The Potential Application of Nanocarriers in Delivering Topical Antioxidants
Abstract
1. Introduction
2. Overview of the Skin Structure and the Related Challenges in Topical Antioxidant Delivery
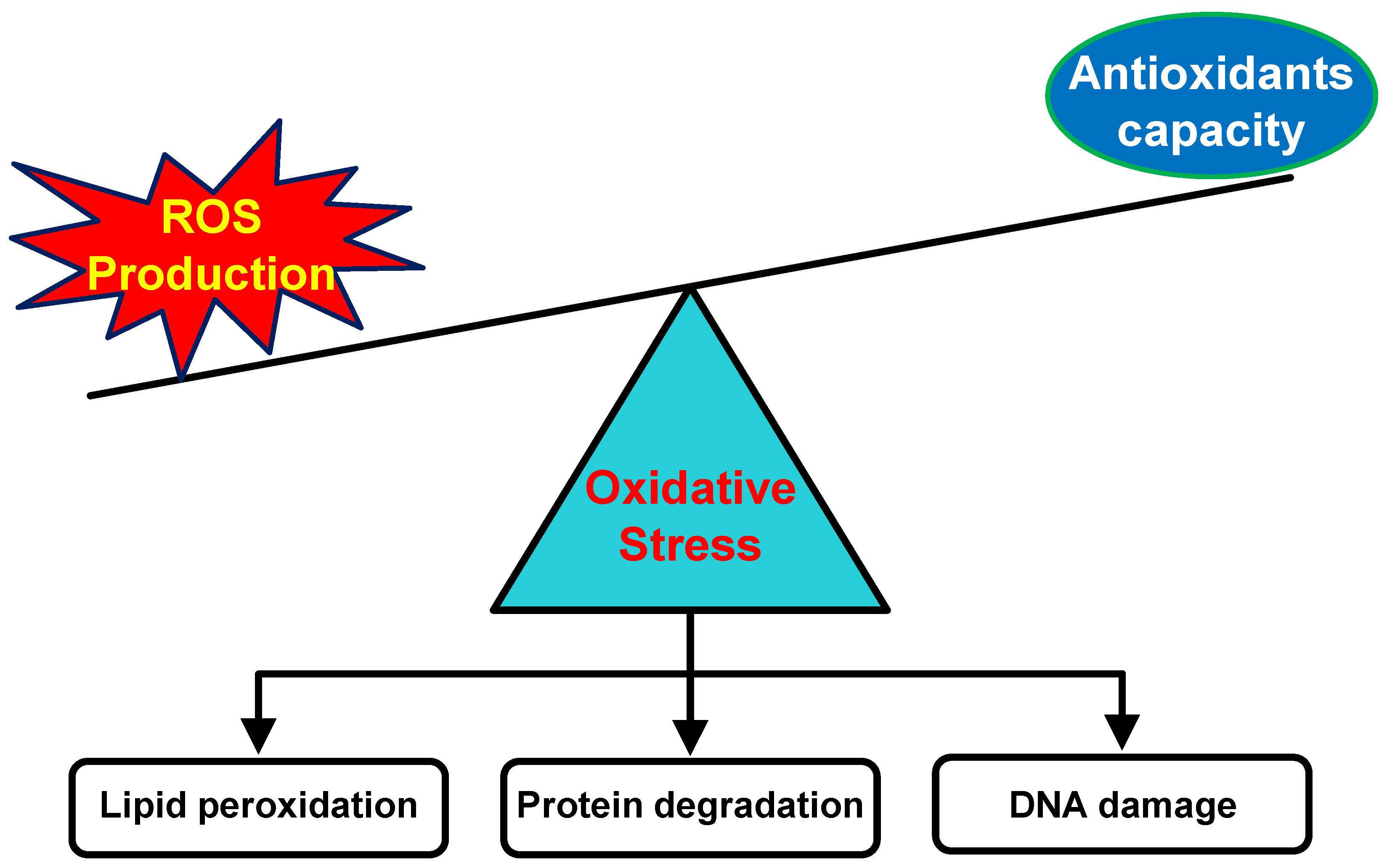
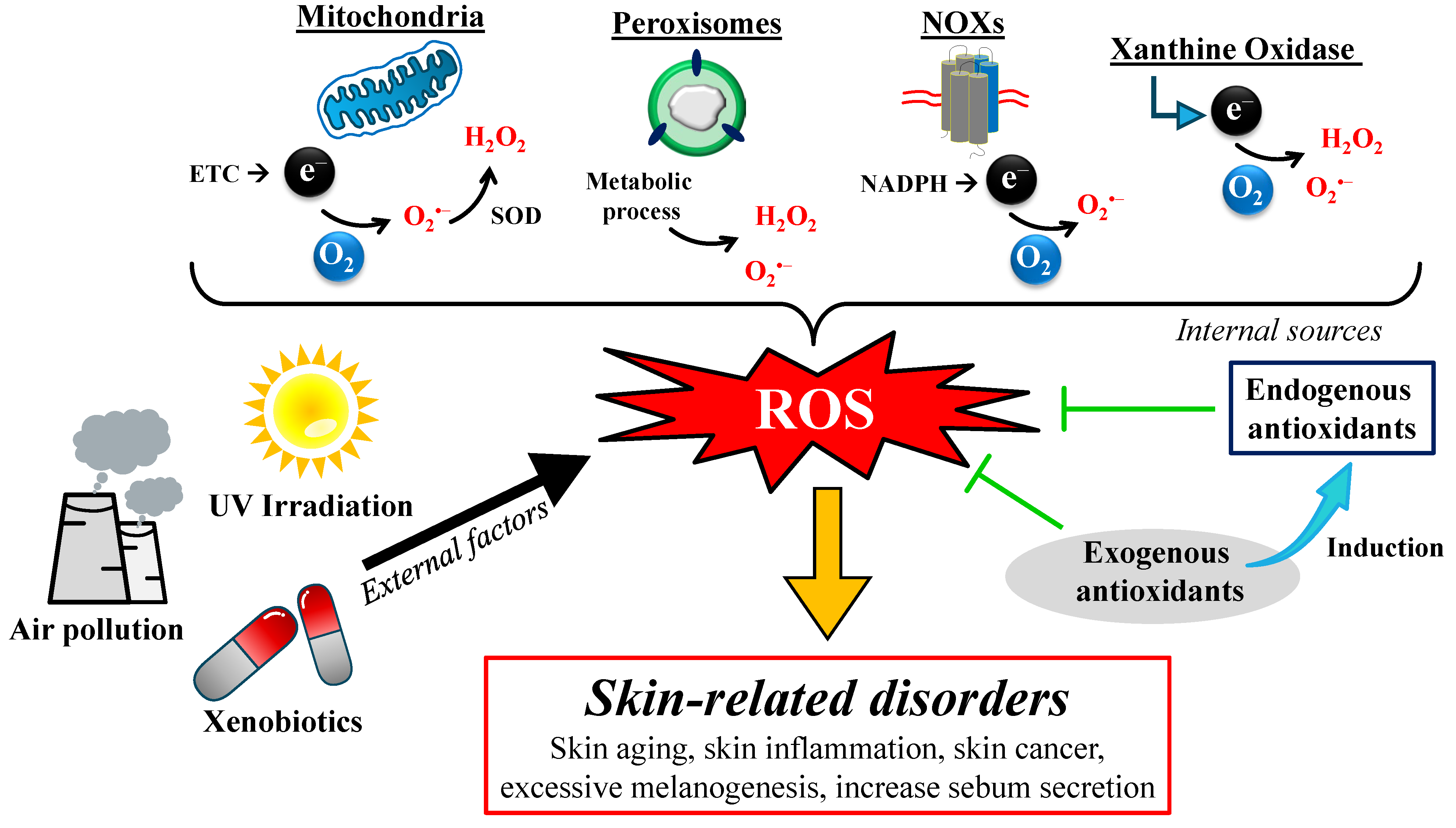
3. The Origin of ROS in the Skin
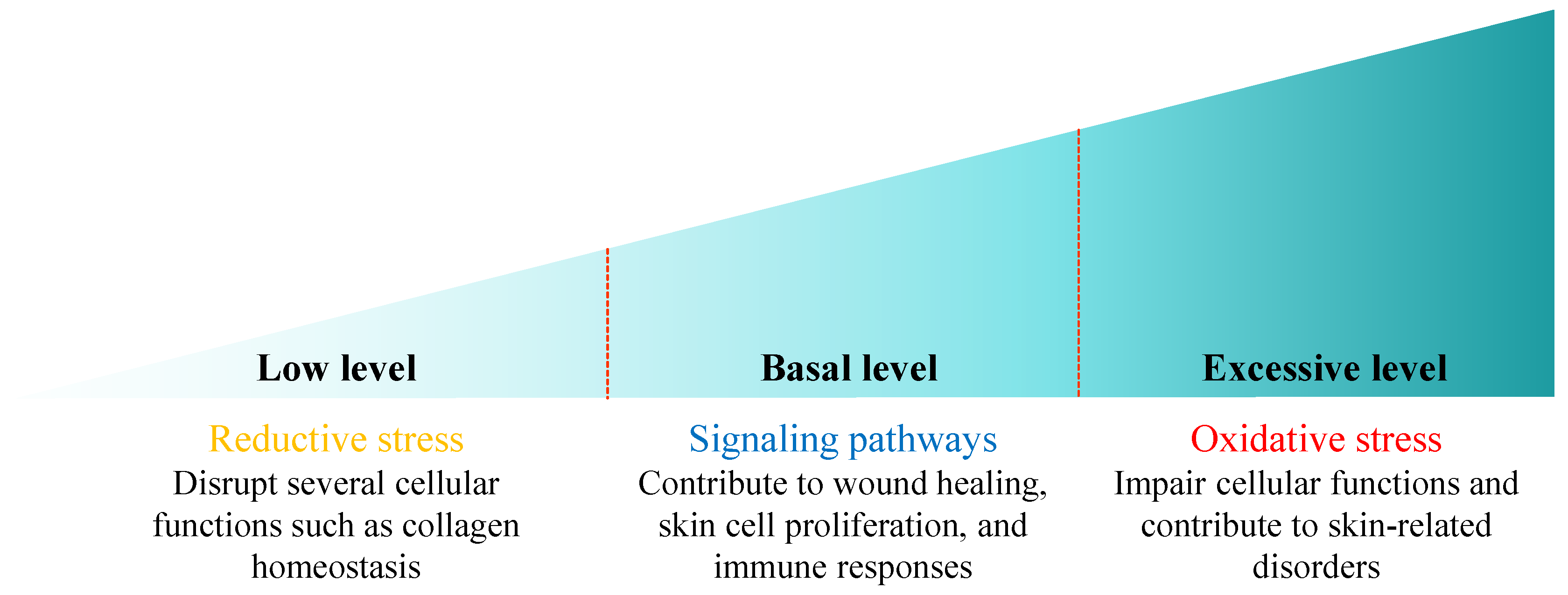
4. The Role of Antioxidant Therapy in Minimizing Harmful Effects of Oxidative Stress
5. Current Clinical Application of Topical Antioxidant Therapy
6. Natural-Resource-Derived Antioxidants
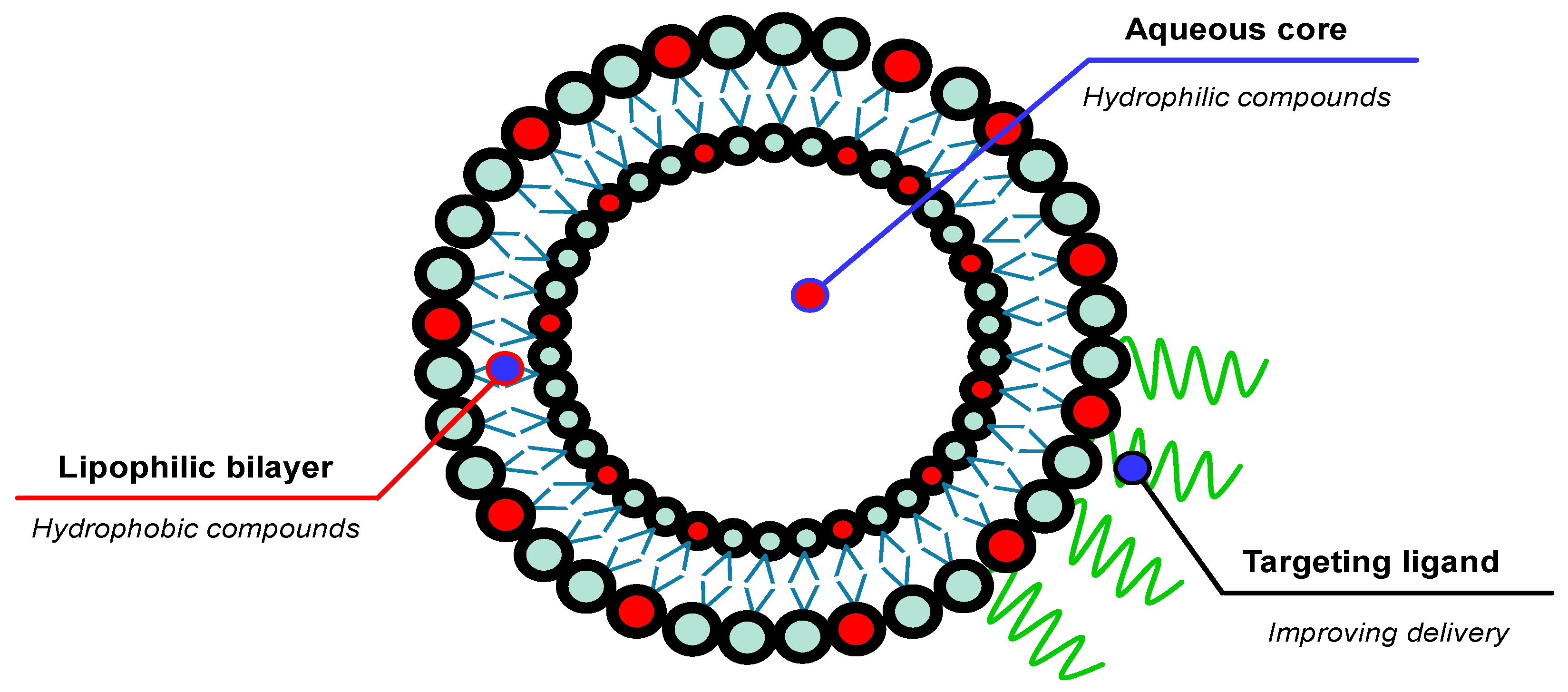
7. The Application of Nanotechnology in Topical Antioxidant Therapy
7.1. Nanoemulsions
| Cargo | Lipid | Surfactant | Result | Refs. |
|---|---|---|---|---|
| Quercetin | Lemon oil and corn oil | Saponin and Tween 80 | Particle size: 52.0 ± 10.0 nm ζ-potential: −41 ± 8 mV | [104] |
| Quercetin | Egg lecithin | Octyldodecanol | Particle size: 197.0 ± 10.0 nm ζ-potential: −27.4 ± 6.0 mV | [105] |
| Achyrocline satureioides extract | Egg lecithin | Octyldodecanol | Particle size: 295.6 ± 9.0 nm ζ-potential: −43.6 ± 2.1 mV | [105] |
| Alpha-lipoic acid | Miglyol 812® | Pluronic® F68 | Particle size: 113.0 ± 12.0 nm ζ-potential: −27.8 ± 2.6 mV | [106] |
| Retinyl palmitate | Labrafac® lipophile | Labrasol® and Plurol® oleique | Particle size: 14.4 ± 1.1 nm ζ-potential: n.a. | [107] |
| Lemon oil | Lemon oil | Tween 80 & Span 80 | Particle size: 64.6 ± 1.6 nm ζ-potential: n.a. | [110] |
| Ginger oil | Ginger oil | Tween 80 and ethanol | Particle size: n.a. ζ-potential: n.a. | [111] |
| Almond oil | Almond oil | Tween 80 | Particle size: 114.1 ± 3.8 nm ζ-potential: −6.8 ± 0.2 mV | [112] |
| Neem oil | Neem oil | Tween 80 | Particle size: 73.4 ± 7.6 nm ζ-potential: −16.2 ± 0.5 mV | [112] |
7.2. Liposomes
7.3. Solid Lipid Nanoparticles (SLNs)
7.4. Nanostructured Lipid Carriers (NLCs)
7.5. Polymeric Nanoparticles
8. The Clinical Implementation of Nanotechnology for Topical Antioxidant Therapy
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| 4nBR | 4-n-butylresorcinol |
| ADP | Adenosine diphosphate |
