In Vivo Assessment of Healing Potential of Ointments Containing Bee Products, Vegetal Extracts, and Polymers on Skin Lesions
Abstract
1. Introduction
2. Results
2.1. Wound Healing Evaluation Parameters
2.1.1. Macroscopic Evaluation
2.1.2. Period of Re-Epithelialization and Wound Contraction Rate
2.1.3. Histopathological Examination
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of the Oily and Hydro-Alcoholic Extracts
4.3. Preparation of the Ointments
4.3.1. Preparation of the Ointment with Vegetable Extracts and Bee Products
4.3.2. Preparation of the Ointment with Vegetable Extracts and Polymers
4.3.3. Preparation of the Ointment with Bee Products and Polymers
4.3.4. Preparation of the Ointment with Bee Products, Vegetable Extracts, and Polymers
4.4. Chemical Reagents, Bee Products, and Polymers
4.5. Experimental Animals and Study Design
4.5.1. Assessment of Degree of Wound Healing
4.5.2. Histopathological Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pattnaik, S.; Mohanty, S.; Sahoo, S.; Mohanty, C. A mechanistic perspective on the role of phytoconstituents-based pharmacotherapeutics and their topical formulations in chronic wound management. J. Drug Deliv. Sci. Technol. 2023, 84, 104546. [Google Scholar] [CrossRef]
- Cedillo-Cortezano, M.; Martinez-Cuevas, L.R.; López, J.A.M.; Barrera López, I.L.; Escutia-Perez, S.; Petricevich, V.L. Use of Medicinal Plants in the Process of Wound Healing: A Literature Review. Pharmaceuticals 2024, 17, 303. [Google Scholar] [CrossRef] [PubMed]
- Kunimitsu, M.; Nakagami, G.; Kitamura, A.; Minematsu, T.; Koudounas, S.; Ogai, K.; Sugama, J.; Takada, C.; Yeo, S.; Sanada, H. Relationship between healing status and microbial dissimilarity in wound and peri-wound skin in pressure injuries. J. Tissue Viability 2023, 32, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, N.K.; Kumar, R.; Mandotra, S.K.; Meena, R.N.; Siddiqui, M.S.; Sawhney, R.C.; Gupta, A. Safety and healing efficacy of sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in rats. Food Chem. Toxicol. 2009, 47, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Ezati, N.; Asadi, E.; Deilami, S.A.; Saber-Samandari, S. Polymers in tissue engineering and regenerative medicine. In Handbook of Polymers in Medicine; Mozafari, M., Pal Singh Chauhan, N., Eds.; Woodhead Publishing: Cambridge, UK; Elsevier: Amsterdam, The Netherlands, 2023; pp. 463–489. [Google Scholar]
- Sun, Y.; Li, D.; Yu, Y.; Zheng, Y. Insights into the Role of Natural Polysaccharide-Based Hydrogel Wound Dressings in Biomedical Applications. Gels 2022, 8, 646. [Google Scholar] [CrossRef]
- Lee, H.; Jung, Y.; Lee, N.; Lee, I.; Lee, J.H. Nature-Derived Polysaccharide-Based Composite Hydrogels for Promoting Wound Healing. Int. J. Mol. Sci. 2023, 24, 16714. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Cao, X.; Wang, Y.; Wang, J.; Zhao, Y. Developing natural polymers for skin wound healing. Bioact. Mater. 2024, 33, 355–376. [Google Scholar] [CrossRef]
