Divergent Hepatic Outcomes of Chronic Ketone Supplementation: Ketone Salts Preserve Liver Health While Ketone Esters and Precursors Drive Inflammation and Steatosis
Abstract
1. Introduction
2. Results
2.1. Histological Analysis Revealed Formulation-Specific Effects on Liver Structure and Markers of Hepatic Inflammation
2.2. Red Blood Cell Area Is Significantly Elevated in 1,3-Butanediol (BD) and KSMCT Groups
2.3. Lipid Accumulation Significantly Increased by KE and MCT Treatments
2.4. Increased TNF-α Levels in BD, KE and MCT Groups, While Decreased Levels in KS and KSMCT Groups
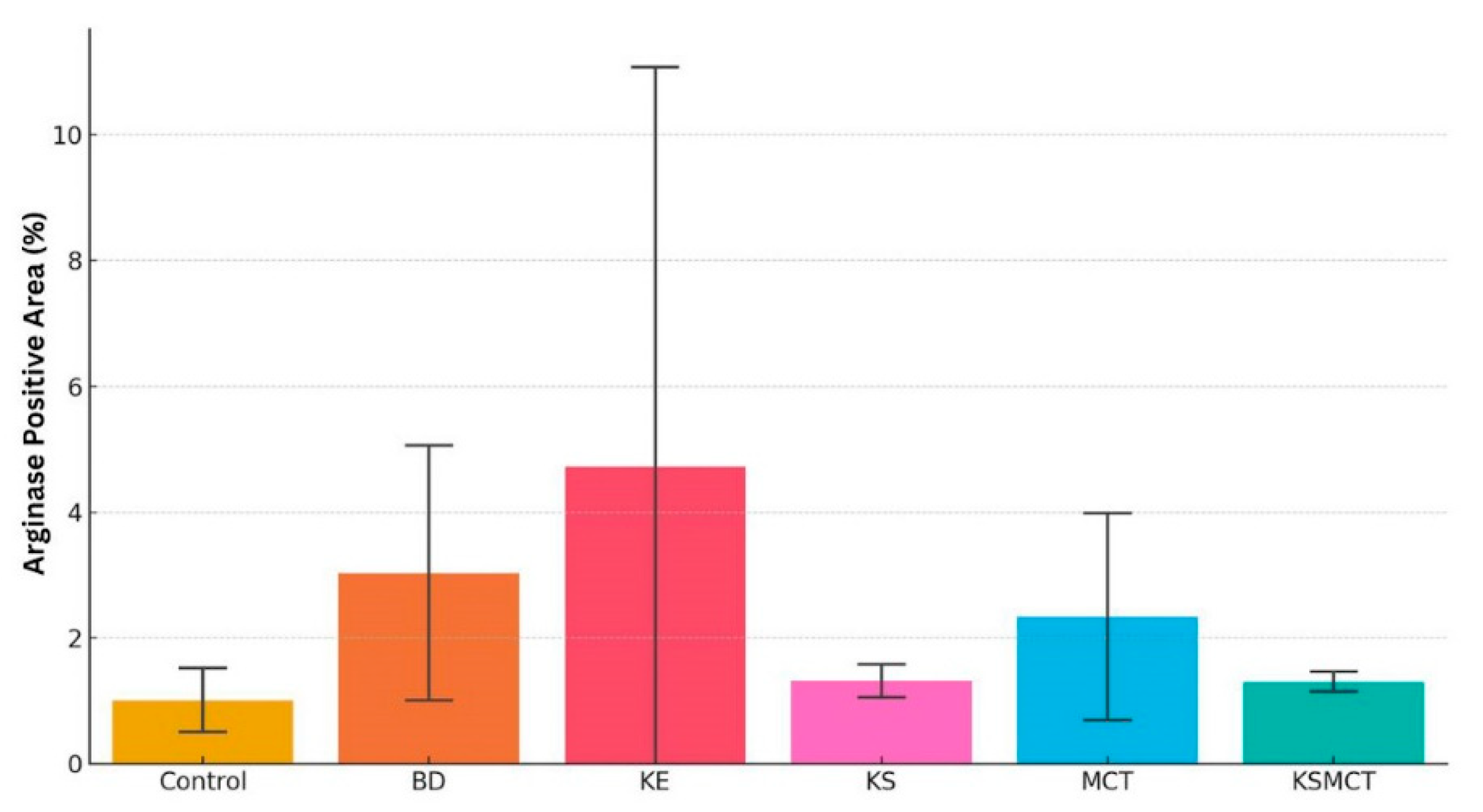
2.5. Elevated Arginase Level in BD and KE Groups
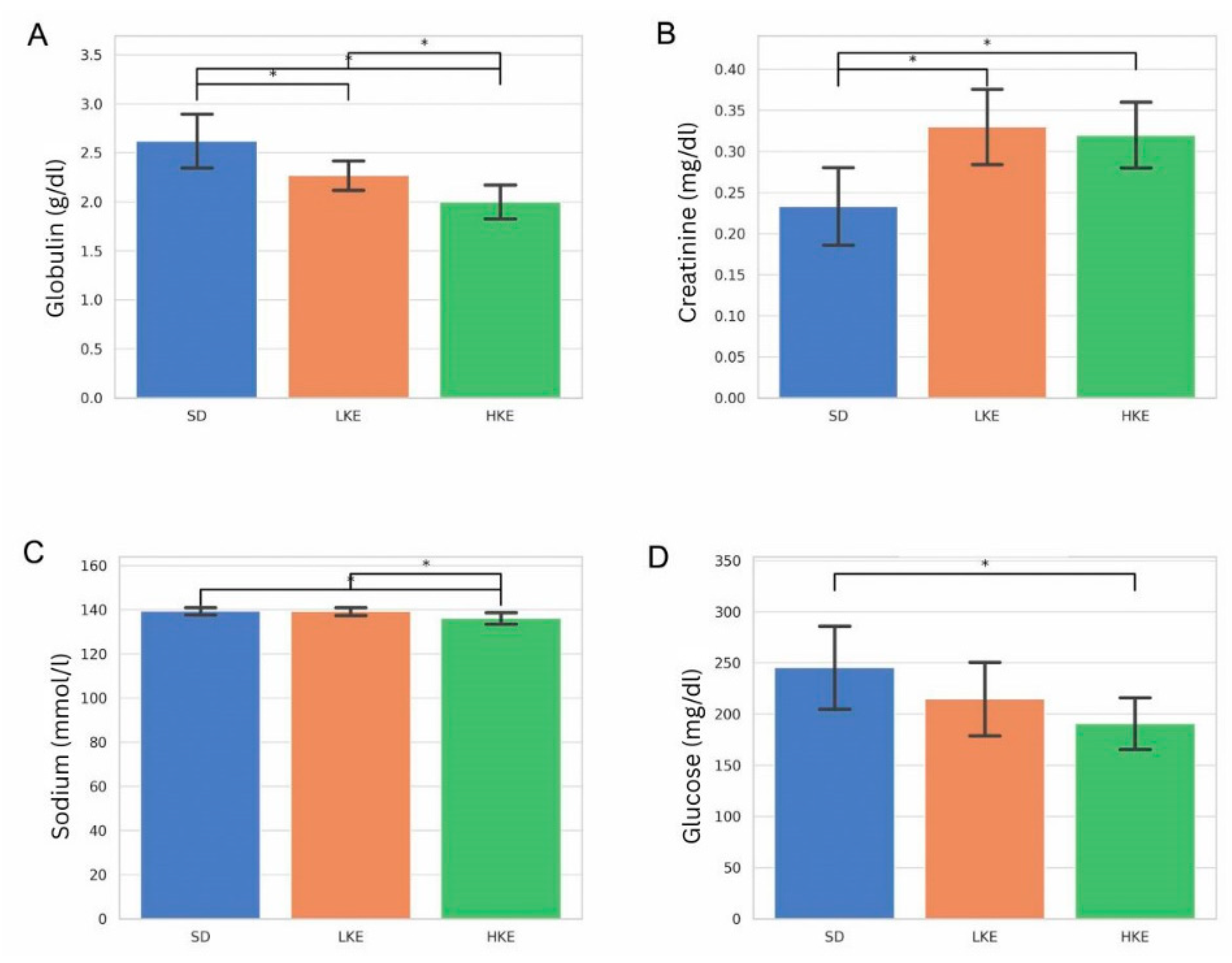
2.6. Blood Chemistry Analysis Revealed Significant Group-Level Differences in Key Biomarkers
3. Discussion
4. Materials and Methods
4.1. Animal Model and Treatment Protocol
4.2. Liver Tissue Collection and Processing
4.3. Hematoxylin and Eosin (H&E) Staining
4.4. Histological Image Analysis
4.5. Immunohistochemistry for TNF-α and Arginase
4.6. Immunohistochemical Staining Quantification for TNF-α and Arginase
4.7. Blood Chemistry Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Courchesne-Loyer, A.; St-Pierre, V.; Vandenberghe, C.; Pierotti, T.; Fortier, M.; Croteau, E.; Castellano, C.A. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2016, 1367, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; McClure, T.S.; Koutnik, A.P.; Egan, B. Exogenous ketone supplements in athletic contexts: Past, present, and future. Sports Med. 2022, 52 (Suppl. S1), 25–67. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Santer, P.; Miller, J.J.; Faull, O.K.; Magor-Elliott, S.; Hiyama, S.; Stirling, M.; Clarke, K. On the metabolism of exogenous ketones in humans. Front. Physiol. 2017, 8, 848. [Google Scholar] [CrossRef] [PubMed]
