1. Introduction
The rise of antibiotic resistance poses a critical global health threat, with an estimated 4.95 million deaths associated with antimicrobial resistance (AMR) in 2019 alone, including 1.27 million deaths directly attributable to AMR, according to the most recent WHO Global Antimicrobial Resistance and Use Surveillance System (GLASS) report [
1]. Among the most critical resistant pathogens, often referred to as “ESKAPE” pathogens, are
Enterococcus faecium,
Staphylococcus aureus,
Klebsiella pneumoniae,
Acinetobacter baumannii,
Pseudomonas aeruginosa, and
Enterobacter species [
2]. Antibiotic-resistant bacteria pose a major health threat worldwide, leading to significant economic loss and high mortality rates, especially in developing countries, including Morocco [
3,
4].
Recent studies have demonstrated the significant therapeutic potential of essential oils in the fight against antibiotic-resistant bacteria [
5,
6]. Investigations into the synergistic effects of essential oils and conventional antibiotics have shown particularly promising results in inhibiting the growth of resistant strains [
7,
8]. In addition, the emergence of innovative technologies, notably nanoencapsulation, has considerably improved the physicochemical stability and antimicrobial efficacy of essential oils in the treatment of infections caused by bacterial strains [
9,
10].
This has led to growing interest in alternative drug sources, especially natural compounds, as numerous existing medications are derived from nature. Essential oils (EOs), which contain a blend of 20 to 100 low-molecular-weight plant secondary metabolites, are typically volatile liquids composed primarily of terpenoids, along with phenylpropanoids, benzenoids, and short-chain aliphatic hydrocarbons [
11,
12]. Known for their diverse bioactive composition, EOs exhibit valuable antimicrobial, antioxidant, antidiabetic, anticancer, and anti-inflammatory effects [
13,
14,
15]. Many EOs also demonstrate significant antibacterial activity against a variety of both gram-positive and gram-negative bacteria, making them strong candidates as antimicrobial agents [
16].
Thymus satureioides Coss., a perennial herb from the Lamiaceae family, is a well-known aromatic plant widely distributed in North Africa, particularly in Morocco, where it holds considerable ethnobotanical and medicinal value. Characterized by its small, woody structure,
T. satureioides grows in arid and semi-arid regions, often on rocky hillsides or mountain slopes. The plant exhibits linear, lanceolate leaves, and small pink to purple flowers that bloom during spring and early summer [
17]. Like many members of the
Thymus genus,
T. satureioides contains a variety of EOs) known for their potent bioactive properties.
In Moroccan traditional medicine,
T. satureioides has long been used as a remedy for various ailments, particularly those associated with respiratory and digestive issues [
18]. It is commonly prepared as an infusion or decoction to treat coughs, colds, bronchitis, and stomach ailments, with locals attributing these effects to its high essential oil content [
19]. The plant is also employed as an antiseptic and is valued for its ability to ward off infections and promote wound healing, highlighting its cultural significance in Moroccan folk medicine.
Scientific studies have corroborated the medicinal properties attributed to
T. satureioides, with a strong focus on its antibacterial activities. Research indicates that the essential oil of
T. satureioides is particularly effective against various pathogenic bacteria, including
Staphylococcus aureus,
Escherichia coli, and
Pseudomonas aeruginosa, which are often resistant to conventional antibiotics [
20].
Lavandula angustifolia Mill., commonly known as lavender, belongs to the Lamiaceae family and is well-regarded for its aromatic and medicinal properties. This perennial, shrub-like plant is characterized by narrow, lanceolate leaves and tall flowering stems topped with clusters of purple-blue flowers, which bloom from late spring through summer. Lavender is native to the Mediterranean region and thrives in dry, rocky soils at moderate altitudes [
21].
In Moroccan ethnobotany,
L. angustifolia has been traditionally employed for a range of therapeutic purposes. It is widely used in Moroccan folk medicine for treating anxiety, insomnia, digestive disorders, and headaches. Lavender essential oil, known for its calming scent, is often used in aromatherapy and is applied topically to soothe skin ailments, highlighting its cultural and medicinal importance in Moroccan communities [
22].
Scientific research has validated the medicinal properties attributed to
L. angustifolia, particularly its antibacterial activities. Lavender essential oil contains various bioactive compounds, with linalool, linalyl acetate, and camphor being the most abundant constituents, responsible for the plant’s antibacterial effects [
23]. Studies have demonstrated that lavender oil exhibits significant antibacterial activity against both gram-positive and gram-negative bacteria, including
S. aureus,
E. coli, and
P. aeruginosa [
24].
Origanum majorana L., commonly known as marjoram, belongs to the Lamiaceae family and is widely cultivated for its aromatic and medicinal properties. This perennial herb features soft, gray-green leaves and small white to pink flowers that bloom in clusters during the summer months. Native to the Mediterranean region,
O. majorana thrives in sunny, well-drained soils and is often grown in dry, rocky landscapes [
25]. In Moroccan ethnobotany,
O. majorana is valued for its therapeutic applications in traditional medicine. Known locally as “mardakouch”, it is used to relieve respiratory ailments, digestive disorders, and muscle pain and is believed to have calming effects when consumed as an infusion. This cultural significance is rooted in its broad application in Moroccan households for both culinary and medicinal purposes, where it is commonly prepared as a tea or used in decoctions [
26].
Studies have shown that marjoram essential oil exhibits potent antibacterial activity against both gram-positive and gram-negative bacteria, including
E. coli,
Salmonella typhi, and
S. aureus [
27]. The antibacterial mechanisms of
O. majorana essential oil are thought to involve the disruption of bacterial cell walls and membranes, ultimately leading to cell lysis [
28].
Beyond their antimicrobial properties, EOs have gained considerable attention for their antioxidant potential. This is particularly relevant given that oxidative stress, characterized by an imbalance between reactive oxygen species (ROS) production and cellular antioxidant defenses, plays a crucial role in the pathogenesis of numerous diseases, including cardiovascular disorders, neurodegenerative conditions, and cancer [
29]. The accumulation of ROS can cause significant damage to cellular components, including lipids, proteins, and DNA, leading to cellular dysfunction and death [
30]. Natural antioxidants, particularly those found in EOs, have emerged as promising therapeutic alternatives due to their ability to neutralize free radicals and protect against oxidative damage [
31,
32].
Research has shown that combining EOs can yield synergistic effects, enhancing efficacy against pathogens more effectively than single applications. However, most studies have focused on simple binary or ternary EO mixtures without optimization. The simplex–centroid-mixture design allows researchers to model and predict the behavior of mixture components and their interactions using a minimal number of experimental runs while maintaining high accuracy [
33,
34]. Unlike traditional one-factor-at-a-time approaches, simplex–centroid designs can efficiently explore the entire mixture space and identify optimal blend proportions, making them particularly valuable for studying EO combinations [
35].
This study employs a simplex–centroid-mixture design to optimize the antibacterial effects of EOs from T. satureioides, L. angustifolia, and O. majorana, three plants with established ethnobotanical significance in Morocco. The antibacterial activities of both individual EOs and their mixtures are assessed through this design approach, while their antioxidant properties are evaluated individually, with the goal of comprehensively characterizing their therapeutic potential.
2. Results and Discussion
2.1. Chemical Profile of the Three EOs
Table 1 provides a comprehensive overview of the chemical profiles, molecular formulas, percentages, and extraction yields of EOs derived from
T. satureioides,
L. angustifolia, and
O. majorana. Detailed analytical data, including the Total Ion Chromatogram (TIC) chromatograms, compositional breakdowns, and retention times, are presented in the
Supplementary Materials (Figures S1–S3 and Tables S1–S3). The extraction yields of the EOs were determined to be 2.34% (
v/
w) for
T. satureioides, 1.68% (
v/
w) for
L. angustifolia, and 3.45% (
v/
w) for
O. majorana. Each essential oil displayed a distinct phytochemical composition, with
T. satureioides containing 29 phytoconstituents,
L. angustifolia comprising 21, and
O. majorana including 23. Collectively, these identified components accounted for 100% of the total chemical composition of the respective EOs.
