilateral NP and required NP surgery with NPS ≥ 3 in 1 nostril and VAS symptom score > 7 | Mepolizumab (6 doses of 750 mg IV, once every 4 wks) | Placebo | 54 | 51 | 105 |
| Phase I Trial NCT02743871 | Danto (2024) [23] | 20 centers in USA | - | CRSwNP (not defined) | PF-06817024 (single dose, 30 mg IV) | Placebo | 11 | 9 | 20 |
| Post hoc of 2 trials [18,21] | De Schryver (2017) [24] | Belgium | - | Same criteria as ID [9,25] | Omalizumab | Placebo | 15 | 8 | 23 |
| Mepolizumab | Placebo | 20 | 10 | 20 | |||||
| OSTRO Phase III Trial NCT03401229 | Bachert (2022) [26]—Main Results | 102 sites in Europe and the US | Jan 2018–Jul 2020 | CRSwNP [Bilateral NP with NPS ≥ 5 “with unilateral score ≥ 2” despite maintenance treatment with INCS for at least 4 weeks before enrollment and a history of SCS use and/or NP surgery] | Benralizumab (30 mg SC; every 4 wks for the 1st 3 doses and every 8 wks after) | Placebo | 207 | 203 | 410 |
| Emson (2024) [27]—Post hoc | |||||||||
| POLYP 1-2 & OLE Phase III Trials NCT03280550, NCT03280537, NCT03478930 | Gevaert (2020) [28]–POLYP 1/2 (Main Results) | North America and Europe | Nov 2017–Mar 2019 | CRSwNP with inadequate response to INCS for 4 weeks before screening with NPS ≥ 5 (≥2 for each nostril) | Omalizumab (75–600 mg (based on total IgE level and body weight) SC injection every 2–4 wks) for 24 wks | Placebo | 72 | 66 | 138 |
| Omalizumab (75–600 mg (based on total IgE level and body weight) SC injection every 2–4 wks) for 52 wks | Placebo | 62 | 65 | 127 | |||||
| Gevaert (2022) [29]—OLE Trial | Omalizumab (75–600 mg (based on total IgE level and body weight) SC injection every 2–4 wks) for 52 wks | Placebo (received omalizumab for 28 wks) | 124 | 125 | 249 | ||||
| Damask (2022) [30]—POLYP 1/2 (Post hoc) | |||||||||
| Han (2022) [31]—POLYP 1/2 (Post hoc) | |||||||||
| Meltzer (2024) [32]—POLYP 1/2 (Post hoc) | |||||||||
| Gevaert (2024) [33]—POLYP 1/2 & OLE | |||||||||
| QUEST Phase III Trial NCT02414854 | Hopkins (2022) [34]—Subgroup analysis | Europe, Western Countries, Asia, and Latin America | May 2015–Sep 2016 | CRSwNP [History at baseline] | Dupilumab (300 mg SC every 2 wks for 52 wks) | Placebo | 123 | 70 | 193 |
| Bourdin (2022) [35]—Post hoc | Dupilumab (200–300 mg SC every 2 wks for 52 wks) | Placebo | 66 | 43 | 107 | ||||
| Maspero (2023) [36]—Post hoc | |||||||||
| SINUS 24 & 52 Phase III Trials | Bachert (2019) [25]—Main Results | 67 centers in 13 countries | Dec 2016–Aug 2017 | Bilateral NP and symptoms of chronic rhinosinusitis despite INCS therapy before enrollment and had received SCS in the previous 2 years or previous NP surgery | Dupilumab (SC 300 mg every 2 wks) for 24 wks | Placebo | 143 | 133 | 276 |
| 117 centers in 14 countries | Nov 2016–Aug 2017 | Dupilumab (300 mg SC, once every 2 wks) for 52 wks | 150 | 153 | 448 | ||||
| Dupilumab (300 mg SC, once every 4 wks) for 52 weeks | 145 | ||||||||
| Desrosiers (2021) [37]—Post hoc | --------Same as Original Trial-------- | ------------------------------- Same as Original Trial -------------------------------- | |||||||