| AGA | Androgenic alopecia |
| ATP | Adenosine triphosphate |
| BHA | Butylated hydroxy anisole |
| BHT | Butylated hydroxy toluene |
| CAT | Catalase |
| CO | Clove essential oil |
| CoQ10 | Coenzyme Q10 |
| DCP | Dicetyl phosphate |
| DNA | Deoxyribonucleic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DSBs | DNA strand breaks |
| ETC | Electron transport chain |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GRAS | Generally recognized as safe |
| GSH | Glutathione |
| GSH-px | Glutathione peroxidase |
| MASI | Melasma area and severity index |
| MDA | Malondialdehyde |
| MMP-9 | Matrix metalloproteinase 9 |
| NLC | Nanostructured lipid carrier |
| NOXs | NADPH oxidases |
| O/W NE | Oil in water nanoemulsion |
| PAHs | Polycyclic aromatic hydrocarbons |
| PCL | Polycaprolactone |
| PEG | Polyethylene glycol |
| PM | Particulate matters |
| Prxs | Peroxiredoxins |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RSV | Resveratrol |
| SLN | Solid lipid nanoparticle |
| SOD | Superoxide dismutase |
| TA | Tranexamic acid |
| UV | Ultraviolet |
| UVA | Ultraviolet A |
| UVB | Ultraviolet B |
| VCO | Virgin coconut oil |
| VOCs | Volatile organic compounds |
| W/O NE | Water in oil nanoemulsion |
| XO | Xanthine oxidase |
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Addor, F.A.S. Antioxidants in dermatology. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef]
- Azevedo Martins, T.E.; Sales de Oliveira Pinto, C.A.; Costa de Oliveira, A.; Robles Velasco, M.V.; Gorriti Guitiérrez, A.R.; Cosquillo Rafael, M.F.; Tarazona, J.P.H.; Retuerto-Figueroa, M.G. Contribution of Topical Antioxidants to Maintain Healthy Skin—A Review. Sci. Pharm. 2020, 88, 27. [Google Scholar] [CrossRef]
- Leppert, W.; Malec-Milewska, M.; Zajaczkowska, R.; Wordliczek, J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules 2018, 23, 681. [Google Scholar] [CrossRef]
- Cosmetic Antioxidants Market Size to Hit USD 253.24 Mn by 2033 n.d. Available online: https://www.precedenceresearch.com/cosmetic-antioxidants-market (accessed on 26 December 2024).