- Kaur, T.; Joshi, A.; Singh, N. Natural cocktail of bioactive factors conjugated on nanofibrous dressing for improved wound healing. Biomat. Adv. 2022, 143, 213163. [Google Scholar] [CrossRef]
- Andritoiu, C.V.; Andriescu, C.E.; Ibanescu, C.; Lungu, C.; Ivanescu, B.; Vlase, L.; Havarneanu, C.; Popa, M. Effects and Characterization of Some Topical Ointments Based on Vegetal Extracts on Incision, Excision, and Thermal Wound Models. Molecules 2020, 25, 5356. [Google Scholar] [CrossRef] [PubMed]
- Andritoiu, C.V.; Andriescu, C.E.; Danu, M.; Lungu, C.; Ivanescu, B.; Havarneanu, C.; Popa, M. Evaluation of the Wound Healing Potential of Some Natural Polymers on Three Experimental Models. Pharmaceuticals 2021, 14, 465. [Google Scholar] [CrossRef] [PubMed]
- Andritoiu, C.V.; Lungu, C.; Danu, M.; Ivanescu, B.; Andriescu, C.E.; Vlase, L.; Havarneanu, C.; Iurciuc Tincu, C.E.; Popa, M. Evaluation of the Healing Effect of Ointments Based on Bee Products on Cutaneous Lesions in Wistar Rats. Pharmaceuticals 2021, 14, 1146. [Google Scholar] [CrossRef] [PubMed]
- Manon-Jensen, T.; Kjeld, N.G.; Karsdal, M.A. Collagen-mediated hemostasis. J. Thromb. Haemost. 2016, 14, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacoter. 2018, 98, 469–483. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, C.; Bueno, I.L.; Quaresma, A.C.M.; Longato, G.B. Healing Potential of Propolis in Skin Wounds Evidenced by Clinical Studies. Pharmaceuticals 2022, 15, 1143. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef] [PubMed]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Lu, K.; Bhat, M.; Basu, S. Plants and their active compounds: Natural molecules to target angiogenesis. Angiogenesis 2016, 19, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W.; Supp, D.M. Role of Arginine and Omega-3 Fatty Acids in Wound Healing and Infection. Adv. Wound Care 2014, 3, 682–690. [Google Scholar] [CrossRef]
- Martí, I.; Líndez, A.A.; Reith, W. Arginine-dependent immune responses. Cell. Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Thakur, M.; Chopra, H.K.; Dar, B.N.; Nanda, V. Assessment of fatty acids, amino acids, minerals, and thermal properties of bee propolis from Northern India using a multivariate approach. J. Food Comp. Anal. 2022, 111, 104624. [Google Scholar] [CrossRef]
- Dwivedi, D.; Dwivedi, M.; Malviya, S.; Singh, V. Evaluation of wound healing, anti-microbial and antioxidant potential of Pongamia pinnata in wistar rats. J. Tradit. Complement. Med. 2016, 7, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Rajinikanth, B.S.; Rajkumar, D.S.R.; Keerthika, K.; Vijayaragavan, V. Chitosan-Based Biomaterial in Wound Healing: A Review. Cureus 2024, 16, e55193. [Google Scholar] [CrossRef] [PubMed]
