Abstract
Infection with the protozoan parasite Trypanosoma cruzi causes human Chagas disease. Benznidazole (BNZ) and nifurtimox are the current drugs for the treatment; however, they induce severe adverse side effects in patients; therefore, there is a need to improve the treatment effectiveness and efficiency of these drugs for its safer use. Background/Objective: Glyburide, glipizide, and gliquidone, hypoglycemic drugs for diabetes treatment, were previously predicted to bind to dihydrofolate reductase-thymidylate synthase from T. cruzi by in silico docking analysis; they also showed antiproliferative effects against T. cruzi epimastigotes, the stage of the insect vector. In the present study, the potential parasiticidal effect of these antidiabetic drugs was tested in monotherapy and bi-therapy with BNZ in human cells in vitro and in animals. Methods: Evaluation was performed in (a) a model of in vitro infection of T. cruzi trypomastigotes using human fibroblasts as host cells and (b) in mice infected with T. cruzi. Results: The antidiabetic drugs in monotherapy showed antiparasitic effects in preventing infection progression (trypomastigotes release), with an IC50 of 8.4–14.3 µM in comparison to that of BNZ (0.26 µM) in vitro. However, in bi-therapy, the presence of just 0.5 or 1 µM of the antidiabetics decreased the BNZ IC50 by 5–10 times to 0.03–0.05 µM. Remarkably, the antidiabetic drugs in monotherapy decreased the infection in mice by 40–60% in a similar extent to BNZ (80%). In addition, the combination of BNZ plus antidiabetics perturbed the antioxidant metabolites in epimastigotes. Conclusions: These results identified antidiabetics as potential drugs in combination therapy with BNZ to treat T. cruzi infection.
1. Introduction
Chagas disease, or American trypanosomiasis, in humans is caused by the infection with the trypanosomatid protozoan parasite Trypanosoma cruzi (T. cruzi) [1,2]. The World Health Organization (WHO) estimates that 7 million people worldwide are infected with the parasite, mainly in Latin American countries, but that 75 million people are at risk of being infected because of living in endemic regions [3]. Furthermore, cases in non-endemic countries in Europe, Asia, Oceania, the USA, and Canada have been reported in immigrant persons. The disease causes around 12,000 deaths annually [3].
The parasite infection initiates by the invasion of skin breaches or mucosa by the trypomastigotes eliminated in the feces or urine of the triatomine vector during the blood feeding, or by organ transplantation and blood transfusion from infected persons, congenital transmission, laboratory accidents, or ingestion of food contaminated with the parasite [4].
The disease progression presents two stages. The acute one, which lasts approximately two months, is characterized by high levels of blood parasitemia without or with mild and unspecific symptoms after parasite infection. The chronic stage can be developed throughout several years after the primary infection, which may occur unnoticed; however, 30–40% of the patients display severe cardiomyopathies and 10% of patients develop inflammation of the gastrointestinal tract (megasyndrome) [1,2] and denervation, particularly from the parasympathetic nervous system [5]. In this stage, cardiac arrhythmias or insufficiency, progressive heart failure, or sudden death occur due to destruction of the innervation of the cardiac muscle [2,5,6].
Benznidazol (BNZ) (Scheme 1) and nifurtimox (Nfx) are recognized by the WHO as efficacious drugs for the treatment of T. cruzi infection, mainly in the acute phase or in early chronic infections [1,3,7,8]. BNZ is preferred over Nfx due to better tolerability and tissue penetration and a lower rate of treatment interruption (9–29%) than nifurtimox (14–75%) [1,7,8]. Adverse side effects have been described for both antichagasic drugs; for BNZ they include skin hypersensibility, edema, muscle and joint pain, and polyneuropathy [9], whereas the most common Nfx side effects are anorexia, weight loss, neurological disorders, nausea, and vomiting [10]. Both drugs are effective for treatment in the acute and early chronic stage phases, showing fewer and moderate adverse effects in children; however, the adverse effects in adults are severe. Moreover, their use in the advanced chronic stage is limited by the health frailty of these patients [11]. For these reasons, new antichagasic treatment strategies with better efficacy and safety are necessary.
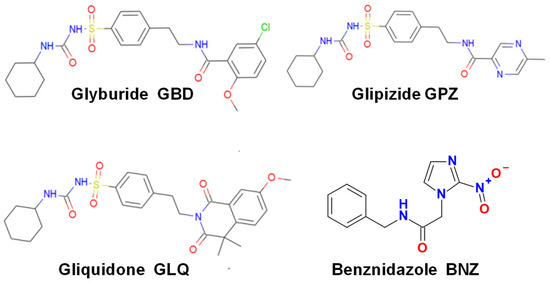
Scheme 1.
Chemical structures of the canonic antichagasic BNZ and the antidiabetic drugs used in the present study.
In recent years, repurposing of drugs of common medical use to treat infectious diseases for which they were not originally designed is a promising clinical alternative, which also saves costs and time in identifying new antiparasitic drugs [12,13,14]. By applying classical docking analysis and protein–ligand interaction profiling of an FDA-approved drug library against the dihydrofolate reductase-thymidylate synthase of T. cruzi, it was predicted that the antidiabetic compounds glipizide (GPZ), gliquidone (GLQ), and glyburide (GBD; also known as glybenclamide) (Scheme 1) are potent and specific enzyme inhibitors [15]. These compounds are ATP-sensitive potassium channel inhibitors of the sulfonylurea type that promote insulin secretion for the treatment of type-2 diabetic patients [16,17,18]. When tested against epimastigotes they showed antiproliferative effects, with an IC50 of 13.4, 12, and 66 µM, for GPZ, GLQ, and GBD, respectively [15].
Previous studies on the use of GBD against Leishmania infection showed that the antidiabetic alone decreased the infection and increased glucantime efficacy in a synergic fashion [19,20,21,22]. It was also predicted by in silico docking analysis that GBD can bind to Leishmania trypanothione synthetase (TryS) with high affinity [23]. TryS is the enzyme responsible for trypanothione [T(SH)2] synthesis; the latter is the specific and main antioxidant metabolite in trypanosomatids, and its related enzymes and metabolism are sites for therapeutic intervention [24,25,26]. Thus, the sulfonylurea-type antidiabetic drugs seem to have potent non-canonical inhibitory effects on the trypanosomatid metabolism and cell functions; therefore, it was foreseen that GBD, GPZ, and GLQ may have potent and specific effects on the parasite, and hence, they might be used for the treatment of T. cruzi infection.
In this study the effect of GBD, GPZ, and GLQ on preventing the progression of the infection with T. cruzi in a model of in vitro infection of human fibroblasts was evaluated in monotherapy and bi-therapy with BNZ. Drug combination therapy may bring about the use of lower doses to thus attenuate the severe side effects of the canonical BNZ treatment. Furthermore, the effect of the antidiabetic drugs on decreasing parasitemia in the T. cruzi acute infection in mice was also analyzed. It was also explored whether treatment with the hypoglycemic compounds alters the thiol pools in T. cruzi.