- Antioxidants Market Size, Share & Value|Global Report [2032] n.d. Available online: https://www.fortunebusinessinsights.com/industry-reports/food-antioxidants-market-100789 (accessed on 26 December 2024).
- Feynman, R.P. There’s Plenty of Room at the Bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Cui, M.; Wiraja, C.; Chew, S.W.T.; Xu, C. Nanodelivery Systems for Topical Management of Skin Disorders. Mol. Pharm. 2021, 18, 491–505. [Google Scholar] [CrossRef]
- Michniak-Kohn, B.; Kohn, J. Overcoming the barrier of skin to drug permeation for localized dermatological therapies. J. Med. Sci. 2023, 92, e926. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Nanocarriers for Delivery of Antioxidants on the Skin. Cosmetics 2015, 2, 342–354. [Google Scholar] [CrossRef]
- Maqsoudlou, A.; Assadpour, E.; Mohebodini, H.; Jafari, S.M. Improving the efficiency of natural antioxidant compounds via different nanocarriers. Adv. Colloid Interface Sci. 2020, 278, 102122. [Google Scholar] [CrossRef] [PubMed]
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin Barriers in Dermal Drug Delivery: Which Barriers Have to Be Overcome and How Can We Measure Them? Pharmaceutics 2020, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.H. Drug delivery to and through the skin. Drug Deliv. Transl. Res. 2024, 14, 2032–2040. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M.H.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Lee, D.H.; Lim, S.; Kwak, S.S.; Kim, J. Advancements in Skin-Mediated Drug Delivery: Mechanisms, Techniques, and Applications. Adv. Healthc. Mater. 2024, 13, 2375. [Google Scholar] [CrossRef]
- Desa, D.E.; Nichols, M.G.; Smith, H.J. Aminoglycosides rapidly inhibit NAD(P)H metabolism increasing reactive oxygen species and cochlear cell demise. J. Biomed. Opt. 2018, 24, 051403. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Magnani, F.; Mattevi, A. Structure and mechanisms of ROS generation by NADPH oxidases. Curr. Opin. Struct. Biol. 2019, 59, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M. New insights into purine metabolism in metabolic diseases: Role of xanthine oxidoreductase activity. Am. J. Physiol.-Endocrinol. Metab. 2020, 319, E827–E834. [Google Scholar] [CrossRef] [PubMed]
- Higa, Y.; Hiasa, M.; Tenshin, H.; Nakaue, E.; Tanaka, M.; Kim, S.; Nakagawa, M.; Shimizu, S.; Tanimoto, K.; Teramachi, J.; et al. The Xanthine Oxidase Inhibitor Febuxostat Suppresses Adipogenesis and Activates Nrf2. Antioxidants 2023, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Richa Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet light induced generation of reactive oxygen species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef]
- Onyango, A.N. Endogenous Generation of Singlet Oxygen and Ozone in Human and Animal Tissues: Mechanisms, Biological Significance, and Influence of Dietary Components. Oxid. Med. Cell Longev. 2016, 2016, 2398573. [Google Scholar] [CrossRef]
- Marrot, L. Pollution and Sun Exposure: A Deleterious Synergy. Mechanisms and Opportunities for Skin Protection. Curr. Med. Chem. 2018, 25, 5469–5486. [Google Scholar] [CrossRef]
- Soeur, J.; Belaïdi, J.-P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C.; Perez, P.; Zanini, M.; Zobiri, O.; Mezzache, S.; et al. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Dermatol. Sci. 2017, 86, 162–169. [Google Scholar] [CrossRef]
- Bayil, S.; Cicek, H.; Cimenci, I.G.; Hazar, M. How volatile organic compounds affect free radical and antioxidant enzyme activity in textile workers. Arh. Hig. Rada Toksikol. 2008, 59, 283–287. [Google Scholar] [CrossRef][Green Version]
- Bayil, S.; Celik, A.; Cicek, H.; Geyikli, I.; Tarakcioglu, M. Free radical and antioxidant enzyme levels at exposure of volatile organic compounds in workers. Saudi Med. J. 2007, 28, 290–291. [Google Scholar]
- Kuang, H.; Li, Z.; Lv, X.; Wu, P.; Tan, J.; Wu, Q.; Li, Y.; Jiang, W.; Pang, Q.; Wang, Y.; et al. Exposure to volatile organic compounds may be associated with oxidative DNA damage-mediated childhood asthma. Ecotoxicol. Environ. Saf. 2021, 210, 111864. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.V.R.; Sauce, R.; de Oliveira, C.A.; de Oliveira Pinto, C.A.S.; Martinez, R.M.; Baah, S.; Almeida, T.S.; Rosado, C.; Baby, A.R. Active ingredients, mechanisms of action and efficacy tests antipollution cosmetic and personal care products. Braz. J. Pharm. Sci. 2018, 54, e01003. [Google Scholar] [CrossRef]
- Patzelt, A.; Antoniou, C.; Sterry, W.; Lademann, J. Skin penetration from the inside to the outside: A review. Drug Discov. Today Dis. Mech. 2008, 5, e229–e235. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Carrasco, E.; Calvo, M.I.; Blázquez-Castro, A.; Vecchio, D.; Zamarrón, A.; de Almeida, I.J.D.; Stockert, J.C.; Hamblin, M.R.; Juarranz, Á.; Espada, J. Photoactivation of ROS Production In Situ Transiently Activates Cell Proliferation in Mouse Skin and in the Hair Follicle Stem Cell Niche Promoting Hair Growth and Wound Healing. J. Investig. Dermatol. 2015, 135, 2611–2622. [Google Scholar] [CrossRef]
- Choi, D.-I.; Park, J.-H.; Choi, J.-Y.; Piao, M.; Suh, M.-S.; Lee, J.-B.; Yun, S.-J.; Lee, S.-C. Keratinocytes-Derived Reactive Oxygen Species Play an Active Role to Induce Type 2 Inflammation of the Skin: A Pathogenic Role of Reactive Oxygen Species at the Early Phase of Atopic Dermatitis. Ann. Dermatol. 2021, 33, 26. [Google Scholar] [CrossRef]
- Carne, N.A.; Bell, S.; Brown, A.P.; Määttä, A.; Flagler, M.J.; Benham, A.M. Reductive Stress Selectively Disrupts Collagen Homeostasis and Modifies Growth Factor-independent Signaling Through the MAPK/Akt Pathway in Human Dermal Fibroblasts. Mol. Cell. Proteom. 2019, 18, 1123–1137. [Google Scholar] [CrossRef]
- Alonso, C.; Rubio, L.; Touriño, S.; Martí, M.; Barba, C.; Fernández-Campos, F.; Coderch, L.; Parra, J.L. Antioxidative effects and percutaneous absorption of five polyphenols. Free Radic. Biol. Med. 2014, 75, 149–155. [Google Scholar] [CrossRef]
- Alonso, C.; Martí, M.; Barba, C.; Carrer, V.; Rubio, L.; Coderch, L. Skin permeation and antioxidant efficacy of topically applied resveratrol. Arch. Dermatol. Res. 2017, 309, 423–431. [Google Scholar] [CrossRef]