- Leonida, M.; Banjade, S.; Vo, T.; Anderle, G.; Haas, G.; Philips, N. Nanocomposite materials with antimicrobial activity based on chitosan. Int. J. Nano Biomat. 2011, 3, 316–334. [Google Scholar] [CrossRef]
- da Silva, M.C.; da Silva, H.N.; Alves Leal Cruz, R.d.C.; Sagoe Amoah, S.K.; de Lima Silva, S.M.; Lia Fook, M.V. N-Acetyl-D-Glucosamine-Loaded Chitosan Filaments Biodegradable and Biocompatible for Use as Absorbable Surgical Suture Materials. Materials 2019, 12, 1807. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Song, S.Y.; Park, E.H.; Kim, E.; Oh, G.C.; Choo, E.H.; Hwang, B.H.; Chang, K.; Oak, M.H. Beneficial Effects of Caffeic Acid Phenethyl Ester on Wound Healing in a Diabetic Mouse: Role of VEGF and NO. Appl. Sci. 2022, 12, 2320. [Google Scholar] [CrossRef]
- Balaha, M.; Cataldi, A.; Ammazzalorso, A.; Cacciatore, I.; De Filippis, B.; Di Stefano, A.; Maccallini, C.; Rapino, M.; Korona-Glowniak, I.; Przekora, A.; et al. CAPE derivatives: Multifaceted agents for chronic wound healing. Arch. Pharm. 2024, 357, e2400165. [Google Scholar] [CrossRef] [PubMed]
- Werneck Cerqueira, A.F.L.; de Mello Brandão, H.; Tavares, G.D.; Rodarte, M.P. Ferulic Acid: A Review of Mechanisms of Action, Absorption, Toxicology, Application on Wound Healing. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2024, 23, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar Gl Lonchin, S. A bio-polymeric scaffold incorporated with p-Coumaric acid enhances diabetic wound healing by modulating MMP-9 and TGF-β3 expression. Colloids Surf. B Biointerfaces 2023, 225, 113280. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.T.B.; Araújo-Filho, H.G.; Barreto, A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S.; Barreto, R.S.S. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef] [PubMed]
- Alecu, M. Molecular Pathology of Skin; Medical Publishing: Bucharest, Romania, 2006; pp. 56–64. [Google Scholar]
- Scepankova, H.; Combarros-Fuertes, P.; Fresno, J.M.; Tornadijo, M.E.; Dias, M.S.; Pinto, C.A.; Saraiva, J.A.; Estevinho, L.M. Role of Honey in Advanced Wound Care. Molecules 2021, 26, 4784. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.P.; Akkol, E.K.; Yılmazer, D.; Baykal, T.; Kırmızıbekmez, H.; Alper, M.; Yeşilada, E. Investigations on the in vivo wound healing potential of Hypericum perforatum L. J. Ethnopharmacol. 2010, 127, 468–477. [Google Scholar] [CrossRef]
- DeMesquita, C.J.G.; Leite, J.A.; Fechine, F.V.; deRocha, J.L.C.; Leite, J.G.; Leite Filho, J.A.; Barbosa Filho, R.A. Effect of imiquimod on partial-thickness burns. Burns 2010, 36, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Agbaje, M.; Rutland, C.; Maboni, G.; Blanchard, A.; Bexon, M.; Stewart, C.; Jone, M.; Totemeyer, S. Novel inflammatory cell infiltration scoring system to investigate healthy and footrot affected ovine interdigital skin. PeerJ 2018, 6, e5097. [Google Scholar] [CrossRef] [PubMed]