In
T. satureioides EO (TSEO), sesquiterpenes are dominant, particularly β-himachalene (42.16%) and α-himachalene (20.04%), which contribute to the oil’s therapeutic profile. Both compounds are widely recognized for their anti-inflammatory and antimicrobial activities, positioning TSEO as a potentially valuable medicinal agent. Caryophyllene, another sesquiterpene, is present at 10.80%, adding to the oil’s bioactivity spectrum with its documented anti-inflammatory properties, as supported by studies showing its efficacy in pain and inflammation management [
36]. Minor compounds like δ-cadinene (1.45%) and tumerone (1.19%) also contribute to the complexity of TSEO’s bioactivity. This profile aligns with studies on
Thymus species in North Africa, where sesquiterpenes are often predominant in
Thymus EOs, particularly under arid conditions, suggesting ecological factors may influence the profile [
37].
The EO of
L. angustifolia (LAEO) is primarily composed of oxygenated monoterpenes, with linalool (19.12%) and 1,8-cineole (15.42%) as major components. Linalool, a compound noted for its calming and antimicrobial effects, significantly contributes to LAEO’s therapeutic potential and is frequently cited in aromatherapy studies [
38]. Similarly, 1,8-cineole has been widely reported in
L. angustifolia oils and is valued for its antimicrobial and respiratory benefits, which makes LAEO a versatile EO for health and wellness applications. In comparison, a study by Guitton et al. (2018) found 1,8-cineole at comparable levels in
Lavandula species, highlighting its consistent occurrence across this genus [
39]. camphor (10.02%) and borneol (5.66%) are also prevalent, both of which are known for antimicrobial, analgesic and stimulant properties [
40,
41,
42].
O. majorana EO (OMEO) is particularly rich in camphor (39.58%) and 3-thujanone (23.03%), indicating its potential use in antimicrobial and anti-inflammatory applications. Camphor, a terpene with antiseptic and mild anesthetic effects, has shown efficacy in various medicinal applications, as noted in research on
Origanum species across the Mediterranean [
43]. The presence of thujone (5.69%) and epiglobulol (5.50%) further enhances OMEO’s unique aroma and potential therapeutic benefits. These compounds provide a balanced profile of oxygenated monoterpenes and monoterpenes, which contributes to OMEO’s popularity in both medicinal and culinary contexts. This profile aligns with findings from Aligiannis et al. (2001) [
44], which similarly observed high camphor content in Mediterranean
Origanum species.
Comparing these oils, some compounds such as β-myrcene, linalool, and γ-terpinene are found across all three EOs, albeit in varying concentrations. For instance, linalool appears most abundantly in LAEO (19.12%), while it is present minimally in TSEO (0.72%), and OMEO (0.80%). Camphor, present in both LAEO and OMEO, contributes to their shared therapeutic versatility, with studies showing its benefits in respiratory and pain-relieving applications [
45]. In contrast, β-himachalene is highly specific to TSEO, emphasizing its distinctive sesquiterpene profile.
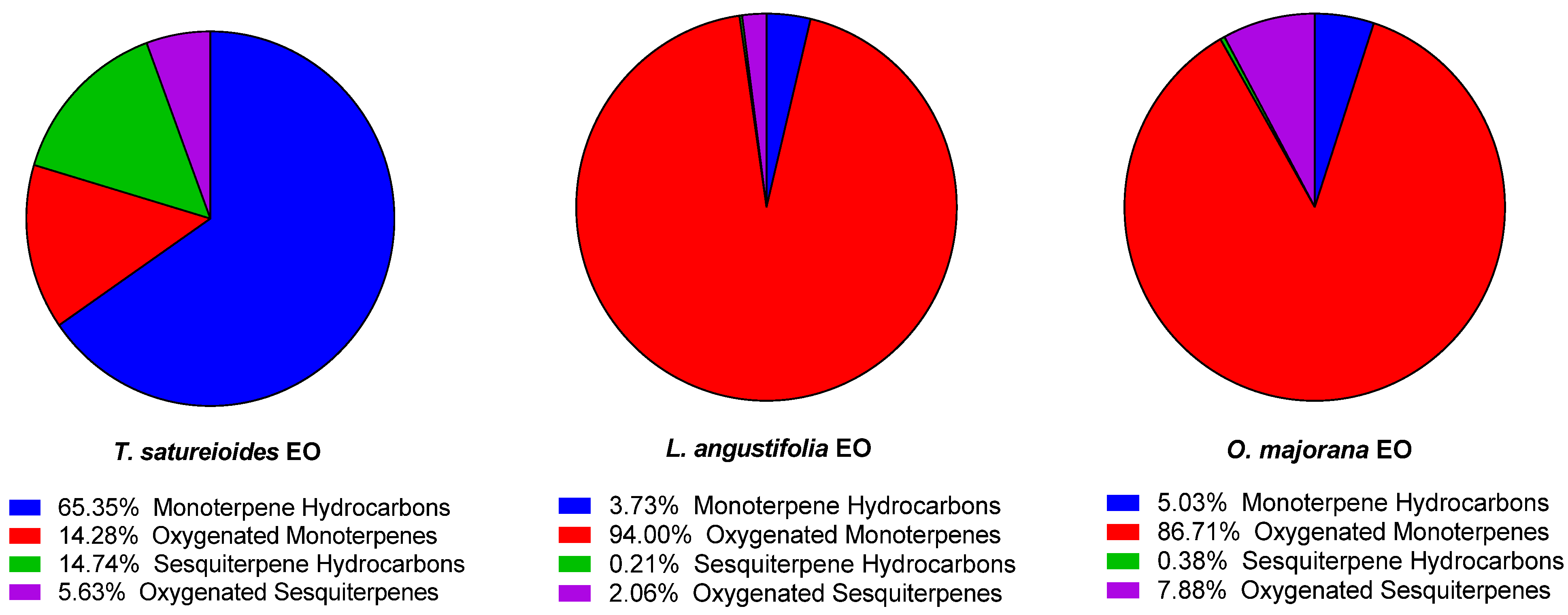
In terms of structural group composition, TSEO is notably rich in monoterpenes hydrocarbons (65.35%), and sesquiterpene hydrocarbons (14.74%), while LAEO and OMEO are mainly dominated by oxygenated monoterpenes (94.00%, and 86.71%, respectively) (
Figure 1). These results are in accordance with previously published studies on these plants [
46,
47,
48]. This distribution reflects their differing ecological adaptations and potential applications. Sesquiterpenes, being more stable and less volatile, are particularly suited for applications requiring prolonged activity, such as their incorporation in smart packaging treatments [
49].
Overall, these investigations highlight the critical role of ecological, climatic, and nutritional factors in shaping both the quantitative and qualitative composition of EOs in plants. They provide compelling evidence that the chemical profiles of EOs are influenced by external and internal factors, including climatic conditions, seasonal fluctuations, soil properties, and intrinsic metabolic pathways. These findings emphasize the complex interplay between environmental conditions and plant physiology in determining the unique chemical characteristics of EOs [
50].
2.2. Antioxidant Activity of Individual EOs
The antioxidant potential of the EOs derived from
T. satureioides,
L. angustifolia, and
O. majorana was assessed using two well-established assays: the DPPH and ABTS radical scavenging methods (
Figure 2). These tests are complementary, as they evaluate antioxidant activity through distinct mechanisms and in varying reaction environments [
51,
52]. All three EOs demonstrated greater antioxidant efficacy in the ABTS assay compared to the DPPH assay. This variation can be attributed to the differences in assay characteristics: ABTS is suitable for both hydrophilic and lipophilic antioxidant systems, whereas DPPH is primarily effective for hydrophobic systems [
53,
54].
To classify the antioxidant activity of essential oils, IC
50 values obtained from the DPPH assay were used as benchmarks. Essential oils with IC
50 values between 200 and 300 µg/mL were categorized as exhibiting moderate antioxidant activity. This classification is supported by previously published studies, including El Hachlafi et al. (2023), which established similar thresholds for evaluating antioxidant potency in essential oils [
55].
T. satureioides EO displayed moderate antioxidant activity, with IC
50 values of 284.67 ± 2.58 µg/mL in the DPPH assay and 239.54 ± 3.29 µg/mL in the ABTS assay. This EO’s chemical composition includes high concentrations of β-himachalene (42.16%), α-himachalene (20.04%), caryophyllene (10.80%), and thymol (9.71%). Both β-himachalene and α-himachalene are sesquiterpenes, and while sesquiterpenes are generally known for their anti-inflammatory and antimicrobial properties, their antioxidant activity can vary. Research on the antioxidant activity of himachalene derivatives is limited; however, sesquiterpenes like these may offer moderate antioxidant effects due to their ability to interact with lipid radicals and slow down oxidative processes [
56]. Caryophyllene is a bicyclic sesquiterpene and is known for its significant antioxidant activity. Studies have shown that caryophyllene can scavenge free radicals effectively, likely due to its unique structure, which allows it to donate electrons and stabilize radical species [
57]. Thymol, a monoterpenoid phenol, is well-known for its potent antioxidant properties, attributed to its ability to donate hydrogen atoms to free radicals and stabilize them. Thymol’s hydroxyl (-OH) group enables it to act as a strong radical scavenger, making it one of the more effective compounds for preventing oxidative damage [
43,
58].
