Fully Automated Production of (((S)-1-Carboxy-5-(6-([18F]fluoro)-2-methoxynicotinamido)pentyl)carbamoyl)-l-glutamic Acid ([18F]JK-PSMA-7) †
Abstract
1. Introduction
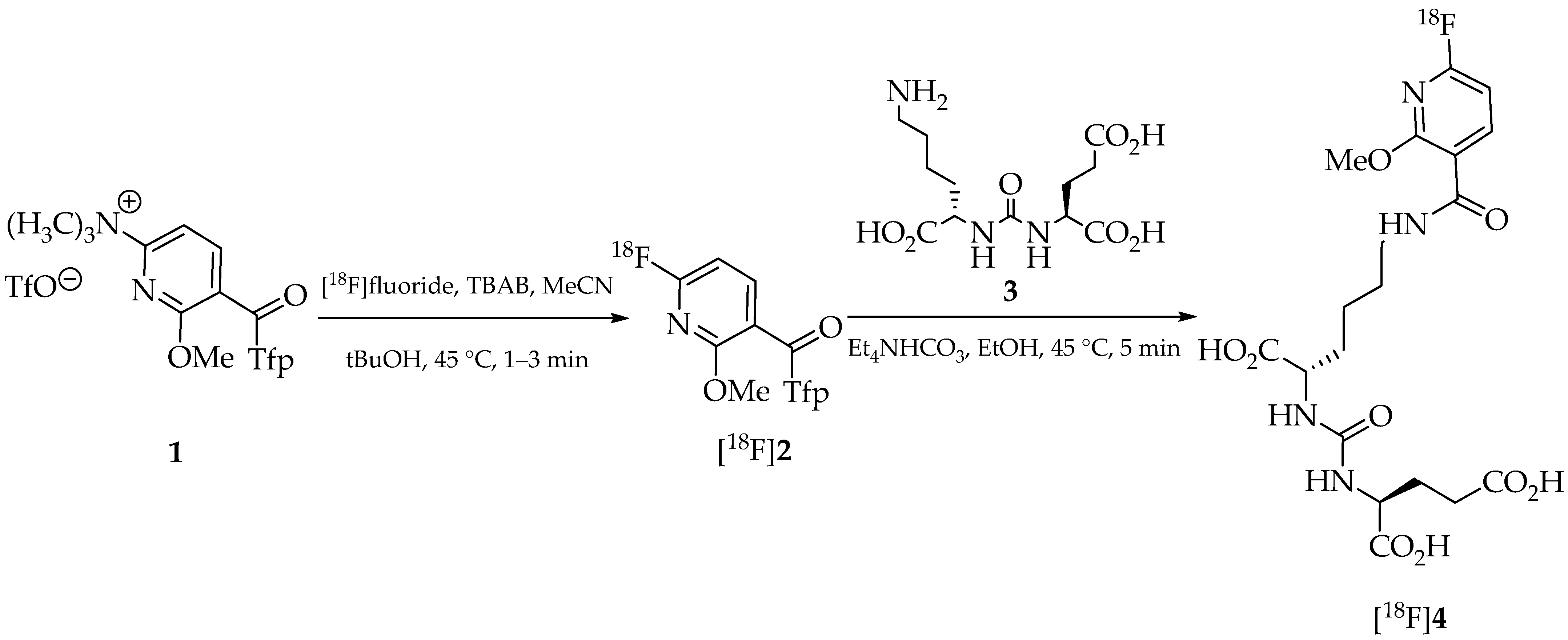
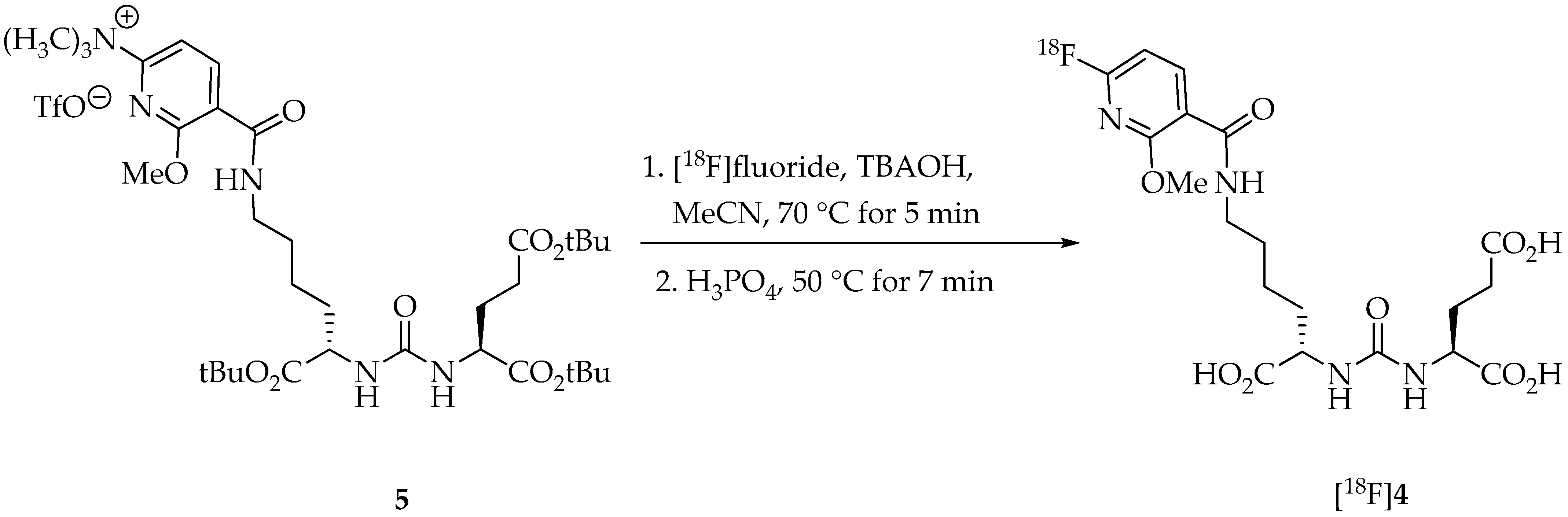
2. Results and Discussion
3. Materials and Methods
3.1. Production of No-Carrier-Added [18F]Fluoride
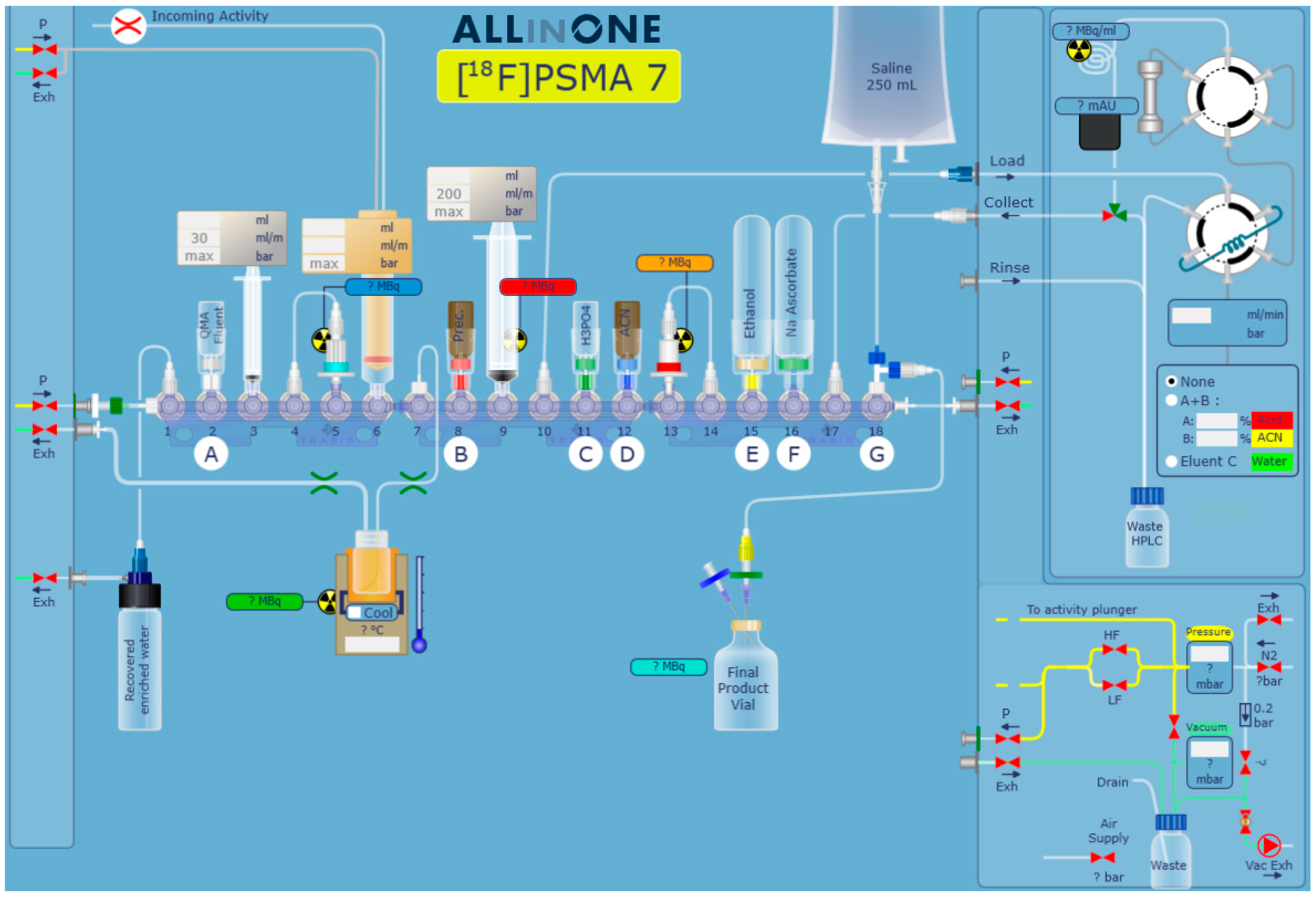
3.2. Automated Synthesis of [18F]JK-PSMA-7 on the Trasis AllInOne Synthesizer
3.2.1. Cassette Preparation
- A vial with tetrabutylammonium hydroxide-30-hydrate (TBAOH; 11.5 mg, 60.1 mmol) in MeCN/water (85/15 v/v%; 1.5 mL) for elution of [18F]fluoride from the QMA cartridge was attached to position 2 (A) of the cassette.
