Characterization and Anti-Allergic Mechanisms of Bioactive Compounds in a Traditional Chinese Medicine Prescription Using UHPLC-Q-TOF-MS/MS, Network Pharmacology and Computational Simulations
Abstract
1. Introduction
2. Results
2.1. Qualitative Study of Chemical Constituents in ACP Based on UHPLC–Q–TOF–MS Technology
2.1.1. Flavonoids
2.1.2. Amino Acids
2.1.3. Phenolic Acids
2.1.4. Saponins
2.1.5. Nucleosides
2.1.6. Iridoids
2.1.7. Other Compounds
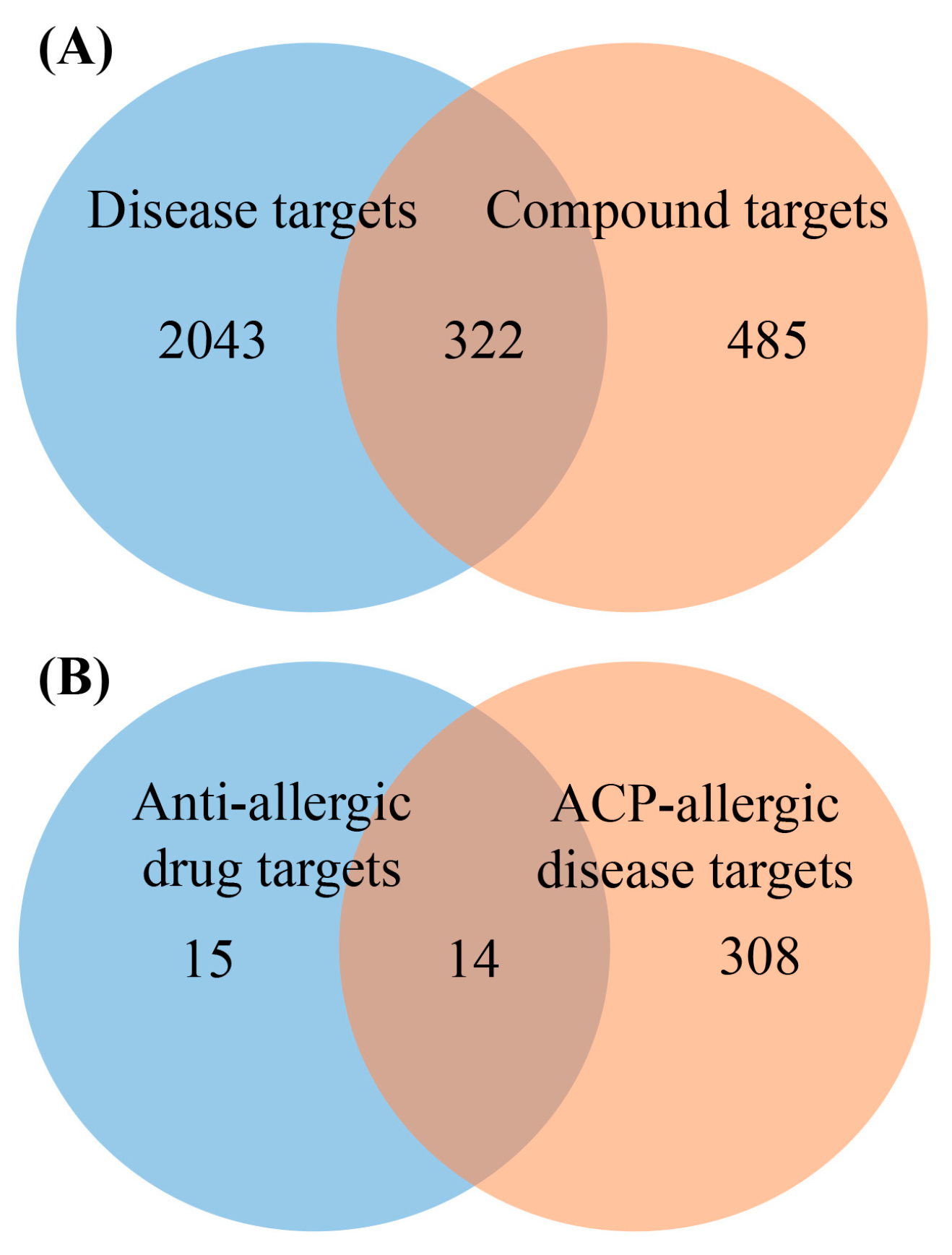
2.2. Target Analysis Results
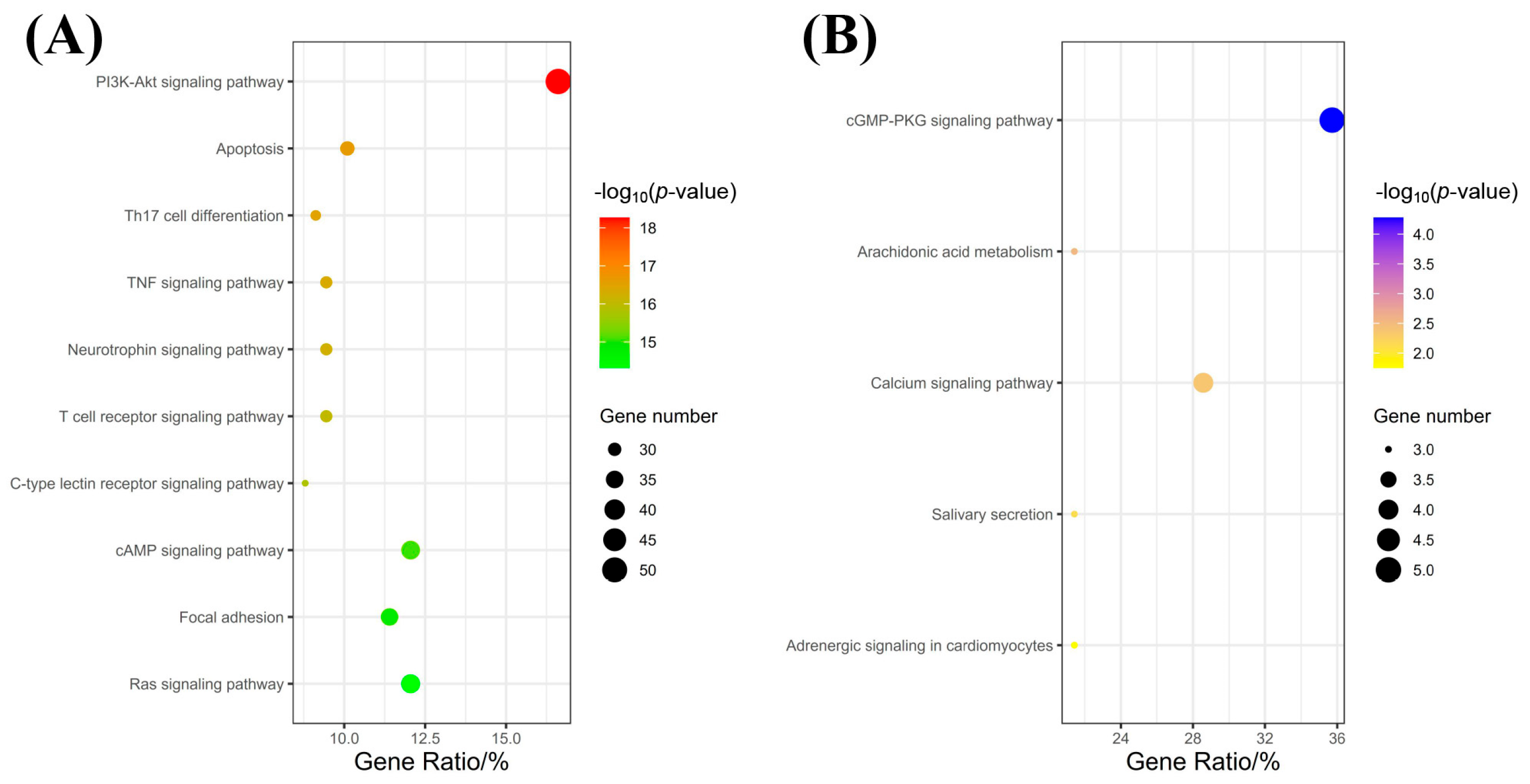
2.3. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis Results
2.4. Network Analysis
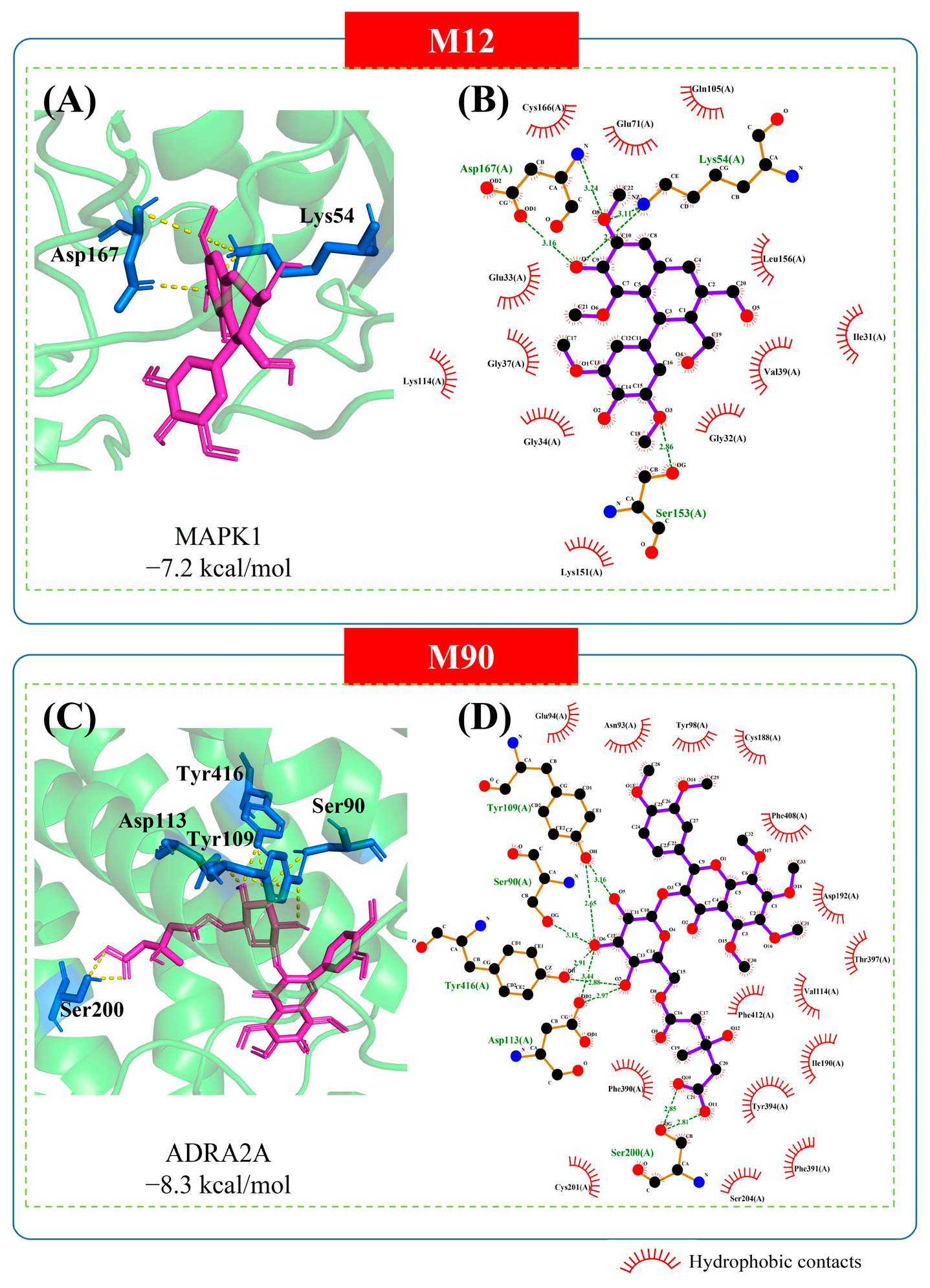
2.5. Molecular Docking Analysis
2.6. MD Simulation
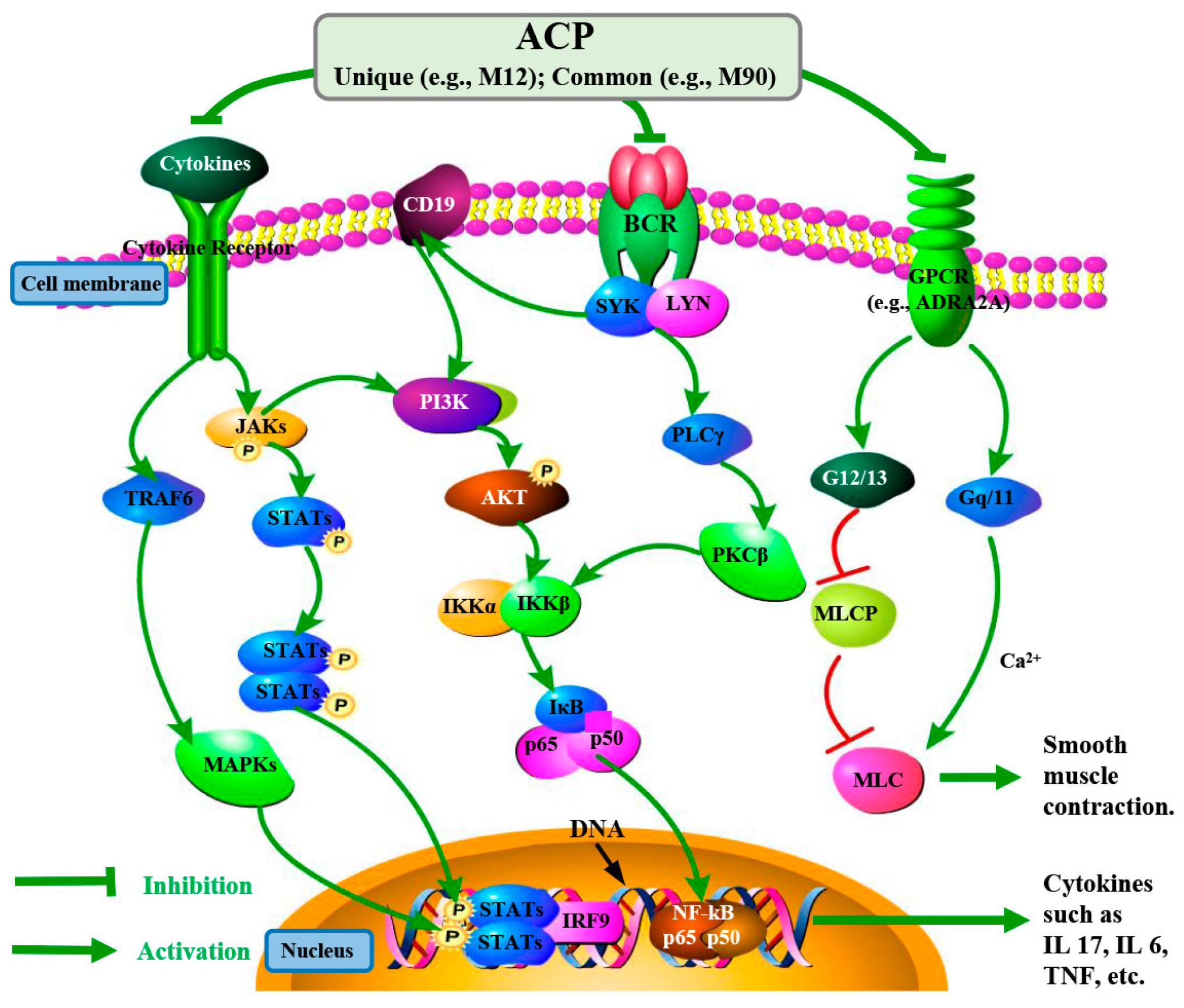
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation for Water-Extraction of ACP
4.3. Chromatography Conditions and MS Parameters
4.4. Network Pharmacology Analysis
4.4.1. Collection of ACP Compound Targets and ACP-Disease Targets
4.4.2. Collection of Anti-Allergic Drugs and Their Targets
4.4.3. Target Intersection
4.4.4. KEGG Pathway Enrichment Analysis
4.4.5. Network Construction
4.5. Molecular Docking
4.6. Molecular Dynamics (MD) Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TCM | Traditional Chinese Medicine |
| ACP | Allergic constitution prescription |
| UHPLC-Q-TOF-MS/MS | Ultra-high performance liquid chromatography quadrupole time-of-flight tandem mass spectrometry |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| WAO | World Allergy Organization |
| WHO | World Health Organization |
| MD | Molecular dynamics |
| RMSD | Root mean squared deviation |
| Rg | Radius of gyration |
| RMSF | Root mean squared fluctuations |
| SASA | Solvent accessible surface area |
| QAMS | Quantitative analysis of multi-components by single-marker |
| DRS | Digital reference standard |
| TRSDMC | Two reference substances for determination of multiple components |
| QASRE | Quantitative analysis by standardized reference extract |
References
- Weinberg, E.G. The WAO white book on allergy 2011–2012. Curr. Allergy Clin. Immunol. 2011, 24, 156–157. [Google Scholar]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Rivas, M.F.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Borges, M.S.; et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef] [PubMed]
- Tanno, L.K.; Demoly, P. Allergy in the World Health Organization’s International Classification of Diseases (ICD)-11. Pediatr. Allergy Immunol. 2022, 33 (Suppl. 27), 5–7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cai, T.; Chen, H.; Chen, L.; Chen, Y.; Gao, X.; Gao, X.; Geng, S.; Guo, Y.; Hao, F.; et al. Expert consensus on the use of omalizumab in chronic urticaria in China. World Allergy Organ. J. 2021, 14, 100610. [Google Scholar] [CrossRef]
- Takaiwa, F. Next-generation allergen-specific immunotherapy for Japanese cedar pollinosis using molecular approaches. Immunotargets Ther. 2021, 10, 213–224. [Google Scholar] [CrossRef]
- Mikhail, I.; Stukus, D.R.; Prince, B.T. Fatal anaphylaxis: Epidemiology and risk factors. Curr. Allergy Asthma Rep. 2021, 21, 28. [Google Scholar] [CrossRef]
- Li, X.T.; Ma, Q.B.; Yin, J.; Zheng, Y.A.; Chen, R.C.; Chen, Y.G.; Li, T.Z.; Wang, Y.Q.; Yang, K.H.; Zhang, H.J.; et al. A clinical practice guideline for the emergency management of anaphylaxis (2020). Front. Pharmacol. 2022, 13, 845689. [Google Scholar] [CrossRef]
- Kapoor, Y.; Kumar, K. Structural and clinical impact of anti-allergy agents: An overview. Bioorg. Chem. 2020, 94, 103351. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, H.M.; Bao, L.; Wang, J.; Wang, Q. Three-grade prevention of the allergic constitution. World Chin. Med. 2016, 11, 1892–1894. [Google Scholar] [CrossRef]
- Zhang, K.L.; Sun, P.C.; Chen, X.M.; Chen, J.X.; Zhang, Y.; Wang, Y.; Wang, J.; Wang, Q. Research on the mechanism of intervention of allergic constitution (TIZHI) by Chinese medicines for expelling wind to relieve superficies based on bioinformatics. Tianjin J. Tradit. Chin. Med. 2021, 38, 273–279. [Google Scholar] [CrossRef]
- Shao, D.M.; Wang, Q.; Sun, P.C.; Wang, J. Analysis of Professor WANG Qi’s constitution three-grade prevention program for allergic asthma. Tianjin J. Tradit. Chin. Med. 2022, 39, 409–414. [Google Scholar] [CrossRef]