Among the tested EOs,
L. angustifolia displayed the most potent antioxidant activity, with IC
50 values of 84.36 ± 2.99 µg/mL in the DPPH assay and 139.61 ± 2.82 µg/mL in the ABTS assay. Notably, in the DPPH assay, the antioxidant performance of LAEO (IC
50 = 84.36 ± 2.99 µg/mL) exceeded that of synthetic antioxidants, such as BHT (IC
50 = 99.72 ± 8.42 µg/mL) and ascorbic acid (AA) (IC
50 = 126.78 ± 5.33 µg/mL). The EO also showed good activity in the ABTS assay (IC
50 = 139.61 ± 2.82 µg/mL). This exceptional activity is consistent with findings from earlier studies, including those by Nikšić et al., who reported a high antioxidant capacity for
L. angustifolia EO, with an IC
50 value of 0.421 ± 0.03 mg/mL [
59].
The superior antioxidant activity of
L. angustifolia EO can be attributed to its unique chemical composition. The EO contains high concentrations of linalool (19.12%), 1,8-cineole (15.42%), thymol (12.57%), and camphor (10.02%), each of which is known for contributing to antioxidant effects. Linalool, a major component of
L. angustifolia EO, is a monoterpene alcohol with established antioxidant properties. Linalool’s hydroxyl (-OH) group can donate hydrogen atoms to neutralize free radicals, thus stabilizing them and reducing oxidative stress. This property has been well-documented in the literature, where linalool is often highlighted for its ability to scavenge radicals, particularly in EOs from lavender species [
23]. Camphor, another component of
L. angustifolia EO, is a bicyclic monoterpene ketone with moderate antioxidant properties. While camphor demonstrates lower radical scavenging capacity compared to linalool and thymol, it may contribute to antioxidant activity through its ability to modulate lipid peroxidation mechanisms [
60].
O. majorana EO demonstrated strong antioxidant potential, with IC
50 values of 231.57 ± 4.57 µg/mL in the DPPH assay and 273.91 ± 4.36 µg/mL in the ABTS assay. This result is consistent with findings from Milos et al. (2000), who reported significant free radical scavenging activity in
O. majorana EO in both DPPH and ABTS assays, attributing its antioxidant properties to a rich chemical profile that includes high concentrations of oxygenated monoterpenes, such as terpinen-4-ol and γ-terpinene [
61].
Camphor, the most abundant component at 39.58%, has been reported to exhibit robust free radical scavenging activity (in DPPH test, an IC
50 = 77.00 ± 8.30 µg/mL; an IC
50 of 96.32 4.15 µg/mL in ABTS test, and an IC
50 of 101.12 ± 4.03 µg/mL in FRAP test), significantly contributing to the EO’s overall antioxidant performance [
62]. This EO also contains 3-thujanone (23.03%), 1,8-cineol (7.42%), and thujone (5.69%), both of which are recognized for their antioxidative effects [
63]. The synergy among these active compounds likely enhances the antioxidant efficacy of
O. majorana EO, positioning it as a valuable natural source of antioxidants for potential applications in food preservation and healthcare.
Terpenoids, including compounds found in these EOs, possess significant antioxidant potential, primarily through their ability to neutralize free radicals. Oxidative stress arises when there’s an imbalance between free radicals, or reactive oxygen species (ROS), and the body’s antioxidant defenses, leading to cellular damage. Terpenoids play a vital role in combating oxidative stress, making them valuable in both traditional and modern therapeutic applications [
64].
2.3. Single Antibacterial Activity
Table 2 highlights the antibacterial activity of three EOs (TSEO, LAEO, OMEO) against
E. coli,
S. aureus, and
P. aeruginosa, with comparisons to the antibiotics Kanamycin and Chloramphenicol. The inhibition zone diameters (IZ) and minimum inhibitory concentrations (MIC) reveal the varying effectiveness of these EOs in inhibiting bacterial growth.
Firstly, examining the inhibition zone diameters, TSEO stands out as the most potent, especially against
S. aureus (35.05 ± 1.95 mm), followed by
E. coli (26.80 ± 0.30 mm), and
P. aeruginosa (30.25 ± 0.75 mm). This aligns with the existing literature suggesting that thyme oil, rich in monoterpene hydrocarbons, is particularly effective against gram-positive bacteria such as
S. aureus due to its ability to disrupt bacterial cell membranes [
28]. In comparison, LAEO shows moderate IZ values, being most effective against
S. aureus (19.75 ± 1.75 mm), and less so against
E. coli (12.70 ± 1.54 mm) and
P. aeruginosa (15.50 ± 3.00 mm). This effect can be attributed to lavender oil’s components, such as linalool and linalyl acetate, which have known antimicrobial properties, especially against gram-positive bacteria [
65]. Meanwhile, OMEO demonstrates limited activity with minimal IZ values across all bacteria, particularly against
S. aureus, with an IZ value of 1.64 ± 0.50 mm. Though oregano oil usually contains oxygenated monoterpenes, with 1,8-cineol, thymol, and camphor, and is effective against bacterial pathogens, as reported by Si et al. (2006) [
66].
The minimum inhibitory concentration (MIC) values further reinforce these findings. TSEO exhibits the lowest MIC values (0.25–0.5% v/v) across all bacterial strains, highlighting its strong antibacterial effect. Lower MIC values for TSEO against S. aureus, and E. coli suggest that even at low concentrations, thyme oil can effectively inhibit bacterial growth, consistent with its high IZ values. LAEO, on the other hand, requires higher concentrations to achieve inhibition (MIC of 1–2% v/v), indicating moderate antibacterial potency. Its MIC values are similar to those of OMEO, suggesting that while LAEO can be effective, it requires higher concentrations than TSEO to achieve similar effects. OMEO, with MIC values of 2% v/v across all bacteria, demonstrates limited antibacterial efficacy, aligning with its small IZ values and indicating that it might not be as potent or might contain fewer active components.
Comparing these results with the antibiotics kanamycin and chloramphenicol, it is clear that the synthetic antimicrobials exhibit stronger antibacterial activity. Chloramphenicol, in particular, shows the lowest MIC values (0.062–0.25%
v/
v) and consistently large IZs across all bacterial strains, underscoring its superior potency. While TSEO’s activity is notable and, in some cases, comparable to kanamycin, the antibiotics’ overall effectiveness is much higher. Nevertheless, with rising antibiotic resistance, EOs like TSEO present a promising area for developing complementary or alternative antimicrobial agents [
67].
The antibacterial activity of these EOs can be attributed to their specific chemical compositions and associated mechanisms of action. These bioactive compounds target bacterial cells through diverse pathways, including membrane disruption, interference with enzymatic activities, and induction of oxidative stress, leading to inhibiting growth and cell death. For instance, β-himachalene is known for its high affinity to the main bacterial protease, suggesting a potential role in inhibiting bacterial protein functions [
68]. Caryophyllene is known to alter membrane permeability and integrity, causing extensive damage to bacterial cell membranes. This leads to leakage of intracellular content, which disrupts cellular homeostasis and results in cell death [
69]. Similarly, carvacrol and thymol are potent antibacterial agents against both gram-positive and gram-negative bacteria, disrupting bacterial membranes and inhibiting membrane ATPases [
70]. Additionally, Linalool has been shown to disrupt the bacterial membrane by inducing oxidative stress, leading to intracellular leakage and cell death [
71]. Furthermore, Oxygenated monoterpenes such as cineol contribute to antibacterial efficacy by damaging bacterial membranes and inducing reactive oxygen species (ROS)-mediated oxidative stress [
72].
These findings support the potential of EOs, particularly TSEO, as natural antimicrobials, especially in applications where antibiotic resistance is a concern. However, further research is necessary to understand the mechanisms of action, synergistic effects with antibiotics, and in vivo efficacy of these oils. Additionally, the variability in efficacy among different EOs suggests a need for standardized extraction and formulation processes to ensure consistent antibacterial performance [
73].
