Gedunin Mitigates Cutibacterium acnes-Induced Skin Inflammation by Inhibiting the NF-κB Pathway
Abstract
1. Introduction
2. Results
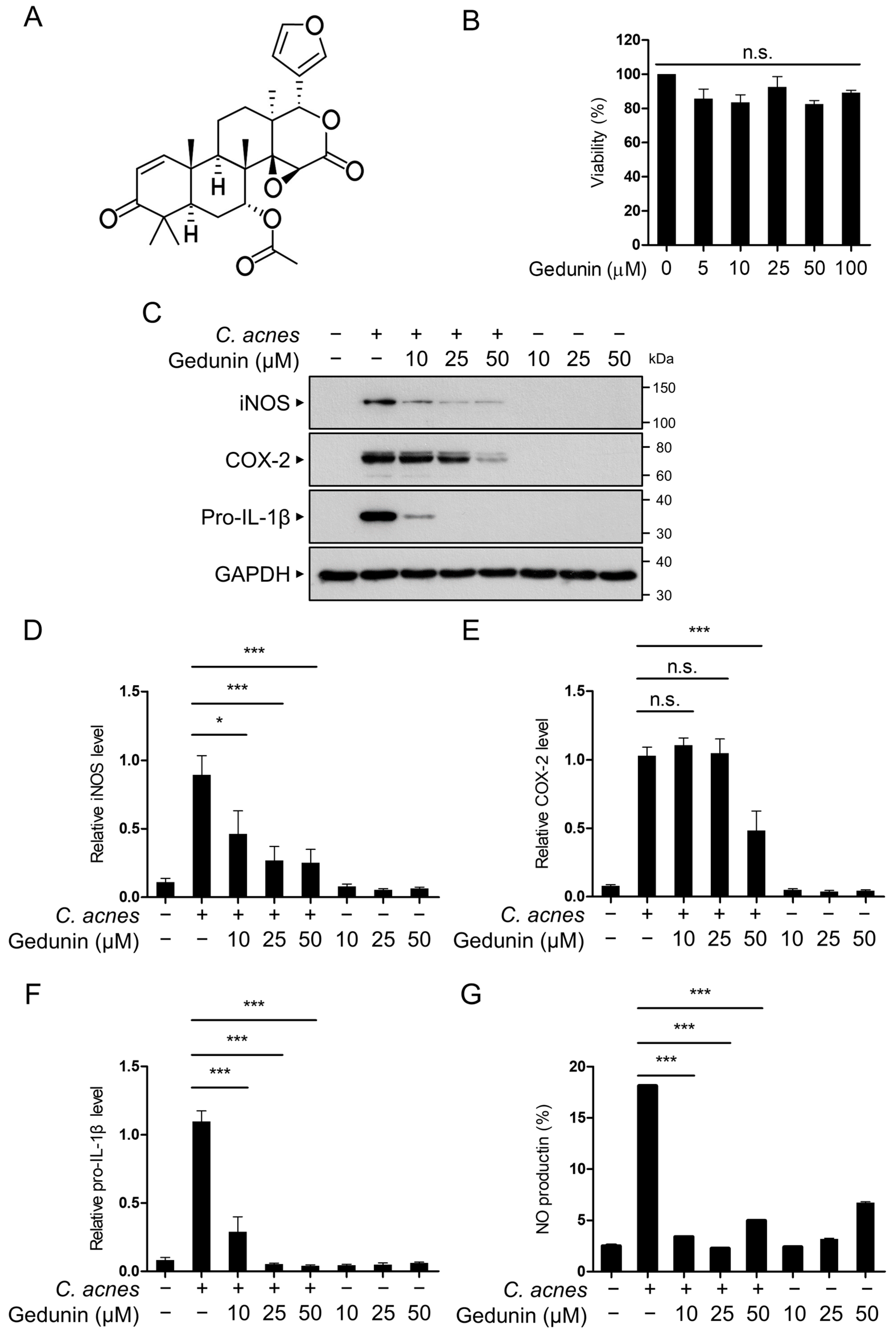
2.1. Gedunin Suppresses C. acnes-Induced Inflammation in RAW 264.7 Macrophages
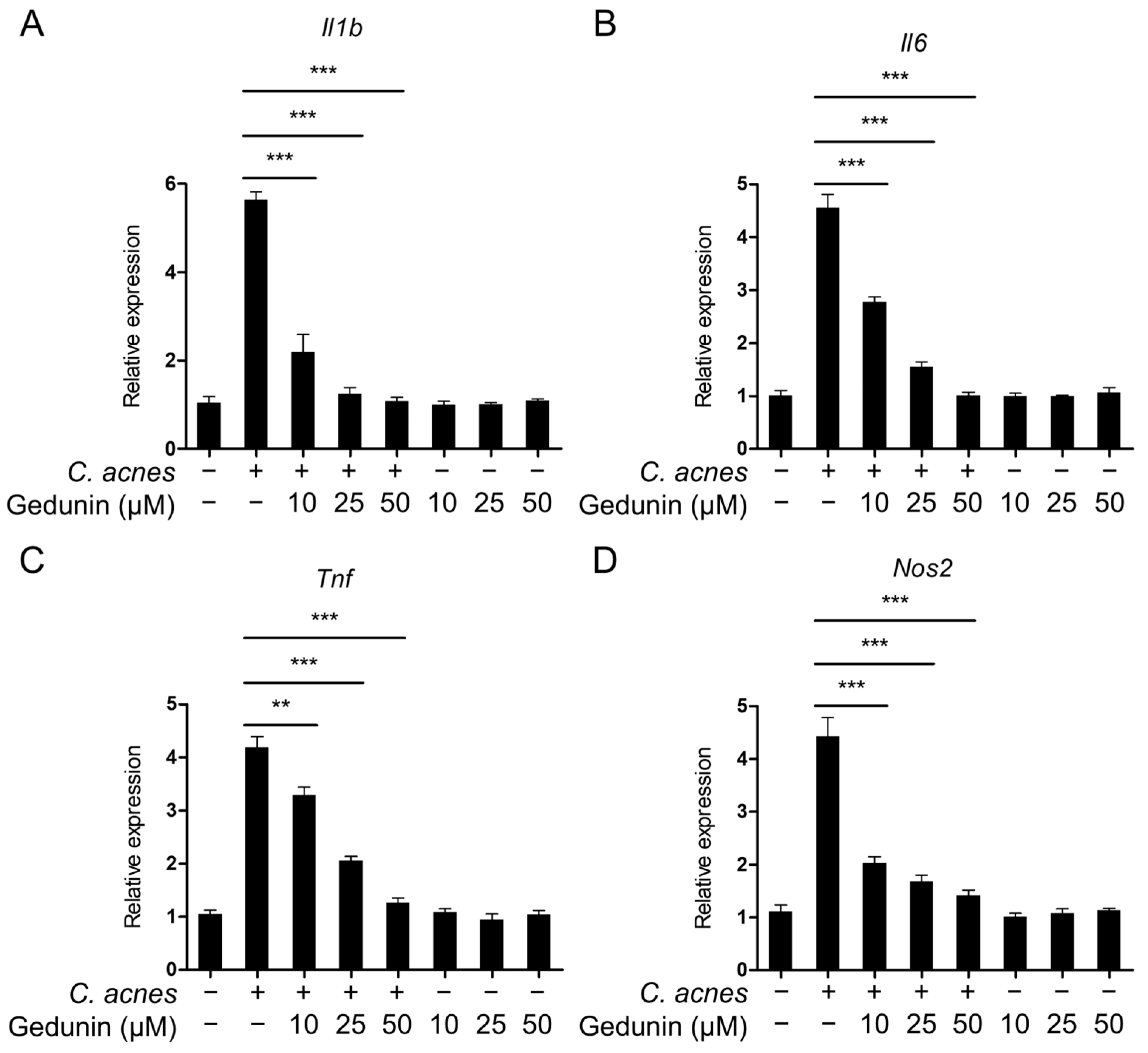
2.2. Gedunin Suppresses mRNA Expression of Inflammatory Mediators Induced by C. acnes
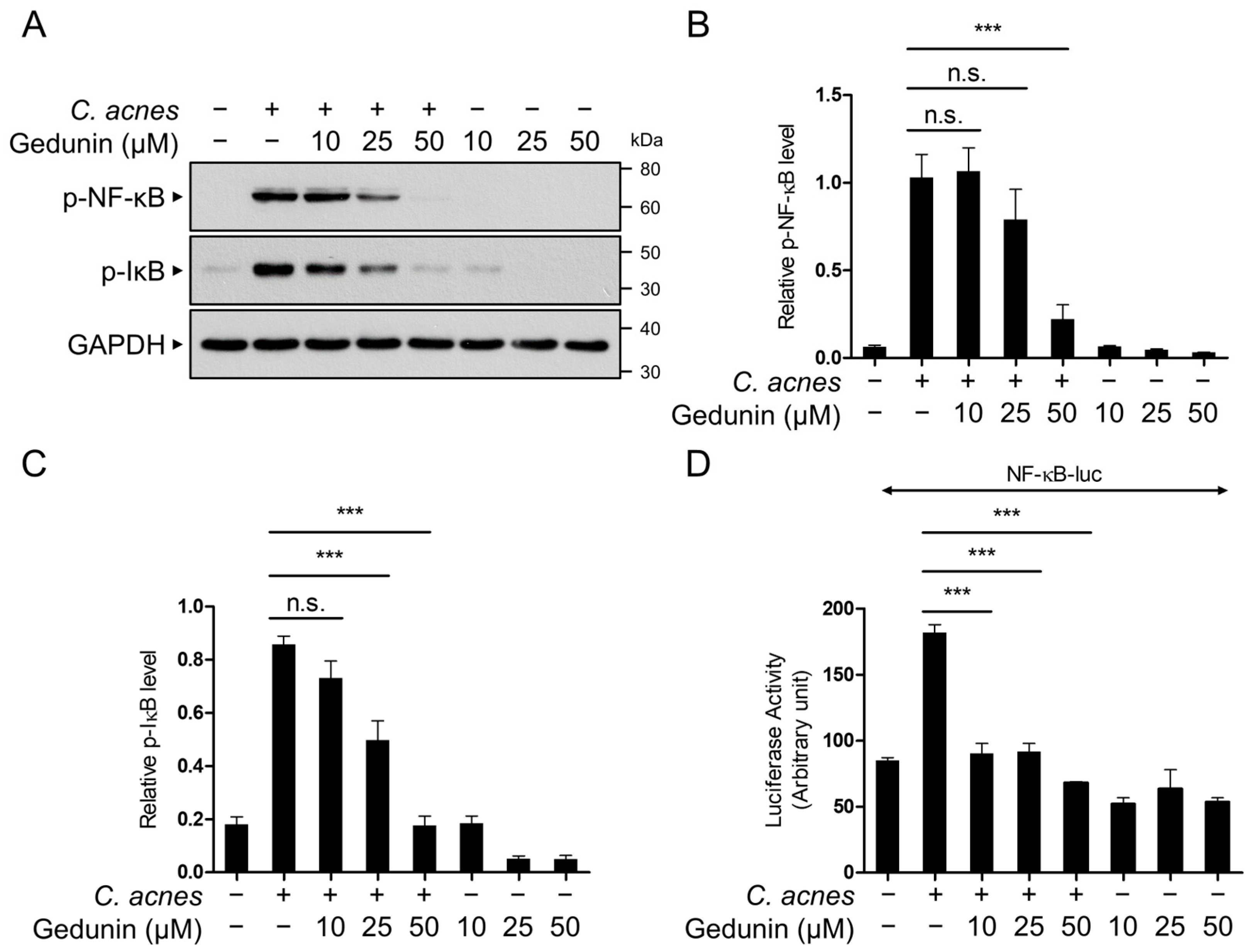
2.3. Gedunin Suppresses the NF-κB Signaling Pathway Induced by C. acnes
2.4. Gedunin Does Not Affect the MAPK Signaling Pathway
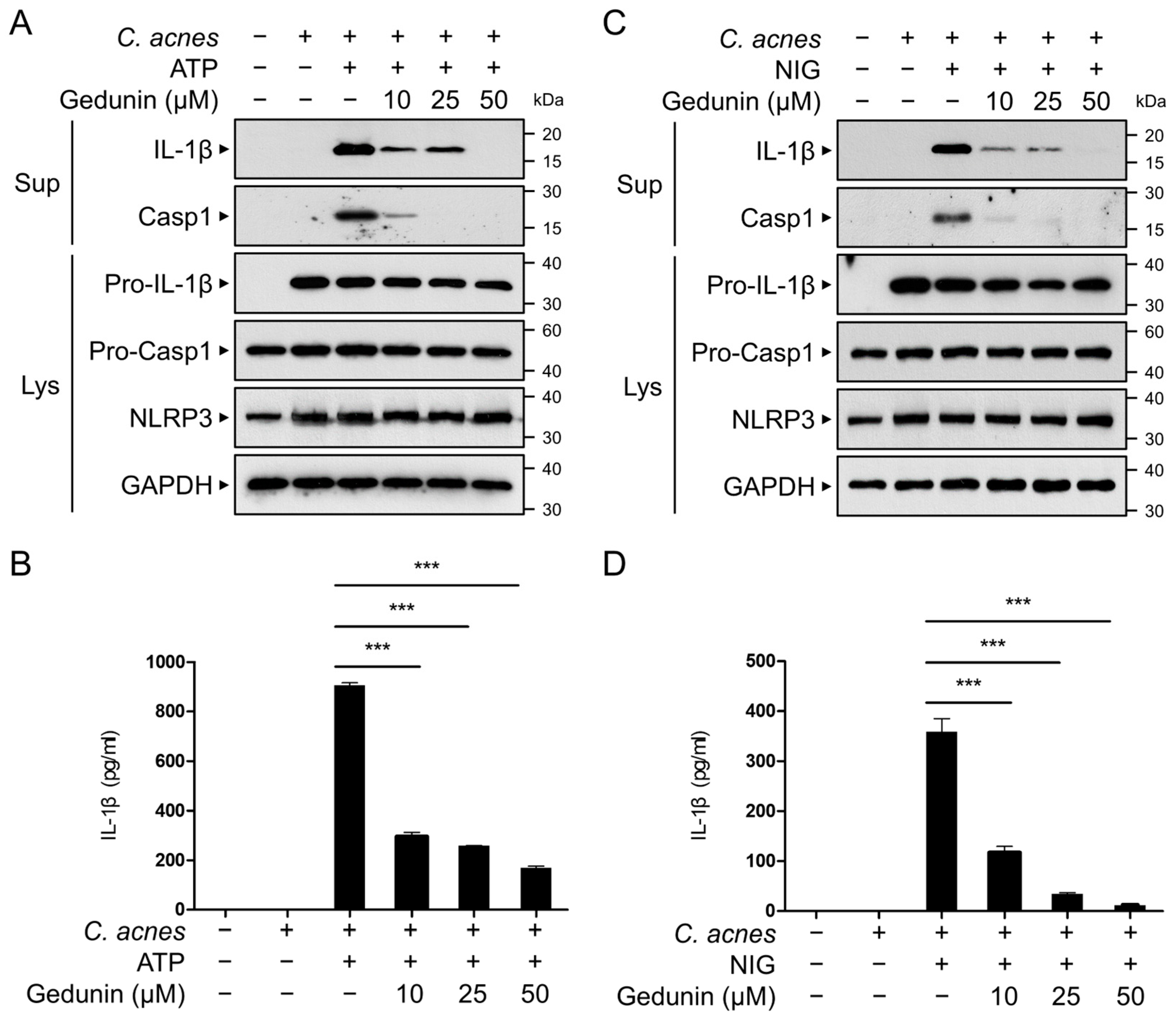
2.5. Gedunin Inhibits C. acnes-Induced Activation of NLRP3 Inflammasome
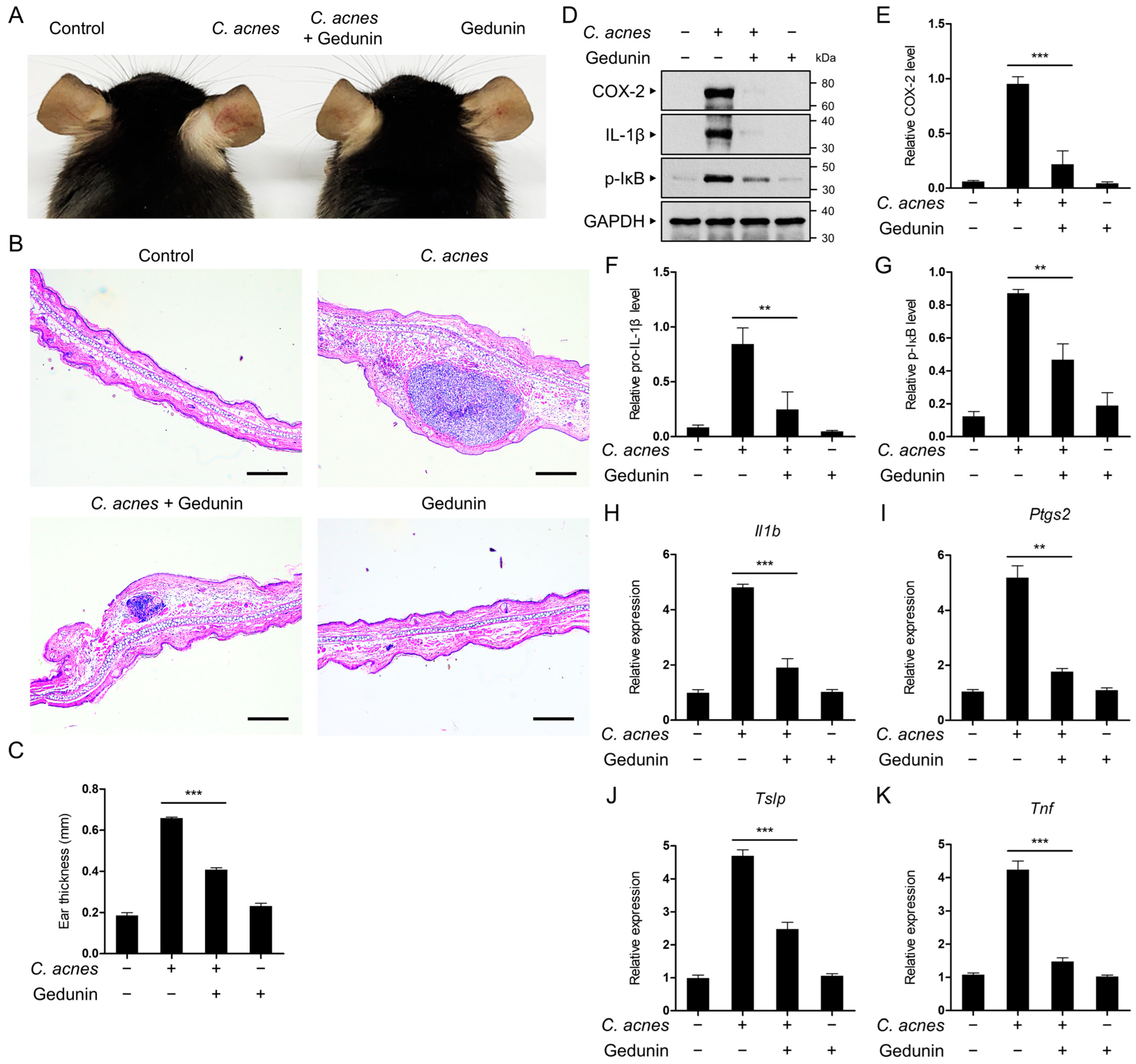
2.6. Gedunin Alleviates C. acnes-Induced Skin Inflammation In Vivo
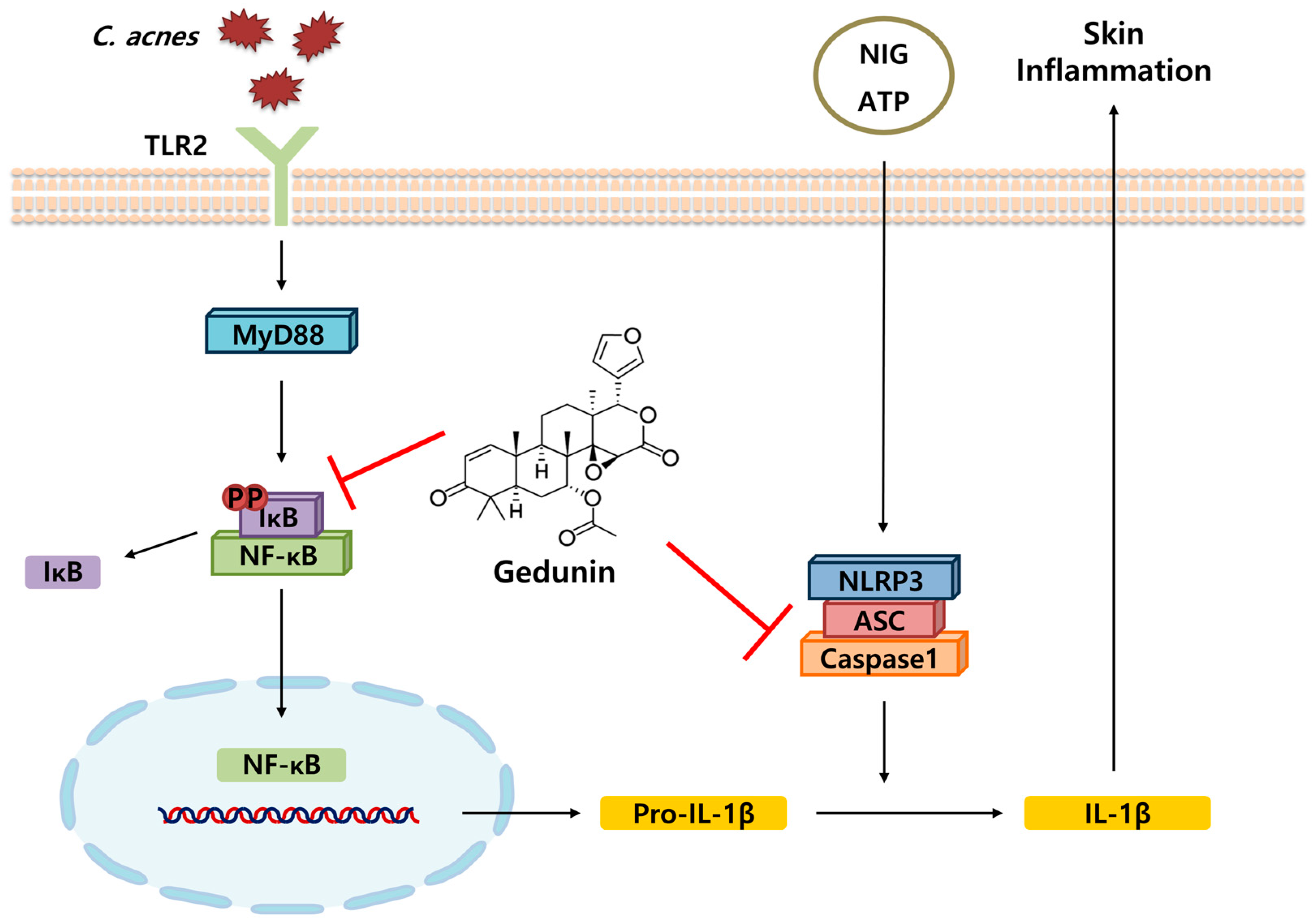
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. C. acnes
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Western Blot Analysis
4.6. NO Assay
4.7. Quantitative Real-Time PCR
4.8. Reporter Gene Assay
4.9. ELISA
4.10. In Vivo Mouse Acne Model
4.11. Histological Analysis