- van Rijt, W.J.; Van Hove, J.L.K.; Vaz, F.M.; Havinga, R.; Allersma, D.P.; Zijp, T.R.; Bedoyan, J.K.; Heiner-Fokkema, M.R.; Reijngoud, D.J.; Geraghty, M.T.; et al. Enantiomer-specific pharmacokinetics of D,L-3-hydroxybutyrate: Implications for the treatment of multiple acyl-CoA dehydrogenase deficiency. J. Inherit. Metab. Dis. 2021, 44, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Kesl, S.L.; Poff, A.M.; Ward, N.P.; Fiorelli, T.N.; Ari, C.; Van Putten, A.J.; Sherwood, J.W.; Arnold, P.; D’Agostino, D.P. Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague-Dawley rats. Nutr. Metab. 2016, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Myette-Côté, É.; Caldwell, H.G.; Ainslie, P.N.; Clarke, K.; Little, J.P. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 1491–1501. [Google Scholar] [CrossRef]
- Ari, C.; Murdun, C.; Goldhagen, C.; Koutnik, A.P.; Bharwani, S.R.; Diamond, D.M.; Kindy, M.; D’Agostino, D.P.; Kovacs, Z. Exogenous ketone supplements improved motor performance in preclinical rodent models. Nutrients 2020, 12, 2459. [Google Scholar] [CrossRef] [PubMed]
- Ari, C.; Kovács, Z.; Juhasz, G.; Murdun, C.; Goldhagen, C.; Koutnik, A.; Kesl, S.; D’Agostino, D. Exogenous ketone supplements reduce anxiety-related behavior in Sprague-Dawley and Wistar Albino Glaxo/Rijswijk rats. Front. Mol. Neurosci. 2016, 9, 137. [Google Scholar] [CrossRef]
- Ari, C.; Kovács, Z.; Murdun, C.; Koutnik, A.; Goldhagen, C.; Rogers, C.; Diamond, D.; D’Agostino, D. Nutritional ketosis delays the onset of isoflurane-induced anesthesia. BMC Anesthesiol. 2018, 18, 54. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Y.; Kou, X.; Jing, Y.; Sun, K.; Sheng, D.; Yu, G.; Yu, D.; Zhao, Q.; Zhao, X.; et al. The protective or damaging effect of tumor necrosis factor-α in acute liver injury is concentration-dependent. Cell Biosci. 2016, 6, 8. [Google Scholar] [CrossRef]