| Mullol (2022) [38]—Post hoc | |||||||||
| Fujieda (2022) [39]—Post hoc | |||||||||
| Lee (2022) [40]—Post hoc | |||||||||
| Bachert (2022) [41]—Post hoc | |||||||||
| Chuang (2022) [31]—Post hoc | |||||||||
| Bachert (2023) [42]—Post hoc | |||||||||
| Gurnell (2024) [43]—Post hoc | |||||||||
| SNAPSE & OLE (treatment-free) Phase III Trials NCT03085797 | Han (2021) [44]—Main Results | 93 centers in 11 countries | May 2017–Dec 2018 | CRSwNP [recurrent, bilateral, refractory, severe NP symptoms with nasal obstruction VAS score >5 and were eligible for repeat NP surgery with NPS ≥ 5] | Mepolizumab (100 mg SC every 4 wks for 52 wks) | Placebo | 206 | 201 | 407 |
| Hopkins (2023) [45]—Post hoc | |||||||||
| Fokkens (2023) [57]—Post hoc | |||||||||
| Chupp (2023) [46]—Post hoc | |||||||||
| Desrosiers (2024) [47]—OLE Trial | 69 | 65 | 134 | ||||||
| Phase IIa Trial NCT01920893 | Bachert (2016) [48]—Main Results | 13 sites in the US and Europe | Aug 2013–Aug 2014 | CRSwNP [bilateral NP and chronic symptoms of rhinosinusitis despite INCS treatment ≥ 2 months, and ≥ 2 rhinosinusitis symptoms (nasal obstruction, nasal discharge, facial pain/pressure, reduction/loss of smell] | Dupilumab (600 SC mg loading dose followed by 300 mg weekly) | Placebo | 30 | 30 | 60 |
| Bachert (2019) [25]—Post hoc | 16 | 19 | 35 | ||||||
| Jonstam (2019) [49]—Post hoc | |||||||||
| Bachert (2020) [50]—Post hoc | |||||||||
| Bachert (2020) [51]—Post hoc | |||||||||
| WAYPOINT NCT04851964 | Lipworth (2025)—Main [9] | 112 sites in 10 countries (Canada, China, Denmark, Germany, Hungary, Japan, Poland, Spain, UK, and USA) | Apr 2021–Aug 2023 | Patients with physician-diagnosed CRSwNP for at least 12 months with NPS of at least 5, NCS of at least 2, and SNOT-22 of at least 30 | Tezepelumab (210 mg) every 4 weeks for 52 weeks | Placebo | 203 | 205 | 408 |
| MERIT NCT04607005 | Fujieda (2024)—Main [8] | 60 centers (Japan, China, Russia) | Feb 2021–Mar 2022 | Patients with blood eosinophil count >2%, endoscopic NPS of at least 5, nasal obstruction VAS score of at least 5, and sinonasal symptoms of at least 2, and either prior NPS or SCS use | Mepolizumab (100 mg SC) every 4 weeks for 52 weeks | Placebo | 84 | 85 | 169 |
| Trial Name | Author (YOP) | Allocation | Comorbid Asthma (%) | Prior NP Surgery (%) | CRSwNP Duration (yr); M (SD) | Gender [M/F] | Age [Mean/SD] | FU [wk] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Definition | Intervention | Control | Intervention | Control | Intervention | Control | Total | Intervention | Control | Total | |||
| BREATHE Phase III Trials (post hoc) NCT01287039/NCT01285323 | Weinstein (2019) [16] | Reslizumab (3 mg/kg, IV) every 4 wks for 52 wks | Placebo | 100 | 100 | Inadequately controlled eosinophilic asthma (≥400 cells/mL) on at least medium-dose ICS | - | - | - | - | - | - | 57/93 | - | - | 51.1 (11.2) | 52 |