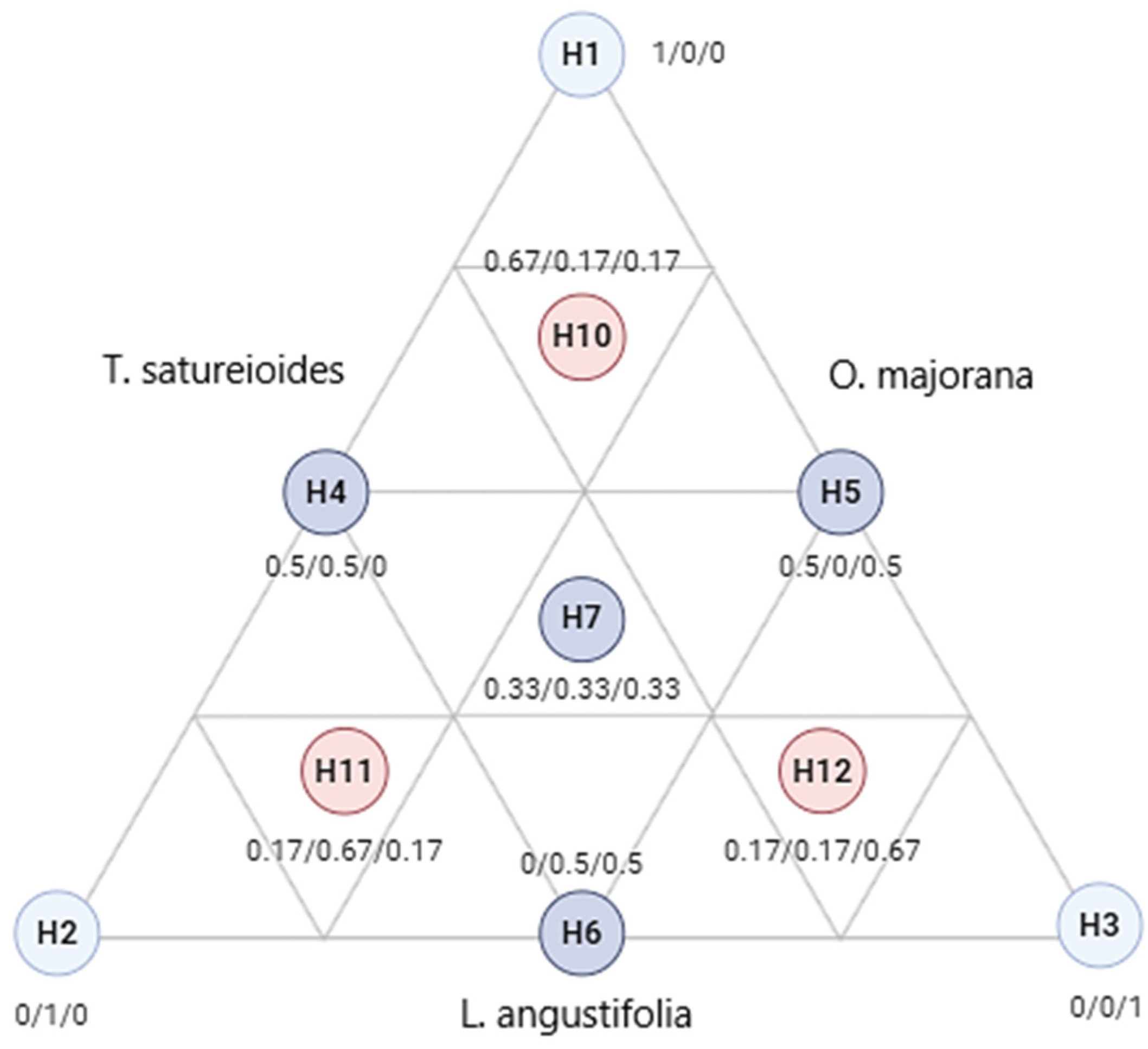
2.4. Simplex Centroid Design
Table 3 provides a detailed examination of the antibacterial effectiveness of various combinations of EOs from
T. satureioides,
L. angustifolia, and
O. majorana against three bacterial strains:
E. coli,
S. aureus, and
P. aeruginosa. This study employs a simplex–centroid design to evaluate the synergistic effects of these oils, with effectiveness quantified by the minimum inhibitory concentration (MIC).
The MIC values across the table suggest varying degrees of antibacterial activity. For
E. coli, the MIC ranges from 0.25 to 0.5, indicating that certain oil combinations can effectively inhibit this gram-negative bacterium. Literature supports this finding, particularly highlighting the efficacy of
O. majorana EO due to its compounds which are known to disrupt bacterial cells [
74].
Against
S. aureus, the MIC values span from 0.25 to 1.0. This variation could be attributed to the different active components in the oils and the unique cell wall properties of this gram-positive bacterium.
L. angustifolia EO is notably effective, potentially due to its linalool content that can penetrate and disrupt bacterial membranes [
23].
The challenge of inhibiting
P. aeruginosa is evident with MIC values ranging from 0.375 to 1.0, reflecting this pathogen’s notorious resistance to many antibiotics. The contribution of
T. satureioides EO, rich in monoterpene hydrocarbons, including thymol and carvacrol, is significant here as these components are known for their ability to compromise the integrity of resistant bacterial membranes [
75].
The study also explores balanced and specialized mixtures of these oils. For instance, mixtures with equal proportions of all three oils show broad-spectrum effectiveness, suggesting potential for general antibacterial applications. Conversely, mixtures tailored with higher proportions of specific oils, such as L. angustifolia for targeting S. aureus, indicate the possibility of optimizing these blends for particular therapeutic uses against resistant strains.
2.5. Variance Analysis of the Fitted Models
Table 4 presents the variance analysis used to evaluate the regression models for antimicrobial activity of essential oil mixtures against
E. coli,
S. aureus, and
P. aeruginosa. The analysis examined degrees of freedom (DF), sum of squares (SS), mean squares (MS), F-statistics, and
p-values for each bacterial species.
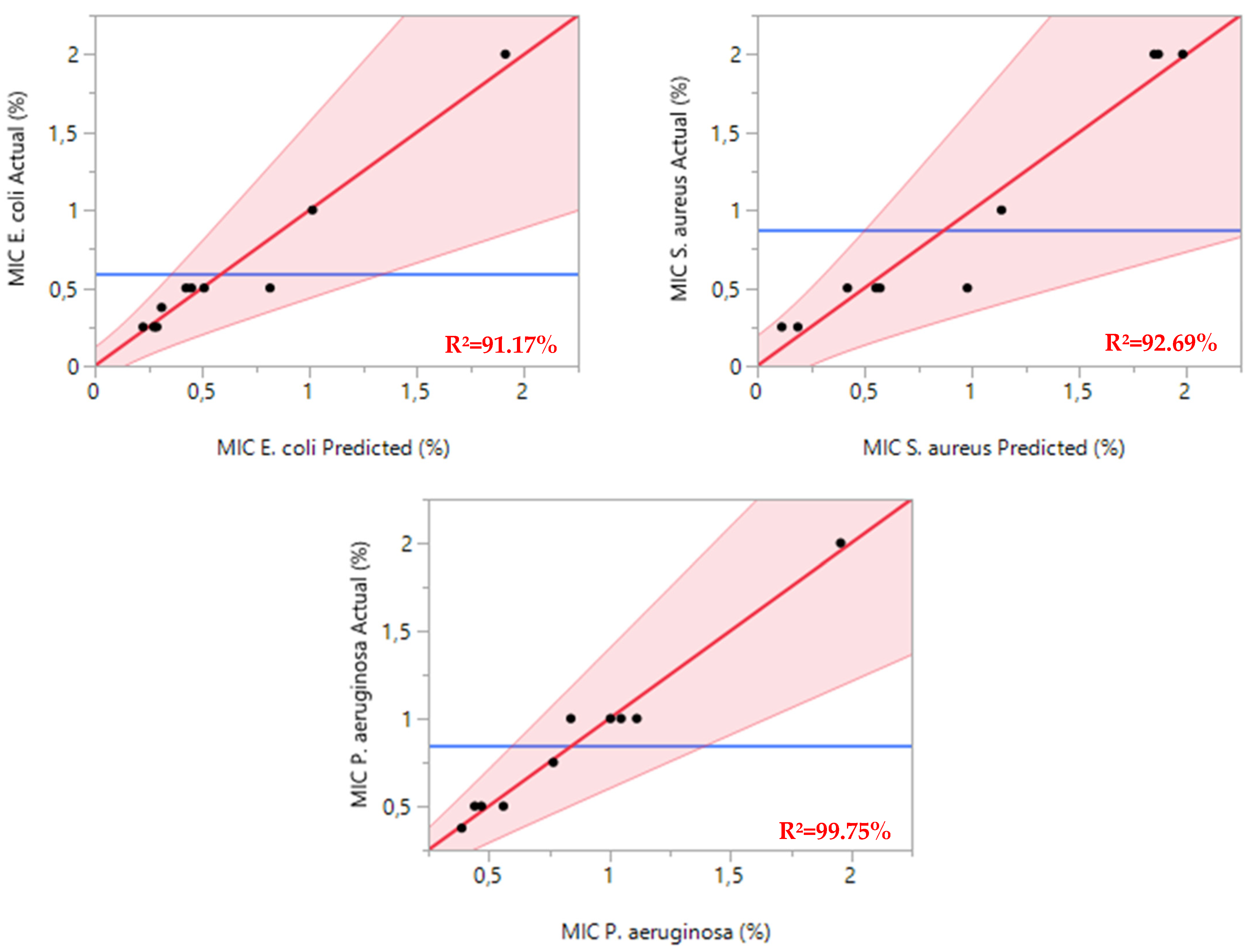
For
E. coli, the regression model showed strong statistical significance with an F-statistic of 16.0996 and a
p-value of 0.0039. The model achieved an R
2 of 0.9117, indicating that it effectively explains over 91% of the variance in minimum inhibitory concentrations (MICs) (
Figure 3.). This high explanatory power confirms the model’s reliability in predicting the antibacterial effects of essential oil combinations against