- A vial with solid labeling precursor 5 (10 mg; 12.1 µmol) was attached to position 8 (B) of the cassette.
- A vial with phosphoric acid (1.1 mL, 85%) was secured to position 11 (C) of the cassette.
- A vial with anhydrous MeCN (1 mL) was coupled to position 12 (D) of the cassette.
- A vial with absolute EtOH (10.35 mL) was attached to position 15 (E) of the cassette.
- A vial filled with 100 mg of sodium ascorbate was secured to position 16 (F) of the cassette.
- A storage bag with sterile saline solution (250 mL) and the final product vial were connected (via a three-way valve) to position 18 (G) of the cassette.
3.2.2. HPLC Unit
3.2.3. Preliminary Steps Before Start of the Synthesis
- The precursor (vial in position B) was dissolved in 1 mL of MeCN (from vial in position D).
- The C-18 cartridge was preconditioned with 5 mL of EtOH (from vial in position E) followed by 16 mL of saline (from storage bag in position G).
- Sodium ascorbate was dissolved in 10 mL of saline (from storage bag in position G) and passed into the final product vial.
- The sodium ascorbate syringe was rinsed with 10 mL of saline (from storage bag in position G) and the saline solution passed into the waste.
- A total of 12.6 mL of saline (from storage bag in position G) was drawn up with the syringe and passed into the now empty sodium ascorbate vial, which was later used to collect and dilute the HPLC product fraction of about 5 mL (see above).
- Finally, the cassette was dried with N2.