- Wohlsein, P.; Peters, M.; Schulze, C.; Baumgärtner, W. Thermal Injuries in Veterinary Forensic Pathology. Vet. Pathol. 2016, 53, 1001–1017. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.L.; Fourcaudot, A.B.; Sebastian, E.A.; Shankar, R.; Brown, A.W.; Leung, K.P. Standardization of deep partial-thickness scald burns in C57BL/6 mice. Int. J. Burn. Trauma 2018, 8, 26–33. [Google Scholar]
- El-Sayed, Y.S. Time Course of Histomorphologic features during chronic burn wound healing. Forensic Med. Anat. Res. 2016, 4, 1–6. [Google Scholar] [CrossRef][Green Version]
- Hawkins, H.K.; Jay, J.; Finnerty, C.C. Pathophysiology of the burn scar. In Total Burn Care; Elsevier: Amsterdam, The Netherlands, 2018; pp. 466–475.e3. [Google Scholar] [CrossRef]
| Experimental Groups | Wound Area (mm2) | WCR (%) (Mean ± SEM) | |||
|---|---|---|---|---|---|
| Day 0 | Day 6 | Day 9 | Day 6 | Day 9 | |
| NC Group | 64.0 (8.0 × 8.0) | 61.6 (7.7 × 8.0) | 56.25 (7.5 × 7.5) | 5.78 ± 1.86 | 14.85 ± 2.95 |
| OB Group | 64.0 (8.0 × 8.0) | 45.5 (6.5 × 7.0) | 40.8 (6.0 × 6.8) | 26.74 ± 2.13 * | 33.79 ± 2.21 * |
| AP Group | 64.0 (8.0 × 8.0) | 7.5 (2.5 × 3.0) | 1.56 (1.2 × 1.3) | 89.33 ± 0.60 | 97.82 ± 0.13 |
| PPo Group | 64.0 (8.0 × 8.0) | 19.5 (3.0 × 3.5) | 2 (1.0 × 2.0) | 84.06 ± 0.47 | 96.61 ± 0.30 |
| APo Group | 64.0 (8.0 × 8.0) | 9.0 (3.0 × 3.0) | 1.5 (1.0 × 1.5) | 86.25 ± 0.31 | 97.50 ± 0.09 |
| APPo Group | 64.0 (8.0 × 8.0) | 5.0 (2.0 × 2.5) | 1.0 (1.0 × 1.0) | 91.35 ± 0.83 | 98.17 ± 0.15 |
| Incision | Excision | Thermal Burn |
|---|---|---|
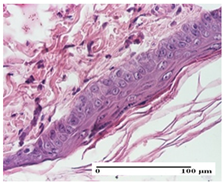 | 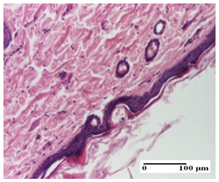 | 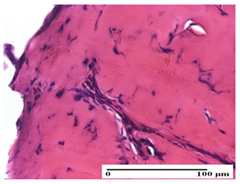 |
| mild inflammatory infiltrate in superficial dermis (S1, G0) | no inflammation, moderate edema (S0, G0) | dermal collagenization, thinning of the epidermis (S0, G0, D1) |
| Experimental Groups | Incision | Excision | Thermal Burn |
|---|---|---|---|
| NC Group |  | 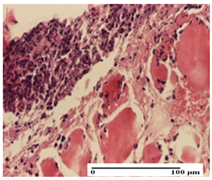 | 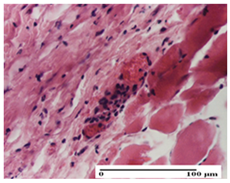 |
| important inflammatory infiltrate in hypodermis and striated muscle (S3, G0) | deep ulceration and polymorphic inflammatory infiltrate near the striated muscle (S3, G0) | important inflammatory infiltrate in hypodermis and striated muscle (S2, G0, D1) | |
| OB Group |  | 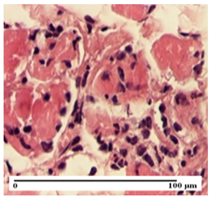 |  |
| relatively important inflammatory infiltrate in hypodermis and striated muscle (S2, G0) | moderate polymorphic inflammatory infiltrate in the interstitium of the striated muscle (S2, G0) | dermal collagenization, hemorrhage (S1, G0, D2) | |
| AP Group | 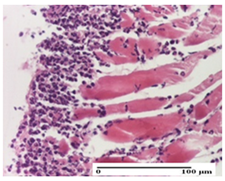 | 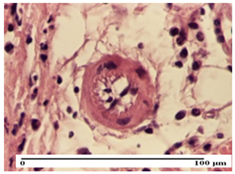 | 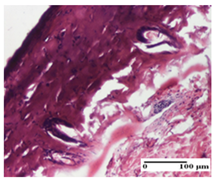 |