- Chew, S.C.; Nyam, K.L. Chapter 6—Refining of edible oils. In Lipids and Edible Oils; Academic Press: Cambridge, MA, USA, 2020; pp. 213–241. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Vale, D.L.; Martinez, R.M.; Medeiros, D.C.; da Rocha, C.; Sfeir, N.; Lopez, R.F.V.; Vicentini, F.T.M.C.; Verri, W.A., Jr.; Georgetti, S.R.; Baracat, M.M.; et al. A topical formulation containing quercetin-loaded microcapsules protects against oxidative and inflammatory skin alterations triggered by UVB irradiation: Enhancement of activity by microencapsulation. J. Drug Target. 2021, 29, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, W.-J.; Choi, J.; Frei, B. Quercetin affects glutathione levels and redox ratio in human aortic endothelial cells not through oxidation but formation and cellular export of quercetin-glutathione conjugates and upregulation of glutamate-cysteine ligase. Redox Biol. 2016, 9, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Buranasudja, V.; Rani, D.; Malla, A.; Kobtrakul, K.; Vimolmangkang, S. Insights into antioxidant activities and anti-skin-aging potential of callus extract from Centella asiatica (L.). Sci. Rep. 2021, 11, 13459. [Google Scholar] [CrossRef]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid. Med. Cell Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef]
- Yi, N.; Chiang, Z. Topical Vitamin C and the Skin. Jcad J. Clin. Aesthetic Dermatol. 2017, 14, 14–17. [Google Scholar]
- Telang, P. Vitamin C in dermatology. Indian Dermatol. Online J. 2013, 4, 143. [Google Scholar] [CrossRef]
- Pehlivan, F.E. Vitamin C: An Antioxidant Agent; Hamza, A.H., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 2. [Google Scholar] [CrossRef]
- Matsuda, S.; Shibayama, H.; Hisama, M.; Ohtsuki, M.; Iwaki, M. Inhibitory effects of a novel ascorbic derivative, disodium isostearyl 2-O-L-ascorbyl phosphate on melanogenesis. Chem. Pharm. Bull. 2008, 56, 292–297. [Google Scholar] [CrossRef]
- Burke, K.E. Interaction of vitamins C and E as better cosmeceuticals. Dermatol. Ther. 2007, 20, 314–321. [Google Scholar] [CrossRef]
- Keen, M.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311. [Google Scholar] [CrossRef]
- Augustyniak, A.; Bartosz, G.; Cipak, A.; Duburs, G.; Horáková, L.; Luczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, E.; et al. Natural and synthetic antioxidants: An updated overview. Free Radic. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef]
- Fujisawa, S.; Kadoma, Y.; Yokoe, I. Radical-scavenging activity of butylated hydroxytoluene (BHT) and its metabolites. Chem. Phys. Lipids 2004, 130, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.Y.; Lee, Y.C. Natural antioxidants from plant extracts in skincare cosmetics: Recent applications, challenges and perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Thakore, K.N. Butylated hydroxyanisole. In Encyclopedia of Toxicology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 364–365. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Liaudanskas, M.; Žvikas, V.; Petrikaitė, V. The Potential of Dietary Antioxidants from a Series of Plant Extracts as Anticancer Agents against Melanoma, Glioblastoma, and Breast Cancer. Antioxidants 2021, 10, 1115. [Google Scholar] [CrossRef]
- Lu, Q.; Summanen, P.H.; Lee, R.; Huang, J.; Henning, S.M.; Heber, D.; Finegold, S.M.; Li, Z. Prebiotic Potential and Chemical Composition of Seven Culinary Spice Extracts. J. Food Sci. 2017, 82, 1807–1813. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Teoh, E.S. Medicinal Orchids of Asia; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–752. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The importance of antioxidants and place in today’s scientific and technological studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Charoensup, W.; Sriyab, S.; Punyoyai, C.; Neimkhum, W. Herbal Extracts as Potential Antioxidant, Anti-Aging, Anti-Inflammatory, and Whitening Cosmeceutical Ingredients. Chem. Biodivers. 2021, 18, e2100245. [Google Scholar] [CrossRef]
- Dienaitė, L.; Pukalskienė, M.; Pukalskas, A.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Isolation of strong antioxidants from paeonia officinalis roots and leaves and evaluation of their bioactivities. Antioxidants 2019, 8, 249. [Google Scholar] [CrossRef]
- Moshari-Nasirkandi, A.; Alirezalu, A.; Alipour, H.; Amato, J. Screening of 20 species from Lamiaceae family based on phytochemical analysis, antioxidant activity and HPLC profiling. Sci. Rep. 2023, 13, 16987. [Google Scholar] [CrossRef]
- Seawan, N.; Vichit, W.; Thakam, A.; Thitipramote, N.; Chaiwut, P.; Pintathong, P.; Thitilertdech, N. Antioxidant Capacities, Phenolic, Anthocyanin and Proanthocyanidin Contents of Pigmented Rice Extracts Obtained by Microwave-Assisted Method. Suranaree J. Sci. Technol. 2014, 21, 301–306. [Google Scholar] [CrossRef]
- Chen, X.; Shang, S.; Yan, F.; Jiang, H.; Zhao, G.; Tian, S.; Chen, R.; Chen, D.; Dang, Y. Antioxidant Activities of Essential Oils and Their Major Components in Scavenging Free Radicals, Inhibiting Lipid Oxidation and Reducing Cellular Oxidative Stress. Molecules 2023, 28, 4559. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Amengual, J. Bioactive Properties of Carotenoids in Human Health. Nutrients 2019, 11, 2388. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Rodríguez-Concepción, M. Carotenoid Biosynthesis in Arabidopsis: A Colorful Pathway. Arabidopsis Book 2012, 10, e0158. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Advances in photosynthesis and respiration. Photosynth. Res. 2012, 111, 327–329. [Google Scholar] [CrossRef][Green Version]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, J. Astaxanthin isomers: Selective distribution and isomerization in aquatic animals. Aquaculture 2020, 520, 734915. [Google Scholar] [CrossRef]
- Šimat, V.; Rathod, N.; Čagalj, M.; Hamed, I.; Generalić Mekinić, I. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Mar. Drugs 2022, 20, 206. [Google Scholar] [CrossRef]
- Visioli, F.; Artaria, C. Astaxanthin in cardiovascular health and disease: Mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct. 2017, 8, 39–63. [Google Scholar] [CrossRef]
- Che, H.; Li, Q.; Zhang, T.; Wang, D.; Yang, L.; Xu, J.; Yanagita, T.; Xue, C.; Chang, Y.; Wang, Y. Effects of Astaxanthin and Docosahexaenoic-Acid-Acylated Astaxanthin on Alzheimer’s Disease in APP/PS1 Double-Transgenic Mice. J. Agric. Food Chem. 2018, 66, 4948–4957. [Google Scholar] [CrossRef]