2. Results
2.1. Effect of BNZ and Antidiabetics in Monotherapy
To evaluate the effects of the antidiabetics alone, a model of in vitro infection with trypomastigotes of monolayers of human foreskin fibroblasts 1 (HFF-1) was used as described in the methodology; the criterium to determine efficacy was the ability to decrease the trypomastigotes’ release after the 4th–5th day post-infection. The T. cruzi Querétaro strain (TBAR/MX/0000/Querétaro) was used, which is classified in the Tc1 group [27], and is characterized by its high degree of virulence [28].
Figure 1 shows the effect of the antidiabetic drugs in monotherapy on the liberation of trypomastigotes, which were effective at 10 µM or lower concentrations (Figure 1A, Table 1). As a control, the cell growth of HFF-1 host cells exposed for five days to the antidiabetic drugs was determined (Figure 1B, Table 1), showing 50% decrease in growth at 5–10 µM for GBD and GLQ, and 35 µM for GPZ. These latter results suggested that cell growth inhibition might partially contribute to the effect of GBD and GLQ on the release of trypomastigotes at concentrations > 5 µM.
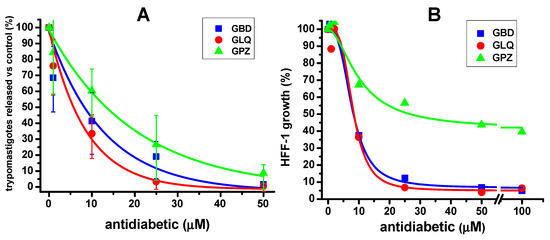
Figure 1.
Effect of antidiabetic drugs in monotherapy in a model of in vitro T. cruzi Querétaro strain infection. (A) The trypomastigotes burst after 5 days of in vitro infection in the presence of increasing concentrations of the antidiabetics is shown. In (B), the effect of the antidiabetics on cell host growth (HFF-1, human foreskin fibroblasts 1) after 5 days is shown. The experimental data points are the average and standard deviation of the number of independent experiments, which was n = 4 in (A) and n = 2 in (B). In (A), 100% trypomastigotes release in the absence of any drug (0 µM) was 1 ± 0.4 × 106 parasites/mL; in (B), 100% cell growth was 1.33 ± 0.0057 × 105 cells/mL. Data were fitted to the dose–response equation using Origin 8 Software.

Table 1.
IC50 values of BNZ and antidiabetics in monotherapy and IC50 of BNZ in bi-therapy with antidiabetics on Queretaro strain release of trypomastigotes from in vitro infection and on HFF-1 growth.
2.2. Effect of BNZ and Antidiabetics in Bi-Therapy
It was further evaluated whether the antidiabetic drugs could decrease the IC50 of BNZ for trypomastigotes bursting (Figure 2 and Table 1). BNZ alone potently decreased the release of trypomastigotes at concentrations up to 0.5 µM, with an IC50 of 0.26 µM (Figure 2A,B). Then, the IC50 of BNZ was determined in the presence of fixed low doses of 0.5 µM and 1 µM of the antidiabetics; these concentrations were selected in which the cell viability and growth of HFF-1 cells were not compromised (Figure 1B). The results indicate that the presence of the antidiabetics at low concentrations significantly decreased the IC50 of BNZ by 5.2–8.6 folds, to 0.05 µM or lower values (Figure 2A,B; Table 1). For instance, the 40% inhibition of infection by 0.2 µM BNZ alone (Figure 2A) was significantly increased up to 70% (Figure 2A) or 80% (Figure 2B) when combined with 0.5 or 1 µM GBD, respectively.
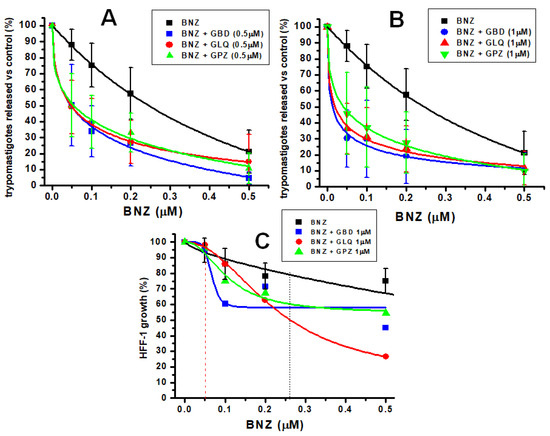
Figure 2.
Effect of BNZ alone or in bi-therapy with antidiabetics on T. cruzi Queretaro strain in vitro infection and on HFF-1 growth. Parasite burst after 5 days of in vitro infection in the presence of variable BNZ concentrations (0 to 0.5 µM) plus fixed 0.5 µM (A) or 1 µM (B) of the antidiabetics. In (C), the effect of variable BNZ concentrations in the absence or presence of 1 µM of antidiabetics on HFF-1 host growth after 5 days is shown. In all the experiments, 0 means no drug added. The experimental data shown represent the mean and standard deviation of the number of independent experiments, which was n = 8 for (A,B), and for (C), n = 3 for BNZ in monotherapy and n = 2 for bi-therapy. The 100% trypomastigote release in the absence of any drug (point 0) was 1 ± 0.4 × 106 parasites/mL. The 100% cell growth in the absence of any drug was 8.4 ± 3.4 × 104 cells/mL. Data in (A,B) were fitted to the dose–response equation; in (C), data were fitted to the logistic equation (with no mechanistic meaning) using Origin 8.0 software.
As a rigorous control, the growth of HFF-1 cells in bi-therapy was also evaluated. BNZ in monotherapy and bi-therapy with antidiabetic showed cytostatic effects (Figure 2C); at the IC50 of BNZ inhibition on release of trypomastigotes in monotherapy (0.26 µM), BNZ decreased the growth of host cells by 20%, whereas in bi-therapy with 1µM antidiabetics, the inhibition increased to 40–50% (Figure 2C black vertical dotted line). However, at the lowered IC50 values of BNZ (0.05 µM) for trypomastigote release in bi-therapy with 1 µM of the antidiabetics, HFF-1 growth was not affected (Figure 2C, red vertical dashed line). These results clearly indicate that the combination of BNZ with low doses of the antidiabetic lowered the BNZ concentration necessary to decrease infection, displacing it to a safer range where no adverse side effects on host cells are promoted. Table 1 summarizes the IC50 of BNZ and antidiabetics in monotherapy and bi-therapy.