| inflammatory infiltrate in the interstitium of the striated muscle (S3, G0) | thick-walled vessel (S1, G0) | dermal collagenization (S1, G0, D2) | |
| PPo Group | 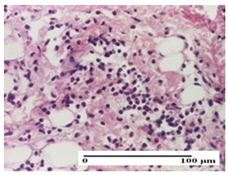 | 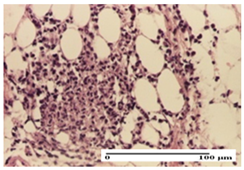 | 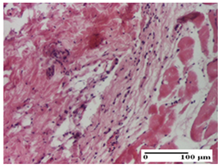 |
| severe polymorphic inflammatory infiltrate in hypodermis (S2, G0) | polymorphic inflammatory infiltrate in hypodermis (S2, G0) | slight inflammatory infiltrate (S1, G0, D0) | |
| APo Group | 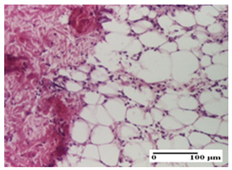 | 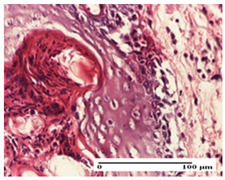 | 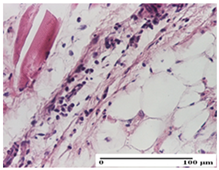 |
| mild inflammatory infiltrate in hypodermis (S1, G0) | abscess in the corneous layer, epidermal acanthosis, moderate polymorphic inflammatory infiltrate in dermis (S2, G0) | mild inflammatory infiltrate in hypodermis and between the striated muscle fibers (S1, G0) | |
| APPo Group | 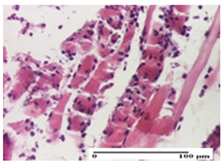 | 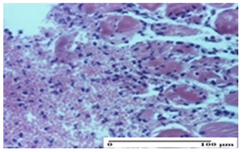 | 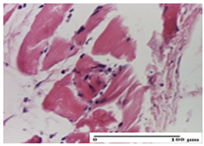 |
| moderate interstitial inflammatory infiltrate (S1, G0) | moderate inflammatory infiltrate in the interstitium of the striated muscle (S1, G0) | inflammatory infiltrate in the striated muscle (S1, G0, D0) |
| Experimental Groups | Incision | Excision | Thermal Burn |
|---|---|---|---|
| NC Group | 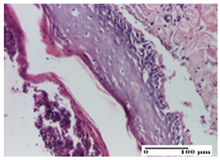 | 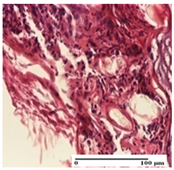 | 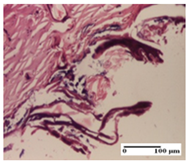 |
| abscess in the corneous layer, epidermal acanthosis, mild inflammatory infiltrate in dermis (S2, G0) | superficial ulceration (S3, G0) | abscess in the corneous layer, mild dermal inflammatory infiltrate (S1, G0, D2) | |
| OB Group | 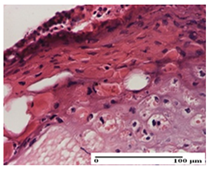 |  | 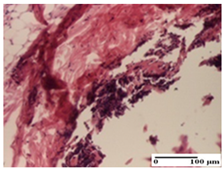 |
| degeneration of keratinocytes, paranucleosis, vacuolar spongiosis (S2, G0) | inflammatory infiltrate in hypodermis (S3, G0) | abscess in the corneous layer (S3, G0, D2) | |
| AP Group | 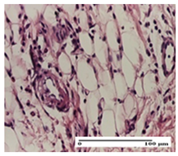 | 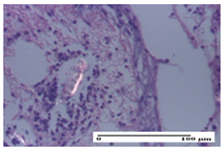 | 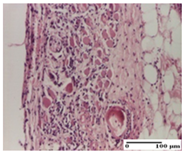 |
| hypodermis with mild inflammatory infiltrate (S1, G0) | lymphocytic inflammatory infiltrate—detail in polarized light microscopy (S2, G0) | fibrosis that embeds striated muscle fibers, mild inflammation (S1, G0, D2) | |
| PPo Group | 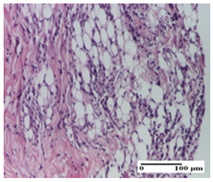 |  | 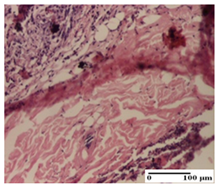 |
| moderate inflammatory infiltrate in the dermis and hypodermis (S2, G0) | moderate inflammatory infiltrate in hypodermis and striated muscle (S2, G0) | abscess in the corneous layer (S2, G0, D2) | |
| APo Group |  | 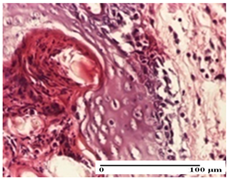 |  |
| moderate inflammatory infiltrate (S2, G0) | abscess in the corneous layer moderate vacuolization (S2, G0) | congestion and hemorrhage (S1, G0, D0) | |
