A Newly Validated HPLC-DAD Method for the Determination of Ricinoleic Acid (RA) in PLGA Nanocapsules
Abstract
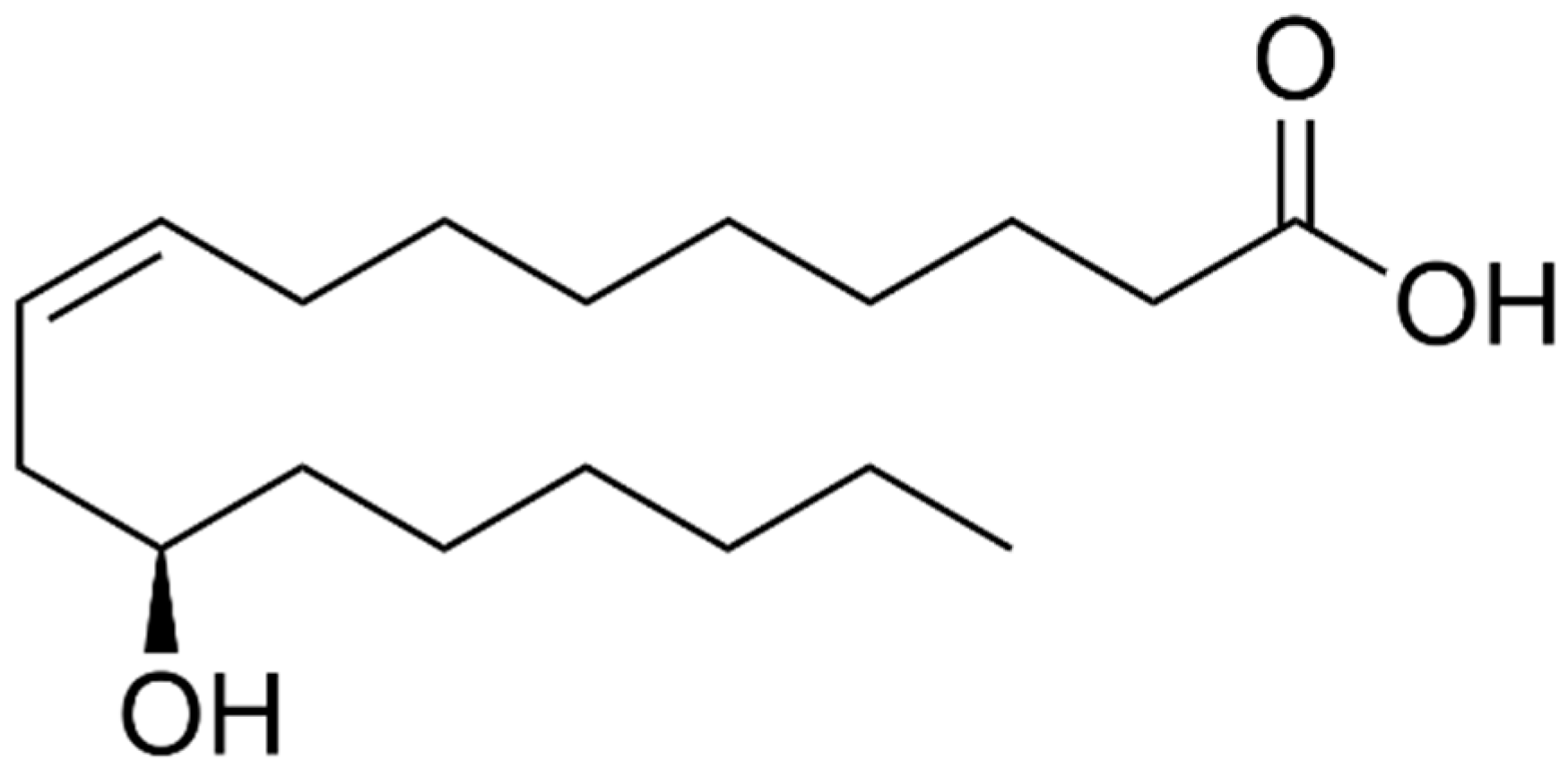
1. Introduction
2. Results and Discussion
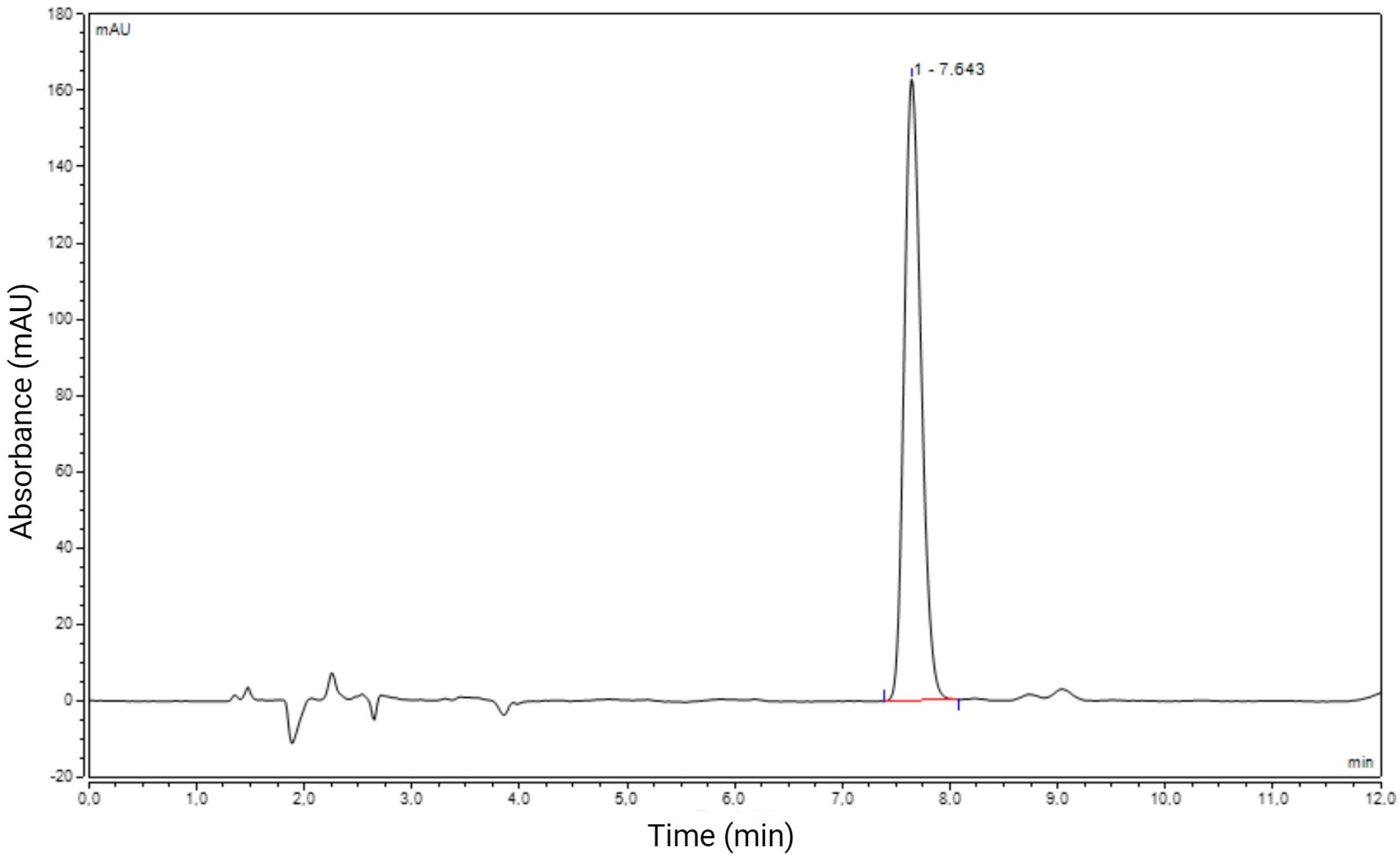
2.1. Chromatography
2.2. Method Validation
2.2.1. Linearity
2.2.2. Limits of Quantification (LOQ) and Detection (LOD)
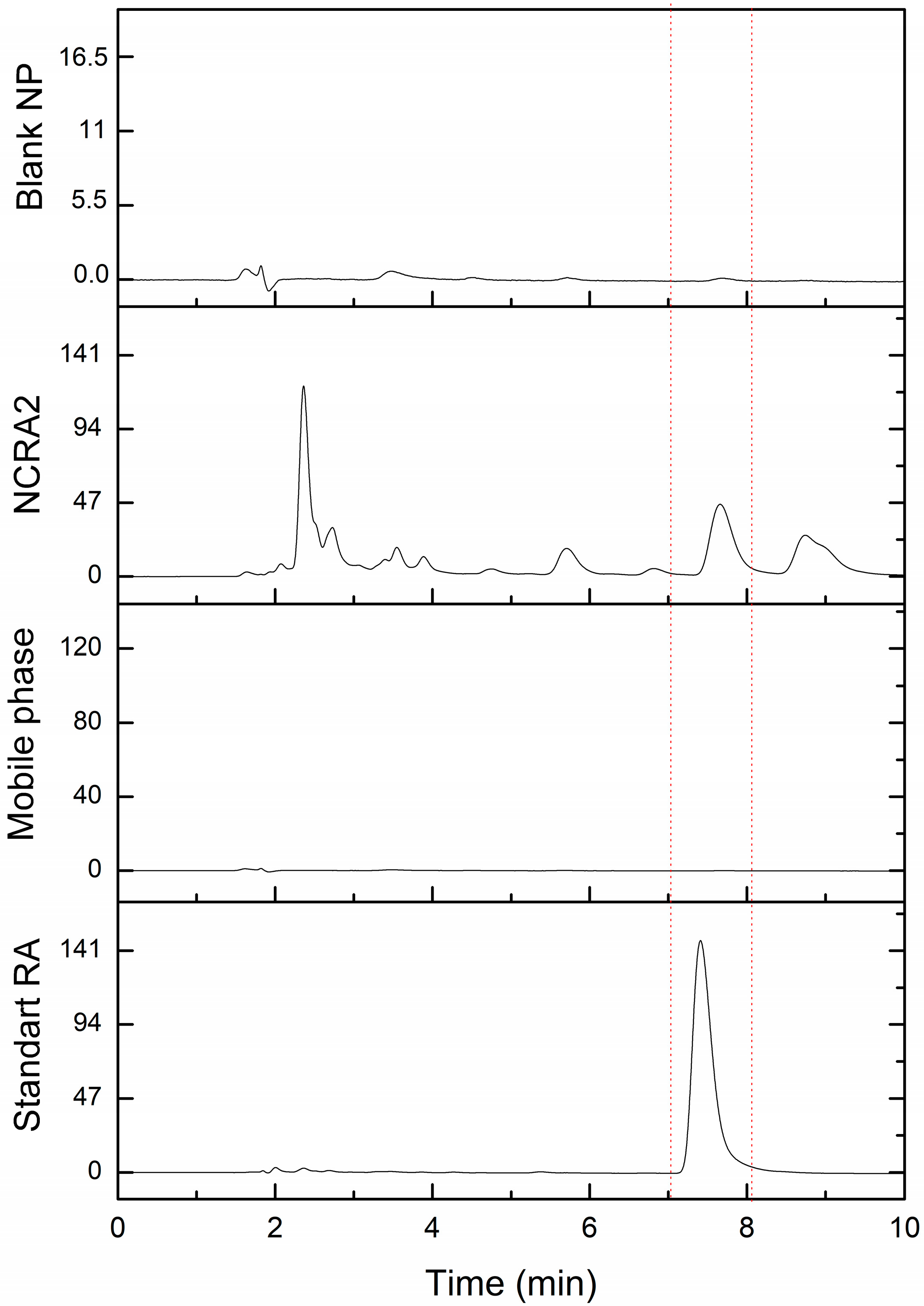
2.2.3. Specificity
2.2.4. Precision
2.2.5. Robustness
2.2.6. Accuracy
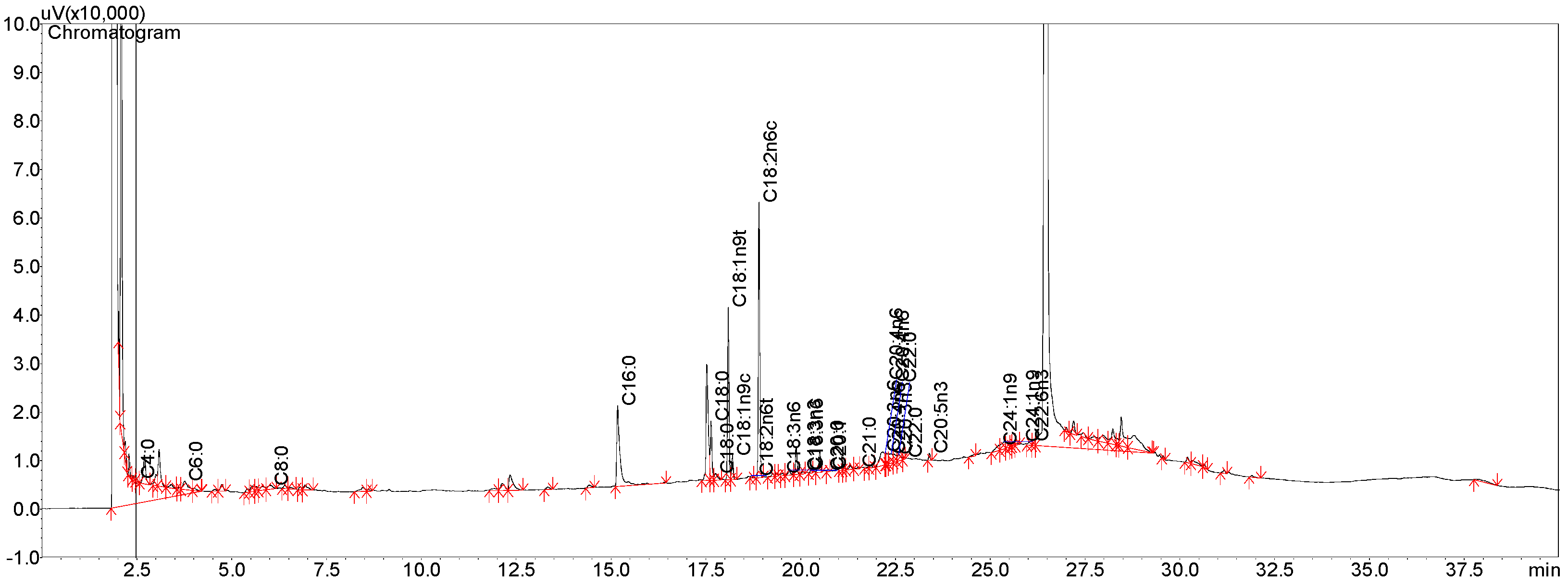
2.3. Gas Chromatography of Ricinoleic Acid (Commercial)
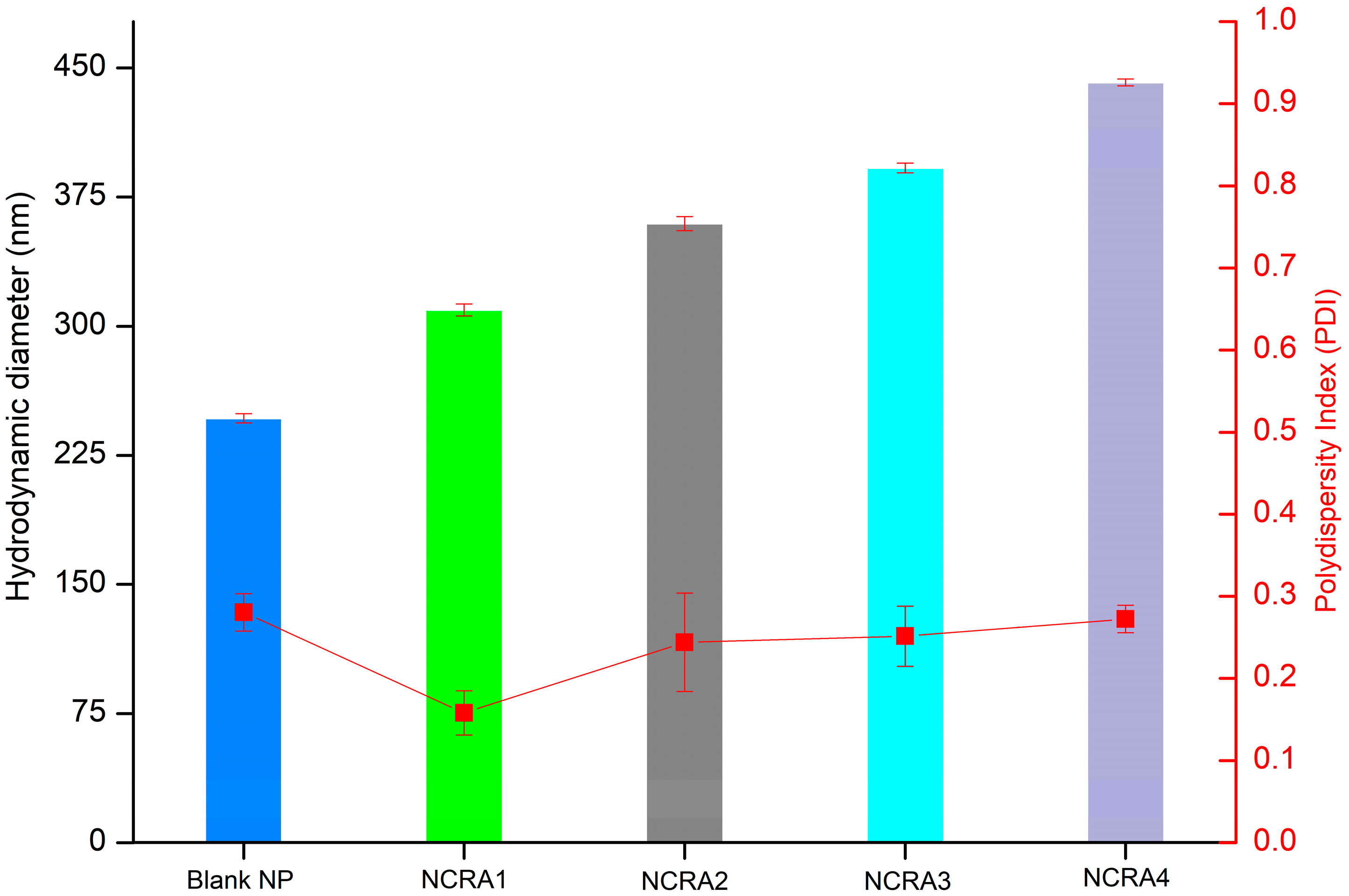
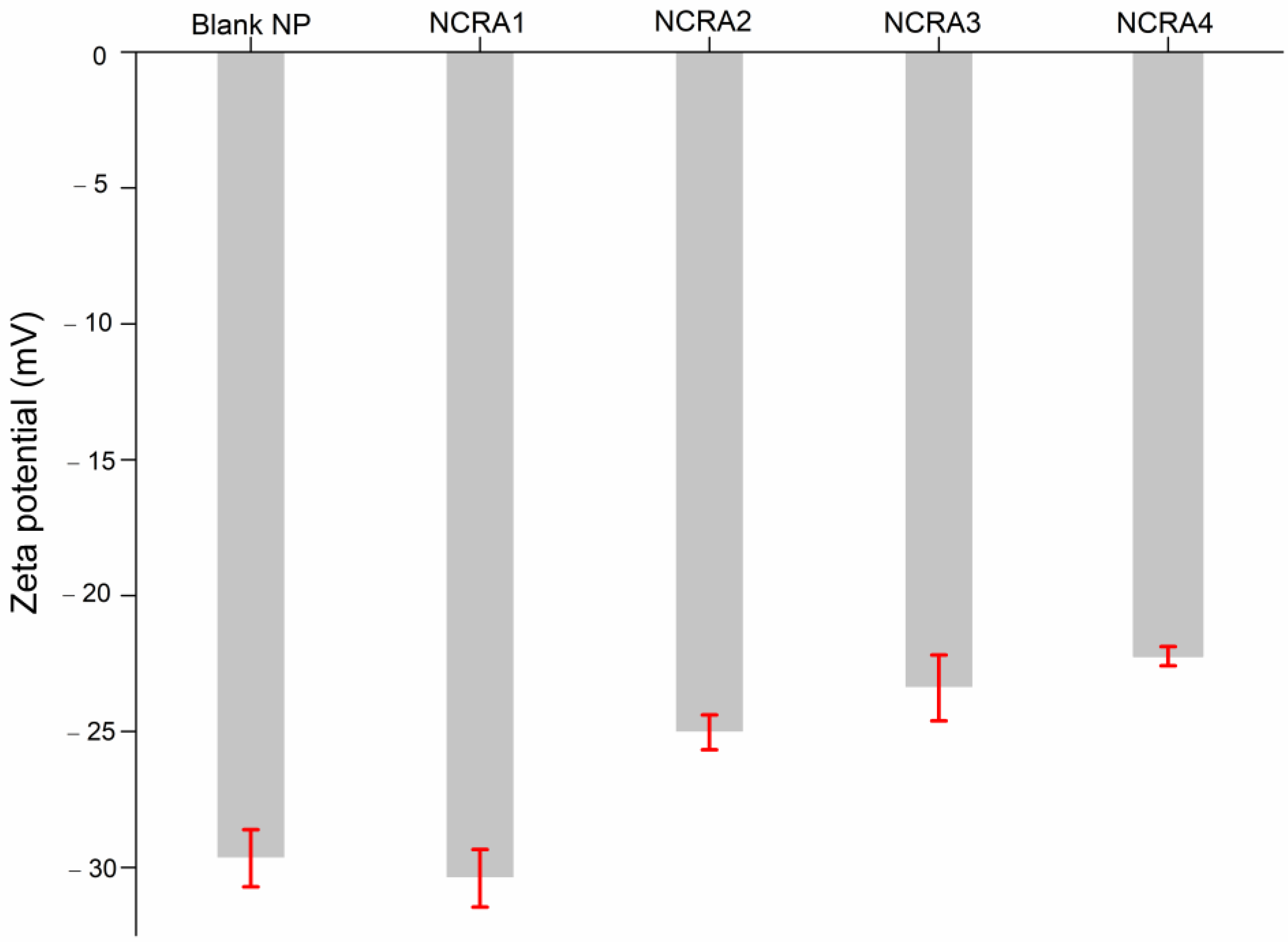
2.4. Particle Size, Polydispersity Index and Zeta Potential of Nanocapsules Containing Ricinoleic Acid (RA)
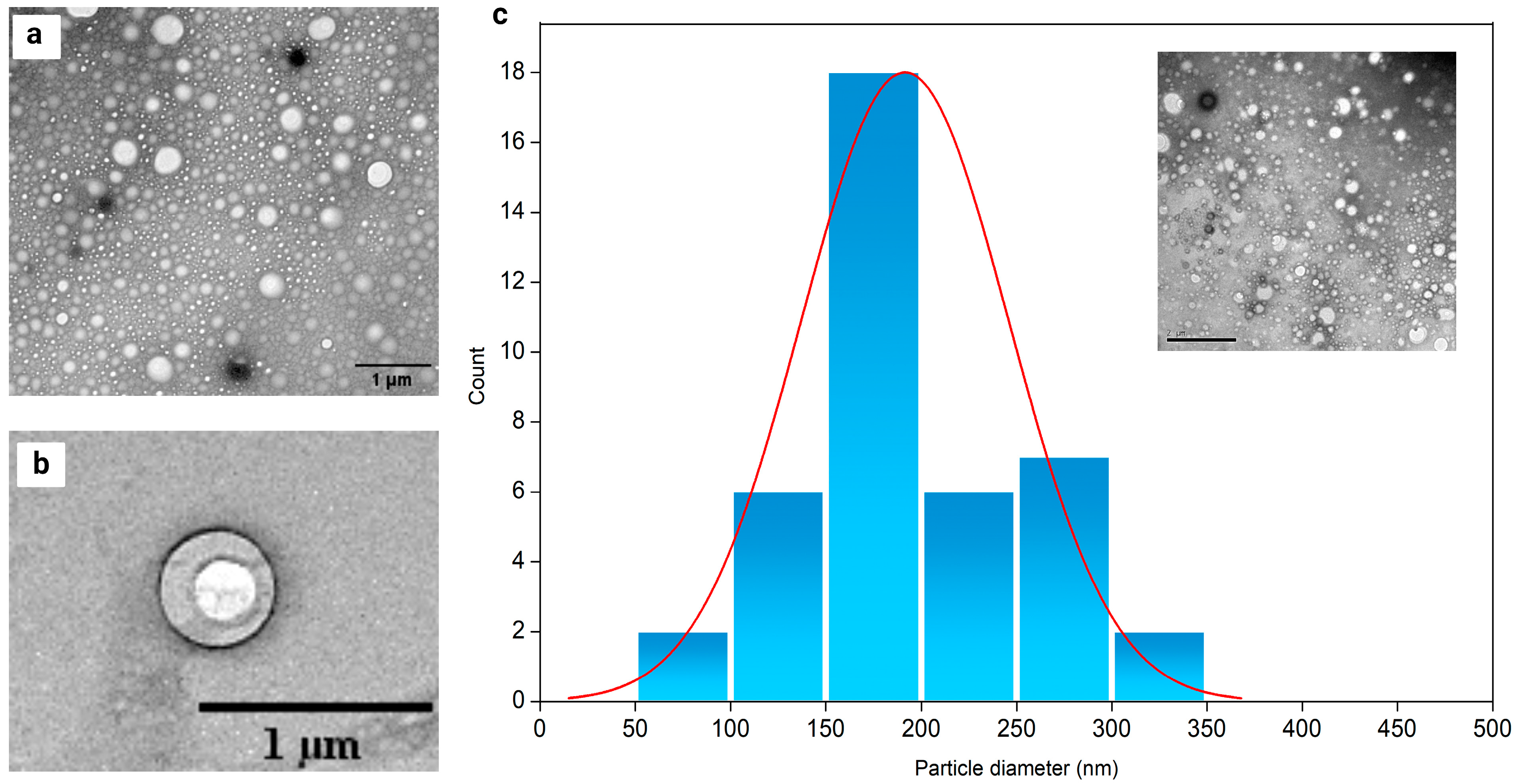
2.5. Nanoparticle Morphology Characterization
3. Materials and Methods
3.1. Materials
3.2. Chromatographic Conditions
3.3. Method Validation
3.3.1. Linearity
3.3.2. Limit of Quantification (LOQ) and Detection (LOD)
3.3.3. Selectivity
3.3.4. Precision
3.3.5. Robustness
3.3.6. Accuracy
3.4. Ricinoleic Acid (RA) Content (Commercial Sample) by Gas Chromatography
3.5. Preparation of Ricinoleic Acid (RA)-Loaded Nanocapsules
3.6. Determination of Particle Size, Polydispersity Index, and Zeta Potential
3.7. Encapsulation Efficiency
3.8. Nanoparticle Morphology Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeboah, A.; Ying, S.; Lu, J.; Xie, Y.; Amoanimaa-Dede, H.; Boateng, K.G.A.; Chen, M.; Yin, X. Castor Oil (Ricinus Communis): A Review on the Chemical Composition and Physicochemical Properties. Food Sci. Technol. 2021, 41, 399–413. [Google Scholar] [CrossRef]