E. coli.
The model for S. aureus demonstrated similar robustness, with an F-statistic of 12.5836 and a p-value of 0.0069. The R2 value of 0.9269 suggests excellent predictive accuracy, validating the model’s ability to characterize how essential oil mixtures affect S. aureus growth inhibition.
The most striking results emerged from the P. aeruginosa analysis, which yielded an F-statistic of 32.0945 and a p-value of 0.0008. The exceptionally high R2 of 0.9975 demonstrates near-perfect alignment between predicted and observed MIC values, confirming the model’s outstanding accuracy in predicting the antimicrobial efficacy of essential oil combinations against this notably antibiotic-resistant pathogen.
2.6. Components Effects and Adjusted Models
The computed regression coefficients for the special model are shown in
Table 5. The associations between all tested parameters and the obtained MIC responses for
E. coli,
S. aureus, and
P. aeruginosa were found using regression models with significant coefficients (
p-values < 0.05).
The MIC response against
E. coli reveals that the binary interaction terms α13 and α23, along with the coefficients representing the effects of individual components (α
2 and α
3), are statistically significant. However, the ternary interaction term (α
123), the binary interaction term α
12, and the linear term (α
1) do not exhibit any significant influence on the E. coli response (
p > 0.05). Consequently, these non-significant coefficients were excluded from the proposed models, and the resulting mathematical model, as expressed in Equation (1), describes the response as a function of the significant components.
Concerning the MIC
S. aureus response, the significant terms were α
1, α
13, and α
23. These results confirm that the binary effect
T. satureioides, and
L. angustifolia EOs have a major influence on the antibacterial activity against
S. aureus. Equation (2) thus expresses the accepted mathematical model:
For the MIC
P. aeruginosa response, the significant terms were α
1, α
2, α
3, α
13, and α
23. These results showcase that linear and binary terms (except for α
12) have a major influence on the antibacterial activity against
P. aeruginosa. Equation (3) thus expresses the accepted mathematical model:
2.7. Desirability Functions and Optimization of the Mixture
The optimization process, guided by experimental design methodology, aims to identify the ideal ratios of the studied components to maximize the response values. While the optimal results predicted by statistically validated mathematical models may not always precisely match those observed in the 12 experimental trials, they reliably estimate response values within the experimental range. To determine the most favorable outcomes, the optimization process begins by evaluating the highest recorded values. Accordingly, the best MIC results were 0.25%, 0.25%, and 0.375% v/v for E. coli, S. aureus, and P. aeruginosa, respectively. Any configurations capable of producing responses at or above these levels were considered acceptable.
2.8. Formulation Profile
The contour plot and the 3D surface graph, depicted as 2D and 3D mixture plots in
Figure 4 and
Figure 5, illustrate the optimal combination of the three EOs—
T. satureioides,
L. angustifolia, and
O. majorana—for maximizing antibacterial activity, measured as MIC responses against
E. coli,
S. aureus, and
P. aeruginosa. These visual tools provide a clear representation of the relationship between the MIC responses and the concentrations of each essential oil. Generated using Design-Expert software v12.0, the plots employ iso-response curves, which are particularly effective for identifying the precise conditions necessary to achieve the most favorable response values. In the visualizations, the color gradients represent varying levels of antibacterial activity. The blue areas correspond to the lowest MIC values, indicating the highest antibacterial efficacy. In contrast, the regions shaded from yellow to dark red indicate progressively higher MIC values, reflecting reduced antibacterial effectiveness.
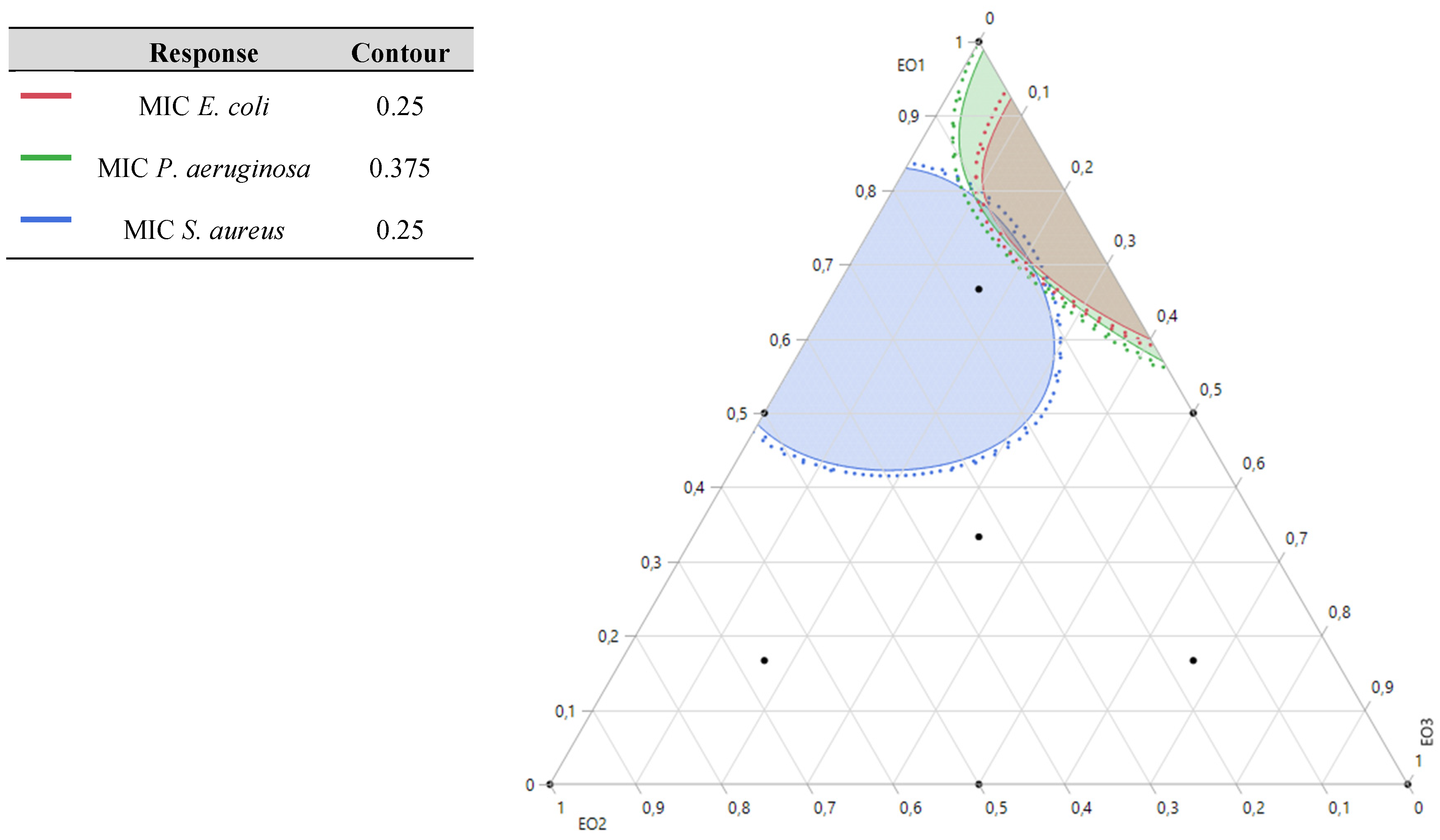
2.8.1. Optimization of MICE. coli Response
The 2D and 3D mixture plots (
Figure 4) reveal that the dark blue region corresponds to the optimal compromise for achieving the lowest MIC value for
E. coli, determined to be 0.20%
v/
v. This optimal result is achieved using a binary combination of
T. satureioides and
O. majorana EOs. The efficacy of this specific mixture is further validated by the desirability test (
Figure 5), which confirms that a MIC value of 0.20%
v/
v, with an impressive desirability score of 99.99%, can be attained when the EOs are combined in the following proportions: 76%