4.12. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zouboulis, C.C. Is acne vulgaris a genuine inflammatory disease? Dermatology 2001, 203, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Poli, F. Epidemiology of Acne. Dermatology 2003, 206, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, C.G.; Burkhart, C.N.; Lehmann, P.F. Acne: A review of immunologic and microbiologic factors. Postgrad. Med. J. 1999, 75, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Leeming, J.P.; Holland, K.T.; Cunliffe, W.J. The pathological and ecological significance of microorganisms colonizing acne vulgaris comedones. J. Med. Microbiol. 1985, 20, 11–16. [Google Scholar] [CrossRef]
- Koreck, A.; Pivarcsi, A.; Dobozy, A.; Kemény, L. The role of innate immunity in the pathogenesis of acne. Dermatology 2003, 206, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Mouser, P.E.; Baker, B.S.; Seaton, E.D.; Chu, A.C. Propionibacterium acnes-reactive T helper-1 cells in the skin of patients with acne vulgaris. J. Investig. Dermatol. 2003, 121, 1226–1228. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ochoa, M.T.; Krutzik, S.R.; Takeuchi, O.; Uematsu, S.; Legaspi, A.J.; Brightbill, H.D.; Holland, D.; Cunliffe, W.J.; Akira, S.; et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 2002, 169, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.F.; Poyner, T.; Cunliffe, B. Clinical reviewAcne vulgarisCommentary: A UK primary care perspective on treating acne. BMJ 2002, 325, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Heymann, W.R. Toll-like receptors in acne vulgaris. J. Am. Acad. Dermatol. 2006, 55, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Needleman, P.; Manning, P.T. Interactions between the inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) pathways: Implications for therapeutic intervention in osteoarthritis. Osteoarthr. Cartil. 1999, 7, 367–370. [Google Scholar] [CrossRef]
- Tsai, H.H.; Lee, W.R.; Wang, P.H.; Cheng, K.T.; Chen, Y.C.; Shen, S.C. Propionibacterium acnes-induced iNOS and COX-2 protein expression via ROS-dependent NF-κB and AP-1 activation in macrophages. J. Dermatol. Sci. 2013, 69, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Ali, P.; Chen, Y.-F.; Sargsyan, E. Bioactive molecules of herbal extracts with anti-infective and wound healing properties. In Microbiology for Surgical Infections; Elsevier: Amsterdam, The Netherlands, 2014; pp. 205–220. [Google Scholar] [CrossRef]