- Arslan, A.; Rancke-Madsen, A.; Brask, J. Enzymatic Synthesis of Estolides from Castor Oil. Catalysts 2020, 10, 835. [Google Scholar] [CrossRef]
- Scholz, V.; da Silva, J.N. Prospects and Risks of the Use of Castor Oil as a Fuel. Biomass Bioenergy 2008, 32, 95–100. [Google Scholar] [CrossRef]
- de Lima Da Silva, N.; Maciel, M.R.W.; Batistella, C.B.; Filho, R.M. Optimization of Biodiesel Production from Castor Oil. Appl. Biochem. Biotechnol. 2006, 130, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Dumancas, G.G.; Kasi Viswanath, L.C.; Maples, R.; Subong, B.J.J. Castor Oil: Properties, Uses, and Optimization of Processing Parameters in Commercial Production. Lipid Insights 2016, 9, LPI-S40233. [Google Scholar] [CrossRef] [PubMed]
- Nitbani, F.O.; Tjitda, P.J.P.; Wogo, H.E.; Detha, A.I.R. Preparation of Ricinoleic Acid from Castor Oil: A Review. J. Oleo Sci. 2022, 71, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.J.E.; Alves, D.C.L.; de Sá, R.O.; Viana, R. da S.; Ferrari, S. Potencial produtivo de híbridos de mamona em diferentes densidades de plantas na segunda safra em ambiente estressante. Rev. Ciências Agroveterinárias 2021, 20, 053–059. [Google Scholar] [CrossRef]
- Data Bridge Market Research Mercado Global de Óleo de Mamona—Tendências Do Setor e Previsão Para 2031. Available online: https://www.databridgemarketresearch.com/pt/reports/global-castor-oil-market (accessed on 17 July 2024).