| Phase IIIb Trial NCT03170271 | Canonica (2022) [58] | Benralizumab (30 mg every 8 wks; first 3 doses every 4 wks) | Placebo | 100 | 100 | Severe, eosinophilic asthma who had experienced ≥2 prior-year exacerbations despite high-dosage inhaled corticosteroid plus additional controller(s) | 71.9 | 71.9 | - | - | 43/53 | 33/24 | 76/77 | 53.1 (12.3) | 52.6 (11.1) | 53 (11.5) | 24 |
| CROWNS-1 Phase II Trial NCT04805398 | Zhang (2023) [59] | CM310 (subcutaneous 300 mg; every 2 wks) for 16 wks | Placebo | 64 | 68 | - | 57 | 68 | 8.9 (9.3) | 8.9 (7.6) | 18/10 | 14/14 | 32/24 | 48.8 (12.2) | 46.4 (12.5) | 47.6 (12.3) | 16 |
| NAVIGATORY Phase III Trial (subgroup) NCT03347279 | Laidlaw (2023) [60] | Tezepelumab (subcutaneous 210 mg every 4 wks) for 52 wks | Placebo | 100 | 100 | Physician-diagnosed asthma, for patients who had received medium- or high-dose inhaled glucocorticoids (daily dose of ≥500 μg of fluticasone propionate or equivalent) for at least 12 months before screening and at least one additional controller medication, with or without oral glucocorticoids, for at least 3 months | - | - | - | - | 25/37 | 28/28 | 53/65 | 51.2 (13.3) | 50.4 (12.6) | 50.8 (12.9) | 52 |
| Phase I Trial | Gevaert (2006) [17] | Reslizumab (single IV infusion at 1 mg/kg) | Placebo | 87.5 | 75 | Asthma history | 25 | 50 | - | - | 6/2 | 6/2 | 16/8 | 43.6 (12.6) | 48 (12.1) | - | 36 |
| Reslizumab (single IV infusion at 3 mg/kg) | Placebo | 62.5 | 75 | - | - | 4/4 | 48.5 (18.1) | 36 | |||||||||
| - | Gevaert (2013) [18] | Omalizumab (4–8 subcutaneous doses; once every 2 wks) for 16 wks | Placebo | 100 | 100 | Severe allergic asthma [based on Global Initiative for Asthma Guidelines and diagnosed with respiratory physicians] | 87 | 75 | - | - | 12/3 | 4/4 | 16/7 | 50 (14.4) | 45 (14.2) | 47.3 (14.3) | 16 |
| Phase II Trial NCT02772419 | Takabayashi (2021) [18] | Benralizumab (single dose 30 mg) | Placebo | 81.8 | 90.9 | Comorbid asthma | 59.1 | 72.7 | - | - | 11/11 | 7/4 | 30/26 | 54 (10.8) | 53.3 (14.8) | 53.7 (12.9) | 12 |
| Benralizumab (3 doses of 30 mg; once every 4 weeks) | 82.6 | 65.2 | - | - | 12/11 | 53 (12.3) | 12 | ||||||||||
| - | Wahba (2019) [19] | Omalizumab (0.016 mg/kg/IgE (IU/mL)); once every 2 wks | Conventional therapy (ABs + corticosteroid) | - | - | - | - | - | 9.2 (3.6) mo | 9.8 (2.8) mo | 28/15 | 26/17 | 54/32 | 37 (11.7) | 37.5 (10.2) | - | 16 |
| NCT03450083 | Tversky (2021) [20] | Benralizumab (30 mg SC) every 4 wks for 20 wks | Placebo | 83 | 100 | Asthma history | number: 3 (2.3) * | 2.3 (1.6) * | 10 (5.1) | 11.1 (14.4) | 7/5 | 7/5 | 14/10 | 49.8 (12.1) | 50.8 (13.1) | - | 20 |
| - | Boiko (2023) [11] | Dupilumab (300 mg SC every 2 wks for 24 wks) | Reslizumab (3 mg/kh IV once every 4 wks for 24 wks) | 100 | 100 | Severe eosinophilic asthma | - | - | (6–29) | (6–28) | 3/7 | 3/6 | 6/13 | 28–69 | 29–58 | - | 24 |
| CRT110178 | Gevaert (2011) [21] | Mepolizumab (750 mg, 2 IV injections 28 days apart) | Placebo | 50 | 30 | Asthma history | 75 | 80 | 10.5 (5.6) | 14.3 (8.23) | 14/6 | 8/2 | 22/8 | 50.05 (8.86) | 45.9 (11.43) | - | 48 |