2.3. Effect of the Antidiabetic Drugs on an Acute Model of T. cruzi Infection in Mice
The effect of the antidiabetic drugs and BNZ in the clearance of blood trypomastigotes in mice infected with T. cruzi in acute drug treatment in monotherapy was evaluated. Trypanosoma cruzi Ninoa strain was used for these experiments; this strain is classified in the Tc1 group like the Querétaro strain [27]; Ninoa is characterized by lower virulence and therefore lower mortality in mice compared to the Querétaro strain [28]. However, epimastigotes of Ninoa strain are more resistant to BNZ [27]. Then, the T. cruzi Ninoa strain seems to be a more appropriate parasitic source to evaluate drug susceptibility in mice. The infected animals were treated at the peak of parasitemia with the drugs at a single dose of 100 mg drug/kg of weight, which is 15–30 folds lower than the lethal doses in mice (1500–3000 mg/kg) [29,30], and the presence of blood trypomastigotes was monitored every 2 h up to 8 h, which is the interval of the half-life of the antidiabetics in humans [17]. BNZ alone efficiently decreased the infection by 80% (Figure 3). GBD and GPZ alone also decreased the parasite infection by 60% in a significant manner with respect to the vehicle and similar to BNZ, whereas GLQ showed a lower effect than BNZ (p ≤ 0.01); the Kruskal–Wallis statistical test allowed these significant differences to be determined. These results indicate that GBD and GPZ were effective for decreasing T. cruzi parasitemia in mice. It is expected that combining BNZ with the antidiabetics may bring about an increased, synergistic inhibitory effect on infection, but this will require further implementation of lengthy treatments and re-dosing of the drugs at lower concentrations.
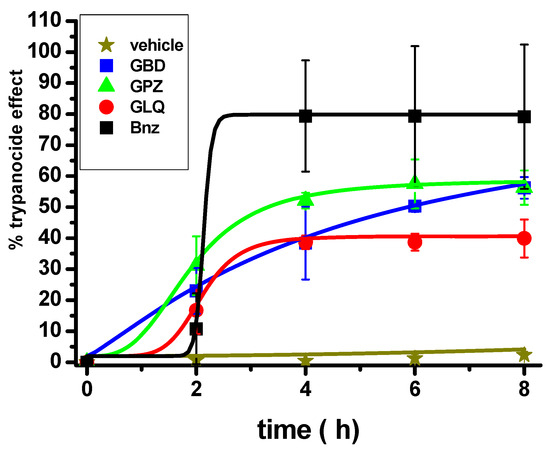
Figure 3.
Effect of BNZ and antidiabetic drugs in monotherapy on T. cruzi Ninoa strain infection in a murine model. After parasitemia was established in the animals (12–18 days post inoculation), they were treated at a single dose of 100 mg/kg weight of BNZ, and antidiabetics and trypomastigotes were counted every 2 h in animals’ blood samples. The vehicle was Arabic gum. The number of mice was 3 for each group. Kruskall–Wallis non parametric analysis was p ≤ 0.01 for BNZ, GBD, and GPZ versus vehicle; p ≤ 0.01 for BNZ versus GLQ.
2.4. Effect on Thiol Metabolites
The effect of monotherapy and bi-therapy in the content of thiol metabolites cysteine (Cys), glutathione (GSH), and trypanothione [T(SH)2] was evaluated. This was done in epimastigotes due to the high amount of biological material necessary for thiol determination. The precursors for T(SH)2 synthesis are Cys, GSH, and spermidine (Scheme 2). We have previously demonstrated that Cys but not spermidine is the limiting precursor for T(SH)2 synthesis [25,31,32]; hence, the analyses were focused on thiol metabolites.
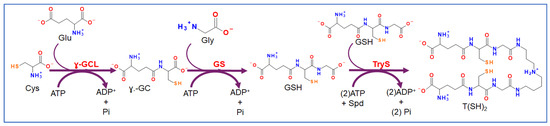
Scheme 2.
Trypanothione synthesis pathway in trypanosomatids. Trypanothione [T(SH)2] is synthesized from two molecules of glutathione (GSH) and one of spermidine (Spd) in a reaction catalyzed by trypanothione synthetase (TryS). In turn, GSH is synthesized from cysteine (Cys), glutamate (Glu), and glycine (Gly) by gamma-glutamylcysteine ligase (γ-GCL) and glutathione synthetase (GS) with intermediate gamma-glutamylcysteine (γ-GC). ATP is used in the three reactions.
BNZ seems to slightly decrease the Cys level for GSH and T(SH)2 synthesis (Figure 4). GBD and GLQ alone decreased the levels of GSH and T(SH)2 and increased those of Cys, suggesting inhibition at the beginning of the pathway. Interestingly, GPZ alone increased the level of T(SH)2 and decreased the levels of Cys and GSH; however, the increase in T(SH)2 cannot explain the near 50% decrease in Cys and GSH. Remarkably, the combination of BNZ with any of the antidiabetics decreased the T(SH)2 levels (Figure 4C). The changes in these antioxidant metabolites suggested perturbation of the antioxidant system in the parasite.
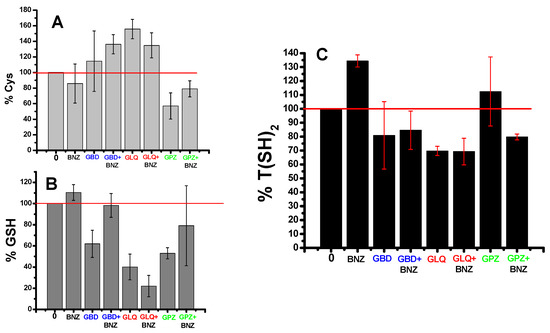
Figure 4.
Effect of BNZ and antidiabetics in mono- and bi- therapy on thiol metabolites of T. cruzi Querétaro strain epimastigotes. Epimastigotes were treated for 24 h with 1 µM of BNZ and 1 µM antidiabetics alone or in combination. Afterwards, thiol metabolites Cys (A), GSH (B) and T(SH)2 (C) were determined by high-performance liquid chromatography (HPLC). Data are the mean ± standard deviation of three independent experiments. The 100% metabolite contents (horizontal red line) correspond to Cys = 5.1 ± 1.5, GSH = 5.6 ± 3.4, and T(SH)2 = 5.8 ± 2.4 nmol/mg cell protein from untreated epimastigotes.
3. Discussion
3.1. Effectiveness of BNZ Alone and in Combination with Antidiabetics
There is an urgent need to develop new therapeutic strategies or to improve the existing ones for the treatment of human infection with T. cruzi. BNZ is an effective drug to diminish or eliminate the infection of the parasite in infected people that is early and promptly diagnosed; however, more treatment difficulties are encountered for patients in the chronic stage [2,33,34,35]. The severe adverse side effects become a factor of concern when deciding on treatments for the infected chronic patient [1,2,7,9,10,11].