- Rissato, S.R.; Galhiane, M.S.; Fernandes, J.R.; Gerenutti, M.; Gomes, H.M.; Ribeiro, R.; de Almeida, M.V. Evaluation of Ricinus Communis L. for the Phytoremediation of Polluted Soil with Organochlorine Pesticides. Biomed. Res. Int. 2015, 2015, 549863. [Google Scholar] [CrossRef] [PubMed]
- Kammili Anjani, K.A. A Re-Evaluation of Castor (Ricinus communis L.) as a Crop Plant. CABI Rev. 2014, 2014, 9038. [Google Scholar] [CrossRef]
- Ramos Morales, E.; Mata Espinosa, M.A.; McKain, N.; Wallace, R.J. Ricinoleic Acid Inhibits Methanogenesis and Fatty Acid Biohydrogenation in Ruminal Digesta from Sheep and in Bacterial Cultures. J. Anim. Sci. 2012, 90, 4943–4950. [Google Scholar] [CrossRef]
- Vieira, C.; Evangelista, S.; Cirillo, R.; Terracciano, R.; Lippi, A.; Maggi, C.A.; Manzini, S. Antinociceptive Activity of Ricinoleic Acid, a Capsaicin-like Compound Devoid of Pungent Properties. Eur. J. Pharmacol. 2000, 407, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, S.; Zabielska, J.; Kula, J.; Kunicka-Styczyńska, A. Synthesis of (R)- and (S)-Ricinoleic Acid Amides and Evaluation of Their Antimicrobial Activity. J. Am. Oil Chem. Soc. 2018, 95, 69–77. [Google Scholar] [CrossRef]
- PubChem. 2,3-Bis(12-Hydroxyoctadec-9-Enoyloxy)Propyl 12-Hydroxyoctadec-9-Enoate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/96919 (accessed on 20 July 2024).
- Steingrub, J.S.; Lopez, T.; Teres, D.; Steingart, R. Amniotic Fluid Embolism Associated with Castor Oil Ingestion. Crit. Care Med. 1988, 16, 642–643. [Google Scholar] [CrossRef]