- Dutta, S.; Kumar, S.P.J.; Banerjee, R. A comprehensive review on astaxanthin sources, structure, biochemistry and applications in the cosmetic industry. Algal Res. 2023, 74, 103168. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Nicod, N.; Parker, R.S. Vitamin E Secretion by Caco-2 Monolayers to APOA1, but Not to HDL, Is Vitamer Selective. J. Nutr. 2013, 143, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols—Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.-Y.; Ward, L.C.; Fong, C.W.; Yap, W.N.; Brown, L. Anti-inflammatory γ- and δ-tocotrienols improve cardiovascular, liver and metabolic function in diet-induced obese rats. Eur. J. Nutr. 2017, 56, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Teo, J.S.M.; Chai, S.F.; Yeap, S.L.; Lau, A.J. Vitamin E analogues differentially inhibit human cytochrome P450 3A (CYP3A)-mediated oxidative metabolism of lithocholic acid: Impact of δ-tocotrienol on lithocholic acid cytotoxicity. Toxicology 2019, 423, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Pahrudin Arrozi, A.; Shukri, S.N.S.; Wan Ngah, W.Z.; Mohd Yusof, Y.A.; Ahmad Damanhuri, M.H.; Jaafar, F.; Makpol, S. Comparative Effects of Alpha- and Gamma-Tocopherol on Mitochondrial Functions in Alzheimer’s Disease In Vitro Model. Sci. Rep. 2020, 10, 8962. [Google Scholar] [CrossRef]
- Salaj, N.; Kladar, N.; Srđenović Čonić, B.; Jeremić, K.; Hitl, M.; Gavarić, N.; Božin, B. Traditional multi-herbal formula in diabetes therapy—Antihyperglycemic and antioxidant potential. Arab. J. Chem. 2021, 14, 103347. [Google Scholar] [CrossRef]
- Farag, R.S.; Abdel-Latif, M.S.; Abd El Baky, H.H.; Tawfeek, L.S. Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnol. Rep. 2020, 28, e00536. [Google Scholar] [CrossRef]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Rosas-Murrieta, N.H.; Millán-Perez-Peña, L.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef]
- Ghafoor, K.; Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Babiker, E.E.; Mohamed Ahmed, I.A. Total phenolics, total carotenoids, individual phenolics and antioxidant activity of ginger (Zingiber officinale) rhizome as affected by drying methods. LWT 2020, 126, 109354. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Letsiou, S.; Bakea, A.; Holefors, A.; Rembiesa, J. In vitro protective effects of Paeonia mascula subsp. hellenica callus extract on human keratinocytes. Sci. Rep. 2020, 10, 19213. [Google Scholar] [CrossRef]
- Elmowafy, M. Skin penetration/permeation success determinants of nanocarriers: Pursuit of a perfect formulation. Colloids Surf. B Biointerfaces 2021, 203, 111748. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Franzè, S.; Minghetti, P.; Casiraghi, A. Emulsion versus nanoemulsion: How much is the formulative shift critical for a cosmetic product? Drug Deliv. Transl. Res. 2018, 8, 414–421. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Ganta, S.; Talekar, M.; Singh, A.; Coleman, T.P.; Amiji, M.M. Nanoemulsions in translational research—Opportunities and challenges in targeted cancer therapy. AAPS PharmSciTech 2014, 15, 694–708. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, R.; Mehta, S.K. Formulation of saponin stabilized nanoemulsion by ultrasonic method and its role to protect the degradation of quercitin from UV light. Ultrason. Sonochem. 2016, 31, 29–38. [Google Scholar] [CrossRef]
- Zorzi, G.K.; Caregnato, F.; Moreira, J.C.F.; Teixeira, H.F.; Carvalho, E.L.S. Antioxidant Effect of Nanoemulsions Containing Extract of Achyrocline satureioides (Lam) D.C.—Asteraceae. AAPS PharmSciTech 2016, 17, 844–850. [Google Scholar] [CrossRef]
- Ruktanonchai, U.; Bejrapha, P.; Sakulkhu, U.; Opanasopit, P.; Bunyapraphatsara, N.; Junyaprasert, V.; Puttipipatkhachorn, S. Physicochemical characteristics, cytotoxicity, and antioxidant activity of three lipid nanoparticulate formulations of alpha-lipoic acid. AAPS PharmSciTech 2009, 10, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Clares, B.; Calpena, A.C.; Parra, A.; Abrego, G.; Alvarado, H.; Fangueiro, J.F.; Souto, E.B. Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: Effect on skin permeation. Int. J. Pharm. 2014, 473, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef]
- Sindle, A.; Martin, K. Art of Prevention: Essential Oils—Natural Products Not Necessarily Safe. Int. J. Womens Dermatol. 2021, 7, 304–308. [Google Scholar] [CrossRef]
- Liu, T.; Gao, Z.; Zhong, W.; Fu, F.; Li, G.; Guo, J.; Shan, Y. Preparation, Characterization, and Antioxidant Activity of Nanoemulsions Incorporating Lemon Essential Oil. Antioxidants 2022, 11, 650. [Google Scholar] [CrossRef]
- Ningsih, I.Y.; Faradisa, H.; Cahyani, M.D.; Rosyidi, V.A.; Hidayat, M.A. The formulation of ginger oil nanoemulsions of three varieties of ginger (Zingiber officinale rosc.) as natural antioxidant. J. Res. Pharm. 2020, 24, 914–924. [Google Scholar] [CrossRef]
- Rinaldi, F.; Hanieh, P.N.; Maurizi, L.; Longhi, C.; Uccelletti, D.; Schifano, E.; Del Favero, E.; Cantù, L.; Ricci, C.; Ammendolia, M.G.; et al. Neem Oil or Almond Oil Nanoemulsions for Vitamin E Delivery: From Structural Evaluation to in vivo Assessment of Antioxidant and Anti-Inflammatory Activity. Int. J. Nanomed. 2022, 17, 6447–6465. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Satrialdi Takano, Y.; Hirata, E.; Ushijima, N.; Harashima, H.; Yamada, Y. An effective in vivo mitochondria-targeting nanocarrier combined with a π-extended porphyrin-type photosensitizer. Nanoscale Adv. 2021, 3, 5919–5927. [Google Scholar] [CrossRef]
- Akbaba, H.; Erel-Akbaba, G.; Başpınar, Y.; Şentürk, Ş. Design of Liposome Formulations for CRISPR/Cas9 Enzyme Immobilization: Evaluation of 5-Alpha-Reductase Enzyme Knockout for Androgenic Disorders. ACS Omega 2023, 8, 46101–46112. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Haloho, S.E.E.; Harashima, H. Intravenous liposomal vaccine enhances CTL generation, but not until antigen presentation. J. Control. Release 2022, 343, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hibino, M.; Yamada, Y.; Fujishita, N.; Sato, Y.; Maeki, M.; Tokeshi, M.; Harashima, H. The Use of a Microfluidic Device to Encapsulate a Poorly Water-Soluble Drug CoQ10 in Lipid Nanoparticles and an Attempt to Regulate Intracellular Trafficking to Reach Mitochondria. J. Pharm. Sci. 2019, 108, 2668–2676. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Pierre, M.B.R.; Dos Santos Miranda Costa, I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch. Dermatol. Res. 2011, 303, 607–621. [Google Scholar] [CrossRef]