Drug repurposing or repositioning appears as a promising strategy to identify new potentially successful antichagasic drugs, thus also saving time and costs. Computationally aided methods on selected targets [36,37] and, recently, in silico screening of FDA-approved drugs on parasite proteins and enzymes have emerged as innovative and promising approaches to repurposing drugs [13,14]. Using such approaches, we previously identified GLB, GLQ, and GPZ as potent inhibitors of T. cruzi epimastigotes growth [15]. The antidiabetics alone showed lower or similar efficacy than BNZ in epimastigotes cultured in vitro. The IC50 values on the growth of epimastigotes were 66, 12, and 13.4 µM for GBD, GLQ, and GPZ, respectively [15], compared to the IC50 of BNZ of 12 ± 2 µM [25] or 9.4 ± 1.3 µM [38]. It is worth noting that both BNZ IC50 determinations were attained under the same experimental conditions and are similar [25,38], and they are comparable to those reported by other research groups in other strains [39,40]. Furthermore, trypomastigotes isolated from infected cells and exposed in vitro for 3 h to BNZ (before transformation into amastigotes) showed a lethal concentration 50 (LC50) to 183 µM BNZ [38].
Here, the effect of BNZ and the antidiabetic drugs on in vitro infection of human mammalian cells by T. cruzi was explored. Although more laborious experimentally than drug evaluation with epimastigotes, the experimental approach used here provided a reliable and robust setup for proof-of-concept evaluation before its testing in animal models. Remarkably, BNZ required an extremely low concentration (0.26 µM, a lower dose than for epimastigotes) to decrease the in vitro cell infection, demonstrating its high efficacy. The antidiabetics alone did not compete with BNZ in its anti-infective effect, requiring up to 30-fold higher concentrations than that of BNZ to attain the same effect. However, the presence of 0.5 µM or 1 µM antidiabetics (which were innocuous on host cell growth) was able to significantly lower the IC50 of BNZ by 5–8 times. These results suggest that the combination of the antidiabetics can decrease the doses of BNZ required to cure the infection and support continued investigation in animal infection models.
Evaluation of the antidiabetic drugs in monotherapy in animals infected with T. cruzi produced encouraging results, showing a parasiticidal effect in an acute treatment and short time monitoring of parasitemia (8 h). Although BNZ was still the most effective drug to decrease the infection in the animals, the antidiabetics also showed parasiticidal effects; particularly, GBD and GPZ showed potent effects and seemed less toxic than GLQ (Figure 2C). This observation suggests that the antidiabetics may be considered as alternative antichagasic drugs to be used in combination with BNZ. It should be pointed out that the plasma concentrations of these drugs (sulfonylureas) that exert antidiabetic effects is in the range of 50–200 nM [18,41], which can be achieved with very low oral drug doses (<5 mg/day or 10 µmol GBD/day). The present results support further studies on combination therapies with BNZ in in vivo assays, both in the long and short term, in order to search for the decrease in the adverse effects of BNZ and, in addition, to determine the modes of action to decrease or eliminate T. cruzi parasitemia.
The T. cruzi Ninoa strain was used for the in vivo model evaluation because there were no in vivo drug-treating studies with the Queretaro strain to make comparisons with the results of the present study, as there are for the Ninoa strain [42,43]. Furthermore, epimastigotes of the T. cruzi Ninoa strain are more resistant to BNZ than those from the Querétaro and even CLBrener strains (IC50 on growth are 8.21, 4.29, and 5.54 µM, respectively [27]). Therefore, it seems of greater clinical interest to carry out the in vivo studies on a parasite strain with higher BNZ resistance.
It remains to be determined in animal models the effect of the bi-therapy BNZ + antidiabetics in short-acute and long-duration treatment protocols followed by post-treatment evaluation with several parasitological and serological tests that effectively demonstrate the elimination of the infection and its null or negligible effect on the physiological functions of the animals.
To our knowledge, this is the first time that antidiabetic drugs of the sulfonylurea type have been repurposed to treat T. cruzi infection. New-generation, safer hypoglycemic drugs such as glimepiride and glycazide have been developed [44] that should also be tested. These results open a possibility for combination therapy with lower doses of BNZ and antidiabetics. On the other hand, recently, metformin, another hypoglycemic compound of the biguanide family with a different mode of action than sulfonylurea, has shown biological effects against Echinococcus [45], Toxoplasma [46], and Acanthamoeba [47] parasites.
3.2. Possible Mode of Action
The most known and studied effect of the sulfonylurea antidiabetics is the inhibition of the ATP-gated potassium channels in pancreatic beta cells. This inhibition induces intracellular potassium build-up, which causes depolarization of the plasma membrane and promotes opening of the calcium channels; the ensuing increase in cytosolic calcium induces insulin secretion [16,17,18]. The previously reported anti-infective effect of GBD (glibenclamide) in Leishmania has been suggested to be through inhibition of ATP-binding cassette MDR (multidrug resistance) transporters; these transporters mediate the sequestration of glucantime into intracellular organelles, hence their inhibition by GBD could promote increasing the drug intracellularly [19,20,21,22]. In this regard, it has been proposed that GBD decreased the glucose uptake in GBD-resistant Leishmania [48]. GBD has also shown anti-helmintic activity on Echinococcus granulosus infection in mice in monotherapy, although there was no synergistic effectiveness in the bi-therapy with albendazole [49].
On the other hand, a previous report of in silico docking analysis in Leishmania TryS suggested that the enzyme can bind GBD; further, Leishmania donovani promastigotes exposed to GBD decreased their growth [23]; however, a direct relationship with inhibition of the antioxidant defense was not experimentally demonstrated. Here, epimastigotes exposed to antidiabetics alone or in combination with BNZ showed a significant decrease in T(SH)2, an effect that was not observed with BNZ alone (Figure 4). It is noted that the GBD and GLQ concentration used was 1 µM, which was much lower than the IC50 value on epimastigotes growth for the compounds in monotherapy (66 and 12 µM, respectively) [15]. GPZ decreased T(SH)2 only in combination with BNZ. These results suggest that antidiabetics can perturb the antioxidant capacity of the parasite, which is necessary to contend with the oxidative stress during host cell infection.
4. Materials and Methods
4.1. Cell Culture
Epimastigotes of the T. cruzi Queretaro strain (TBAR/MX/0000/Queretaro) [27,50] were cultured at 28 °C in LIT medium (liver infusion-tryptose: 0.5% tryptose, 0.5% liver infusion, 0.2% glucose, 0.4% NaCl, 0.04% KCl, 0.42% Na2HPO4) supplemented with 10% fetal bovine serum FBS (Biowest; Nuaillie, France); antibiotic mixture, 100 U penicillin/mL and 100 µg streptomycin/mL (Sigma-Aldrich, Darmstadt, Germany) and 25 µg/mL hemin (Santa Cruz; Dallas, TX, USA). The parasites were harvested at the exponential growth phase (4 days) by centrifugation at 2630× g and washed with phosphate-buffered saline pH 7.4 (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4 pH 7.4) and centrifuged again prior to experiments. For infection, epimastigotes were resuspended in DMEM-2% FBS.