- Tan, Q.G.; Luo, X.D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef] [PubMed]
- Tom, S.; Rane, A.; Katewa, A.S.; Chamoli, M.; Matsumoto, R.R.; Andersen, J.K.; Chinta, S.J. Gedunin Inhibits Oligomeric Aβ(1-42)-Induced Microglia Activation Via Modulation of Nrf2-NF-κB Signaling. Mol. Neurobiol. 2019, 56, 7851–7862. [Google Scholar] [CrossRef]
- Conte, F.P.; Ferraris, F.K.; Costa, T.E.; Pacheco, P.; Seito, L.N.; Verri, W.A., Jr.; Cunha, F.Q.; Penido, C.; Henriques, M.G. Effect of gedunin on acute articular inflammation and hypernociception in mice. Molecules 2015, 20, 2636–2657. [Google Scholar] [CrossRef]
- Ferraris, F.K.; Moret, K.H.; Figueiredo, A.B.; Penido, C.; Henriques, M. Gedunin, a natural tetranortriterpenoid, modulates T lymphocyte responses and ameliorates allergic inflammation. Int. Immunopharmacol. 2012, 14, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Borges, P.V.; Moret, K.H.; Raghavendra, N.M.; Maramaldo Costa, T.E.; Monteiro, A.P.; Carneiro, A.B.; Pacheco, P.; Temerozo, J.R.; Bou-Habib, D.C.; das Graças Henriques, M.; et al. Protective effect of gedunin on TLR-mediated inflammation by modulation of inflammasome activation and cytokine production: Evidence of a multitarget