- Goksor, E.; Loid, P.; Alm, B.; Aberg, N.; Wennergren, G. The allergic march comprises the coexistence of related patterns of allergic disease not just the progressive development of one disease. Acta Paediatr. 2016, 105, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.A.; Vonk, J.M.; Baurecht, H.; Marenholz, I.; Tian, C.; Hoffman, J.D.; Helmer, Q.; Tillander, A.; Ullemar, V.; van Dongen, J.; et al. Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat. Genet. 2017, 49, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yin, C.L.; Qin, Y.; Cheng, Z.H.; Chen, D.F. Approach to the study of flavone di-C-glycosides by high performance liquid chromatography-tandem ion trap mass spectrometry and its application to characterization of flavonoid composition in Viola yedoensis. J. Mass Spectrom. 2014, 49, 1010–1024. [Google Scholar] [CrossRef]
- Qin, Y.; Zhou, R.R.; Huang, J.H.; Jin, J.; Zhou, Q.Y.J.; Liu, H.; Xiao, J.; Zhao, Y.H.; Shu, J.; Zhang, S.H.; et al. Comprehensive comparison of the anti-inflammatory activity and chemical consistency of traditional Chinese medicine formula granules with Ge-Gen decoction as a representative sample. Biomed. Chromatogr. 2019, 33, e4689. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, M.; Tang, C.; Fu, K.; Li, X.L.; Song, Y.L.; Zhang, J.W.; Wang, Z. The study of chemical components in Qishiwei Zhenzhu pills and its anti-apoptotic mechanism in cerebral ischemic based on LC-MS and network pharmacology. J. Ethnopharmacol. 2023, 302, 115891. [Google Scholar] [CrossRef]
- Qin, Y.; Zhou, R.R.; Jin, J.; Xie, J.; Liu, H.; Cai, P.; Shu, J.; Zhao, Y.H.; Huang, L.Q.; Zhang, S.H. UPLC-ESI-Q-TOF-MS/MS analysis of anticancer fractions from Ophiocordyceps xuefengensis and Ophiocordyceps sinensis. Biomed. Chromatogr. 2020, 34, e4841. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.; Jin, J.; Huang, J.H.; Li, J.Y.; Zhou, Q.Y.J.; Zhou, R.R.; Zhang, S.H. Screening and identification of antioxidants from Ophiocordyceps xuefengensis (Ascomycetes) by using DPPH-HPLC-DAD-Q-TOF-MS/MS. Int. J. Med. Mushrooms 2018, 20, 887–899. [Google Scholar] [CrossRef]
- Qin, Y.; Jin, J.; Zhou, R.R.; Fang, L.Z.; Liu, H.; Zhong, C.; Xie, Y.; Liu, P.A.; Qin, Y.H.; Zhang, S.H. Integrative analysis of metabolome and transcriptome provide new insights into the bitter components of Lilium lancifolium and Lilium brownii. J. Pharm. Biomed. Anal. 2022, 215, 114778. [Google Scholar] [CrossRef]
- Montero, L.; Ibáñez, E.; Russo, M.; di Sanzo, R.; Rastrelli, L.; Piccinelli, A.L.; Celano, R.; Cifuentes, A.; Herrero, M. Metabolite profiling of licorice (Glycyrrhiza glabra) from different locations using comprehensive two-dimensional liquid chromatography coupled to diode array and tandem mass spectrometry detection. Anal. Chim. Acta 2016, 913, 145–159. [Google Scholar] [CrossRef]
- Wu, W.Y.; Lan, W.N.; Jiao, X.; Shao, A.X.; Wu, P.; Wang, K.; Zhan, S.F. Mechanisms underlying the therapeutic effects of Gang Huo Qing wen granules in the treatment of influenza based on network pharmacology, molecular docking and molecular dynamics. Sci. Rep. 2024, 14, 15853. [Google Scholar] [CrossRef]
- Lobanov, M.Y.; Bogatyreva, N.S.; Galzitskaya, O.V. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. 2008, 42, 623–628. [Google Scholar] [CrossRef]
- Zare, F.; Ataollahi, E.; Mardaneh, P.; Sakhteman, A.; Keshavarz, V.; Solhjoo, A.; Emami, L. A combination of virtual screening, molecular dynamics simulation, MM/PBSA, ADMET, and DFT calculations to identify a potential DPP4 inhibitor. Sci. Rep. 2024, 14, 7749. [Google Scholar] [CrossRef] [PubMed]
- Ghahremanian, S.; Rashidi, M.M.; Raeisi, K.; Toghraie, D. Molecular dynamics simulation approach for discovering potential inhibitors against SARS-CoV-2: A structural review. J. Mol. Liq. 2022, 354, 118901. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; An, N.; Yang, L.; Ren, X.D.; Lu, S.L.; Ji, H.; Wang, Q.L.; Dong, J. Molecular dynamics simulation of the interactions between sesamol and myosin combined with spectroscopy and molecular docking studies. Food Hydrocoll. 2022, 131, 107801. [Google Scholar] [CrossRef]