- Briuglia, M.-L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Farzaneh, H.; Ebrahimi Nik, M.; Mashreghi, M.; Saberi, Z.; Jaafari, M.R.; Teymouri, M. A study on the role of cholesterol and phosphatidylcholine in various features of liposomal doxorubicin: From liposomal preparation to therapy. Int. J. Pharm. 2018, 551, 300–308. [Google Scholar] [CrossRef]
- Serrano, G.; Almudéver, P.; Serrano, J.M.; Milara, J.; Torrens, A.; Expósito, I.; Cortijo, J. Phosphatidylcholine liposomes as carriers to improve topical ascorbic acid treatment of skin disorders. Clin. Cosmet. Investig. Dermatol. 2015, 8, 591–599. [Google Scholar] [CrossRef]
- Min, M.; Egli, C.; Bartolome, R.; Sivamani, R. Ex vivo Evaluation of a Liposome-Mediated Antioxidant Delivery System on Markers of Skin Photoaging and Skin Penetration. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1481–1494. [Google Scholar] [CrossRef]
- Luo, F.; Wang, S.; Zhang, X.; Liu, Z.; Zhu, R.; Xue, W. Extraction of Astaxanthin from Haematococcus pluvialis and Preparation of Astaxanthin Liposomes. Molecules 2024, 29, 3320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Z.; Zhang, W.; Wang, L.; Zhao, P.; Lv, X.; Guo, P.; Chen, J. Surface modification by chitosan for improving stability and antioxidative activity of astaxanthin-loaded liposomes. LWT 2024, 198, 116033. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Perera, A.D.L.C.; Karunaratne, V.; Amaratunga, G.A.J.; Karunaratne, D.N. Improved Delivery of Caffeic Acid through Liposomal Encapsulation. J. Nanomater. 2016, 2016, 9701870. [Google Scholar] [CrossRef]
- Gokce, E.H.; Korkmaz, E.; Tuncay-Tanrıverdi, S.; Dellera, E.; Sandri, G.; Bonferoni, M.C.; Ozer, O. A comparative evaluation of coenzyme Q10-loaded liposomes and solid lipid nanoparticles as dermal antioxidant carriers. Int. J. Nanomedicine 2012, 7, 5109. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Liu, H.; Guo, L.; Jiang, X.; Wang, S.; Tian, R.; Huang, Y.; Jiang, X.; Gou, M. Improving the transdermal delivery of vitamin C by 3D-printed microneedle particles for alleviating skin photodamage. Int. J. Bioprint 2024, 10, 1285. [Google Scholar] [CrossRef]
- DePhillipo, N.N.; Aman, Z.S.; Kennedy, M.I.; Begley, J.P.; Moatshe, G.; LaPrade, R.F. Efficacy of Vitamin C Supplementation on Collagen Synthesis and Oxidative Stress After Musculoskeletal Injuries: A Systematic Review. Orthop. J. Sports Med. 2018, 6, 2325967118804544. [Google Scholar] [CrossRef]
- Sorice, A.; Guerriero, E.; Capone, F.; Colonna, G.; Castello, G.; Costantini, S. Ascorbic acid: Its role in immune system and chronic inflammation diseases. Mini Rev. Med. Chem. 2014, 14, 444–452. [Google Scholar] [CrossRef]
- Ablon, G. Nutraceuticals. Dermatol. Clin. 2021, 39, 417–427. [Google Scholar] [CrossRef]
- Rangsimawong, W.; Opanasopit, P.; Rojanarata, T.; Duangjit, S.; Ngawhirunpat, T. Skin transport of hydrophilic compound-loaded PEGylated lipid nanocarriers: Comparative study of liposomes, niosomes, and solid lipid nanoparticles. Biol. Pharm. Bull. 2016, 39, 1254–1262. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C 2016, 68, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W. Solid lipid nanoparticles Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-Based Cosmeceuticals. ISRN Dermatol. 2014, 2014, 843687. [Google Scholar] [CrossRef]
- Gokce, E.H.; Korkmaz, E.; Dellera, E.; Sandri, G.; Cristina Bonferoni, M.; Ozer, O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int. J. Nanomed. 2012, 7, 1841–1850. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability and performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef]
- Shah, K.A.; Date, A.A.; Joshi, M.D.; Patravale, V.B. Solid lipid nanoparticles (SLN) of tretinoin: Potential in topical delivery. Int. J. Pharm. 2007, 345, 163–171. [Google Scholar] [CrossRef]
- Jeon, H.S.; Seo, J.E.; Kim, M.S.; Kang, M.H.; Oh, D.H.; Jeon, S.O.; Jeong, S.H.; Choi, Y.W.; Lee, S. A retinyl palmitate-loaded solid lipid nanoparticle system: Effect of surface modification with dicetyl phosphate on skin permeation in vitro and anti-wrinkle effect in vivo. Int. J. Pharm. 2013, 452, 311–320. [Google Scholar] [CrossRef]
- Montenegro, L.; Sinico, C.; Castangia, I.; Carbone, C.; Puglisi, G. Idebenone-loaded solid lipid nanoparticles for drug delivery to the skin: In vitro evaluation. Int. J. Pharm. 2012, 434, 169–174. [Google Scholar] [CrossRef]
- Montenegro, L.; Panico, A.M.; Santagati, L.M.; Siciliano, E.A.; Intagliata, S.; Modica, M.N. Solid Lipid Nanoparticles Loading Idebenone Ester with Pyroglutamic Acid: In Vitro Antioxidant Activity and In Vivo Topical Efficacy. Nanomaterials 2018, 9, 43. [Google Scholar] [CrossRef]
- Becker Peres, L.; Becker Peres, L.; de Araújo, P.H.H.; Sayer, C. Solid lipid nanoparticles for encapsulation of hydrophilic drugs by an organic solvent free double emulsion technique. Colloids Surf. B Biointerfaces 2016, 140, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Himawan, E.; Belhadj, S.; Pérez García, R.O.; Paquet Durand, F.; Schipper, N.; Buzgo, M.; Simaite, A.; Marigo, V. Efficient Delivery of Hydrophilic Small Molecules to Retinal Cell Lines Using Gel Core-Containing Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, 131–155. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Houacine, C.; Adams, D.; Singh, K.a.m.a.l.i.n.d.e.r.K. Impact of liquid lipid on development and stability of trimyristin nanostructured lipid carriers for oral delivery of resveratrol. J. Mol. Liq. 2020, 316, 113734. [Google Scholar] [CrossRef]
- Hu, F.-Q.; Jiang, S.-P.; Du, Y.-Z.; Yuan, H.; Ye, Y.-Q.; Zeng, S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf. B Biointerfaces 2005, 45, 167–173. [Google Scholar] [CrossRef]
- Abla, M.J.; Banga, A.K. Formulation of tocopherol nanocarriers and in vitro delivery into human skin. Int. J. Cosmet. Sci. 2014, 36, 239–246. [Google Scholar] [CrossRef]
- Yue, Y.; Zhou, H.; Liu, G.; Li, Y.; Yan, Z.; Duan, M. The advantages of a novel CoQ10 delivery system in skin photo-protection. Int. J. Pharm. 2010, 392, 57–63. [Google Scholar] [CrossRef]
- Guo, C.Y.; Yang, C.F.; Li, Q.L.; Tan, Q.; Xi, Y.W.; Liu, W.N.; Zhai, G.X. Development of a Quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int. J. Pharm. 2012, 430, 292–298. [Google Scholar] [CrossRef]
- Li, B.; Ge, Z.Q. Nanostructured lipid carriers improve skin permeation and chemical stability of idebenone. AAPS PharmSciTech 2012, 13, 276–283. [Google Scholar] [CrossRef]