- Moshiri, M.; Hamid, F.; Etemad, L. Ricin Toxicity: Clinical and Molecular Aspects. Rep. Biochem. Mol. Biol. 2016, 4, 60–65. [Google Scholar]
- Burdock, G.A.; Carabin, I.G.; Griffiths, J.C. Toxicology and Pharmacology of Sodium Ricinoleate. Food Chem. Toxicol. 2006, 44, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Camacho, R.M.; Haro, I.; Gómara, M.J.; Espina, M.; Fonseca, J.; Martins-Gomes, C.; Camins, A.; Silva, A.M.; García, M.L.; Souto, E.B. Cell Penetrating Peptides-Functionalized Licochalcone-A-Loaded PLGA Nanoparticles for Ocular Inflammatory Diseases: Evaluation of in Vitro Anti-Proliferative Effects, Stabilization by Freeze-Drying and Characterization of an in-Situ Forming Gel. Int. J. Pharm. 2023, 639, 122982. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Espina, M.; Auladell, C.; Folch, J.; Kühne, B.A.; Barenys, M.; Sánchez-López, E.; Souto, E.B.; García, M.L.; et al. Epigallocatechin-3-Gallate PEGylated Poly(Lactic-Co-Glycolic) Acid Nanoparticles Mitigate Striatal Pathology and Motor Deficits in 3-Nitropropionic Acid Intoxicated Mice. Nanomedicine 2021, 16, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine Loaded PLGA PEGylated Nanoparticles for Alzheimer’s Disease: In Vitro and in Vivo Characterization. J. Nanobiotechnol 2018, 16, 32. [Google Scholar] [CrossRef]
- Oliveira, D.M.L.; Rezende, P.S.; Barbosa, T.C.; Andrade, L.N.; Bani, C.; Tavares, D.S.; Da Silva, C.F.; Chaud, M.V.; Padilha, F.; Cano, A.; et al. Double Membrane Based on Lidocaine-Coated Polymyxin-Alginate Nanoparticles for Wound Healing: In Vitro Characterization and in Vivo Tissue Repair. Int. J. Pharm. 2020, 591, 120001. [Google Scholar] [CrossRef]