- Alenazy, L.A.; Al Enazy, S.; Castells, M. Drug desensitization update: Key concepts and mechanisms. Curr. Treat. Options Allerg. 2023, 10, 458–472. [Google Scholar] [CrossRef]
- Ai, C.Y.; Zou, Y.; Liu, H.; Yang, Z.Q.; Xi, J.L. Traditional Chinese herbal medicine for allergic diseases: A review. Am. J. Chin. Med. 2023, 51, 779–806. [Google Scholar] [CrossRef]
- Su, X.X. Chinese medicine knowledge on allergic constitution. Chin. J. Clin. Ration. Drug Use 2016, 9, 150–151. [Google Scholar] [CrossRef]
- Li, X.M.; Wang, Q.F.; Schofield, B.; Lin, J.; Huang, S.K.; Wang, Q. Modulation of antigen-induced anaphylaxis in mice by a traditional Chinese medicine formula, Guo Min Kang. Am. J. Chin. Med. 2009, 37, 113–125. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Zhao, H.H.; Wang, T.S.; Zhao, X.S.; Wang, J.; Wang, Q. Anti-inflammatory and anti-asthmatic effects of TMDCT decoction in eosinophilic asthma through Treg/Th17 balance. Front. Pharmacol. 2022, 13, 819728. [Google Scholar] [CrossRef]
- Cheng, J.J.; Zhang, M.L.; Zheng, Y.F.; Wang, J.; Wang, Q. Integrative analysis of network pharmacology and proteomics to identify key targets of Tuomin-Zhiti-Decoction for allergic rhinitis. J. Ethnopharmacol. 2022, 296, 115448. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, C.; Gan, Q.Y.; Wang, Z.X.; Jiang, R.W. Chemical composition-based characterization of the anti-allergic ef- fect of Guominkang Formula on IgE-mediated mast cells activation and passive cutaneous anaphylaxis. Chin. J. Nat. Med. 2022, 20, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.M.; Hu, L.H.; Zhang, H.L.; Zhang, H.Y.; Liu, J.T.; Zhao, X.S.; Wang, J.; Wang, Q. Guominkang formula alleviate inflammation in eosinophilic asthma by regulating immune balance of Th1/2 and Treg/Th17 cells. Front. Pharmacol. 2022, 13, 978421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Zhang, H.Y.; Wang, L.; Zhang, Y.H.; Hu, L.H.; Liu, J.T.; Zhou, Y.M.; Wang, J. Bioinformatics and Network Pharmacology Identify the Therapeutic Role of Guominkang in Allergic Asthma by Inhibiting PI3K/Akt Signaling. J. Inflamm. Res. 2025, 18, 2805–2821. [Google Scholar] [CrossRef]
- Kono, R.; Nakamura, M.; Nomura, S.; Kitano, N.; Kagiya, T.; Okuno, Y.; Inada, K.; Tokuda, A.; Utsunomiya, H.; Ueno, M. Biological and epidemiological evidence of anti-allergic effects of traditional Japanese food ume (Prunus mume). Sci. Rep. 2018, 8, 11638. [Google Scholar] [CrossRef]
- Yang, Y.; Lai, Q.Z.; Wang, C.Y.; Zhou, G.L. Protective effects of herba Houttuyniae Aqueous extract against OVA-induced airway hyperresponsiveness and inflammation in asthmatic mice. Evid.-Based Complement Altern. Med. 2022, 2022, 7609785. [Google Scholar] [CrossRef]
- Makino, T.; Furata, Y.; Wakushima, H.; Fujii, H.; Saito, K.; Kano, Y. Anti-allergic effect of Perilla frutescens and its active constituents. Phytother. Res. 2003, 17, 240–243. [Google Scholar] [CrossRef]
- Tsugawa, H.; Rai, A.; Saito, K.; Nakabayashi, R. Metabolomics and complementary techniques to investigate the plant phytochemical cosmos. Nat. Prod. Rep. 2021, 38, 1729–1759. [Google Scholar] [CrossRef]
- Wang, C.; Chao, I.C.; Qin, Y.; Zhang, W.X.; Zhao, J.; Zhang, Q.W.; Li, S.P. Comparison for quantification of eight components in Alpinia officinarum Hance by using high-performance liquid chromatography coupled with diode array detector and charged aerosol detector with individual and substitute reference compound. J. Pharm. Biomed. Anal. 2022, 210, 114545. [Google Scholar] [CrossRef]
- Wang, Q.J.; Sun, L.; Liu, F.; Jin, H.Y.; Yu, J.D.; Dai, Z.; Ma, S.C. Progress and challenges of reference standard and its new form: Digital reference standard. Chin. Med. 2016, 7, 77–91. [Google Scholar] [CrossRef]
- Chen, A.Z.; Sun, L.; Yuan, H.; Wu, A.Y.; Lu, J.G.; Ma, S.C. Simultaneous qualitative and quantitative analysis of 11 active compounds in rhubarb using two reference substances by UHPLC. J. Sep. Sci. 2018, 41, 3686–3696. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Qiao, C.F.; Chen, Y.W.; Zhao, J.; Cui, X.M.; Zhang, Q.W.; Liu, X.M.; Hu, D.J. A novel strategy with standardized reference extract qualification and single compound quantitative evaluation for quality control of Panax notoginseng used as a functional food. J. Chromatogr. A 2013, 1313, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Feng, X.; Wang, D.F.; Liu, L.; Liu, Y.Q.; Liu, B.; Zhu, L.; Zhang, C.J.; Yang, W.P. Mechanism of Sishen Pills-Tongxie Yaofang in the treatment of ulcerative colitis based on network pharmacology and experimental verification. Sci. Tradit. Chin. Med. 2024, 2, 224–236. [Google Scholar] [CrossRef]