| NCT01362244 | Bachert (2017) [22] | Mepolizumab (6 doses of 750 mg IV, once every 4 wks) | Placebo | 81 | 75 | Asthma history | - | - | - | - | 34/17 | 41/13 | 75/30 | 51 (11) | 50 (10) | - | 25 |
| Phase I Trial NCT02743871 | Danto (2024) [23] | PF-06817024 (single dose, 30 mg IV) | Placebo | - | - | - | - | - | - | - | 8/3 | 5/4 | 13/7 | 54.4 (6.2) | 42.8 (10.7) | - | 3 |
| Post hoc of 2 trials [ID [9,17] | De Schryver (2017) [24] | Omalizumab | Placebo | 100 | 100 | - | - | - | - | - | 12/3 | 4/4 | 16/7 | 50 (14.4) | 45 (14.2) | - | 8 |
| Mepolizumab | Placebo | 50 | 30 | 14/6 | 8/2 | 22/8 | 50.05 (8.86) | 45.9 (11.43) | |||||||||
| OSTRO Phase III Trial NCT03401229 | Bachert (2022) [26]—Main Results | Benralizumab (30 mg SC; every 4 wks for the 1st 3 doses and every 8 wks after) | Placebo | 68.6 | 67 | Comorbid asthma | 72.9 | 73.4 | 6.93 (6.45) | 6.95 (5.46) | 142/65 | 121/82 | 263/147 | 50.1 (12.4) | 50.2 (13.9) | - | 40 (extended to 56) |
| Emson (2024) [27]—Post hoc | |||||||||||||||||
| POLYP 1-2 & OLE Phase III Trials NCT03280550, NCT03280537, NCT03478930 | Gevaert (2020) [28]—POLYP 1/2 (Main Results) | Omalizumab (75–600 mg (based on total IgE level and body weight) SC injection every 2–4 wks) for 24 wks | Placebo | 58.3 | 48.5 | Comorbid asthma | 54.2 | 60.6 | - | - | 47/25 | 41/25 | 88/50 | 50 (14.5) | 52.2 (11.6) | - | 24 |
| Omalizumab (75–600 mg (based on total IgE level and body weight) SC injection every 2–4 wks) for 52 wks | Placebo | 61.3 | 60 | 62.9 | 61.5 | 39/23 | 44/21 | 83/44 | 49 (1.9) | 51 (12) | 24 | ||||||
| Gevaert (2022) [29]—OLE Trial | Omalizumab (75–600 mg (based on total IgE level and body weight) SC injection every 2–4 wks) for 52 wks | Placebo (received omalizumab for 28 wks) | 59.7 | 54.4 | 56.5 | 61.6 | 79/45 | 81/44 | 160/89 | 50 (13.1) | 51.5 (11.8) | 52 | |||||
| Damask (2022) [30]—POLYP 1/2 (Post hoc) | 24–52 | ||||||||||||||||
| Han (2022) [31]—POLYP 1/2 (Post hoc) | |||||||||||||||||
| Meltzer (2024) [32]—POLYP 1/2 (Post hoc) | |||||||||||||||||
| Gevaert (2024) [33]—POLYP 1/2 & OLE | |||||||||||||||||
| QUEST Phase III Trial NCT02414854 | Hopkins (2022) [34]—Subgroup analysis | Dupilumab (300 mg SC every 2 wks for 52 wks) | Placebo | 100 | 100 | Uncontrolled moderate-to-severe asthma | - | - | 19.8 | - | 48/75 | 22/48 | 70/123 | 52.7 (13.5) | 49.6 (11.8) | 51.6 (13) | 52 |
| Bourdin (2022) [35]—Post hoc | Dupilumab (200–300 mg SC every 2 wks for 52 wks) | Placebo | 100 | 100 | Type 2 asthma (with high-dose ICS) | - | - | - | - | - | - | - | |||||
| Maspero (2023) [36]—Post hoc | |||||||||||||||||
| SINUS 24 & 52 Phase III Trials | Bachert (2019) [25]—Main Results | Dupilumab (SC 300 mg every 2 wks) for 24 wks | Placebo | 59 | 57 | Asthma | 69 | 74 | 11.42 (9.69) | 10.77 (8.57) | 88/55 | 70/63 | 158/118 | 52 (13.9) | 50 (14.6) | - | e+Y36---------- Sam |
| Dupilumab (300 mg SC, once every 2 wks) for 52 wks | 57 | 59 | 59 | 58 | 11.28 (10.38) | 10.88 (9.4)0 | 97/53 | 95/58 | 279/169 | 51 (14.2) | 53 (14.4) | ||||||
| Dupilumab (300 mg SC, once every 4 wks) for 52 weeks | 63 | 59 | 10.67 (9.12) | 87/58 | 53 (14.2) | ||||||||||||