- Ataide, J.A.; Gérios, E.F.; Cefali, L.C.; Fernandes, A.R.; Teixeira, M.D.C.; Ferreira, N.R.; Tambourgi, E.B.; Jozala, A.F.; Chaud, M.V.; Oliveira-Nascimento, L.; et al. Effect of Polysaccharide Sources on the Physicochemical Properties of Bromelain–Chitosan Nanoparticles. Polymers 2019, 11, 1681. [Google Scholar] [CrossRef]
- Tanjung, Y.; Dewi, M.; Gatera, V.; Barliana, M.; Joni, I.M.; Chaerunisaa, A. Factors Affecting the Synthesis of Bovine Serum Albumin Nanoparticles Using the Desolvation Method. NSA 2024, 17, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Fatima, W.; Batool, S.R.; Mushtaq, F.; Aslam, M.; Raza, Z.A.; Nazeer, M.A. Controlled Release of Doxorubicin from Gelatin-Based Nanoparticles: Theoretical and Experimental Approach. Mater. Adv. 2024, 5, 2347–2358. [Google Scholar] [CrossRef]
- Cañadas, C.; Alvarado, H.; Calpena, A.C.; Silva, A.M.; Souto, E.B.; García, M.L.; Abrego, G. In Vitro, Ex Vivo and in Vivo Characterization of PLGA Nanoparticles Loading Pranoprofen for Ocular Administration. Int. J. Pharm. 2016, 511, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Cinu, T.A.; Sebastian, R.; Shoja, M.H.; Aleykutty, N.A.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B. Transferrin-Conjugated Docetaxel–PLGA Nanoparticles for Tumor Targeting: Influence on MCF-7 Cell Cycle. Polymers 2019, 11, 1905. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Egea, M.A.