- Xu, W.N.; Wu, J.Y.; Yang, D.Y.; Chen, Y.X.; Wu, X.Y.; Wen, R.; Yan, L.P.; Li, C.; Yu, H. Anti-acute gastric ulcer resistance of Aurantii Fructus Immaturus juice processing Atractylodis Macrocephalae Rhizoma by regulating PTGS2, MAPK1, and KDR targets based on metabolomics and integrated network pharmacology analysis. Sci. Tradit. Chin. Med. 2024, 2, 121–137. [Google Scholar] [CrossRef]
- Liu, J.H.; Li, C.; Zhang, C.H.; Zhang, Z.H. LncRNA-CASC7 enhances corticosteroid sensitivity via inhibiting the PI3K/AKT signaling pathway by targeting miR-21 in severe asthma. Pulmonology 2020, 26, 18–26. [Google Scholar] [CrossRef]
- Li, H.; Bradbury, J.A.; Dackor, R.T.; Edin, M.L.; Graves, J.P.; DeGraff, L.M.; Wang, P.M.; Bortner, C.D.; Maruoka, S.; Lih, F.B.; et al. Cyclooxygenase-2 regulates Th17 cell differentiation during allergic lung inflammation. Am. J. Respir. Crit. Care Med. 2011, 184, 37–49. [Google Scholar] [CrossRef]
- Bracke, M.; van de Graaf, E.; Lammers, J.W.J.; Coffer, P.J.; Koenderman, L. In vivo priming of Fc alpha R functioning on eosinophils of allergic asthmatics. J. Leukoc. Biol. 2000, 68, 655–661. [Google Scholar] [CrossRef]
- Chen, X.X.; Zhao, X.L.; Hu, Y.Z.; Zhang, B.W.; Zhang, Y.; Wang, S. Lactobacillus rhamnosus GG alleviates beta-conglycinin-induced allergy by regulating the T cell receptor signaling pathway. Food Funct. 2020, 11, 10554–10567. [Google Scholar] [CrossRef]
- Conejero, L.; Higaki, Y.; Baeza, M.L.; Fernández, M.; Varela-Nieto, I.; Zubeldia, J.M. Pollen-induced airway inflammation, hyper-responsiveness and apoptosis in a murine model of allergy. Clin. Exp. Allergy 2007, 37, 331–338. [Google Scholar] [CrossRef]
- Quinn, K.D.; Schedel, M.; Nkrumah-Elie, Y.; Joetham, A.; Armstrong, M.; Cruickshank-Quinn, C.; Reisdorph, R.; Gelfand, E.W.; Reisdorph, N. Dysregulation of metabolic pathways in a mouse model of allergic asthma. Allergy 2017, 72, 1327–1337. [Google Scholar] [CrossRef]
- Chiffoleau, E. C-type lectin-like receptors as emerging orchestrators of sterile inflammation represent potential therapeutic targets. Front. Immunol. 2018, 9, 227. [Google Scholar] [CrossRef]
- Jiang, T.Y.; He, F.; Han, S.W.; Chen, C.; Zhang, Y.N.; Che, H.L. Characterization of cAMP as an anti-allergic functional factor in Chinese jujube (Ziziphus jujuba Mill.). J. Funct. Food. 2019, 60, 103414. [Google Scholar] [CrossRef]
- Sutanto, H. Mechanobiology of Type 1 hypersensitivity: Elucidating the impacts of mechanical forces in allergic reactions. Mechanobiol. Med. 2024, 2, 100041. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Kulis, M.; Pons, L.; Zhong, X.P.; Burks, A.W. Peanut allergen Ara h 2-specific T cells are activated via Ras-Erk MAP kinase pathway signalling and identified by CD154 expression. Food Agric. Immunol. 2011, 22, 335–344. [Google Scholar] [CrossRef]
- Ekstedt, S.; Georén, S.K.; Cardell, L.O. Effects of MP-AzeFlu enhanced by activation of bitter taste receptor TAS2R. Allergy Asthma Clin. Immunol. 2020, 16, 45. [Google Scholar] [CrossRef]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef]
- Pelletier, L.; Savignac, M. Calcium signaling components as potential therapeutic targets in allergic asthma. Rev. Fr. Allergol. 2013, 53, 129–132. [Google Scholar] [CrossRef]
- Morris, S.C.; Perkins, C.; Potter, C.; Parsons, D.; Schuman, R.; Khodoun, M.V.; Samavedam, U.; Strait, R.; Finkelman, F.D. Optimizing drug inhibition of IgE-mediated anaphylaxis in mice. J. Allergy Clin. Immunol. 2022, 149, 671–684. [Google Scholar] [CrossRef]
- Elad, S.; Heisler, S.; Shalit, M. Saliva secretion in patients with allergic rhinitis. Int. Arch. Allergy Immunol. 2006, 141, 276–280. [Google Scholar] [CrossRef]
- Platt, M. Pharmacotherapy for allergic rhinitis. Int. Forum Allergy Rhinol. 2014, 4, S35–S40. [Google Scholar] [CrossRef]