Human foreskin fibroblasts HFF-1 (HFF-1 SCRC-1041) and kidney fibroblasts of Rhesus monkey (LLC-MK2) were cultured in an incubator at 37 °C under 5% CO2-air atmosphere in Petri dishes or 25 cm2 flasks in Dulbecco’s modified Eagle medium (DMEM) from GIBCO (Grand Island, NY, USA) supplemented with 10% FBS (GIBCO, Billings, MT, USA) and antibiotic mixture (SIGMA; St. Louis, MO, USA). At confluence, the cells were detached by trypsinization, collected by centrifugation at 562× g, and used for experimentation.
4.2. Trypomastigotes Obtention
The protocol of infection was performed as previously described [25]. Briefly, a primary infection was performed where 20,000 HFF-1 cells/mL were cultured in 25 cm2 flasks in DMEM-10% FBS. After 24 h, the medium was changed to DMEM supplemented with 2% FBS (DMEM-2% FBS), and the cells were infected with 1 × 107 epimastigotes for 48 h. Thereafter, the medium with unbound epimastigotes was thoroughly mixed, removed, and changed for fresh DMEM-2% FBS, and the cells were incubated as described; the infection was washed daily with the same medium until trypomastigote release at the 4–5th day post infection. Subsequently, 1 × 106 of the released trypomastigotes of the first infection were used for a secondary infection using LLC-MK2 fibroblasts following the same protocol. The trypomastigotes released from this secondary infection were used for the experiments with antichagasic and antidiabetics.
4.3. Evaluation of the Mono and Bi-Therapy in the In Vitro Infection
In 24-well tissue culture plates, 2 × 104 HFF-1 cells in 0.5 mL of DMEM + 10% FBS were deposited in each well and incubated at 37 °C for 24 h for adherence. Next, the incubation medium was changed to DMEM + 2% FBS (0.5 mL), and 3 × 105 trypomastigotes were added to infect cells for 2 h at 37 °C. After the incubation, the cells were vigorously washed three times with DMEM + 2% FBS to remove uninternalized trypomastigotes, taking care to preserve the monolayer integrity. Then, the infected cells were incubated in DMEM + 2% FBS for 4–5 days in the absence or presence of BNZ (0, 0.05, 0.1, 0.2, 0.5 µM) and antidiabetics (0, 1, 10, 25, 50 µM) in monotherapy; the drug compounds were purchased from SIGMA. For bi-therapy, the same concentrations of BNZ were used in the presence of 0.5 or 1 µM of antidiabetics. It was previously determined that these antidiabetic concentrations in monotherapy did not significantly affect the HFF-1 viability or the monolayer integrity. In every independent assay, a control of infected cells without BNZ or antidiabetics was always included, which served as no treatment. The number of released mobile trypomastigotes on the 5th day was determined by light microscopy using a Neubauer chamber. The IC50 is the drug concentration that decreases by 50% the number of released trypomastigotes.
For HFF-1 IC50 evaluation, BNZ and the antidiabetic were varied as described above for the infection cultures, and cell proliferation was determined on the 5th day of growth, and the viability was determined by 0.2% Trypan blue exclusion (Corning Inc.; Corning, NY, USA). Briefly, the cells were washed twice with PBS-EDTA and later trypsinized for detachment. The cell suspension was homogenized with 150 µL of DMEM + 2% FBS, and an aliquot of 50 µL was diluted 1:1 with the solution of Trypan blue. The number of viable cells was counted using a Neubauer chamber under a microscope.
For the IC50 calculation, individual data curves were fitted to the dose–response equation:
where A1 < A2; p > 0; bottom asymptote: A1 = 1; top asymptote: A2 = 2; center: logx0 = −1; hill slope: p = 0.2; or logistic equation
where A1 = initial value, A2 = final value, x0 = center and p = power using the Origin 8 Software. The IC50 values were manually selected from the curves fit.
4.4. Short-Term Antidiabetics Monotherapy in Animals Infected with T. cruzi
Mice of the CD1 strain were infected with Ninoa strain (MHOM/MX/1994/Ninoa) [27,28] following modified protocols previously reported [51,52,53,54]. Briefly, blood trypomastigotes obtained from an infected mouse were diluted with 0.9% saline solution at a density of 1 × 105 trypomastigotes/mL, and 0.1 mL were intraperitoneally inoculated in mice weighing 30–40 g. The lower dose of parasites and higher animal weight allowed better control of the infection and decreased animal mortality. After reaching a parasitemia peak of 22 × 106 blood trypomastigotes/mL (12–18 days post-inoculation), the mice were separated into 5 groups of 3 mice each for drug treatment (for bioethical requirements, the number of animals used was kept at a minimum, and 3 mice per group was sufficient to perform statistical analysis). BNZ and antidiabetic drugs at a concentration of 100 mg/kg weight; doses of 3.16–3.79 mg (depending on the weight of the mice) were administered, diluted in 0.5 mL of vehicle (Arabic gum at 4%, diluted in purified water) using an intragastric tube. Then, 5 µL of blood were immediately extracted from the caudal vein (t = 0), and blood sampling was performed every 2 h up to 8 h. Trypomastigotes were counted using a modified protocol of that reported by Pizzi–Brenner, as reported before [53]. Briefly, the blood sample was placed on an 18 × 18 mm slide, covered with a cover slip, and observed under a microscope at 40× amplification and bright field; mobile trypomastigotes were counted in 15 random fields instead of the 100 random fields used by the Pizzi–Brener method; no statistical difference was observed between the two counting methods. The criterion for evaluating efficacy was the percentage of trypanocidal effect at 8 h compared to time 0.
4.5. Effect of the Mono and Bi-Therapy on Thiol Metabolites
Since thiol metabolite determination requires high amounts of biological material, the thiol determination content was performed in epimastigotes of the Queretaro strain. A 0.2 L culture of LIT medium was inoculated with epimastigotes at a density of 0.7 × 106 parasites/mL. The culture was incubated for 72 h at 28 °C, reaching, at the exponential growth phase, a concentration of 3 × 106 parasites/mL. The culture was separated into 8 aliquots of 25 mL, and 1 µM BNZ was added alone or in combination with 1 µM of the antidiabetic drugs. The cultures were incubated for 24 h at 28 °C. Subsequently, 1 mL of each sample was separated for protein determination, and the rest was used for thiol determination. Both samples were centrifuged at 20,817× g for 10 min; the pellets were washed twice with PBS. The pellets for protein were resuspended in 100 µL of Lowry’s solution A (2% Na2CO3, 0.4% NaOH, 0.16% Na-K tartrate, 1% SDS) and stored at −70 °C until protein determination using the Lowry method [55]. The parasite pellets for thiol metabolite determination were immediately lysed by incubation in liquid nitrogen and stored at −70 °C.