compound. Pharmacol. Res. 2017, 115, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.S.; LaBraico, J.M.; Stern, R.S. Epidemiology of isotretinoin exposure during pregnancy. J. Am. Acad. Dermatol. 1992, 26, 599–606. [Google Scholar] [CrossRef]
- Gollnick, H.; Cunliffe, W.; Berson, D.; Dreno, B.; Finlay, A.; Leyden, J.J.; Shalita, A.R.; Thiboutot, D. Management of acne: A report from a Global Alliance to Improve Outcomes in Acne. J. Am. Acad. Dermatol. 2003, 49, S1–S37. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.H. Antibacterial therapy for acne: A guide to selection and use of systemic agents. Am. J. Clin. Dermatol. 2003, 4, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Haque, E.; Hameed, R.; Maier, P.N.; Irfan, S.; Kamil, M.; Nazir, A.; Mir, S.S. Hsp90 inhibitor gedunin causes apoptosis in A549 lung cancer cells by disrupting Hsp90:Beclin-1:Bcl-2 interaction and downregulating autophagy. Life Sci. 2020, 256, 118000. [Google Scholar] [CrossRef]
- Ong, Y.S.; Khaw, K.Y.; Tan, L.T.-H.; Yew, P.-N.; Tan, K.-B.; Yap, W.H.; Tang, S.Y.; Low, L.E.; Lee, L.-H.; Goh, B.-H. An Overview of the Bioactivities of Gedunin. In Bioactive Natural Products for Pharmaceutical Applications; Springer: Cham, Switzerland, 2021; pp. 563–586. [Google Scholar] [CrossRef]