- Youssef, M.M. Natural Antioxidants: Functions and Benefits- An Article. Glob. J. Nutr. Food Sci. 2019, 2, 2–3. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Silva, L.B.; Kawakami, C.M.; Araújo, M.M.; Del Lama, M.P.F.M.; Naal, R.M.Z.G.; Maria-Engler, S.S.; Gaspar, L.R.; Marcato, P.D. Topical formulation of quercetin encapsulated in natural lipid nanocarriers: Evaluation of biological properties and phototoxic effect. J. Drug Deliv. Sci. Technol. 2019, 53, 101148. [Google Scholar] [CrossRef]
- Paolino, D.; Stancampiano, A.H.S.; Cilurzo, F.; Cosco, D.; Puglisi, G.; Pignatello, R. Nanostructured Lipid Carriers (NLC) for the Topical Delivery of Lutein. Drug Deliv. Lett. 2011, 1, 32–39. [Google Scholar] [CrossRef]
- Calderon-jacinto, R.; Matricardi, P.; Gueguen, V.; Pavon-djavid, G.; Pauthe, E.; Rodriguez-ruiz, V. Dual Nanostructured Lipid Carriers/Hydrogel System for Delivery of Curcumin for Topical Skin Applications. Biomolecules 2022, 12, 780. [Google Scholar] [CrossRef]
- Satrialdi Husna, N.N.; Rihad, Q.R.; Utami, A.R. The Incorporation of Clove Essential Oil into Nanostructured Lipid Carrier for Improvement of the Delivery and Antioxidant Effects on the Fibroblast Cells. Pharm. Nanotechnol. 2024, 12, 1–11. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Shi, Z.; Yang, Y.; Xie, X.; Lee, S.M.; Wang, Y.; Leong, K.W.; Chen, M. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017, 58, 349–364. [Google Scholar] [CrossRef]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Suwannateep, N.; Banlunara, W.; Wanichwecharungruang, S.P.; Chiablaem, K.; Lirdprapamongkol, K.; Svasti, J. Mucoadhesive curcumin nanospheres: Biological activity, adhesion to stomach mucosa and release of curcumin into the circulation. J. Control. Release 2011, 151, 176–182. [Google Scholar] [CrossRef]
- Ashjari, M.; Khoee, S.; Mahdavian, A.R. Controlling the morphology and surface property of magnetic/cisplatin-loaded nanocapsules via W/O/W double emulsion method. Colloids Surf. A Physicochem. Eng. Asp. 2012, 408, 87–96. [Google Scholar] [CrossRef]
- Mangraviti, A.; Tzeng, S.Y.; Kozielski, K.L.; Wang, Y.; Jin, Y.; Gullotti, D.; Pedone, M.; Buaron, N.; Liu, A.; Wilson, D.R.; et al. Polymeric nanoparticles for nonviral gene therapy extend brain tumor survival in vivo. ACS Nano 2015, 9, 1236–1249. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric Nanoparticles, Nanospheres and Nanocapsules, for Cutaneous Applications. Drug Target Insights 2007, 2, 117739280700200. [Google Scholar] [CrossRef]
- Kim, D.G.; Jeong YIl Choi, C.; Roh, S.H.; Kang, S.K.; Jang, M.K.; Nah, J.W. Retinol-encapsulated low molecular water-soluble chitosan nanoparticles. Int. J. Pharm. 2006, 319, 130–138. [Google Scholar] [CrossRef]
- Park, C.-E.; Park, D.-J.; Kim, B.-K. Effects of a chitosan coating on properties of retinol-encapsulated zein nanoparticles. Food Sci. Biotechnol. 2015, 24, 1725–1733. [Google Scholar] [CrossRef]
- Wu, T.; Yen, F.; Lin, L.; Tsai, T.; Lin, C.; Cham, T. Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles. Int. J. Pharm. 2008, 346, 160–168. [Google Scholar] [CrossRef]
- Suwannateep, N.; Wanichwecharungruang, S.; Haag, S.F.; Devahastin, S.; Groth, N.; Fluhr, J.W.; Lademann, J.; Meinke, M.C. Encapsulated curcumin results in prolonged curcumin activity in vitro and radical scavenging activity ex vivo on skin after UVB-irradiation. Eur. J. Pharm. Biopharm. 2012, 82, 485–490. [Google Scholar] [CrossRef]
- Inoue, Y.; Ishizawa, M.; Itakura, S.; Tanikawa, T.; Todo, H. Verification of nanoparticle formation, skin permeation, and apoptosis using nobiletin as a methoxyflavonoid derivative. AAPS Open 2022, 8, 17. [Google Scholar] [CrossRef]
- Ai, M.; Kanai, R.; Todo, H.; Tomita, J.; Tanikawa, T.; Inoue, Y. Characterization, stability, and skin application of astaxanthin particulates. AAPS Open 2024, 10, 9. [Google Scholar] [CrossRef]
- Santos LP dos Caon, T.; Battisti, M.A.; Silva CHB da Simões, C.M.O.; Reginatto, F.H.; de Campos, A.M. Antioxidant polymeric nanoparticles containing standardized extract of Ilex paraguariensis A. St.-Hil. for topical use. Ind. Crops Prod. 2017, 108, 738–747. [Google Scholar] [CrossRef]
- Babbush, K.M.; Babbush, R.A.; Khachemoune, A. Treatment of melasma: A review of less commonly used antioxidants. Int. J. Dermatol. 2021, 60, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, F.; Banihashemi, M.; Zabolinejad, N.; Salehi, M.; Jaafari, M.R.; Marhamati, H.; Golnouri, F.; Dorri, M. Comparison of therapeutic effects of conventional and liposomal form of 4% topical hydroquinone in patients with melasma. J. Cosmet. Dermatol. 2019, 18, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Banihashemi, M.; Zabolinejad, N.; Jaafari, M.R.; Salehi, M.; Jabari, A. Comparison of therapeutic effects of liposomal Tranexamic Acid and conventional Hydroquinone on melasma. J. Cosmet. Dermatol. 2015, 14, 174–177. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Yang, J.H.; Shin, J.-W.; Park, K.-C.; Huh, C.-H.; Na, J.-I. Efficacy of liposome-encapsulated 4-n-butylresorcinol and resveratrol cream in the treatment of melasma. J. Cosmet. Dermatol. 2020, 19, 891–895. [Google Scholar] [CrossRef]
- Queiroz Schmidt, F.M.; Serna González, C.V.; Mattar, R.C.; Lopes, L.B.; Santos, M.F.; de Gouveia Santos, V.L.C. Topical application of a cream containing nanoparticles with vitamin E for radiodermatitis prevention in women with breast cancer: A randomized, triple-blind, controlled pilot trial. Eur. J. Oncol. Nurs. 2022, 61, 102230. [Google Scholar] [CrossRef]
- Hatem, S.; Nasr, M.; Moftah, N.H.; Ragai, M.H.; Geneidi, A.S.; Elkheshen, S.A. Clinical cosmeceutical repurposing of melatonin in androgenic alopecia using nanostructured lipid carriers prepared with antioxidant oils. Expert Opin. Drug Deliv. 2018, 15, 927–935. [Google Scholar] [CrossRef]
- Vaz, S.; Silva, R.; Amaral, M.H.; Martins, E.; Sousa Lobo, J.M.; Silva, A.C. Evaluation of the biocompatibility and skin hydration potential of vitamin E-loaded lipid nanosystems formulations: In vitro and human in vivo studies. Colloids Surf. B Biointerfaces 2019, 179, 242–249. [Google Scholar] [CrossRef]
- Hugo Infante, V.; Maria Maia Campos, P.; Darvin, M.; Lohan, S.; Schleusener, J.; Schanzer, S.; Lademann, J.; Meinke, M. Cosmetic Formulations with Melaleuca alternifolia Essential Oil for the Improvement of Photoaged Skin: A Double-Blind, Randomized, Placebo-Controlled Clinical Study. Photochem. Photobiol. 2023, 99, 176–183. [Google Scholar] [CrossRef]