Thiol determination was performed as described before [25,31]. Cell lysates were thawed and resuspended in 90 µL of lysis buffer (20 mM Hepes, 1 mM EDTA, 0.15 mM KCl at pH 7), 20 mM DTT was added to each sample, and three cycles of freezing in liquid nitrogen and thawing at 37 °C were performed. The samples were centrifuged at 20,817× g for 10 min, and the supernatant was reduced with 2% sodium borohydride (NaBH4) and incubated for 10 min on ice. Then, perchloric acid was added at a final concentration of 3%, and the sample was centrifuged at 20,187× g for 2 min. The supernatant was filtered through a syringe-driven filter unit (0.45 µm) (Millipore; Carrigtwohill, County Cork, Ireland) and 20 µL of the eluate was injected in a high-performance liquid chromatography (HPLC) apparatus (Waters; Milford, MA, USA). The metabolites were separated in a C18 reverse-phase column (Altima, Columbia, MD, USA) using a mixture of acetonitrile—0.1% trifluoroacetic acid and a flux of 0.5 mL/min. The thiol metabolites were post-column derivatized with 0.1 mM di-thio-bis nitrobenzoic acid (DTNB), and the absorbance was detected at 412 nm with a UV-Vis detector coupled to the HPLC apparatus.
5. Conclusions
Antidiabetic drugs that can lower the effective concentration of BNZ to decrease the T. cruzi infection were identified. The antidiabetics drugs GBD, GPZ, and GLQ showed trypanocidal activity in initial studies in an animal in vivo model, which should be experimentally analyzed in further detail to delve into the synergy mechanisms. This combinatory therapy may lower the doses of BNZ necessary to eliminate the infection in the host and thus diminish the undesirable adverse side effects of BNZ, which are the main cause of treatment abandonment, particularly in adult patients. Our findings open a new venue to investigate these low-cost, commercially available antidiabetics to improve BNZ treatment against. T. cruzi infection. If combinatory therapy is successful, translational studies will be necessary before clinical trial evaluation.
Author Contributions
Conceptualization, G.R., B.N.-T. and E.S.; data curation, C.V., R.E. and E.S.; formal analysis, C.V., R.E., R.G.-E. and E.S.; funding acquisition, E.S.; investigation, C.V., R.E., I.J.-G., R.G.-E., B.N.-T. and E.S.; methodology, C.V., R.E., R.G.-E., B.N.-T. and E.S.; project administration, E.S.; resources, E.S.; supervision, C.V., R.E., R.G.-E. and E.S.; validation, C.V., R.E., R.G.-E. and E.S.; visualization, C.V. and E.S.; writing—original draft, C.V., R.E. and E.S.; writing—review and editing, C.V., G.R., B.N.-T. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the following grants: CONAHCyT-Bioseguridad 264292, CONAHCyT-Ciencia Básica 282663, and CONAHCyT-CBF-2023-2024-2740 to E.S. This study was funded in part by the Instituto Nacional de Cardiología Ignacio Chávez, Mexico City, Mexico.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics and Research Committee of Instituto Politécnico Nacional (protocol code ZOO-006-2020 and date of approval 22 December 2020) for studies involving animals.
Data Availability Statement
All data are contained within the article.
Acknowledgments
C.V. acknowledges the Instituto Politécnico Nacional, where she is pursuing her doctorate studies, and CONAHCyT for scholarship 581433. I.J.-G. received a pre-graduate scholarship from CONAHCyT grant 282663. Open Access funding for this article was supported by Instituto Nacional de Cardiología Ignacio Chávez.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
BNZ, benznidazole; GBD, glyburide; GLQ, gliquidone; GPZ, glipizide; HFF-1, human foreskin fibroblasts 1; Nfx, nifurtimox.
References
- Pérez-Molina, J.A.; Molina, I. Chagas Disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Echavarría, N.G.; Echeverría, L.E.; Stewart, M.; Gallego, C.; Saldarriaga, C. Chagas Disease: Chronic Chagas Cardiomyopathy. Curr. Probl. Cardiol. 2021, 46, 100507. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chagas Disease (also Known as American Trypanosomiasis). 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 8 October 2024).
- Coura, J.R. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions—A comprehensive review. Mem. Inst. Oswaldo Cruz. 2015, 110, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A.; Marcondes de Rezende, J.; Luquetti, A.O.; Rassi, A. Clinical Phases and Forms of Chagas Disease. In American Trypanosomiasis; Telleria, J., Tibayrenc, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 709–741. [Google Scholar] [CrossRef]
- Echeverría, L.E.; Rojas, L.Z.; Rueda-Ochoa, O.L.; Gómez-Ochoa, S.A.; González Rugeles, C.I.; Díaz, M.L.; Marcus, R.; Morillo, C.A. Circulating Trypanosoma cruzi load and major cardiovascular outcomes in patients with chronic Chagas cardiomyopathy: A prospective cohort study. Trop. Med. Int. Health 2020, 25, 1534–1541. [Google Scholar] [CrossRef]
- Mills, R.M. Chagas Disease: Epidemiology and Barriers to Treatment. Am. J. Med. 2020, 133, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Losada Galván, I.; Alonso-Padilla, J.; Cortés-Serra, N.; Alonso-Vega, C.; Gascón, J.; Pinazo, M.J. Benznidazole for the treatment of Chagas disease. Expert Rev. Anti-Infect. Ther. 2021, 19, 547–556. [Google Scholar] [CrossRef]