3.2.4. Preparation of Dispensation
3.2.5. Production of [18F]JK-PSMA-7
3.2.6. Quality Control and Validation of Analytical Procedures
HPLC for Identification, Chemical and Radiochemical Purity Determination
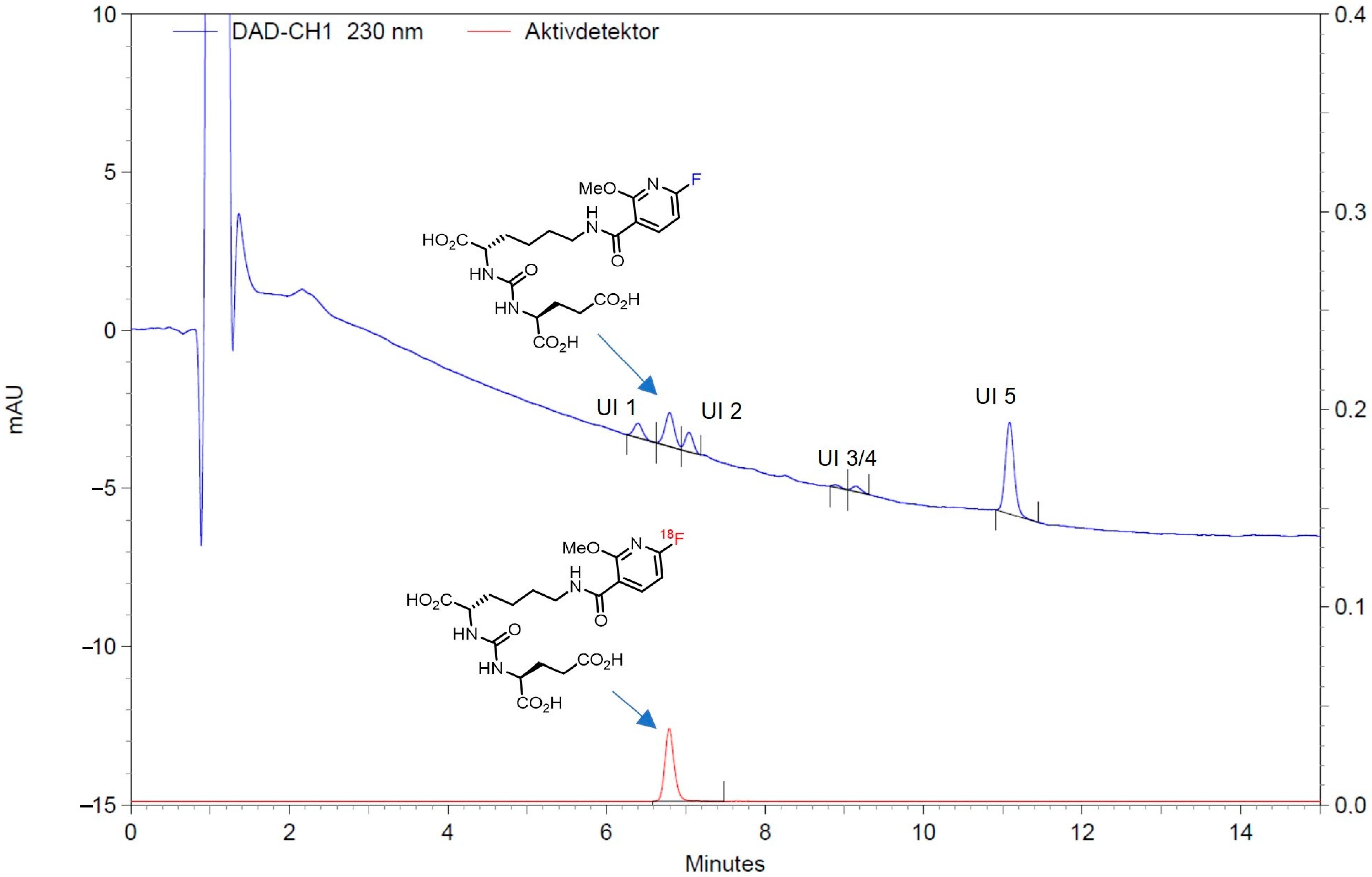
Validation of Chemical Impurities (UV Detector)
Validation of Radiochemical Impurities (Radiodetector)
GC for Residual Solvent and Ethanol Content Determination
Validation of GC Analysis of Residual Solvents
Thin Layer Chromatography Tests
Bacterial Endotoxin Test and Sterility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Foss, C.A.; Mease, R.C.; Cho, S.Y.; Kim, H.J.; Pomper, M.G. GCPII Imaging and Cancer. Curr. Med. Chem. 2012, 19, 1346–1359. [Google Scholar] [CrossRef]
- Capasso, G.; Stefanucci, A.; Tolomeo, A. A systematic review on the current status of PSMA-targeted imaging and radioligand therapy. Eur. J. Med. Chem. 2024, 263, 115966. [Google Scholar] [CrossRef]
- Alberts, I.L.; Seifert, R.; Werner, R.A.; Rowe, S.P.; Afshar-Oromieh, A. Prostate-specific Membrane Antigen: Diagnostics. PET Clin. 2024, 19, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Gholamrezanejhad, A.; Mirpour, S.; Mariani, G. Future of nuclear medicine: SPECT versus PET. J. Nucl. Med. 2009, 50, 16N–18N. [Google Scholar]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. 2013, 40, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Kesch, C.; Yun, M.; Cardinale, J.; Haberkorn, U.; Kopka, K.; Kratochwil, C.; Hadaschik, B.A. 18F-PSMA-1007 PET/CT Detects Micrometastases in a Patient With Biochemically Recurrent Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, e497–e499. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.T.; Kesch, C.; Cardinale, J.; Flechsig, P.; Floca, R.; Eiber, M.; Bonekamp, D.; Radtke, J.P.; Kratochwil, C.; Kopka, K.; et al. Simultaneous whole-body 18F-PSMA-1007-PET/MRI with integrated high-resolution multiparametric imaging of the prostatic fossa for comprehensive oncological staging of patients with prostate cancer: A pilot study. Eur. J. Nucl. Med. 2018, 45, 340–347. [Google Scholar] [CrossRef]
- Neels, O.C.; Kopka, K.; Liolios, C.; Afshar-Oromieh, A. Radiolabeled PSMA Inhibitors. Cancers 2021, 13, 6255. [Google Scholar] [CrossRef]
- Hodolič, M. Imaging of Prostate Cancer Using 18F-Choline PET/Computed Tomography. PET Clin. 2017, 12, 173–184. [Google Scholar] [CrossRef]
- Bouchelouche, K.; Choyke, P.L. Advances in prostate-specific membrane antigen PET of prostate cancer. Curr. Opin. Oncol. 2018, 30, 189–196. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Werner, R.A.; Solnes, L.B.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A.; Rowe, S.P. Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer: An Update on Important Pitfalls. Semin. Nucl. Med. 2019, 49, 255–270. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Haberkorn, U.; Eder, M.; Eisenhut, M.; Zechmann, C. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur. J. Nucl. Med. 2012, 39, 1085–1086. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef]