T. satureioides and 24%
O. majorana.
These findings highlight the efficiency of this binary mixture in delivering significant antibacterial activity against E. coli, emphasizing the importance of precise component ratios in optimizing response values. The integration of contour and desirability analysis provides robust evidence supporting the optimal formulation, which holds potential for targeted antibacterial applications.
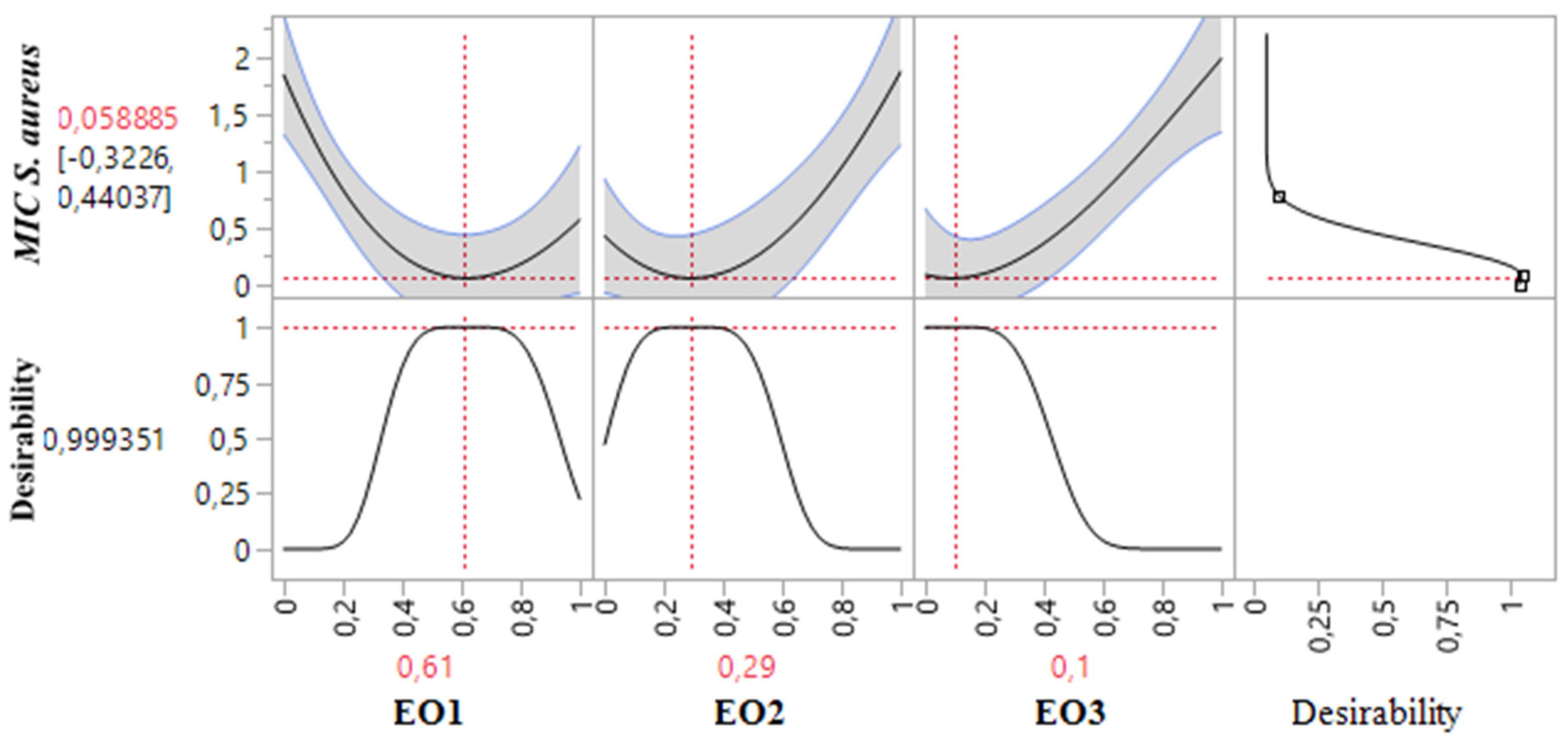
2.8.2. Optimization of MICS. aureus Response
The 2D and 3D mixture plots (
Figure 6) illustrate the dark blue region as the optimal compromise zone for achieving the lowest MIC value for
S. aureus. Experimental MIC values for
S. aureus ranged from 0.25% to 2%
v/
v, as shown in
Table 3. Analysis of the contour and surface plots (
Figure 6) indicates that an optimized MIC value of 0.058%
v/
v can be achieved through a ternary combination of EOs comprising 61% TSEO, 29% LAEO, and 10% OMEO.
This optimization is further supported by the desirability function (
Figure 7), which demonstrates a 99.93% probability of attaining the ideal MIC value (0.058%
v/
v) with this specific combination. These findings highlight the effectiveness of ternary mixtures in achieving superior antibacterial activity against
S. aureus and emphasize the value of statistical and graphical tools in identifying optimal component ratios for enhanced response outcomes.