| Desrosiers (2021) [37]—Post hoc | --------Same like Original Trial------- | ----------------------------------------------- Same like Original Trial -------------------------------------------------------------- | |||||||||||||||
| Mullol (2022) [38]—Post hoc | |||||||||||||||||
| Fujieda (2022) [39]—Post hoc | |||||||||||||||||
| Lee (2022) [40]—Post hoc | |||||||||||||||||
| Bachert (2022) [41]—Post hoc | |||||||||||||||||
| Chuang (2022) [31]—Post hoc | |||||||||||||||||
| Bachert (2023) [42]—Post hoc | |||||||||||||||||
| Gurnell (2024) [43]—Post hoc | |||||||||||||||||
| SNAPSE & OLE (treatment-free) Phase III Trials NCT03085797 | Han (2021) [44]—Main Results | Mepolizumab (100 mg SC every 4 wks for 52 wks) | Placebo | 68 | 74 | Asthma | 100 | 100 | 11.4 (8.5) | 11.5 (8.3) | 139/67 | 125/76 | 264/143 | 48.6 (13.6) | 48.9 (12.5) | - | 52 |
| Hopkins (2023) [45]—Post hoc | |||||||||||||||||
| Fokkens (2023) [57]—Post hoc | |||||||||||||||||
| Chupp (2023) [46]—Post hoc | |||||||||||||||||
| Desrosiers (2024) [47]—OLE Trial | - | - | - | - | - | - | - | - | - | - | - | - | - | 76 | |||
| Phase IIa Trial NCT01920893 | Bachert (2016) [48]—Main Results | Dupilumab (600 SC mg loading dose followed by 300 mg weekly) | Placebo | 53.3 | 63.3 | Comorbid asthma | 53.3 | 63.3 | 7.6 (6.1) | 11.5 (8.7) | 16/14 | 18/12 | 34/26 | 49.3 (9.1) | 47.4 (9.8) | - | 16 |
| Bachert (2019) [25]—Post hoc | 31.3 | 42.1 | Aspirin-sensitive asthma | - | - | 11.32 (8.93) | 8.95 (6.33) | 7/9 | 7/12 | 14/21 | 51.4 (7.6) | 47.7 (9.9) | |||||
| Jonstam (2019) [49]—Post hoc | |||||||||||||||||
| Bachert (2020) [50]—Post hoc | |||||||||||||||||
| Bachert (2020) [51]—Post hoc | |||||||||||||||||
| WAYPOINT NCT04851964 | Lipworth (2025)—Main [9] | Tezepelumab (210 mg) every 4 weeks for 52 weeks | Placebo | 60.1 | 61.5 | Coexisting asthma | 70.9 | 71.7 | 12.71 (10.43) | 12.8 (10.34) | 126/77 | 140/65 | 266/142 | 50.1 (13.6) | 49.4 (13.7) | 49.7 (13.6) | 52 |
| MERIT NCT04607005 | Fujieda (2024)—Main [8] | Mepolizumab (100 mg SC) every 4 weeks for 52 weeks | Placebo | 79 | 79 | Concurrent asthma | 65 | 64 | 11.9 (9.09) | 10.9 (9.08) | 53/31 | 56/29 | 109/60 | 52 (10.5) | 52 (13.2) | - | 52 |
| Rank | Best | 2nd Rank | 3rd Rank | 4th Rank | 5th Rank | 6th Rank | 7th Rank | Worst |
|---|---|---|---|---|---|---|---|---|
| Any AE | PF-06817024 | CM310 | Dupilumab | Placebo | Mepolizumab | Omalizumab | Benralizumab | PF-06817024 |
| SAE | PF-06817024 | CM310 | Dupilumab | Dupilumab | Dupilumab | Placebo | Placebo | Benralizumab |
| AE leading to discontinuation | Tezepelumab | Dupilumab | Dupilumab | Placebo | Placebo | Placebo | Benralizumab | PF-06817024 |
| AE leading to death | CM310 | Tezepelumab | Mepolizumab | Placebo | Placebo | Omalizumab | - | Omalizumab |
| MCID (NCS ≥ 1) | CM310 | Dupilumab | Dupilumab | Omalizumab | - | - | - | Placebo |
| MCID (NPS ≥ 2) | CM310 | Dupilumab | Tezepelumab | Tezepelumab | Omalizumab | Mepolizumab | - | Placebo |
| MCID (NPS ≥ 1) | CM310 | Dupilumab | Dupilumab | Omalizumab | Mepolizumab | Benralizumab | Placebo | Placebo |