- Duan, W.; Wong, W.S.F. Targeting mitogen-activated protein kinases for asthma. Curr. Drug Targets 2006, 7, 691–698. [Google Scholar] [CrossRef]
- Fruman, D.A. The role of class I phosphoinositide 3-kinase in T-cell function and autoimmunity. Biochem. Soc. Trans. 2007, 35, 177–180. [Google Scholar] [CrossRef]
- Kitaura, J.; Asai, K.; Maeda-Yamamoto, M.; Kawakami, Y.; Kikkawa, U.; Kawakami, T. Akt-dependent cytokine production in mast cells. J. Exp. Med. 2000, 192, 729–739. [Google Scholar] [CrossRef]
- Haenisch, B.; Walstab, J.; Herberhold, S.; Bootz, F.; Tschaikin, M.; Ramseger, R.; Bönisch, H. Alpha-adrenoceptor agonistic activity of oxymetazoline and xylometazoline. Fundam. Clin. Pharmacol. 2010, 24, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Wang, J.L.; Liu, C.D.; Song, Y.; Zhang, S.W.; Zi, J.C.; Zhan, J.X.; Masilamani, M.; Cox, A.; Nowak-Wegrzyn, A.; et al. Berberine and limonin suppress IgE production by human B cells and peripheral blood mononuclear cells from food-allergic patients. Ann. Allergy Asthma Immunol. 2014, 113, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.J.; Pei, J.X.; Zeng, X.; Yang, Y.X.; Zhang, Y.M.; Li, F.L.; Deng, Y. Phellopterin alleviates atopic dermatitis-like inflammation and suppresses IL-4-induced STAT3 activation in keratinocytes. Int. Immunopharmacol. 2022, 112, 109270. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chang, C.W.; Wang, C.C.; Chang, M.S.; Yang, L.L. Byakangelicol, isolated from Angelica dahurica, inhibits both the activity and induction of cyclooxygenase-2 in human pulmonary epithelial cells. J. Pharm. Pharmacol. 2002, 54, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, N.; Shim, W.S. Caffeic acid phenethyl ester inhibits pseudo-allergic reactions via inhibition of MRGPRX2/MrgprB2-dependent mast cell degranulation. Arch. Pharm. Res. 2022, 45, 644–657. [Google Scholar] [CrossRef]
- Murata, K.; Deguchi, T.; Fujita, T.; Matsuda, H. Improvement in blood fluidity by Kaempferia parviflora rhizome. J. Nat. Med. 2013, 67, 719–724. [Google Scholar] [CrossRef]
- Lee, E.J.; Ji, G.E.; Sung, M.K. Quercetin and kaempferol suppress immunoglobulin E-mediated allergic inflammation in RBL-2H3 and Caco-2 cells. Inflamm. Res. 2010, 59, 847–854. [Google Scholar] [CrossRef]
- Han, S.W.; Sun, L.; He, F.; Che, H.L. Anti-allergic activity of glycyrrhizic acid on IgE-mediated allergic reaction by regulation of allergy-related immune cells. Sci. Rep. 2017, 7, 7222. [Google Scholar] [CrossRef]
- Han, L.L.; Sakane, I.; Mizuno, M. Synergistic anti-allergy activity using a combination of Enterococcus faecalis IC-1 and luteolin. Food Biosci. 2021, 41, 100924. [Google Scholar] [CrossRef]
- Koval’Skii, I.V.; Krasnyuk, I.I.; Krasnyuk, I.I., Jr.; Nikulina, O.I.; Belyatskaya, A.V.; Kharitonov, Y.Y.; Feldman, N.B.; Lutsenko, S.V. Mechanisms of rutin pharmacological action (review). Pharm. Chem. J. 2014, 48, 73–76. [Google Scholar] [CrossRef]
- Takasugi, M.; Shimada, K.; Yamada, K.; Arai, H. Effects of soybean isoflavones on the release of chemical mediators from rat peritoneal exudate cells by allergic reaction in Vitro. Food Sci. Technol. Res. 2014, 20, 725–729. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Yan, Y.X.; Li, H.L.; Feng, Y.R.; Huang, C.; Fan, S.J. Nomilin and its analogues in citrus fruits: A review of its health promotion effects and potential application in medicine. Molecules 2023, 28, 269. [Google Scholar] [CrossRef]
- Chen, Y.T.; Guo, S.; Chen, G.R.; Liu, C.; Zhang, M.B.; Wang, X.B. Nomilin attenuates lipopolysaccharide-Induced inflammatory response by binding with myeloid differentiation protein-2. Comb. Chem. High Throughput Screen. 2023, 26, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Parise, R.; Pastrello, M.; Camerlingo, C.E.P.; Silva, G.J.; Agostinho, L.A.; de Souza, T.; Magri, F.M.M.; Ribeiro, R.R.; Brandt, C.A.; Polli, M.C. The anti-inflammatory activity of dillapiole and some semisynthetic analogues. Pharm. Biol. 2011, 49, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.B.; Kreutz, T.; Limberger, R.P.; da Veiga, V.F.; Koester, L.S. Development, validation and application of a gas chromatography method for the determination of dillapiole from Piper aduncum essential oil in skin permeation samples. Biomed. Chromatogr. 2023, 37, e5544. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Y.; Wang, S.W.; Ma, J.N.; Yu, J.; Yue, X.; Zhang, Y.; Wang, X.Q. Screening of metabolic markers present in Oxytropis by UHPLC-Q-TOF/MS and preliminary pharmacophylogenetic investigation. Front. Plant Sci. 2022, 13, 958460. [Google Scholar] [CrossRef]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.A.; Iqbal, M.; Anwer, M.K.; Al Hoshani, A.R.; Attia, S.M.; Ahmad, S.F. Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-κB activation against LPS-induced acute lung injury in mice. Pharmacol. Res. 2015, 102, 1–11. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, J.Y.; Hong, S.H.; Lee, J.Y. Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-κB activation in mouse peritoneal macrophages. Am. J. Chin. Med. 2011, 39, 171–181. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, K.; Duraisamy, K.; Allam, A.A.; Ajarem, J.; Chow, B.K.C. Protective effect of genistein against compound 48/80 induced anaphylactoid shock via inhibiting Mas related G protein-coupled receptor X2 (MRGPRX2). Molecules 2020, 25, 1028. [Google Scholar] [CrossRef] [PubMed]
- Camoretti-Mercado, B.; Lockey, R.F. The β-adrenergic theory of bronchial asthma: 50 years later. J. Allergy Clin. Immunol. 2019, 144, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.L.; Sun, M.H.; Yin, W.F.; Yang, L.; Kong, L.Y. Avicularin suppresses cartilage extracellular matrix degradation and inflammation via TRAF6/MAPK activation. Phytomedicine 2021, 91, 153657. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.; Kwa, M.; Gold, L.S.; Lim, H.W. Janus kinase inhibitors in dermatology: Part I. A comprehensive review. J. Am. Acad. Dermatol. 2022, 86, 406–413. [Google Scholar] [CrossRef]
- Morbach, H.; Schickel, J.N.; Cunningham-Rundles, C.; Conley, M.E.; Reisli, I.; Franco, J.L.; Meffre, E. CD19 controls Toll-like receptor 9 responses in human B cells. J. Allergy Clin. Immunol. 2016, 137, 889–898. [Google Scholar] [CrossRef]
- Pogue, S.L.; Kurosaki, T.; Bolen, J.; Herbst, R. B cell antigen receptor-induced activation of Akt promotes B cell survival and is dependent on Syk kinase. J. Immunol. 2000, 165, 1300–1306. [Google Scholar] [CrossRef]
- Mao, H.M.; Zhao, X.C.; Sun, S.C. NF-κB in inflammation and cancer. Cell. Mol. Immunol. 2025, 22, 811–839. [Google Scholar] [CrossRef]
- Zindel, D.; Mensat, P.; Vol, C.; Homayed, Z.; Charrier-Savournin, F.; Trinquet, E.; Banères, J.L.; Pin, J.P.; Pannequin, J.; Roux, T.; et al. G protein-coupled receptors can control the Hippo/YAP pathway through Gq signaling. FASEB J. 2021, 35, e21668. [Google Scholar] [CrossRef]
- Leach, K.; Hannan, F.M.; Josephs, T.M.; Keller, A.N.; Moller, T.C.; Ward, D.T.; Kallay, E.; Mason, R.S.; Thakker, R.V.; Riccardi, D.; et al. International Union of Basic and Clinical Pharmacology. CVIII. Calcium-Sensing Receptor Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2020, 72, 558–604. [Google Scholar] [CrossRef]
- Albert, A.P.; Greenberg, H.Z.E. Role of the calcium-sensing receptor in regulating vascular function. J. Cell Commun. Signal 2025, 19, e70004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, P.; He, M.; Hong, L.; Cao, Q. Hyphenated chromatography detection and compound-target-disease investigation on herb-pair Chuanxiong Rhizoma—Xiangfu Rhizoma. J. Ethnopharmacol. 2019, 243, 112125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Zhang, D.F.; Wang, B.Y.; Zhao, W.B.; Zhang, T.Y.; Sutcharitchan, C.; Li, S. Network pharmacology: Advancing the application of large language models in traditional Chinese medicine research. Sci. Tradit. Chin. Med. 2025, 3, 113–123. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. Swiss Target Prediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Jiao, X.; Sherman, B.T.; Huang, D.W.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. DAVID-WS: A stateful web service to facilitate gene/protein list analysis. Bioinformatics 2012, 28, 1805–1806. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 2024, 53, D672–D677. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Van der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Tian, L. Sobtop 1.0 (dev5). Available online: http://sobereva.com/soft/Sobtop/ (accessed on 10 January 2025).