- Gould, S.; Templin, M.V. Off target toxicities and links with physicochemical properties of medicinal products, including antibiotics, oligonucleotides, lipid nanoparticles (with cationic and/or anionic charges). Data review suggests an emerging pattern. Toxicol. Lett. 2023, 384, 14–29. [Google Scholar] [CrossRef]
- Mladenov, M.; Lubomirov, L.; Grisk, O.; Avtanski, D.; Mitrokhin, V.; Sazdova, I.; Keremidarska-Markova, M.; Danailova, Y.; Nikolaev, G.; Konakchieva, R.; et al. Oxidative Stress, Reductive Stress and Antioxidants in Vascular Pathogenesis and Aging. Antioxidants 2023, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, G.; Wang, D.; Gounder, S.S.; Fernandes, J.; Litovsky, S.H.; Whitehead, K.; Radhakrishnan, R.K.; Franklin, S.; Hoidal, J.R.; Kensler, T.W.; et al. Reductive Stress Causes Pathological Cardiac Remodeling and Diastolic Dysfunction. Antioxid. Redox Signal 2020, 32, 1293–1312. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Reductive Stress in Inflammation-Associated Diseases and the Pro-Oxidant Effect of Antioxidant Agents. Int. J. Mol. Sci. 2017, 18, 2098. [Google Scholar] [CrossRef]
- Monteiro, P.F.; Travanut, A.; Conte, C.; Alexander, C. Reduction-responsive polymers for drug delivery in cancer therapy—Is there anything new to discover? WIREs Nanomed. Nanobiotechnol. 2021, 13, e1678. [Google Scholar] [CrossRef]
- Makoni, P.A.; Wa Kasongo, K.; Walker, R.B. Short Term Stability Testing of Efavirenz-Loaded Solid Lipid Nanoparticle (SLN) and Nanostructured Lipid Carrier (NLC) Dispersions. Pharmaceutics 2019, 11, 397. [Google Scholar] [CrossRef]
- Home|ClinicalTrials.gov n.d. Available online: https://clinicaltrials.gov/ (accessed on 26 December 2024).
- Study Details|Effect of Oxidant Gel and Anti-Oxidant Gel on Wound Healing After Gingival Depigmentation|ClinicalTrials.gov n.d. Available online: https://clinicaltrials.gov/study/NCT05837416?cond=Skin%20Condition&intr=Antioxidants&limit=100&page=1&rank=27 (accessed on 26 December 2024).

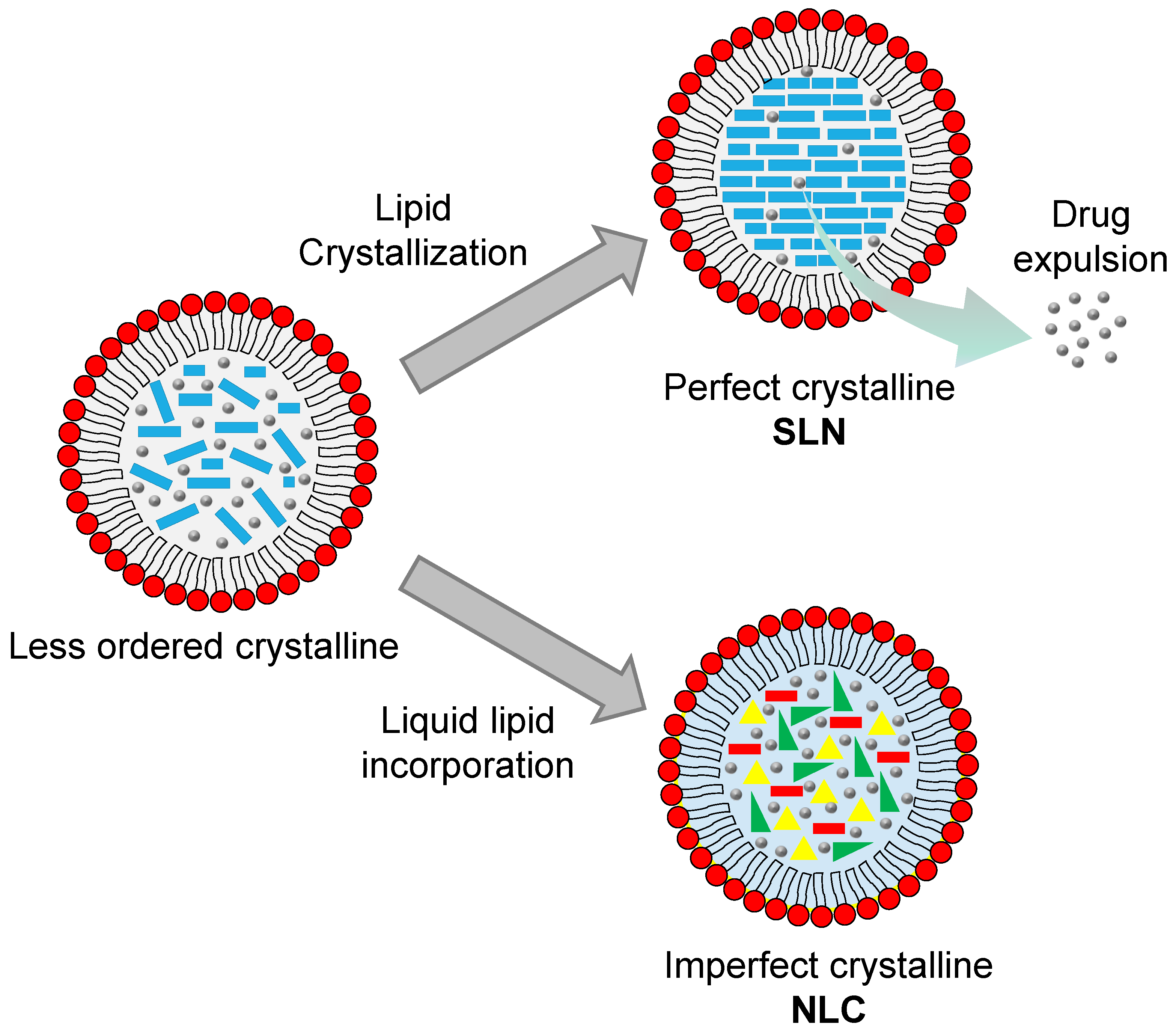
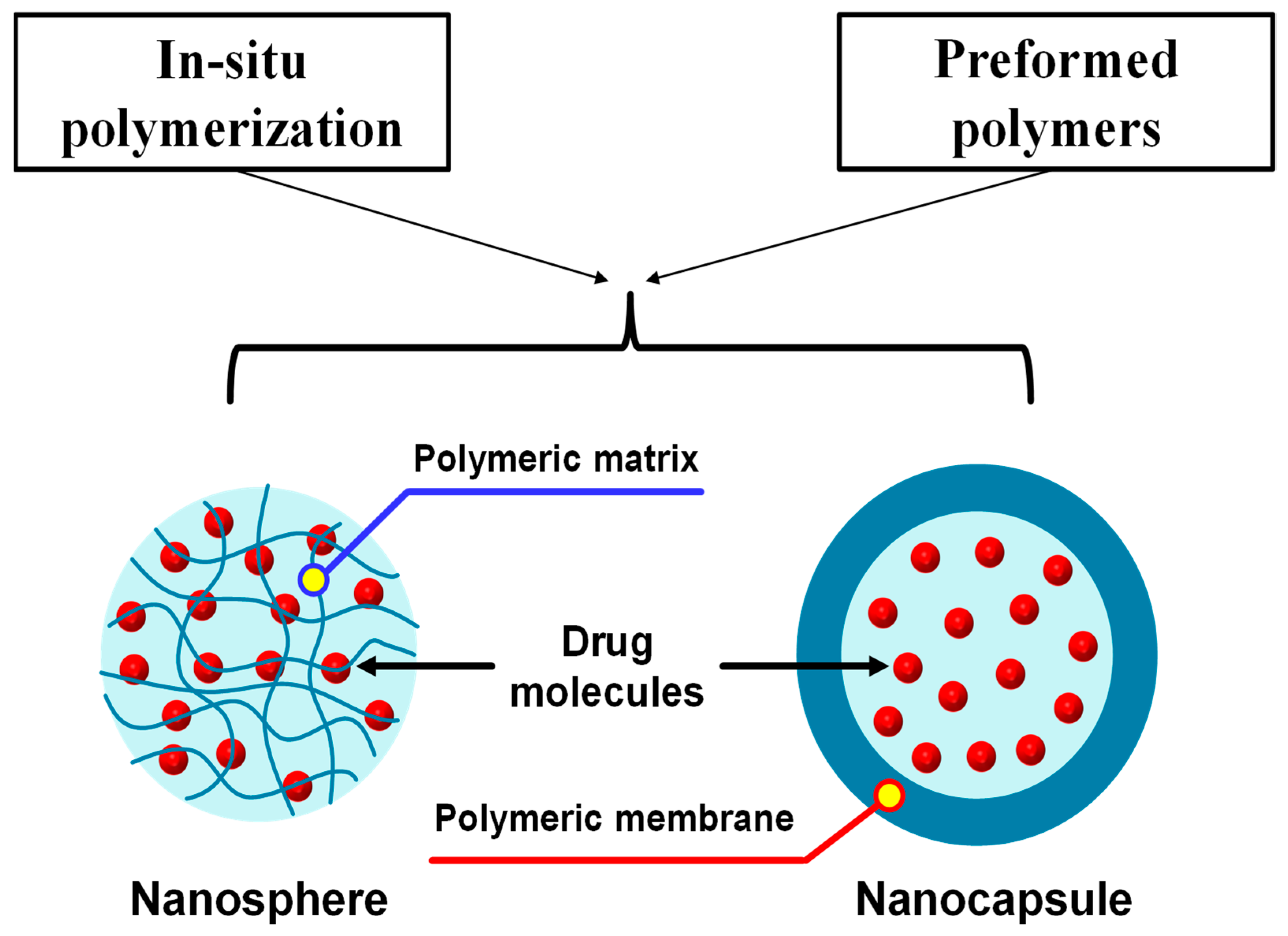
| Name of Plant | Extraction Solvent | Extraction Method | Identified Responsible Compounds | Refs. |
|---|---|---|---|---|
| Phyllanthus emblica | Water | Infusion | Ascorbic acid | [68] |
| Rosa damascene | Water | Infusion | Polyphenols | [68] |
| Stevia rebaudiana | Water | Infusion | Phenolics and flavonoids | [68] |
| Paeonia officinalis | Methanol | Accelerated solvent extraction | Gallic acid derivatives | [69] |
| Salvia nemorosa | Methanol/water (80%, v/v) | Ultrasound-assisted extraction | Rosmarinic acid | [70] |
| Salvia macrochlamys | Methanol/water (80%, v/v) | Ultrasound-assisted extraction | Rosmarinic acid | [70] |
| Homnin black rice and Munpu red rice | Acidified ethanol | Microwave-assisted extraction | Phenolic, anthocyanins, and proanthocyanidins | [71] |
| Cinnamomum sp. | n.a. | n.a. | Eugenol | [72] |
| Syzygium aromaticum | n.a. | n.a. | Eugenol | [72] |
| Centella asiatica | 50% ethanol | Sonication | Kaempferol, quercetin, rutin | [46] |
| Antioxidant | Liposome Composition | Preparation Method | Outcomes | Refs. |
|---|---|---|---|---|
| Vitamin C | Soybean lecithin and sodium cholate | Not defined | Improves skin penetration and the photoprotective effects of vitamin C | [124] |
| Antioxidant complex | Phosphatidylcholine | Thin-film hydration | Restores pro-inflammatory cytokines of photoaging skin | [125] |
| Astaxanthin | Soy lecithin and cholesterol | Ethanol injection | Improves the stability of astaxanthin during storage | [126] |
| Astaxanthin | Soy lecithin and cholesterol, coated with chitosan | Thin-film hydration coupled with high-pressure homogenization | Improves the stability of astaxanthin and its antioxidant activity | [127] |
| Caffeic acid | Egg phosphatidylcholine and cholesterol | Reverse-phase evaporation | Enhances skin penetration by caffeic acid and retain its antioxidant activity | [128] |
| CoQ10 | Phospholipid-Lipoid S100 and cholesterol | Thin-film hydration | Enhances cell proliferation under stress oxidative conditions | [129] |
| Retinyl palmitate | l-α-phosphatidylcholine | Thin-film hydration | Prolongs skin retention and provides a skin hydration effect | [107] |
| Antioxidant | Lipid | Surfactant | Result | Refs. |
|---|---|---|---|---|
| Retinyl palmitate | Compritol® 888 ATO | Sodium lauryl sulphate and Span® 80 | Improves photostability and is dominantly retained in the superficial skin layer | [107] |
| Resveratrol | Compritol® 888 ATO | Poloxamer 188 and Tween® 80 | Dominantly accumulated in the epidermis | [140] |
| Alpha-lipoic acid | Apifil | Pluronic® F68 | Sustained drug release profile | [106] |
| Lutein | Carnauba wax | Plantacare® 810 | Strong drug photoprotective effect | [141] |
| Tretinoin | Glyceryl monostearate | Epikuron 200 | Improves tretinoin photostability and reduces skin irritation | [142] |
| Retinyl palmitate | Glyceryl palmitostearate | PEG-32 glyceryl stearate | Improves skin delivery by a factor of 4.8 | [143] |
| Idebenone | Cetyl palmitate | Isoceteth and glyceryl oleate | Most of the drugs remain accumulated in the upper skin layers | [144] |
| Idebenone (pyroglutamic acid ester) | Cetyl palmitate | Glyceryl oleate | Improves skin hydration effects | [145] |
| Nanocarrier Type | Advantages | Limitations |
|---|---|---|
| Nanoemulsions |
|
|
| Liposomes |
|
|
| SLNs |
|
|
| NLCs |
|
|
| Polymeric nanoparticles |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zazuli, Z.; Hartati, R.; Rowa, C.R.; Asyarie, S.; Satrialdi. The Potential Application of Nanocarriers in Delivering Topical Antioxidants. Pharmaceuticals 2025, 18, 56. https://doi.org/10.3390/ph18010056
Zazuli Z, Hartati R, Rowa CR, Asyarie S, Satrialdi. The Potential Application of Nanocarriers in Delivering Topical Antioxidants. Pharmaceuticals. 2025; 18(1):56. https://doi.org/10.3390/ph18010056
Chicago/Turabian StyleZazuli, Zulfan, Rika Hartati, Cornelia Rosasepti Rowa, Sukmadjaja Asyarie, and Satrialdi. 2025. "The Potential Application of Nanocarriers in Delivering Topical Antioxidants" Pharmaceuticals 18, no. 1: 56. https://doi.org/10.3390/ph18010056
APA StyleZazuli, Z., Hartati, R., Rowa, C. R., Asyarie, S., & Satrialdi. (2025). The Potential Application of Nanocarriers in Delivering Topical Antioxidants. Pharmaceuticals, 18(1), 56. https://doi.org/10.3390/ph18010056