| APPo Group | 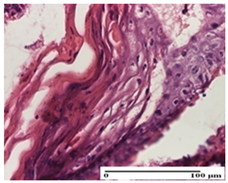 | 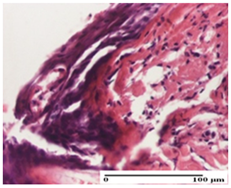 | 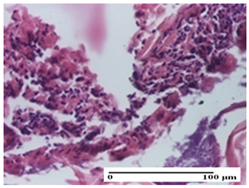 |
| severe inflammatory infiltrate in the dermis, micro-ulceration of the epidermis with hematoleukocyte surface deposit (S3, G0) | inflammatory infiltrate (S1, G0) | Inflammatory infiltrate (S3, G0, D2) |
| Experimental Groups | Incision | Excision | Thermal Burn |
|---|---|---|---|
| NC Group | 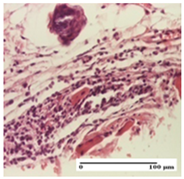 | 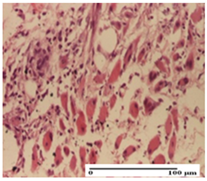 |  |
| important inflammatory infiltrate in hypodermis (S3, G0) | edema (striated muscle) (S1, G0) | abscess, ulceration (S2, G0, D2) | |
| OB Group | 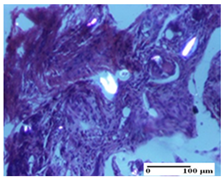 | 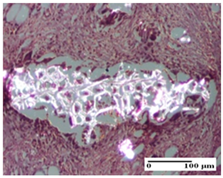 | 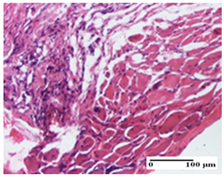 |
| foreign body-type giant cell response to keratin (S1, G0) | foreign body-type giant cell response to keratin—detail in polarized light microscopy (S1, G0) | mild inflammatory infiltrate (S1, G0, D0) | |
| AP Group | 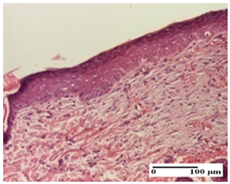 | 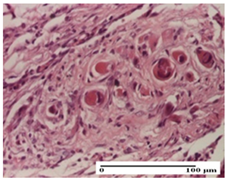 |  |
| dermal collagenization (S0, G0) | severe dermal collagenization that included the muscle layer (S1, G1) | severe dermal collagenization (S1, G0, D0) | |
| PPo Group | 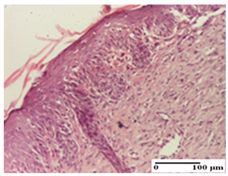 | 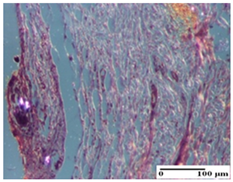 | 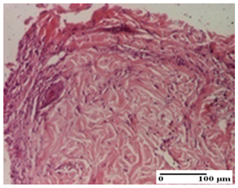 |
| dermal collagenization (S0, G0) | dermal collagenization—detail in polarized light microscopy (S0, G0) | dermal collagenization (S0,G0, D0) | |
| APo Group | 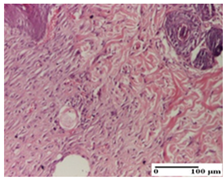 | 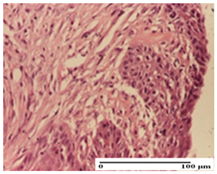 | 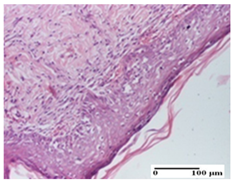 |
| dermal collagenization (S0, G0) | dermal collagenization, epidermal acanthosis (S0, G0) | moderate dermal collagenization, mild inflammatory infiltrate (S1, G0, D0) | |
| APPo Group | 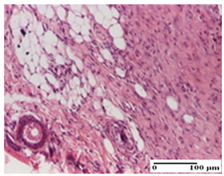 | 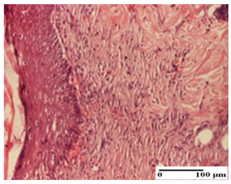 | 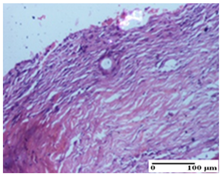 |
| Dermo-hypodermic collagenization, mild inflammatory infiltrate (S1, G0) | dermal collagenization (S0, G0) | dermal collagenization, rare inflammatory elements—detail in polarized light microscopy (S1, G0, D0) |
| Experimental Groups | Incision | Excision | Thermal Burn |
|---|---|---|---|
| NC Group | 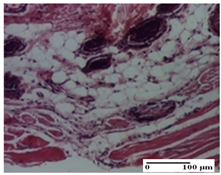 | 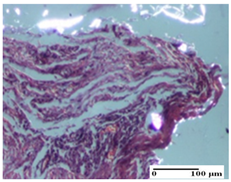 | 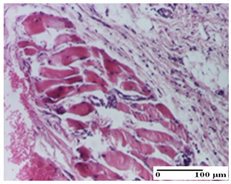 |
| inflammatory infiltrate in hypodermis and muscle tissue (S1, G0) | ulceration, important inflammatory infiltrate (S2, G0)— detail in polarized light microscopy | striated muscle with interstitial inflammatory infiltrate (S1, G0, D0) | |
| OB Group | 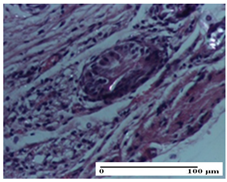 |  | 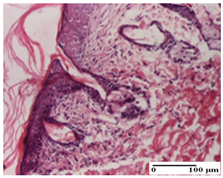 |