- Wang, H.; Mehal, W.; Nagy, L.E.; Rotman, Y. Immunological mechanisms and therapeutic targets of fatty liver diseases. Cell Mol. Immunol. 2021, 18, 73–91. [Google Scholar] [CrossRef]
- Caldwell, R.W.; Rodriguez, P.C.; Toque, H.A.; Narayanan, S.P.; Caldwell, R.B. Arginase: A multifaceted enzyme important in health and disease. Physiol. Rev. 2018, 98, 641–665. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Ren, Y.; Huang, Y.; Liu, W.; Lv, Z.; Qian, L.; Yu, Y.; Xiong, Y. Arginase: Shedding light on the mechanisms and opportunities in cardiovascular diseases. Cell Death Discov. 2022, 8, 413. [Google Scholar] [CrossRef]
- Gopalasingam, N.; Moeslund, N.; Christensen, K.H.; Berg-Hansen, K.; Seefeldt, J.; Homilius, C.; Nielsen, E.N.; Dollerup, M.R.; Alstrup Olsen, A.K.; Johannsen, M.; et al. Enantiomer-Specific Cardiovascular Effects of the Ketone Body 3-Hydroxybutyrate. J. Am. Heart Assoc. 2024, 13, e033628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takahashi, Y.; Fukusato, T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 15539–15548. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Neudorf, H.; Islam, H.; Falkenhain, K.; Oliveira, B.; Jackson, G.S.; Moreno-Cabañas, A.; Madden, K.; Singer, J.; Walsh, J.J.; Little, J.P. Effect of the ketone beta-hydroxybutyrate on markers of inflammation and immune function in adults with type 2 diabetes. Clin. Exp. Immunol. 2024, 216, 89–103. [Google Scholar] [CrossRef]