- Ponnusamy, S.; Haldar, S.; Mulani, F.; Zinjarde, S.; Thulasiram, H.; RaviKumar, A. Gedunin and Azadiradione: Human Pancreatic Alpha-Amylase Inhibiting Limonoids from Neem (Azadirachta indica) as Anti-Diabetic Agents. PLoS ONE 2015, 10, e0140113. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, S.; Durst, T.; Arnason, J.T.; Angerhofer, C.; Pezzuto, J.; Sanchez-Vindas, P.E.; Poveda, L.J.; Gbeassor, M. Antimalarial activity of tropical Meliaceae extracts and gedunin derivatives. J. Nat. Prod. 1997, 60, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.; Zhang, J.; MacKinnon, S.; Leaman, D.; Durst, T.; Philogene, B.J.; Arnason, J.T.; Sanchez-Vindas, P.E.; Poveda, L.; Tamez, P.A.; et al. Traditionally-used antimalarials from the Meliaceae. Curr. Top. Med. Chem. 2003, 3, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kahkhaie, K.R.; Mirhosseini, A.; Aliabadi, A.; Mohammadi, A.; Mousavi, M.J.; Haftcheshmeh, S.M.; Sathyapalan, T.; Sahebkar, A. Curcumin: A modulator of inflammatory signaling pathways in the immune system. Inflammopharmacology 2019, 27, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Shamsnia, H.S.; Roustaei, M.; Ahmadvand, D.; Butler, A.E.; Amirlou, D.; Soltani, S.; Momtaz, S.; Jamialahmadi, T.; Abdolghaffari, A.H.; Sahebkar, A. Impact of curcumin on p38 MAPK: Therapeutic implications. Inflammopharmacology 2023, 31, 2201–2212. [Google Scholar] [CrossRef]
- de Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [PubMed]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Abou Chahla, M.N.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, S.S.; Seo, S.R. Pyrrolidine Dithiocarbamate Suppresses Cutibacterium acnes-Induced Skin Inflammation. Int. J. Mol. Sci. 2023, 24, 4444. [Google Scholar] [CrossRef]

| Gene | Primer Sequence | Accession No. | Product Size (bp) |
|---|---|---|---|
| Il1b (IL-1β) | F: GCCACCTTTTGACAGTGATGAG | NM_008361.4 | 165 |
| R: AGTGATACTGCCTGCCTGAAG | |||
| Il6 (IL-6) | F: TACCACTTCACAAGTCGGAGGC | NM_031168.2 | 116 |
| R: CTGCAAGTGCATCATCGTTGTTC | |||
| Tnf (TNF-α) | F: CCCTCACACTCACAAACCAC | NM_001278601.1 | 133 |
| R: ACAAGGTACAACCCATCGGC | |||
| Nos2 (iNOS) | F: TCCTGGACATTACGACCCCT | NM_001313922.1 | 148 |
| R: AGGCCTCCAATCTCTGCCTA | |||
| Ptgs2 (Cox-2) | F: TTGGAGGCGAAGTGGGTTTT | NM_011198.5 | 148 |