- Wang, J.M.; Cieplak, P.; Kollman, P.A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 2000, 21, 1049–1074. [Google Scholar] [CrossRef]

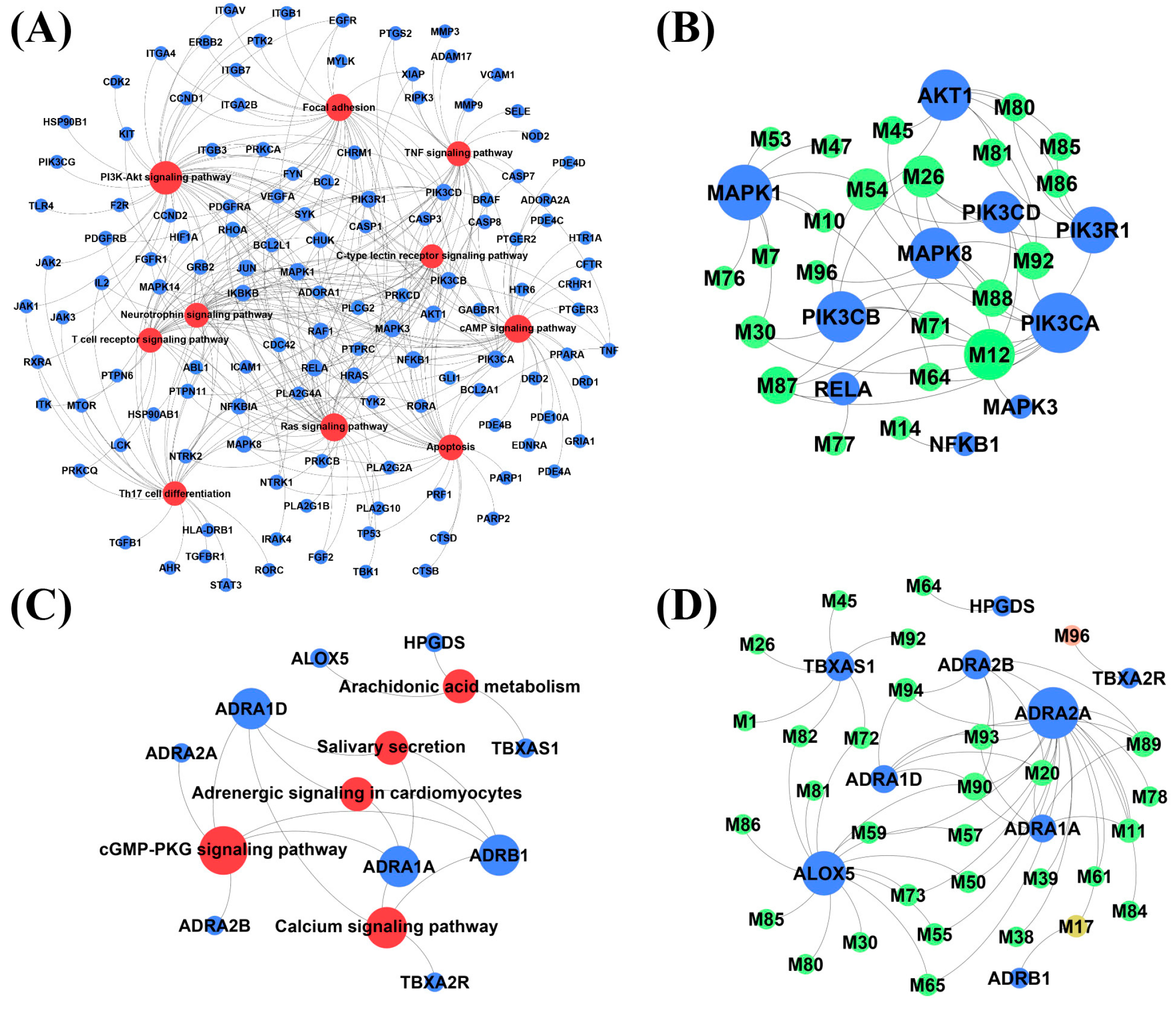
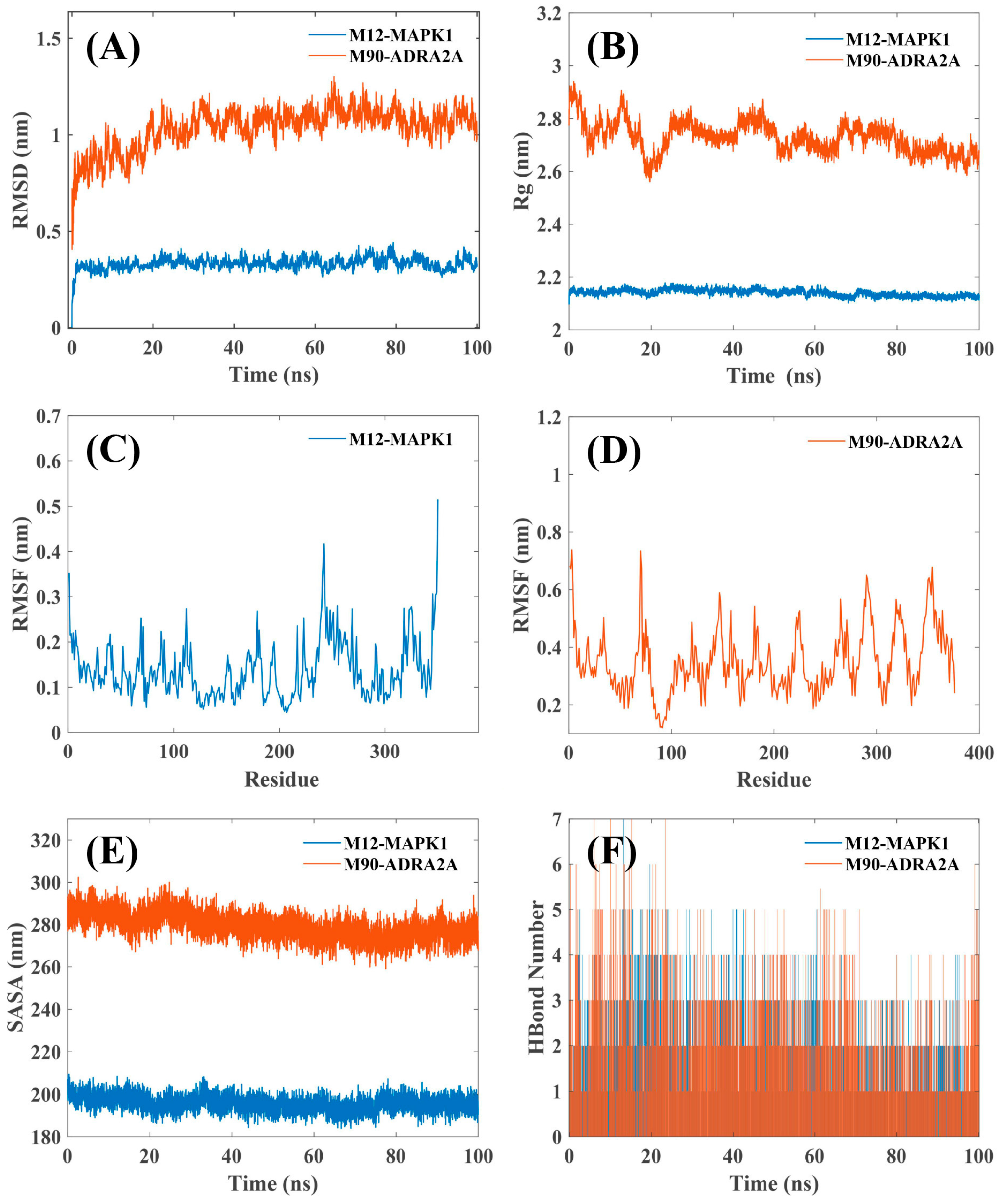
| No.-Unique | Gene Nane | Degree | No.-Common | Gene Nane | Degree |
|---|---|---|---|---|---|
| 1 | Mitogen-activated protein kinase 1 (MAPK1) | 9 | 1 | Alpha-1D adrenergic receptor (ADRA1D) | 4 |
| 2 | Mitogen-activated protein kinase 3 (MAPK3) | 9 | 2 | Beta-1 adrenergic receptor (ADRB1) | 4 |
| 3 | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (PIK3CD) | 9 | 3 | Alpha-1A adrenergic receptor (ADRA1A) | 4 |
| 4 | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PIK3CB) | 9 | 4 | Alpha-2B adrenergic receptor (ADRA2B) | 1 |
| 5 | RAC-alpha serine/threonine-protein kinase (AKT1) | 8 | 5 | Alpha-2A adrenergic receptor (ADRA2A) | 1 |
| 6 | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA) | 8 | 6 | Hematopoietic prostaglandin D synthase (HPGDS) | 1 |
| 7 | Phosphatidylinositol 3-kinase regulatory subunit alpha (PIK3R1) | 8 | 7 | Polyunsaturated fatty acid 5-lipoxygenase (ALOX5) | 1 |
| 8 | Transcription factor p65 (RELA) | 8 | 8 | Thromboxane-A synthase (TBXAS1) | 1 |
| 9 | Nuclear factor NF-kappa-B p105 subunit (NFKB1) | 8 | 9 | Thromboxane A2 receptor (TBXA2R) | 1 |
| 10 | Mitogen-activated protein kinase 8 (MAPK8) | 8 |
| No.-Unique | Mol ID | Degree | Compounds | No.-Common | Mol ID | Degree | Compounds |
|---|---|---|---|---|---|---|---|
| 1 | M12 | 7 | Lyoniresinol | 1 | M90 | 5 | Naringin-3-O-(3-hydroxy-3-methylglutarate)-glucoside |
| 2 | M54 | 5 | Limonin | 2 | M93 | 4 | Glycyrrhizic acid |
| 3 | M26 | 5 | 7-Acetoxy-2-methylisoflavone | 3 | M20 | 4 | Heptopyranosides |
| 4 | M92 | 4 | Apiole | 4 | M89 | 4 | Licorice-saponin G2 |
| 5 | M88 | 4 | Phellopterin | 5 | M94 | 3 | Uralsaponin A |
| 6 | M87 | 4 | Byakangelicol | 6 | M11 | 3 | Phenylalanine |
| 7 | M30 | 3 | Caffeic acid | 7 | M17 | 2 | Tryptophan |