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.B.; Silva, A.M.; García, M.L. PEGylated PLGA Nanospheres Optimized by Design of Experiments for Ocular Administration of Dexibuprofen—In Vitro, Ex Vivo and in Vivo Characterization. Colloids Surf. B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduño-Ramirez, M.L.; García, M.L.; Calpena, A.C.; Souto, E.B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef]
- GUIDELINE, ICH Harmonised Tripartite et al. Impurities in New Drug Products. Q3B (R2), Current Step, v. 4, p. 1-5. 2006. Available online: https://database.ich.org/sites/default/files/Q3B%28R2%29%20Guideline.pdf (accessed on 17 July 2024).
- Chew, Y.-L.; Khor, M.-A.; Lim, Y.-Y. Choices of Chromatographic Methods as Stability Indicating Assays for Pharmaceutical Products: A Review. Heliyon 2021, 7, e06553. [Google Scholar] [CrossRef] [PubMed]
- Imanulkhan, I.; Setyaningsih, W.; Rohman, A.; Palma, M. Development and Validation of HPLC-DAD Method for Simultaneous Determination of Seven Food Additives and Caffeine in Powdered Drinks. Foods 2020, 9, 1119. [Google Scholar] [CrossRef] [PubMed]
- Hollá, M.; Bílková, A.; Jakubec, P.; Košková, S.; Kočová Vlčková, H.; Šatínský, D.; Švec, F.; Sklenářová, H. Benefits and Pitfalls of HPLC Coupled to Diode-Array, Charged Aerosol, and Coulometric Detections: Effect of Detection on Screening of Bioactive Compounds in Apples. Molecules 2021, 26, 3246. [Google Scholar] [CrossRef]