| R: TGGGAGGCACTTGCATTGAT | |||
| Tslp (TSLP) | F: CCCTTCACTCCCCGACAAAA | NM_021367.2 | 61 |
| R: GCAGTGGTCATTGAGGGCTT | |||
| Actb (β-actin) | F: AGAGGGAAATCGTGCGTGAC | NM_007393.5 | 138 |
| R: CGATAGTGATGACCTGACCGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.K.; Heo, Y.J.; Shin, J.H.; Kim, S.S.; Seo, S.R. Gedunin Mitigates Cutibacterium acnes-Induced Skin Inflammation by Inhibiting the NF-κB Pathway. Pharmaceuticals 2025, 18, 71. https://doi.org/10.3390/ph18010071
Sim JK, Heo YJ, Shin JH, Kim SS, Seo SR. Gedunin Mitigates Cutibacterium acnes-Induced Skin Inflammation by Inhibiting the NF-κB Pathway. Pharmaceuticals. 2025; 18(1):71. https://doi.org/10.3390/ph18010071
Chicago/Turabian StyleSim, Ju Kyoung, Ye Ji Heo, Jin Hak Shin, Seon Sook Kim, and Su Ryeon Seo. 2025. "Gedunin Mitigates Cutibacterium acnes-Induced Skin Inflammation by Inhibiting the NF-κB Pathway" Pharmaceuticals 18, no. 1: 71. https://doi.org/10.3390/ph18010071
APA StyleSim, J. K., Heo, Y. J., Shin, J. H., Kim, S. S., & Seo, S. R. (2025). Gedunin Mitigates Cutibacterium acnes-Induced Skin Inflammation by Inhibiting the NF-κB Pathway. Pharmaceuticals, 18(1), 71. https://doi.org/10.3390/ph18010071