- Kopka, K.; Benešová, M.; Bařinka, C.; Haberkorn, U.; Babich, J. Glu-Ureido–Based Inhibitors of Prostate-Specific Membrane Antigen: Lessons Learned During the Development of a Novel Class of Low-Molecular-Weight Theranostic Radiotracers. J. Nucl. Med. 2017, 58, 17S–26S. [Google Scholar] [CrossRef]
- Chen, Y.; Pullambhatla, M.; Foss, C.A.; Byun, Y.; Nimmagadda, S.; Senthamizhchelvan, S.; Sgouros, G.; Mease, R.C.; Pomper, M.G. 2-(3-{1-Carboxy-5-[(6-[18F]Fluoro-Pyridine-3-Carbonyl)-Amino]-Pentyl}-Ureido)-Pentanedioic Acid, [18F]DCFPyL, a PSMA-Based PET Imaging Agent for Prostate Cancer. Clin. Cancer Res. 2011, 17, 7645–7653. [Google Scholar] [CrossRef]
- Cardinale, J.; Roscher, M.; Schaefer, M.; Geerlings, M.; Benešová, M.; Bauder-Wüst, U.; Remde, Y.; Eder, M.; Nováková, Z.; Motlová, L.; et al. Development of PSMA-1007-Related Series of 18F-Labeled Glu-Ureido-Type PSMA Inhibitors. J. Med. Chem. 2020, 63, 10897–10907. [Google Scholar] [CrossRef]
- Cardinale, J.; Martin, R.; Remde, Y.; Schäfer, M.; Hienzsch, A.; Hübner, S.; Zerges, A.-M.; Marx, H.; Hesse, R.; Weber, K.; et al. Procedures for the GMP-Compliant Production and Quality Control of [18F]PSMA-1007: A Next Generation Radiofluorinated Tracer for the Detection of Prostate Cancer. Pharmaceuticals 2017, 10, 77. [Google Scholar] [CrossRef]
- Cardinale, J.; Schäfer, M.; Benešová, M.; Bauder-Wüst, U.; Leotta, K.; Eder, M.; Neels, O.C.; Haberkorn, U.; Giesel, F.L.; Kopka, K. Preclinical Evaluation of 18F-PSMA-1007, a New Prostate-Specific Membrane Antigen Ligand for Prostate Cancer Imaging. J. Nucl. Med. 2017, 58, 425–431. [Google Scholar] [CrossRef]
- Allach, Y.; Banda, A.; van Gemert, W.; de Groot, M.; Derks, Y.; Schilham, M.; Hoepping, A.; Perk, L.; Gotthardt, M.; Janssen, M.; et al. An Explorative Study of the Incidental High Renal Excretion of [18F]PSMA-1007 for Prostate Cancer PET/CT Imaging. Cancers 2022, 14, 2076. [Google Scholar] [CrossRef] [PubMed]
- Zlatopolskiy, B.D.; Endepols, H.; Krapf, P.; Guliyev, M.; Urusova, E.A.; Richarz, R.; Hohberg, M.; Dietlein, M.; Drzezga, A.E.; Neumaier, B. Discovery of 18F-JK-PSMA-7, a PET probe for the detection of small PSMA-positive lesions. J. Nucl. Med. 2019, 60, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Dietlein, F.; Hohberg, M.; Kobe, C.; Zlatopolskiy, B.D.; Krapf, P.; Endepols, H.; Täger, P.; Hammes, J.; Heidenreich, A.; Neumaier, B.; et al. An 18F-labeled PSMA ligand for PET/CT of prostate cancer: First-in-humans observational study and clinical experience with 18F-JK-PSMA-7 during the first year of application. J. Nucl. Med. 2020, 61, 202–209. [Google Scholar] [CrossRef]