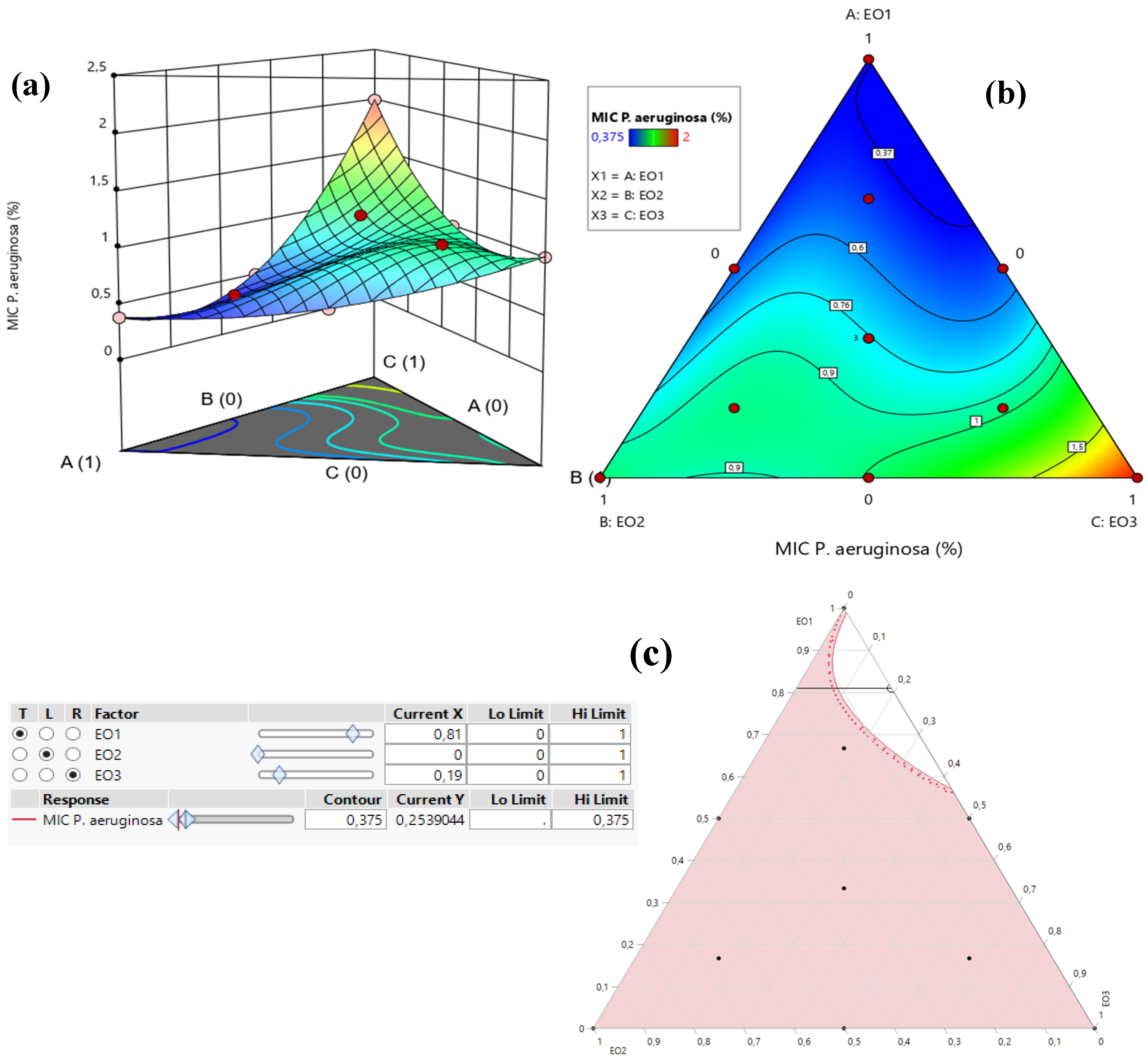
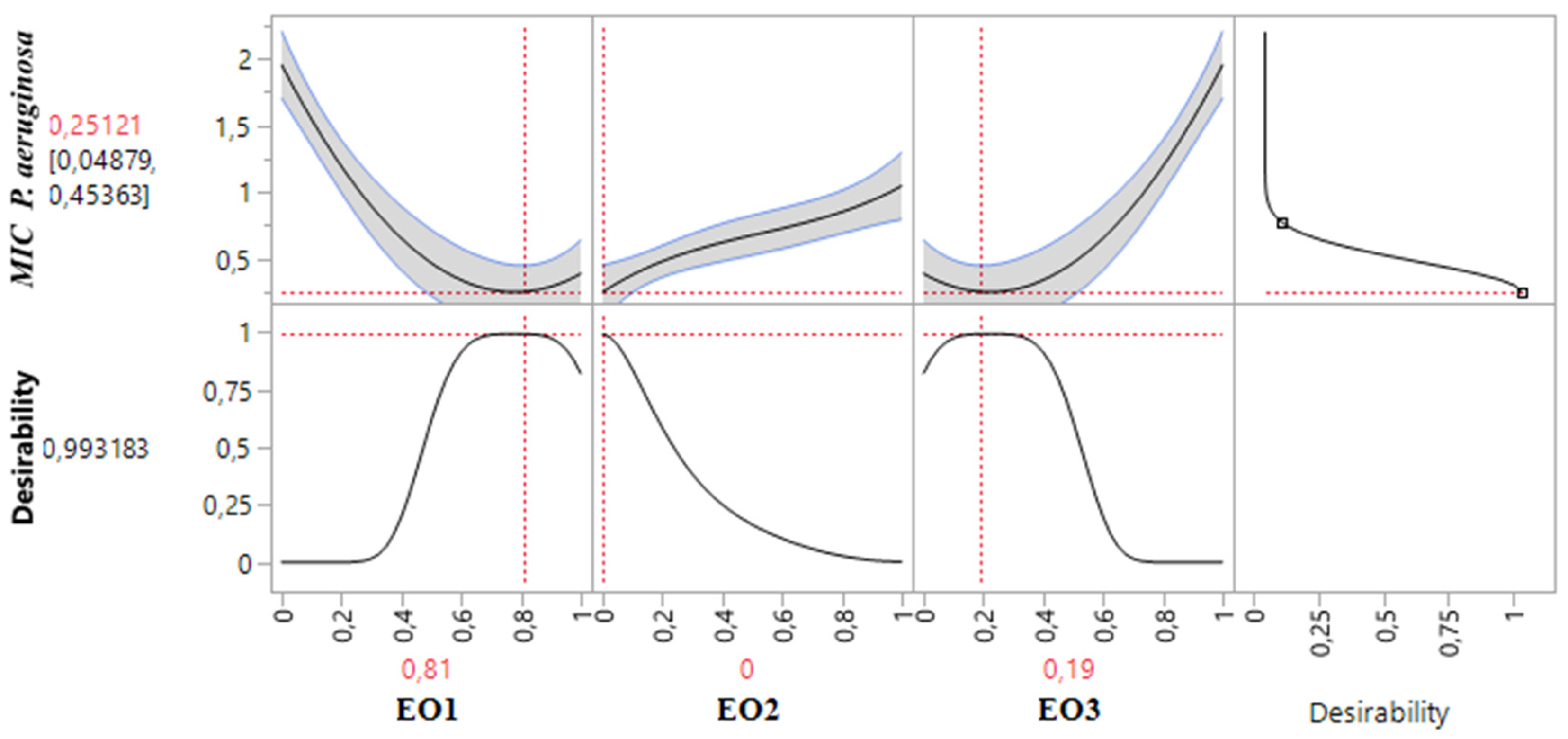
2.8.3. Optimization of MICP. aeruginosa Response
Table 3 demonstrates that the MIC response for
P. aeruginosa varied between 0.375% and 2%. An analysis of the contour and surface plots (
Figure 8) indicates that achieving a significantly low MIC value of 0.02% necessitates a binary mixture of TSEO and OMEO. This highlights the efficacy of these two EOs in combination for strong antibacterial action against
P. aeruginosa.
Additionally, the desirability function analysis (
Figure 9) provides further insights, suggesting a 99.31% probability of achieving an optimal MIC value of 0.25% by utilizing a binary mixture primarily composed of 81% TSEO and 19% OMEO. These results underscore the importance of precise ratio optimization in enhancing the antibacterial potency of essential oil mixtures, providing a practical framework for achieving superior outcomes against