- Kelty, T.J.; Krause, A.A.; Rector, R.S. Ketone metabolites in metabolic dysfunction-associated steatotic liver disease progression: Optimizing keto-therapeutic strategies. Am. J. Physiol. Endocrinol. Metab. 2025, 329, E290–E301. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, E.P. Hepatic metabolism and ketone production in metabolic dysfunction-associated steatotic liver disease. Curr. Opin. Gastroenterol. 2025, 41, 81–86. [Google Scholar] [CrossRef]
- Kelty, T.J.; Kerr, N.R.; Chou, C.H.; Shryack, G.E.; Taylor, C.L.; Krause, A.A.; Knutson, A.R.; Bunten, J.; Childs, T.E.; Meers, G.M.; et al. Cognitive impairment caused by compromised hepatic ketogenesis is prevented by endurance exercise. J. Physiol. 2025. [Google Scholar] [CrossRef]
- Nelson, A.B.; Queathem, E.D.; Puchalska, P.; Crawford, P.A. Metabolic messengers: Ketone bodies. Nat. Metab. 2023, 5, 2062–2074. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Hao, S.; Wang, J.; Chen, W.; Dai, S.; Hou, Z.; Chen, B.; Zhang, Y.; Liu, D. Inversed albumin-to-globulin ratio and underlying liver disease severity as a prognostic factor for survival in hepatocellular carcinoma patients undergoing transarterial chemoembolization. Diagn. Interv. Radiol. 2023, 29, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Ávila, M.; Mora Sánchez, M.G.; Bernal Amador, A.S.; Paniagua, R. The metabolism of creatinine and its usefulness to evaluate kidney function and body composition in clinical practice. Biomolecules 2025, 15, 41. [Google Scholar] [CrossRef]
- Ari, C.; Murdun, C.; Koutnik, A.; Goldhagen, C.; Rogers, C.; Park, C.; Bharwani, S.; Diamond, D.; Kindy, M.; D’Agostino, D.; et al. Exogenous ketones lower blood glucose level in rested and exercised rodent models. Nutrients 2019, 11, 2330. [Google Scholar] [CrossRef]