- Hohberg, M.; Kobe, C.; Krapf, P.; Täger, P.; Hammes, J.; Dietlein, F.; Zlatopolskiy, B.D.; Endepols, H.; Wild, M.; Neubauer, S.; et al. Biodistribution and radiation dosimetry of [18F]-JK-PSMA-7 as a novel prostate-specific membrane antigen-specific ligand for PET/CT imaging of prostate cancer. EJNMMI Res. 2019, 9, 66. [Google Scholar] [CrossRef]
- Van Simaeys, G.; Doumont, G.; De Maeseneire, C.; Passon, N.; Lacroix, S.; Lentz, C.; Horion, A.; Warnier, C.; Torres, D.; Martens, C.; et al. [18F]-JK-PSMA-7 and [18F]-FDG tumour PET uptake in treated xenograft human prostate cancer model in mice. Eur. J. Nucl. Med. 2021, 48, 1773–1784. [Google Scholar] [CrossRef]
- Decristoforo, C.; Schwarz, S.W. Radiopharmacy: Regulations and legislations in relation to human applications. Drug Discov. Today Technol. 2011, 8, e71–e77. [Google Scholar] [CrossRef]
- Gillings, N.; Hjelstuen, O.; Behe, M.; Decristoforo, C.; Elsinga, P.H.; Ferrari, V.; Kiss, O.C.; Kolenc, P.; Koziorowski, J.; Laverman, P.; et al. EANM guideline on quality risk management for radiopharmaceuticals. Eur. J. Nucl. Med. 2022, 49, 3353–3364. [Google Scholar] [CrossRef]
- Moya, E.; Cerrato, C.; Bedoya, L.M.; Guerra, J.A. Radiopharmaceutical small-scale preparation in Europe: Will we be able to harmonize the situation? EJNMMI Radiopharm. Chem. 2024, 9, 64. [Google Scholar] [CrossRef]
- Krasikova, R. PET Radiochemistry Automation: State of the Art and Future Trends in 18F-nucleophilic Fluorination. Curr. Org. Chem. 2013, 17, 2097–2107. [Google Scholar] [CrossRef]
- Allott, L.; Aboagye, E.O. Chemistry Considerations for the Clinical Translation of Oncology PET Radiopharmaceuticals. Mol. Pharm. 2020, 17, 2245–2259. [Google Scholar] [CrossRef]
- Bogni, A.; Laera, L.; Cucchi, C.; Iwata, R.; Seregni, E.; Pascali, C. An improved automated one-pot synthesis of O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET) based on a purification by cartridges. Nucl. Med. Biol. 2019, 72–73, 11–19. [Google Scholar] [CrossRef]
- Joshi, R.K.; Goud, N.S.; Nagaraj, C.; Kumar, D.; Gopinath, R.; Rao, N.P.; Dhawan, A.; Bhattacharya, A.; Mangalore, S.; Bharath, R.D.; et al. Radiosynthesis challenges of 11C and 18F-labeled radiotracers in the FX2C/N tracerlab and their validation through PET-MR imaging. Appl. Radiat. Isot. 2021, 168, 109486. [Google Scholar] [CrossRef]
- Coenen, H.H.; Ermert, J. 18F-labelling innovations and their potential for clinical application. Clin. Transl. Imaging 2018, 6, 169–193. [Google Scholar] [CrossRef]