- Viotti, R.; Vigliano, C.; Lococo, B.; Alvarez, M.G.; Petti, M.; Bertocchi, G.; Armenti, A. Side effects of benznidazole as treatment in chronic Chagas disease: Fears and realities. Expert Rev. Anti-Infect. Ther. 2009, 7, 157–163. [Google Scholar] [CrossRef]
- Jackson, Y.; Alirol, E.; Getaz, L.; Wolff, H.; Combescure, C.; Chappuis, F. Tolerance and safety of nifurtimox in patients with chronic Chagas disease. Clin. Infect. Dis. 2010, 51, e69–e75. [Google Scholar] [CrossRef]
- Ramos, L.G.; de Souza, K.R.; Júnior, P.A.S.; Câmara, C.C.; Castelo-Branco, F.S.; Boechat, N.; Carvalho, S.A. Tackling the challenges of human Chagas disease: A comprehensive review of treatment strategies in the chronic phase and emerging therapeutic approaches. Acta Trop. 2024, 256, 107264. [Google Scholar] [CrossRef]
- Torchelsen, F.K.V.D.S.; Mazzeti, A.L.; Mosqueira, V.C.F. Drugs in preclinical and early clinical development for the treatment of Chagas’s disease: The current status. Expert Opin. Investig. Drugs 2024, 33, 575–590. [Google Scholar] [CrossRef]
- Porta, E.O.J.; Kalesh, K.; Steel, P.G. Navigating drug repurposing for Chagas disease: Advances, challenges, and opportunities. Front. Pharmacol. 2023, 14, 1233253. [Google Scholar] [CrossRef]
- Bellera, C.L.; Alberca, L.N.; Sbaraglini, M.L.; Talevi, A. In silico Drug Repositioning for Chagas Disease. Curr. Med. Chem. 2020, 27, 662–675. [Google Scholar] [CrossRef]
- Juárez-Saldivar, A.; Schroeder, M.; Salentin, S.; Haupt, V.J.; Saavedra, E.; Vázquez, C.; Reyes-Espinosa, F.; Herrera-Mayorga, V.; Villalobos-Rocha, J.C.; García-Pérez, C.A.; et al. Computational Drug Repositioning for Chagas Disease Using Protein-Ligand Interaction Profiling. Int. J. Mol. Sci. 2020, 21, 4270. [Google Scholar] [CrossRef]
- Proks, P.; Reimann, F.; Green, N.; Gribble, F.; Ashcroft, F. Sulfonylurea stimulation of insulin secretion. Diabetes 2002, 51 (Suppl. S3), S368–S376. [Google Scholar] [CrossRef]
- Sola, D.; Rossi, L.; Schianca, G.P.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; Derosa, G. Sulfonylureas and their use in clinical practice. Arch. Med. Sci. 2015, 11, 840–848. [Google Scholar] [CrossRef]
- Lv, W.; Wang, X.; Xu, Q.; Lu, W. Mechanisms and Characteristics of Sulfonylureas and Glinides. Curr. Top. Med. Chem. 2020, 20, 37–56. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Campos, Y.; Fernandez, M.; Moll, H.; Mendoza-León, A. Leishmania sp.: Growth and survival are impaired by ion channel blockers. Exp. Parasitol. 1998, 88, 11–19. [Google Scholar] [CrossRef]
- Serrano-Martín, X.; Payares, G.; Mendoza-León, A. Glibenclamide, a blocker of K+ATP channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2006, 50, 4214–4216. [Google Scholar] [CrossRef]
- Padrón-Nieves, M.; Díaz, E.; Machuca, C.; Romero, A.; Ponte, A. Glibenclamide modulates glucantime activity and disposition in Leishmania major. Exp. Parasitol. 2009, 121, 331–337. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Novais, F.O.; Paixão, C.S.; de Oliveira, C.I.; Machado, P.R.L.; Carvalho, L.P.; Scott, P.; Carvalho, E.M. Glyburide, a NLRP3 Inhibitor, Decreases Inflammatory Response and Is a Candidate to Reduce Pathology in Leishmania braziliensis Infection. J. Investig. Dermatol. 2020, 140, 246–249.e2. [Google Scholar] [CrossRef]
- Rub, A.; Shaker, K.; Kashif, M.; Arish, M.; Dukhyil, A.A.B.; Alshehri, B.M.; Alaidarous, M.A.; Banawas, S.; Amir, K. Repurposing Glyburide as Antileishmanial Agent to Fight Against Leishmaniasis. Protein Pept. Lett. 2019, 26, 371–376. [Google Scholar] [CrossRef]
- Talevi, A.; Carrillo, C.; Comini, M. The Thiol-polyamine Metabolism of Trypanosoma cruzi: Molecular Targets and Drug Repurposing Strategies. Curr. Med. Chem. 2019, 26, 6614–6635. [Google Scholar] [CrossRef]
- González-Chávez, Z.; Vázquez, C.; Mejia-Tlachi, M.; Márquez-Dueñas, C.; Manning-Cela, R.; Encalada, R.; Rodríguez-Enríquez, S.; Michels, P.A.M.; Moreno-Sánchez, R.; Saavedra, E. Gamma-glutamylcysteine synthetase and tryparedoxin 1 exert high control on the antioxidant system in Trypanosoma cruzi contributing to drug resistance and infectivity. Redox Biol. 2019, 26, 101231. [Google Scholar] [CrossRef]
- Saavedra, E.; González-Chávez, Z.; Moreno-Sánchez, R.; Michels, P.A.M. Drug Target Selection for Trypanosoma cruzi Metabolism by Metabolic Control Analysis and Kinetic Modeling. Curr. Med. Chem. 2019, 26, 6652–6671. [Google Scholar] [CrossRef]
- Martínez, I.; Nogueda, B.; Martínez-Hernández, F.; Espinoza, B. Microsatellite and mini-exon analysis of Mexican human DTU I Trypanosoma cruzi strains and their susceptibility to nifurtimox and benznidazole. Vector Borne Zoonotic Dis. 2013, 13, 181–187. [Google Scholar] [CrossRef]
- Espinoza, B.; Rico, T.; Sosa, S.; Oaxaca, E.; Vizcaino-Castillo, A.; Caballero, M.L.; Martínez, I. Mexican Trypanosoma cruzi T. cruzi I strains with different degrees of virulence induce diverse humoral and cellular immune responses in a murine experimental infection model. J. Biomed. Biotechnol. 2010, 2010, 890672. [Google Scholar] [CrossRef]
- Pfizer. Material Safety Data Sheet: Glyburide. Available online: https://cdn.pfizer.com/pfizercom/products/material_safety_data/MICRONASE.pdf (accessed on 26 December 2024).
- Cayman Chemical. Safety Data Sheet: Glipizide. Available online: https://s3-us-west-2.amazonaws.com/drugbank/msds/DB01067.pdf?1561061159 (accessed on 6 December 2024).