- Falkenhain, K. Ketones and insulin: A paradoxical interplay with implications for glucose metabolism. J. Endocr. Soc. 2025, 9, bvaf101. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Gao, W.; Loor, J.J.; Aboragah, A.; Fang, Z.; Du, X.; Zhang, M.; Song, Y.; Liu, G.; Li, X. Reducing hepatic endoplasmic reticulum stress ameliorates the impairment in insulin signaling induced by high levels of β-hydroxybutyrate in bovine hepatocytes. J. Dairy. Sci. 2021, 104, 12845–12858. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Shippy, D.C.; Wilhelm, C.; Viharkumar, P.A.; Raife, T.J.; Ulland, T.K. β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflamm. 2020, 17, 280. [Google Scholar] [CrossRef]
- Wakana, H.; Kono, H.; Fukushima, H.; Nakata, Y.; Akazawa, Y.; Maruyama, S.; Hagio, K.; Fujii, H.; Ichikawa, D. Effects of medium-chain triglycerides administration in chemically-induced carcinogenesis in mice. Anticancer. Res. 2019, 39, 6653–6660. [Google Scholar] [CrossRef] [PubMed]
- Mourad, S.; Abdualkader, A.M.; Li, X.; Jani, S.; Ceddia, R.B.; Al Batran, R. A high-fat diet supplemented with medium-chain triglycerides ameliorates hepatic steatosis by reducing ceramide and diacylglycerol accumulation in mice. Exp. Physiol. 2024, 109, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.; Bargut, T.C.L.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Medium-chain triglyceride reinforce the hepatic damage caused by fructose intake in mice. Prostaglandins Leukot. Essent. Fatty Acids 2019, 140, 64–71. [Google Scholar] [CrossRef]
- Anekwe, C.V.; Chandrasekaran, P.; Stanford, F.C. Ketogenic diet-induced elevated cholesterol, elevated liver enzymes and potential non-alcoholic fatty liver disease. Cureus 2020, 12, e6605. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.; Sankar, P.; Varacallo, M.A. Physiology, blood plasma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531504/ (accessed on 15 February 2025).