P. aeruginosa.
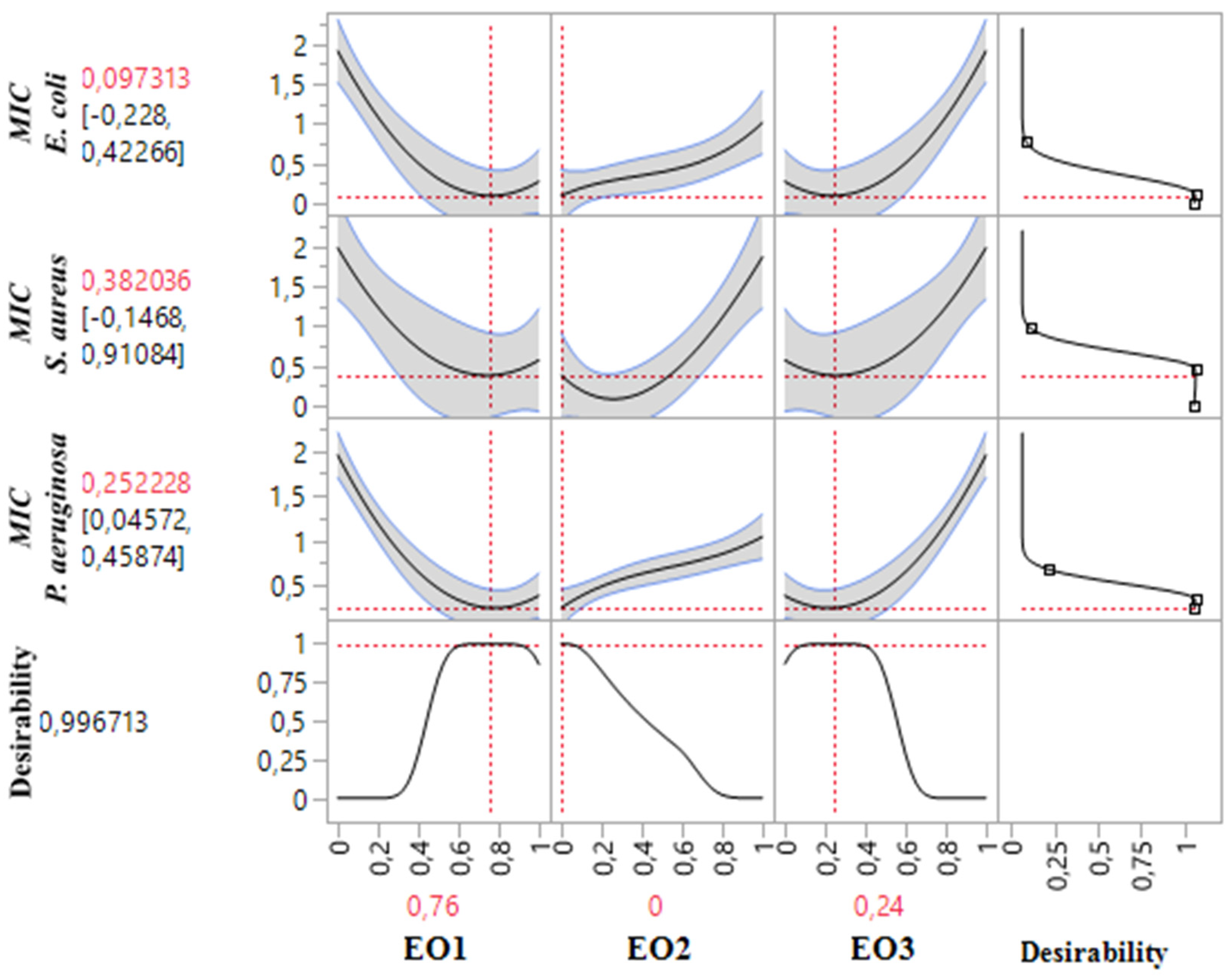
2.9. Simultaneous Optimization of All Responses
The results of simultaneous optimization are particularly evident in the contour plots illustrating the MIC responses for S. aureus, P. aeruginosa, and E. coli, influenced by the combinations of TSEO, LAEO, and OMEO. Notably, the optimal formulations derived from this study demonstrated significantly enhanced antibacterial activity compared to the individual pure EOs, underscoring the efficacy of these optimized mixtures.
These findings validate the performance of the formulated combinations. For
S. aureus, the desired compromise region to achieve the target MIC requires a ternary mixture predominantly composed of TSEO, LAEO, and OMEO. In contrast, for
E. coli and
P. aeruginosa, the optimal antibacterial activity is achieved with a binary mixture primarily comprising TSEO and OMEO. These results, depicted in
Figure 10 and
Figure 11, highlight the critical role of precise component ratios in achieving superior antibacterial responses across multiple bacterial strains.
The use of mixture design methodology has become increasingly popular among researchers in various fields, particularly for creating essential oil (EO) blends. For instance, Benkhaira et al. [
76], demonstrated the synergistic effects of EOs from
Ruta montana L.,
Clinopodium nepeta (L.) Kuntze., and
Dittrichia viscosa (L.) Greuter against
S. aureus, and
P. aeruginosa as innovative antiadhesive agents for 3D-printed materials. Similarly, Kachkoul et al. [
77], highlighted the synergy of EOs from
Rosmarinus officinalis L.,
Eucalyptus camaldulensis Dehnh., and
Mentha pulegium L. against bacteria associated with lithiasis infection. Ouedrhiri et al. [
78], have also reported enhanced antibacterial activity against
Bacillus subtilis, and
S. aureus using a ternary mixture of
Origanum compactum Benth.,
Origanum majorana L., and
Thymus serpyllum L.
The mixtures studied included optimal formulations featuring two predominant classes of compounds: oxygenated monoterpenes (thymol, camphor, linalool, 1,8-cineole, and 3-thujanone) and sesquiterpene hydrocarbons (α-himachalene, β-himachalene, and caryophyllene). These bioactive components target different sites within bacterial cells [
79]. While hydrocarbon monoterpenes display comparatively weaker antibacterial effects, oxygenated terpenoids—containing hydroxyl (-OH) groups—exhibit stronger antibacterial properties [
80]. Key compounds in the EOs, such as thymol and linalool, are known to disrupt membrane permeability by interacting with phospholipids, resulting in a fluidifying effect [
58,
81]. Thymol, in particular, disrupts bacterial citrate metabolism and inhibits ATP-synthesizing enzymes [
82,
83]. Furthermore, Burt [
28], suggested that the synergistic or additive effects among EOs may stem from interactions between major and minor components. Numerous studies in the literature have investigated the synergistic antibacterial properties of EOs derived from various plants, as well as the interactions among their individual components [
76,
78,
84,
85].
Our research supports the effectiveness of combined natural antibacterial therapies in controlling pathogenic bacteria. However, to our knowledge, no prior study has demonstrated the synergistic activity of these EOs against S. aureus, E. coli, and P. aeruginosa.
2.10. Experimental Verification of the Assumed Model
Table 6 provides a comprehensive assessment of cubic models used to evaluate the antibacterial effects of essential oil (EO) combinations from
T. satureioides,
L. angustifolia, and
O. majorana. This analysis is crucial for verifying the accuracy of these models in predicting antibacterial activities against three bacterial strains. The model’s reliability is supported by the close alignment of experimental results with predicted outcomes, demonstrating their strong correlation and confirming the model’s effectiveness in practical applications.
In particular, for the combination containing 76% T. satureioides, and 24% O. majorana, the experimental MICE. coli value was recorded at 0.10 ± 0.00% (v/v), which closely aligns with the predicted value of 0.097 ± 0.00% (v/v). A mixture of 61% T. satureioides, 29% L. angustifolia, and 10% O. majorana showed an experimental MICS.aureus value of 0.06 ± 0.00% (v/v), closely matching the predicted value of 0.058 ± 0.00% (v/v). Additionally, the experimental MICP. aeruginosa for a mixture of 81% T. satureioides, and 19% O. majorana was recorded at 0.25 ± 0.00% (v/v), precisely matching the predicted value of 0.25 ± 0.00% (v/v). These findings underscore the model’s capability to accurately forecast the antibacterial potential of these EO combinations under the conditions tested.
The validation of these results is essential, as it not only verifies the robustness of the modeling approach but also enhances the understanding of how specific EO proportions can synergistically improve antibacterial effects.
4. Conclusions
This study effectively optimized the antibacterial and antioxidant properties of essential oil (EO) mixtures from T. satureioides, L. angustifolia, and O. majorana using a simplex–centroid design. Chemical analysis revealed that TSEO was predominantly composed of β-himachalene (42.16%), α-himachalene (20.04%), and caryophyllene (10.80%), while LAEO contained mainly linalool (19.12%), 1,8-cineol (15.42%), and thymol (12.57%). OMEO was characterized by high levels of camphor (39.58%), 3-thujanone (23.03%), and 1,8-Cineol (7.42%).
The resulting formulations demonstrated significant antibacterial activity, with minimum inhibitory concentrations (MIC) of 0.097% (v/v) against E. coli, 0.058% (v/v) against S. aureus, and 0.250% (v/v) against P. aeruginosa. The optimized mixture, consisting of 76% T. satureioides, and 24% O. majorana, achieved a desirability of 99.99%, showcasing broad-spectrum antibacterial efficacy. In antioxidant evaluations, L. angustifolia EO exhibited superior performance, with IC50 values of 84.36 µg/mL, and 139.61 µg/mL in DPPH and ABTS assays, respectively. This performance surpasses that of T. satureioides, and O. majorana, underscoring its potential in mitigating oxidative stress.
These results suggest promising applications for these EO formulations across various industries. In healthcare, they may serve as natural alternatives or adjuncts to conventional antibiotics, especially against multidrug-resistant pathogens. In the food industry, these formulations could act as natural preservatives, extending shelf life and enhancing safety. Additionally, their potent antioxidant properties position them as potential candidates for incorporation into cosmetic products to combat oxidative damage and aging. However, several limitations must be considered before scaling up these formulations for commercial use. The stability of the essential oil mixtures may be influenced by factors such as temperature, light, and storage conditions, which could affect their efficacy over time. Furthermore, the cost of sourcing and processing these essential oils may limit their widespread use in large-scale applications. Developing more cost-effective methods for extraction and ensuring long-term stability through formulation adjustments or controlled-release systems are essential steps to enhance their practical applicability.