- Vázquez, C.; Mejia-Tlachi, M.; González-Chávez, Z.; Silva, A.; Rodríguez-Zavala, J.S.; Moreno-Sánchez, R.; Saavedra, E. Buthionine sulfoximine is a multitarget inhibitor of trypanthione synthesis in Trypanosoma cruzi. FEBS Lett. 2017, 591, 3881–3894. [Google Scholar] [CrossRef]
- Vázquez, C.; Encalada, R.; Belmont-Díaz, J.; Rivera, M.; Alvarez, S.; Nogueda-Torres, B.; Saavedra, E. Metabolic control analysis of the transsulfuration pathway and the compensatory role of the cysteine transport in Trypanosoma cruzi. Biosystems 2023, 234, 105066. [Google Scholar] [CrossRef]
- Rodriques Coura, J.; de Castro, S.L. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz. 2002, 97, 3–24. [Google Scholar] [CrossRef]
- Guedes, P.M.; Silva, G.K.; Gutierrez, F.R.; Silva, J.S. Current status of Chagas disease chemotherapy. Expert Rev. Anti-Infect. Ther. 2011, 9, 609–620. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Crespillo-Andújar, C.; Bosch-Nicolau, P.; Molina, I. Trypanocidal treatment of Chagas disease. Enferm. Infecc. Microbiol. Clin. 2020, 39, 458–470. [Google Scholar] [CrossRef]
- Beltran-Hortelano, I.; Alcolea, V.; Font, M.; Pérez-Silanes, S. Examination of multiple Trypanosoma cruzi targets in a new drug discovery approach for Chagas disease. Bioorg. Med. Chem. 2022, 58, 116577. [Google Scholar] [CrossRef] [PubMed]
- Laureano de Souza, M.; Lapierre, T.J.W.J.D.; Vitor de Lima Marques, G.; Ferraz, W.R.; Penteado, A.B.; Henrique Goulart Trossini, G.; Murta, S.M.F.; de Oliveira, R.B.; de Oliveira Rezende, C., Jr.; Ferreira, R.S. Molecular targets for Chagas disease: Validation, challenges and lead compounds for widely exploited targets. Expert Opin. Ther. Targets 2023, 27, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, C.; Matus-Meza, A.S.; Nuñez-Moreno, O.; Barbosa-Sánchez, B.M.; Farías-Gutiérrez, V.M.; Mendoza-Conde, M.; Hernández-Luis, F.; Saavedra, E. Exploring Quinazoline Nitro-Derivatives as Potential Antichagasic Agents: Synthesis and In vitro Evaluation. Molecules 2024, 29, 4501. [Google Scholar] [CrossRef]
- Maciel Diogo, G.; Andrade, J.S.; Sales Junior, P.A.; Maria Fonseca Murta, S.; Dos Santos, V.M.R.; Taylor, J.G. Trypanocidal Activity of Flavanone Derivatives. Molecules 2020, 25, 397. [Google Scholar] [CrossRef]
- Acosta, N.; Yaluff, G.; López, E.; Bobadilla, C.; Ramírez, A.; Fernández, I.; Escobar, P. In vitro susceptibility to benznidazole, nifurtimox and posaconazole of Trypanosoma cruzi isolates from Paraguay (Sensibilidad in vitro a benznidazol, nifurtimox y posaconazol de cepas de Trypanosoma cruzi de Paraguay). Biomed. Rev. Inst. Nac. Salud 2020, 40, 749–763. [Google Scholar] [CrossRef]
- Groop, L.C.; Barzilai, N.; Ratheiser, K.; Luzi, L.; Wåhlin-Boll, E.; Melander, A.; DeFronzo, R.A. Dose-dependent effects of glyburide on insulin secretion and glucose uptake in humans. Diabetes Care 1991, 14, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Palos, I.; Lara-Ramirez, E.E.; Lopez-Cedillo, J.C.; Garcia-Perez, C.; Kashif, M.; Bocanegra-Garcia, V.; Nogueda-Torres, B.; Rivera, G. Repositioning FDA Drugs as Potential Cruzain Inhibitors from Trypanosoma cruzi: Virtual Screening, In Vitro and In Vivo Studies. Molecules 2017, 22, 1015. [Google Scholar] [CrossRef]
- Gómez-Escobedo, R.; Méndez-Álvarez, D.; Vázquez, C.; Saavedra, E.; Vázquez, K.; Alcántara-Farfán, V.; Cordero-Martínez, J.; Gonzalez-Gonzalez, A.; Rivera, G.; Nogueda-Torres, B. Molecular Docking-Based Virtual Screening of FDA-Approved Drugs Using Trypanothione Reductase Identified New Trypanocidal Agents. Molecules 2024, 29, 3796. [Google Scholar] [CrossRef]
- Al-Saleh, Y.; Sabico, S.; Al-Furqani, A.; Jayyousi, A.; Alromaihi, D.; Ba-Essa, E.; Alawadi, F.; Alkaabi, J.; Hassanein, M.; Al-Sifri, S.; et al. Sulfonylureas in the Current Practice of Type 2 Diabetes Management: Are They All the Same? Consensus from the Gulf Cooperation Council (GCC) Countries Advisory Board on Sulfonylureas. Diabetes Ther. 2021, 12, 2115–2132. [Google Scholar] [CrossRef]
- Loos, J.A.; Negro, P.S.; Ortega, H.H.; Salinas, F.J.; Arán, M.; Pellizza, L.; Salerno, G.L.; Cumino, A.C. Anti-echinococcal effect of metformin in advanced experimental cystic echinococcosis: Reprogrammed intermediary carbon metabolism in the parasite. Antimicrob. Agents Chemother. 2024, 68, e0094124. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Nabil El Achy, S.; Hezema, N.N. Could metformin modulate the outcome of chronic murine toxoplasmosis? Acta Trop. 2024, 258, 107339. [Google Scholar] [CrossRef]
- Ozpinar, N.; Karaman, U.; Ozpinar, H.; Dag, S. Do antidiabetic drugs prevent the transformation of Acanthamoeba trophozoite into cyst form? Pathog. Glob. Health 2023, 117, 674–680. [Google Scholar] [CrossRef]
- Uzcategui, N.L.; Figarella, K.; Camacho, N.; Ponte-Sucre, A. Substrate preferences and glucose uptake in glibenclamide-resistant Leishmania parasites. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 395–402. [Google Scholar] [CrossRef]
- Loos, J.A.; Churio, M.S.; Cumino, A.C. Anthelminthic activity of glibenclamide on secondary cystic echinococcosis in mice. PLoS Negl. Trop. Dis. 2017, 11, e0006111. [Google Scholar] [CrossRef]
- López-Olmos, V.; Pérez-Nasser, N.; Piñero, D.; Ortega, E.; Hernandez, R.; Espinoza, B. Biological characterization and genetic diversity of Mexican isolates of Trypanosoma cruzi. Acta Trop. 1998, 69, 239–254. [Google Scholar] [CrossRef]
- Romanha, A.J.; de Castro, S.L.; de Nazaré Correia Soeiro, M.; Lannes-Vieira, J.; Ribeiro, I.; Talvani, A.; Bourdin, B.; Blum, B.; Olivieri, B.; Zan, C.; et al. In Vitro and In Vivo Experimental Models for Drug Screening and Development for Chagas Disease. Mem. Inst. Oswaldo Cruz 2010, 105, 233–238. [Google Scholar] [CrossRef]
- Bustamante, J.M.; Craft, J.M.; Crowe, B.D.; Ketchie, S.A.; Tarleton, R.L. New, combined, and reduced dosing treatment protocols cure Trypanosoma cruzi infection in mice. J. Infect. Dis. 2014, 209, 150–162. [Google Scholar] [CrossRef]
- Brener, Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. Sao Paulo 1962, 4, 389–396. [Google Scholar]
- Simões-Silva, M.R.; De Araújo, J.S.; Oliveira, G.M.; Demarque, K.C.; Peres, R.B.; D’Almeida-Melo, I.; Batista DG, J.; Da Silva, C.F.; Cardoso-Santos, C.; Da Silva, P.B.; et al. Drug repurposing strategy against Trypanosoma cruzi infection: In vitro and in vivo assessment of the activity of metronidazole in mono- and combined therapy. Biochem. Pharmacol. 2017, 145, 46–53. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).