- Carvalho, D.; Jesus, Â.; Pinho, C.; Oliveira, R.F.; Moreira, F.; Oliveira, A.I. Validation of an HPLC-DAD Method for Quercetin Quantification in Nanoparticles. Pharmaceuticals 2023, 16, 1736. [Google Scholar] [CrossRef]
- Rigon, R.B.; Fachinetti, N.; Severino, P.; Durazzo, A.; Lucarini, M.; Atanasov, A.G.; El Mamouni, S.; Chorilli, M.; Santini, A.; Souto, E.B. Quantification of Trans-Resveratrol-Loaded Solid Lipid Nanoparticles by a Validated Reverse-Phase HPLC Photodiode Array. Appl. Sci. 2019, 9, 4961. [Google Scholar] [CrossRef]
- Silva, L.S.; de Oliveira, M.G.; Martins, C.F.; Borges, L.L.; Fiuza, T.S.; da Conceição, E.C.; Paula, J.R. de Validation HPLC-DAD Method for Quantification of Gallic and Ellagic Acid from Eugenia punicifolia Leaves, Extracts and Fractions. J. Braz. Chem. Soc. 2023, 34, 401–413. [Google Scholar] [CrossRef]
- Plante, M.; Bailey, B.; Acworth, I. The Use of Charged Aerosol Detection with HPLC for the Measurement of Lipids. Methods Mol. Biol. 2009, 579, 469–482. [Google Scholar] [CrossRef]
- Council of Europe Strasbourg. European Pharmacopoeia 7.1, Edition 2011; Council of Europe Strasbourg: Strasbourg, France, 2024. [Google Scholar]
- Zuy, Y.; Sweck, S.O.; Dockery, C.R.; Potts, G.E. HPLC Detection of Organic Gunshot Residues Collected with Silicone Wristbands. Anal. Methods 2020, 12, 85–90. [Google Scholar] [CrossRef]
- International Conference on Harmonization (ICH), Q2A: [15] Guidelines for Collaborative Study Procedure to Validate Text on Validation of Analytical Procedures, US FDA Characteristics of a Method of Analysis. J. Assoc. Off. Anal. Federal Register. 1995, 60, 11260.