- Dahl, K.; Jussing, E.; Bylund, L.; Moein, M.M.; Samén, E.; Tran, T. Fully automated production of the fibroblast activation protein radiotracer [18F]FAPI-74. J. Label. Compd. Radiopharm. 2021, 64, 346–352. [Google Scholar] [CrossRef]
- Faivre-Chauvet, A.; Bourdeau, C.; Bourgeois, M. Radiopharmaceutical good practices: Regulation between hospital and industry. Front. Nucl. Med. 2022, 2, 990330. [Google Scholar] [CrossRef]
- Ermert, J.; Krapf, P.; Wicher, T.; Zlatopolskiy, B.; Neumaier, B. Fully automated and GMP-compliant synthesis of [18F]JK-PSMA-7 on a Trasis AllinOne module. In Procceding of the 24th International Symposium on Radiopharmaceutical Sciences, iSRS 2022, Nantes, France, 29 May–3 June 2022. [Google Scholar]
- Collet, C.; Otabashi, M.; Giacomelli, F.; Veran, N.; Karcher, G.; Chapleur, Y.; Lamandé-Langle, S. Fully automated production of sodium [18F]fluoride on AllInOne and miniAllInOne synthesizers. Appl. Radiat. Isot. 2015, 102, 87–92. [Google Scholar] [CrossRef]
- Cybulska, K.A.; Bloemers, V.; Perk, L.R.; Laverman, P. Optimised GMP-compliant production of [18F]DPA-714 on the Trasis AllinOne module. EJNMMI Radiopharm. Chem. 2021, 6, 20. [Google Scholar] [CrossRef]
- Richarz, R.; Krapf, P.; Zarrad, F.; Urusova, E.A.; Neumaier, B.; Zlatopolskiy, B.D. Neither azeotropic drying, nor base nor other additives: A minimalist approach to 18F-labeling. Org. Biomol. Chem. 2014, 12, 8094–8099. [Google Scholar] [CrossRef]

| Test | Method | Specifications at Release | Typical Results of a Production Batch (Here 24 August 2024) |
|---|---|---|---|
| At product release | |||
| Appearance | Visual inspection | Clear and colorless, free from visible particles | Corresponds to specification |
| (Radio)chemical identity of [18F]JK-PSMA-7 | HPLC (UV, γ-ray detectors) | [18F]JK-PSMA-7 peak in the radiochromatogram is consistent with the retention time of the reference standard to the nearest ± 0.5 min, taking detector delay into account | Corresponds to specification |
| Radionuclide identity of fluorine-18 (half-life measurement) | Dose calibrator | 110 ± 5 min | 109.6 min |
| Radiochemical identity of [18F]JK-PSMA-7 | HPLC (γ-ray detector) | Retention time of [18F]JK-PSMA-7 consistent with retention time of JK-PSMA-7 (±0.5 min) | Corresponds to specification |
| Radiochemical purity of [18F]JK-PSMA-7 | HPLC (γ-ray detector) | ≥95% of total fluorine-18 activity | 99.8% |
| pH | pH meter | 4.5–8.5 | 6.31 |
| Chemical concentration of JK-PSMA-7 | HPLC (UV detector) | ≤10 µg/mL | 0.54 µg/mL |
| Sum of JK-PSMA-7 and all impurities above disregard limit | HPLC (UV detector) | ≤5 µg/mL | 4.65 µg/mL |
| Activity concentration | Dose calibrator | ≥30 MBq/mL | 1068 MBq/mL |
| Chemical concentration of tetrabutylammonium (TBA) | TLC spot test | <260 μg/mL | <260 μg/mL |
| Bacterial endotoxins | Kinetic chromogenic LAL method | ≤17.5 IU/mL | <3.9 IU/mL |