- Ravaut, G.; Carneiro, A.; Mounier, C. Exploring the impacts of ketogenic diet on reversible hepatic steatosis: Initial analysis in male mice. Front. Nutr. 2024, 11, 1290540. [Google Scholar] [CrossRef]
- Ari, C.; D’Agostino, D.P.; Cha, B.J. Neuroregeneration Improved by Sodium-D,L-Beta-Hydroxybutyrate in Primary Neuronal Cultures. Pharmaceuticals 2024, 17, 1160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poffé, C.; Ramaekers, M.; Bogaerts, S.; Hespel, P. Exogenous ketosis impacts neither performance nor muscle glycogen breakdown in prolonged endurance exercise. J. Appl. Physiol. 2020, 128, 1643–1653. [Google Scholar] [CrossRef]
- Emanuele, F.; Biondo, M.; Tomasello, L.; Arnaldi, G.; Guarnotta, V. Ketogenic Diet in Steatotic Liver Disease: A Metabolic Approach to Hepatic Health. Nutrients 2025, 17, 1269. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Iavicoli, I.; Di Paola, R.; Koverech, A.; Cuzzocrea, S.; Rizzarelli, E.; Calabrese, E.J. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim. Biophys. Acta 2012, 1822, 753–783. [Google Scholar] [CrossRef]
- Milder, J.B.; Patel, M. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. 2012, 100, 295–303. [Google Scholar] [CrossRef]
- Torres, J.A.; Holznecht, N.; Asplund, D.A.; Kroes, B.C.; Amarlkhagva, T.; Haeffner, M.M.; Sharpe, E.H.; Koestner, S.; Strubl, S.; Schimmel, M.F.; et al. β-Hydroxybutyrate recapitulates the beneficial effects of ketogenic metabolic therapy in polycystic kidney disease. iScience 2024, 27, 110773. [Google Scholar] [CrossRef] [PubMed]