| MCID (SNOT-22 ≥ 8.9) | Dupilumab | Benralizumab | Benralizumab | Mepolizumab | - | - | - | Placebo |
| SCS Use | Reslizumab | Dupilumab | Tezepelumab | Mepolizumab | Mepolizumab | Benralizumab | - | Placebo |
| Nasal Polyp Surgery | Tezepelumab | Reslizumab | Dupilumab | Mepolizumab | Mepolizumab | Benralizumab | - | Placebo |
| Endoscopic NPS | Dupilumab | Tezepelumab | Tezepelumab | Omalizumab | Mepolizumab | Benralizumab | Placebo | Reslizumab |
| NCS | Dupilumab | Tezepelumab | CM310 | Omalizumab | - | - | - | Placebo |
| Lund-Mackay CT score | CM310 | Dupilumab | Tezepelumab | Omalizumab | Mepolizumab | Benralizumab | - | Placebo |
| UPSIT score | Dupilumab | CM310 | Mepolizumab | Omalizumab | - | - | - | Placebo |
| TNSS score | CM310 | Omalizumab | - | - | - | - | - | Placebo |
| QoL (EQ-5D VAS) | Dupilumab | Mepolizumab | - | - | - | - | - | Placebo |
| SNOT-22 (total score) | Dupilumab | Dupilumab | Mepolizumab | Mepolizumab | Omalizumab | Benralizumab | - | Placebo |
| SNOT-22 (emotion score) | Mepolizumab | Omalizumab | Dupilumab | Tezepelumab | - | - | - | Placebo |
| SNOT-22 (function score) | Dupilumab | Tezepelumab | - | - | - | - | - | Placebo |
| SNOT-22 (sleep score) | Mepolizumab | Dupilumab | Omalizumab | Tezepelumab | - | - | - | Placebo |
| SNOT-22 (ear/facial pain score) | Omalizumab | Mepolizumab | Dupilumab | Tezepelumab | - | - | - | Placebo |
| SNOT-22 (nasal symptom score) | Omalizumab | Mepolizumab | Dupilumab | Tezepelumab | - | - | - | Placebo |
| VAS (nasal congestion/blockade score) | Mepolizumab | Dupilumab | Dupilumab | Placebo | - | - | - | Placebo |
| VAS (loss of smell score) | Benralizumab | Dupilumab | Mepolizumab | Mepolizumab | Omalizumab | - | - | Placebo |
| VAS (rhinorrhea score) | Mepolizumab | Omalizumab | Placebo | - | - | - | - | Placebo |
| Mepolizumab | Omalizumab | Dupiluzumab | Benralizumab | Reslizumab | Tezepelumab | CM310 | PF-06814024 | Placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | |
| Acute sinusitis | 6.3 | 3–9.6 | 2 | 0–5 | - | - | - | - | - | - | - | - | - | - | - | - | 6.6 | 3.2–10 |
| Allergic reaction | 6.5 | 1.8–11.2 | 7 | 6–19 | - | - | - | - | - | - | - | - | - | - | - | - | 4.26 | 0–14.5 |
| Arterial thrombotic event | - | - | 1 | 1–2 | - | - | - | - | - | - | - | - | - | - | - | - | 0.4 | 0.006–1.4 |
| Arthralgia | 6.1 | 3.2–9.1 | 2 | 1–4 | - | - | - | - | - | - | 3.4 | 1–6 | - | - | - | - | 2.18 | 1.2–4.5 |
| Asthma (new-onset) | 1.9 | 0.4–3.3 | - | - | 2 | 1–5 | 9 | 5–13 | - | - | 0.5 | 0–1.5 | 2 | 1–6 | - | - | 7.58 | 5.7–12.8 |
| Asthma (exacerbation) | - | - | 8 | 0–16 | - | - | 3 | 3–8 | - | - | - | - | - | - | - | - | 4.2 | 1.1–7.2 |
| Back pain | 3.8 | 0–7.5 | 4 | 1–7 | 10 | 1–21 | - | - | - | - | 4.9 | 1.9–7.9 | - | - | 18 | 5–41 | 4.7 | 1.8–7.6 |
| Bronchitis | 6.9 | 0–14.9 | - | - | 3 | 1–10 | - | - | - | - | - | - | - | - | - | - | 4.07 | 3.6–10 |
| Common cold | 25 | 6–44 | 53 | 28–79 | - | - | 6 | 2–10 | - | - | - | - | - | - | - | - | 7.3 | 1.4–19 |