| 8 | M64 | 2 | Nomilin | 8 | M73 | 2 | Diosmin |
| 9 | M71 | 2 | Naringenin | 9 | M50 | 2 | Luteolin-7-O-β-D-glucoside |
| 10 | M45 | 2 | Dillapiol | 10 | M59 | 2 | Hyperoside |
| 11 | M86 | 2 | Nortangeretin | 11 | M55 | 2 | Leucoside |
| 12 | M80 | 2 | Luteolin | 12 | M65 | 2 | Kaempferol 3-O-robinobioside |
| 13 | M81 | 2 | Kaempferol | 13 | M57 | 2 | Rutin |
| 14 | M85 | 2 | Quercetin | 14 | M72 | 2 | Genistein |
| 15 | M82 | 2 | Daidzein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, L.; Qin, Y.; Ip, C.; Xu, W.; Zeng, H.; Duan, X.; Wang, J.; Zhao, J.; Wang, Q.; Li, S. Characterization and Anti-Allergic Mechanisms of Bioactive Compounds in a Traditional Chinese Medicine Prescription Using UHPLC-Q-TOF-MS/MS, Network Pharmacology and Computational Simulations. Pharmaceuticals 2025, 18, 1444. https://doi.org/10.3390/ph18101444
Hong L, Qin Y, Ip C, Xu W, Zeng H, Duan X, Wang J, Zhao J, Wang Q, Li S. Characterization and Anti-Allergic Mechanisms of Bioactive Compounds in a Traditional Chinese Medicine Prescription Using UHPLC-Q-TOF-MS/MS, Network Pharmacology and Computational Simulations. Pharmaceuticals. 2025; 18(10):1444. https://doi.org/10.3390/ph18101444
Chicago/Turabian StyleHong, Liang, You Qin, Chiwai Ip, Wenfei Xu, Haoxuan Zeng, Xiu Duan, Ji Wang, Jing Zhao, Qi Wang, and Shaoping Li. 2025. "Characterization and Anti-Allergic Mechanisms of Bioactive Compounds in a Traditional Chinese Medicine Prescription Using UHPLC-Q-TOF-MS/MS, Network Pharmacology and Computational Simulations" Pharmaceuticals 18, no. 10: 1444. https://doi.org/10.3390/ph18101444
APA StyleHong, L., Qin, Y., Ip, C., Xu, W., Zeng, H., Duan, X., Wang, J., Zhao, J., Wang, Q., & Li, S. (2025). Characterization and Anti-Allergic Mechanisms of Bioactive Compounds in a Traditional Chinese Medicine Prescription Using UHPLC-Q-TOF-MS/MS, Network Pharmacology and Computational Simulations. Pharmaceuticals, 18(10), 1444. https://doi.org/10.3390/ph18101444