| inflammatory infiltrate and fibrosis in hypodermis, foreign body-type giant cell response to keratin (S2, G0) | relatively important inflammatory infiltrate (S2, G0) | congestion and edema, mild dermal collagenization (S1, G0, D0) | |
| AP Group | 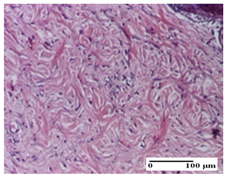 | 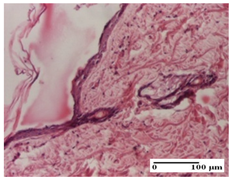 | 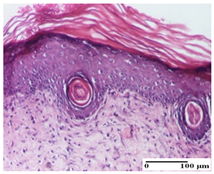 |
| important collagenization of the dermis (S1, G0) | dermal collagenization, mild edema of the dermis (S1, G0) | severe collagenization of the dermis (S1, G0, D0) | |
| PPo Group | 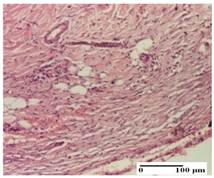 | 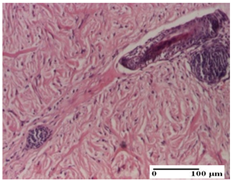 | 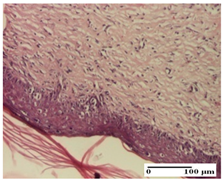 |
| dermal collagenization (S1, G0) | dermal collagenization (S1, G0) | dermal collagenization (S1, G0, D0) | |
| APo Group | 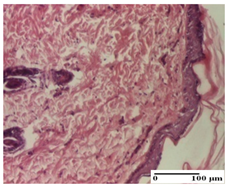 | 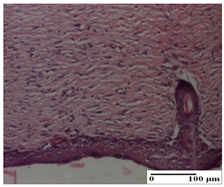 |  |
| dermal collagenization (S0, G0) | dermal collagenization, the hair follicle with atrophy of the sebaceous glands (S1, G0) | dermal collagenization (S1, G0, D0) | |
| APPo Group | 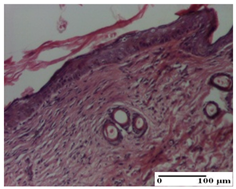 | 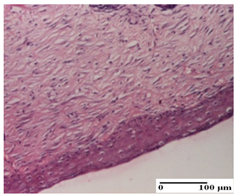 | 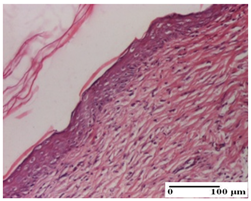 |
| dermal collagenization, atrophy of skin appendages (S1, G0) | dermal collagenization (S0, G0) | dermal collagenization (S1, G0, D0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andritoiu, C.V.; Lungu, C.; Iurciuc, C.E.; Andriescu, C.E.; Havarneanu, C.; Popa, M.; Cuciureanu, M.; Tarţău, L.M.; Ivanescu, B. In Vivo Assessment of Healing Potential of Ointments Containing Bee Products, Vegetal Extracts, and Polymers on Skin Lesions. Pharmaceuticals 2025, 18, 65. https://doi.org/10.3390/ph18010065
Andritoiu CV, Lungu C, Iurciuc CE, Andriescu CE, Havarneanu C, Popa M, Cuciureanu M, Tarţău LM, Ivanescu B. In Vivo Assessment of Healing Potential of Ointments Containing Bee Products, Vegetal Extracts, and Polymers on Skin Lesions. Pharmaceuticals. 2025; 18(1):65. https://doi.org/10.3390/ph18010065
Chicago/Turabian StyleAndritoiu, Calin Vasile, Cristina Lungu, Camelia Elena Iurciuc (Tincu), Corina Elena Andriescu, Corneliu Havarneanu, Marcel Popa, Magdalena Cuciureanu, Liliana Mititelu Tarţău, and Bianca Ivanescu. 2025. "In Vivo Assessment of Healing Potential of Ointments Containing Bee Products, Vegetal Extracts, and Polymers on Skin Lesions" Pharmaceuticals 18, no. 1: 65. https://doi.org/10.3390/ph18010065
APA StyleAndritoiu, C. V., Lungu, C., Iurciuc, C. E., Andriescu, C. E., Havarneanu, C., Popa, M., Cuciureanu, M., Tarţău, L. M., & Ivanescu, B. (2025). In Vivo Assessment of Healing Potential of Ointments Containing Bee Products, Vegetal Extracts, and Polymers on Skin Lesions. Pharmaceuticals, 18(1), 65. https://doi.org/10.3390/ph18010065





.png)