| Cough | 3.7 | 1.4–6 | - | - | 2 | 1–6 | - | - | - | - | - | - | - | - | 18 | 5–41 | 6.1 | 3.4–8.9 |
| COVID-19 | - | - | - | - | - | - | - | - | - | - | 23.2 | 17.4–29 | - | - | - | - | 18.97 | - |
| CRSwNP | - | - | - | - | - | - | - | - | - | - | 5.4 | 2.3–8.5 | - | - | - | - | 22.93 | - |
| Disk herniation (pre-existing) | 5 | 0–15 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.5 | 1.7–16.9 |
| Diverticulitis (pre-existing) | 5 | 0–15 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.5 | 1.7–16.9 |
| Dizziness | 2.4 | 0–5.6 | 1 | 1–2 | 10 | 1–21 | - | - | - | - | - | - | - | - | 4 | 3–15 | 4.5 | 0.6–5.7 |
| Dyspnea | 7.4 | 0.4–14.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3.9 | 1.4–9.2 |
| Ear pain | 9.3 | 1.5–17 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 1.8–5.8 |
| Epistaxis | 7.5 | 4.3–10.7 | 3 | 0–6 | 13 | 4–30 | - | - | - | - | 5.9 | 2.7–9.2 | - | - | - | - | 4.36 | 2.5–8.1 |
| Fatigue | 7.4 | 0.4–14.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 1.8–5.8 |
| Fever | 3 | 0–5 | - | - | - | - | 13 | 2–27 | - | - | - | - | - | - | - | - | 5.35 | 1.7–10.2 |
| Fracture | 5 | 0–14.6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.5 | 0.7–16.9 |
| Gastroenteritis | - | - | 7 | 6–19 | - | - | - | - | - | - | - | - | - | - | - | - | 5.6 | 1.9–20.5 |
| General myalgia | - | - | 7 | 6–19 | - | - | - | - | - | - | - | - | - | - | - | - | 5.6 | 1–20.5 |
| Headache | 13.8 | 3–24.7 | 13 | 7–33 | 12 | 0–23 | 7 | 1–14 | - | - | 8.4 | 4.6–12.2 | - | - | 9 | 8–26 | 8.42 | 5.3–13.9 |
| Increased serum cholesterol | - | - | - | - | - | - | - | - | - | - | - | - | 7 | 2–17 | - | - | 7.1 | 2.4–16.7 |
| Increased serum TAG | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 1–6 | - | - | 1.7 | 0.3–6.5 |
| Any infection | 44 | 33.4–54.7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 41.18 | - |
| Injection site reaction | - | - | 2 | 1–4 | 22 | 11–55 | 1 | 1–3 | - | - | - | - | - | - | 18 | 5–41 | 22.2 | 4.9–49.4 |
| Insomnia | 6 | 1–12 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 0.1–3.6 |
| Jaundice | - | - | 3 | 2–12 | - | - | - | - | - | - | - | - | - | - | - | - | 12.5 | 10.4–35.4 |
| Laryngeal pain | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 1–6 | - | - | 7.1 | 2.4–16.7 |
| Left ulnar hypoesthesia | - | - | 7 | 6–19 | - | - | - | - | - | - | - | - | - | - | - | - | 5.6 | 0.9–20.5 |
| Malignant neoplasm | - | - | 1 | 0–2 | - | - | - | - | - | - | - | - | - | - | - | - | 0.4 | 0.4–1.1 |
| Mild increase in thyroid hormones | 5 | 5–15 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.5 | 0.7–16.9 |
| Nasal congestion | - | - | 3 | 0–6 | - | - | - | - | - | - | - | - | - | - | 27 | 1–54 | 0.6 | 0.02–4 |
| Nasal obstruction | - | - | 20 | 0–40 | - | - | - | - | - | - | - | - | - | - | - | - | 37.5 | 4–71 |
| Nasal polyps | 4 | 1–7 | 3 | 0–6 | 3 | 1–4 | - | - | - | - | - | - | - | - | - | - | 7.5 | 2.7–12.2 |
| Nasopharyngitis | 18 | 8–29 | 6 | 2–10 | 28 | 5–62 | 20 | 3–36 | - | - | 17.7 | 12.5–23 | - | - | - | - | 12.9 | 10.2–21.3 |