| Sterile filter integrity | Pressure holding test | <20% | Corresponds to specification |
| Acetonitrile content | Gas chromatography | ≤0.41 mg/mL | ≤0.03 mg/mL |
| After product decay | |||
| Radionuclide impurities with half-life > 2 h | γ-ray spectrometer | No signal higher than 5 times background noise | Corresponds to specification |
| Sterility | Direct inoculation | Sterile | Sterile |
| Manifold Position | Reagents or Materials Attached |
|---|---|
| 1 | PE tubing to [18O]water recovery vial |
| 2 | Eluents for QMA elution |
| 3 | Sterile 3 mL syringe with Luer Lock fitting |
| 4 | Silicone tubing connected to QMA cartridge in position 5 |
| 5 | Preconditioned QMA cartridge |
| 6 | Sterile 20 mL syringe with Luer Lock fitting for collection of 18F-activity from cyclotron |
| 7 | Connector to 6 mL reactor with 2 tubes (male connector to cassette, female to exhaust) |
| 8 | Vial with precursor 5 (10 mg) |
| 9 | Sterile 20 mL syringe with Luer Lock fitting |
| 10 | PE tubing (OD 2 mm, length 150 mm) connected to Load port of the HPLC unit |
| 11 | Vial with phosphoric acid (1.1 mL) |
| 12 | Vial with MeCN (1 mL) |
| 13 | Sep-Pak C18 Plus cartridge |
| 14 | PE tubing (OD 2 mm) connected to Sep-Pak C18 Plus cartridge in position 13 |
| 15 | Vial with EtOH (10.35 mL) |
| 16 | Vial with sodium ascorbate solution (10 mL) |
| 17 | PE tubing (OD 2 mm) connected to Collect port of the HPLC unit |
| 18 | Three-way valve connected to 0.9% NaCl for injection bottle and product vial |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krapf, P.; Wicher, T.; Zlatopolskiy, B.D.; Ermert, J.; Neumaier, B. Fully Automated Production of (((S)-1-Carboxy-5-(6-([18F]fluoro)-2-methoxynicotinamido)pentyl)carbamoyl)-l-glutamic Acid ([18F]JK-PSMA-7). Pharmaceuticals 2025, 18, 119. https://doi.org/10.3390/ph18010119
Krapf P, Wicher T, Zlatopolskiy BD, Ermert J, Neumaier B. Fully Automated Production of (((S)-1-Carboxy-5-(6-([18F]fluoro)-2-methoxynicotinamido)pentyl)carbamoyl)-l-glutamic Acid ([18F]JK-PSMA-7). Pharmaceuticals. 2025; 18(1):119. https://doi.org/10.3390/ph18010119
Chicago/Turabian StyleKrapf, Philipp, Thomas Wicher, Boris D. Zlatopolskiy, Johannes Ermert, and Bernd Neumaier. 2025. "Fully Automated Production of (((S)-1-Carboxy-5-(6-([18F]fluoro)-2-methoxynicotinamido)pentyl)carbamoyl)-l-glutamic Acid ([18F]JK-PSMA-7)" Pharmaceuticals 18, no. 1: 119. https://doi.org/10.3390/ph18010119
APA StyleKrapf, P., Wicher, T., Zlatopolskiy, B. D., Ermert, J., & Neumaier, B. (2025). Fully Automated Production of (((S)-1-Carboxy-5-(6-([18F]fluoro)-2-methoxynicotinamido)pentyl)carbamoyl)-l-glutamic Acid ([18F]JK-PSMA-7). Pharmaceuticals, 18(1), 119. https://doi.org/10.3390/ph18010119