- Mehlman, M.A.; Tobin, R.B.; Hahn, H.K.; Kleager, L.; Tate, R.L. Metabolic fate of 1,3-butanediol in the rat: Liver tissue slices metabolism. J. Nutr. 1971, 101, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Plecko, B.; Stoeckler-Ipsiroglu, S.; Schober, E.; Harrer, G.; Mlynarik, V.; Gruber, S.; Moser, E.; Moeslinger, D.; Silgoner, H.; Ipsiroglu, O. Oral β-hydroxybutyrate supplementation in two patients with hyperinsulinemic hypoglycemia: Monitoring of β-hydroxybutyrate levels in blood and cerebrospinal fluid, and in the brain by in vivo magnetic resonance spectroscopy. Pediatr. Res. 2002, 52, 301–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frye, G.D.; Chapin, R.E.; Vogel, R.A.; Mailman, R.B.; Kilts, C.D.; Mueller, R.A.; Breese, G.R. Effects of acute and chronic 1,3-butanediol treatment on central nervous system function: A comparison with ethanol. J. Pharmacol. Exp. Ther. 1981, 216, 306–314. [Google Scholar] [CrossRef] [PubMed]
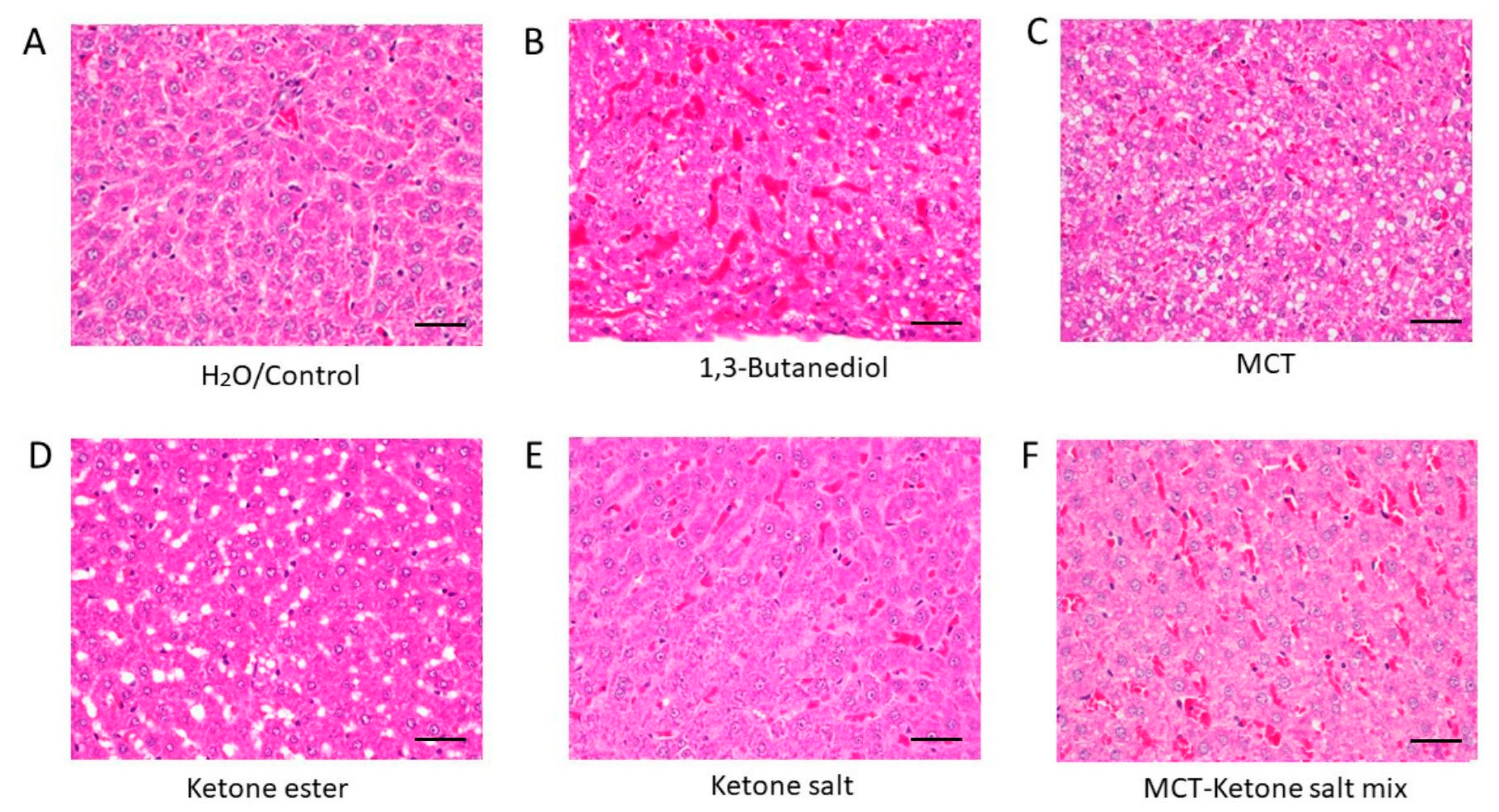
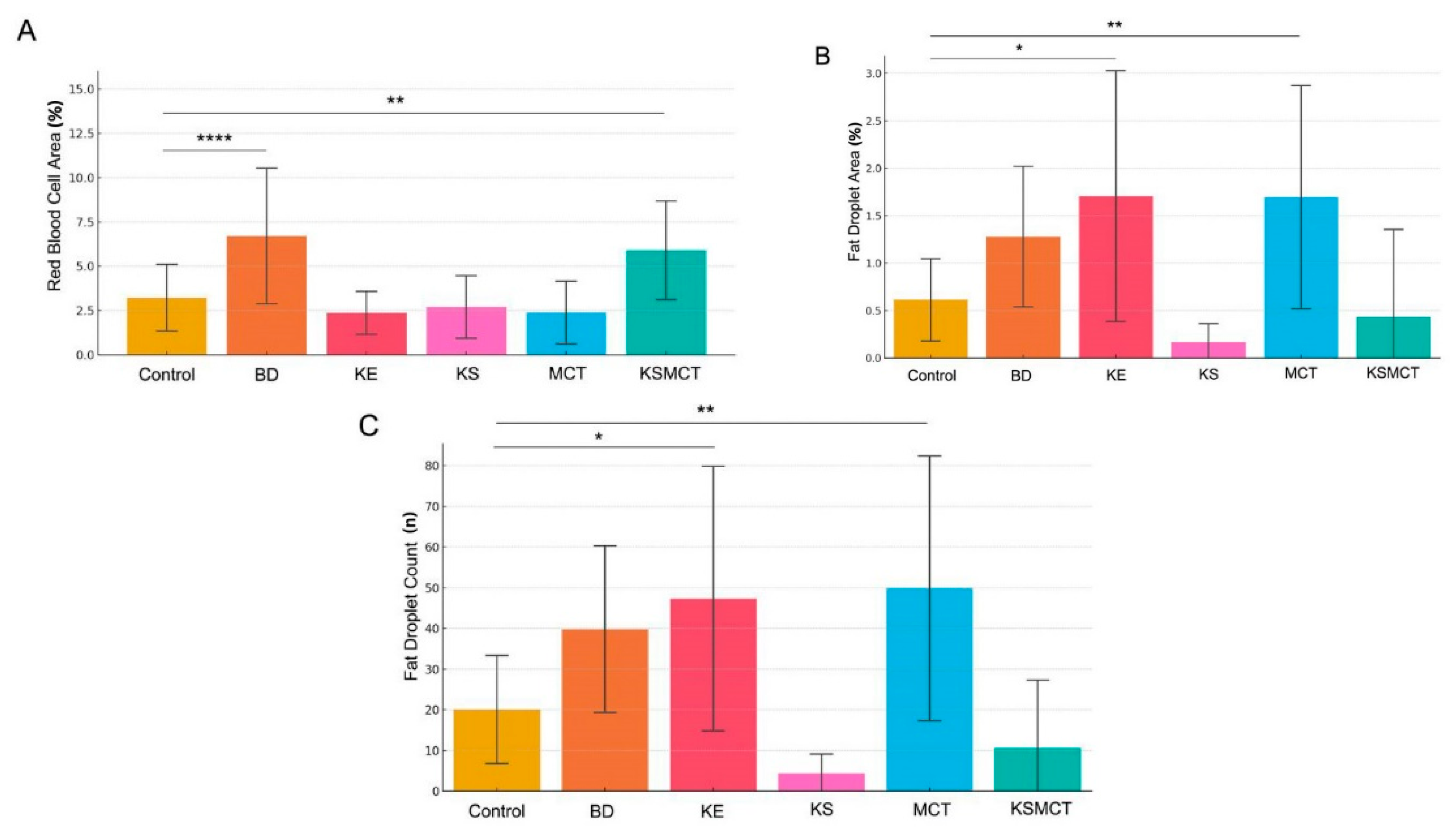
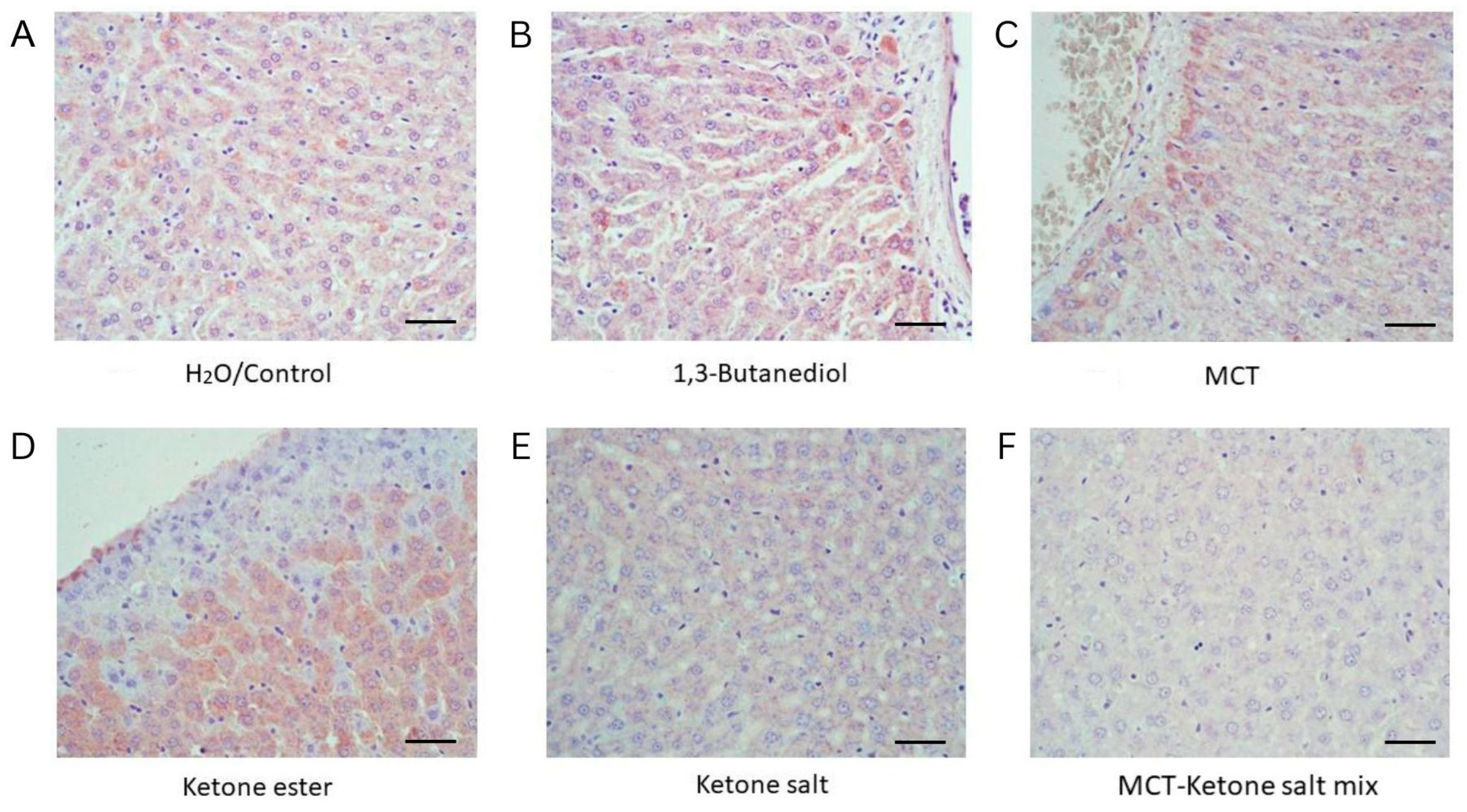
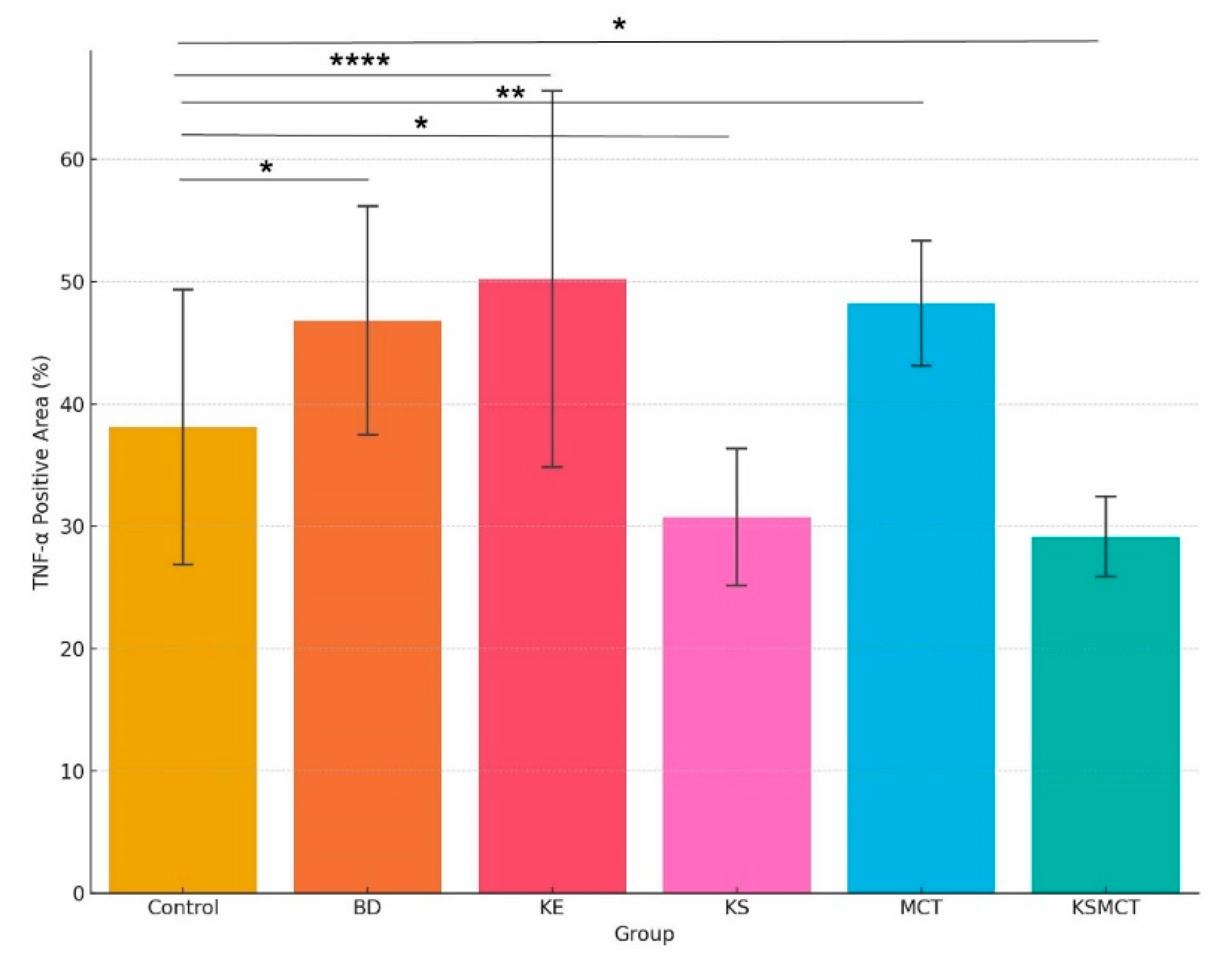
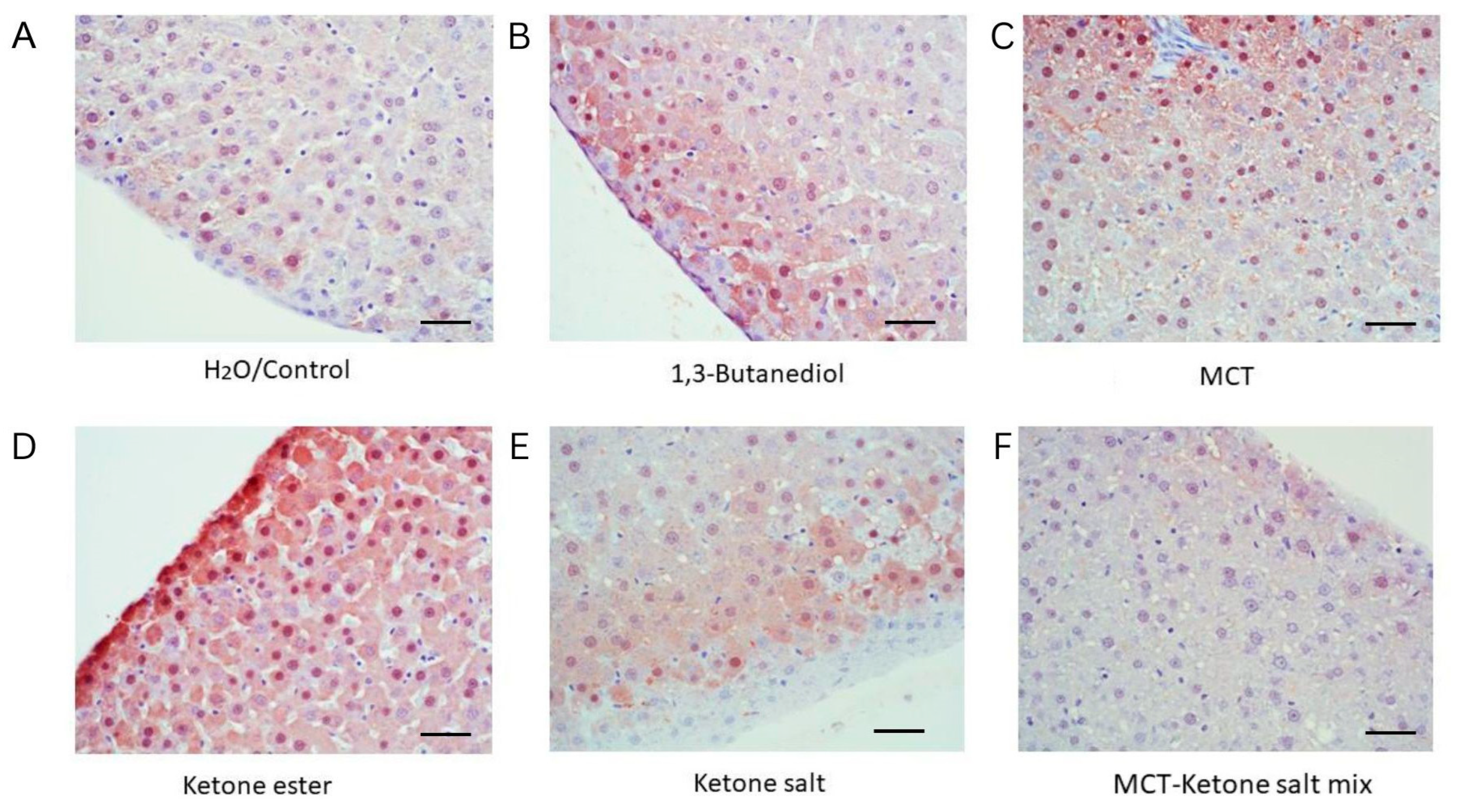
| Treatment | Fat Deposits | RBC Congestion | Histological Interpretation |
|---|---|---|---|
| H2O/Control | None | Minimal | Normal liver histology |
| 1,3-Butanediol | Lipid accumulation | High | Steatosis and hepatic stress |
| MCT | Ballooning lipid vacuoles | Low | Modest hepatic stress, steatosis |
| Ketone Ester | Macrovesicular steatosis, fat vacuoles | Low | Pronounced steatosis and hepatic stress |
| Ketone Salt | None | Low | Preserved structure; best overall histological profile |
| MCT–Ketone Salt Mix | Microvesicular steatosis | High | Early steatosis with moderate vascular changes |
| Treatment | TNF-α Expression | Interpretation |
|---|---|---|
| H2O/Control | Minimal | Baseline inflammatory state |
| 1,3-Butanediol | High | Strong hepatic inflammatory response |
| MCT | High | Immune activation likely due to lipid stress |