| Nausea | 7 | 0–14 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3.9 | 1.4–9.2 |
| Oropharyngeal pain | 8 | 4–11 | - | - | 23 | 8–38 | - | - | - | - | - | - | - | - | - | - | 6.1 | 2.7–9.5 |
| Otitis media | 3 | 0–5 | 13 | 4–31 | - | - | 4 | 3–14 | - | - | - | - | - | - | - | - | 5.1 | 2.3–7.9 |
| Pain in extremity | 5 | 5–15 | - | - | - | - | - | - | - | - | - | - | - | - | 9 | 8–26 | 6.3 | 4.3–16.8 |
| Pharyngitis | 5 | 4–15 | - | - | - | - | - | - | - | - | 4.4 | 1.6–7.3 | - | - | - | - | 2.49 | 0.7–16.9 |
| Pneumonia | - | - | - | - | - | - | 4 | 2–14 | - | - | - | - | 2 | 1–6 | - | - | 7.5 | 7–15.6 |
| Procedural pain | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 18 | 5–41 | 11.1 | 9.4–31.6 |
| Red swollen eyes | 2 | 1–9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 10 | 8.6–28.6 |
| Rhinitis | - | - | 3 | 0–6 | - | - | - | - | - | - | - | - | - | - | - | - | 3 | 0.1–5.8 |
| Rhinorrhea | 6 | 1–12 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 0.1–3.6 |
| Shortness of breath | 5 | 4–15 | 13 | 4–31 | - | - | - | - | - | - | - | - | - | - | - | - | 6.3 | 4.5–17.2 |
| Shoulder pain | - | - | 7 | 6–19 | - | - | - | - | - | - | - | - | - | - | - | - | 5.6 | 0.9–20.5 |
| Sinusitis | 5 | 2–8 | 3 | 1–12 | - | - | 9 | 4–15 | - | - | - | - | - | - | 27 | 1–54 | 6.3 | 2.1–10.6 |
| Tinnitus | - | - | - | - | - | - | - | - | - | - | - | - | 7 | 2–17 | - | - | 1.7 | 0.3–6.5 |
| Toothache | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 1–6 | - | - | 7.1 | 2.4–16.7 |
| Upper GI pain | - | - | 1 | 1–2 | - | - | - | - | - | - | - | - | - | - | - | - | 3 | 1–5.8 |
| URTI | 6 | 3–9 | - | - | 13 | 1–25 | 3 | 1–5 | 62 | 13–75 | 9.4 | 5.4–13.4 | 18 | 4–32 | 27 | 1–54 | 5.83 | 3.5–9.2 |
| UTI | - | - | - | - | - | - | 8 | 7–24 | - | - | - | - | - | - | - | - | 3.8 | 0.6–14.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safia, A.; Khater, A.; Abd Elhadi, U.; Merchavy, S.; Karam, M. Optimizing Biologic Treatment Selection in Chronic Rhinosinusitis with Nasal Polyps: A Network Meta-Analysis of Efficacy and Safety Across 22 RCTs. Pharmaceuticals 2025, 18, 1455. https://doi.org/10.3390/ph18101455
Safia A, Khater A, Abd Elhadi U, Merchavy S, Karam M. Optimizing Biologic Treatment Selection in Chronic Rhinosinusitis with Nasal Polyps: A Network Meta-Analysis of Efficacy and Safety Across 22 RCTs. Pharmaceuticals. 2025; 18(10):1455. https://doi.org/10.3390/ph18101455
Chicago/Turabian StyleSafia, Alaa, Ashraf Khater, Uday Abd Elhadi, Shlomo Merchavy, and Marwan Karam. 2025. "Optimizing Biologic Treatment Selection in Chronic Rhinosinusitis with Nasal Polyps: A Network Meta-Analysis of Efficacy and Safety Across 22 RCTs" Pharmaceuticals 18, no. 10: 1455. https://doi.org/10.3390/ph18101455
APA StyleSafia, A., Khater, A., Abd Elhadi, U., Merchavy, S., & Karam, M. (2025). Optimizing Biologic Treatment Selection in Chronic Rhinosinusitis with Nasal Polyps: A Network Meta-Analysis of Efficacy and Safety Across 22 RCTs. Pharmaceuticals, 18(10), 1455. https://doi.org/10.3390/ph18101455