| Ketone Ester | High | Elevated inflammation, likely metabolic in origin |
| Ketone Salt | Low | Low inflammatory signal, well tolerated |
| Ketone Salt + MCT Mix | Low | Low inflammation |
| Treatment | Arginase Expression | Interpretation |
|---|---|---|
| H2O/Control | Low | Normal hepatic metabolic activity |
| 1,3-Butanediol | High | Elevated metabolic stress |
| MCT | Moderate | Moderate hepatic adaptation |
| Ketone Ester | High | High metabolic activity and possible stress |
| Ketone Salt | Low | Well tolerated with minimal hepatic activation |
| Ketone Salt + MCT Mix | Mild | Low hepatic activation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ari, C.; D’Agostino, D.P. Divergent Hepatic Outcomes of Chronic Ketone Supplementation: Ketone Salts Preserve Liver Health While Ketone Esters and Precursors Drive Inflammation and Steatosis. Pharmaceuticals 2025, 18, 1436. https://doi.org/10.3390/ph18101436
Ari C, D’Agostino DP. Divergent Hepatic Outcomes of Chronic Ketone Supplementation: Ketone Salts Preserve Liver Health While Ketone Esters and Precursors Drive Inflammation and Steatosis. Pharmaceuticals. 2025; 18(10):1436. https://doi.org/10.3390/ph18101436
Chicago/Turabian StyleAri, Csilla, and Dominic P. D’Agostino. 2025. "Divergent Hepatic Outcomes of Chronic Ketone Supplementation: Ketone Salts Preserve Liver Health While Ketone Esters and Precursors Drive Inflammation and Steatosis" Pharmaceuticals 18, no. 10: 1436. https://doi.org/10.3390/ph18101436
APA StyleAri, C., & D’Agostino, D. P. (2025). Divergent Hepatic Outcomes of Chronic Ketone Supplementation: Ketone Salts Preserve Liver Health While Ketone Esters and Precursors Drive Inflammation and Steatosis. Pharmaceuticals, 18(10), 1436. https://doi.org/10.3390/ph18101436