- Hassan, M.E.; Hetta, M.H. Validated HPTLC Analysis Method for Quantification of Ricinoleic Acid and Oleic Acid Content in Castor Oil, Olive Oil and Selected Market Formulations. Act. Scie Pharma 2019, 3, 57–67. [Google Scholar] [CrossRef]
- AOAC Association of Official Analytical Chemists. Official Methods of Analysis, 20th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2016. [Google Scholar]
- US Pharmacopeia 23. Chromatography Section (621); United States Pharmacopeial Convention: Rockville, MD, USA, 1994; p. 1776. [Google Scholar]
- Perlatti, B.; da Silva, M.F.d.G.F.; Fernandes, J.B.; Forim, M.R. Validation and Application of HPLC–ESI-MS/MS Method for the Quantification of RBBR Decolorization, a Model for Highly Toxic Molecules, Using Several Fungi Strains. Bioresour. Technol. 2012, 124, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, P.; Marie, J.M.; Maria Xavier, A.J. Castor Oil-Derived Monomer Ricinoleic Acid Based Biodegradable Unsaturated Polyesters. Polym. Degrad. Stab. 2019, 170, 109016. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Preparation of Size-Tunable Sub-200 Nm PLGA-Based Nanoparticles with a Wide Size Range Using a Microfluidic Platform. PLoS ONE 2022, 17, e0271050. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Long, X.; Du, J.; Teng, M.; Zhang, W.; Wang, Y.; Wang, X.; Wang, Z.; Zhang, P.; Li, J. Enhanced Curcumin Solubility and Antibacterial Activity by Encapsulation in PLGA Oily Core Nanocapsules. Food Funct. 2020, 11, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to Improve the Scientific Value of Zeta-Potential Measurements in nanoEHS. Environ. Sci Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Indocyanine Green-Loaded Biodegradable Nanoparticles: Preparation, Physicochemical Characterization and in Vitro Release. Int. J. Pharm. 2004, 278, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Abd El Hady, W.E.; El-Emam, G.A.; Saleh, N.E.; Hamouda, M.M.; Motawea, A. The Idiosyncratic Efficacy of Spironolactone-Loaded PLGA Nanoparticles Against Murine Intestinal Schistosomiasis. Int. J. Nanomed. 2023, 18, 987–1005. [Google Scholar] [CrossRef] [PubMed]
- Schaffazick, S.R.; Guterres, S.S.; Freitas, L.d.L.; Pohlmann, A.R. Caracterização e estabilidade físico-química de sistemas poliméricos nanoparticulados para administração de fármacos. Quím. Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- Bharate, S.S.; Kumar, V.; Vishwakarma, R.A. Determining Partition Coefficient (Log P), Distribution Coefficient (Log D) and Ionization Constant (pKa) in Early Drug Discovery. Comb. Chem. High. Throughput Screen. 2016, 19, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Froiio, F.; Ginot, L.; Paolino, D.; Lebaz, N.; Bentaher, A.; Fessi, H.; Elaissari, A. Essential Oils-Loaded Polymer Particles: Preparation, Characterization and Antimicrobial Property. Polymers 2019, 11, 1017. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and Characterization of PLGA Nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef]
- Abraham, J. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals For Human Use. In Handbook of Transnational Economic Governance Regimes; Tietje, C., Brouder, A., Eds.; Brill: Leiden, The Netherlands, 2010; pp. 1041–1053. ISBN 978-90-04-18156-4. [Google Scholar]
- Moosavi, S.M.; Ghassabian, S. Linearity of Calibration Curves for Analytical Methods: A Review of Criteria for Assessment of Method Reliability. In Calibration and Validation of Analytical Methods—A Sampling of Current Approaches; Stauffer, M.T., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-084-0. [Google Scholar]
- Ahmed, S.A.; Saleem, M.F.; Hassanzadeh, H. Optimization of Solvent Evaporation Method in Liposomal Nanocarriers Loaded-garlic Essential Oil (Allium sativum): Based on the Encapsulation Efficiency, Antioxidant Capacity, and Instability. IET Nanobiotechnology 2023, 17, 438–449. [Google Scholar] [CrossRef] [PubMed]

| Validation Parameters | Results |
|---|---|
| Intra-assay precision (Repeatability) (n = 6 determinations) Theoretical concentration (µg/mL) Concentration obtained (µg/mL) | 1.93% |
| 25.00 | |
| 26.01 ± 0.48 | |
| Inter-assay precision (n = 12 determinations) Theoretical concentration (µg/mL) Concentration obtained (µg/mL) | 2.43% |
| 25.00 | |
| 27.05 ± 0.65 |
| Parameters | Variation | Concentration (µg/mL) | Area | RSD (%) Area | Ret. Time (min) | RSD (%) Ret. Time | Symetry Factor | RSD (%) Symettry Factor |
|---|---|---|---|---|---|---|---|---|
| Flow (mL/min) | 1.0 | 23.55 | 19.745 | 1.379 | 6.02 | 1.053 | 1.68 | 0.907 |
| 0.8 * | 28.72 | 23.532 | 0.764 | 7.49 | 0.231 | 1.71 | 0.336 | |
| 0.6 | 38.34 | 31.702 | 1.005 | 10.04 | 0.304 | 1.73 | 0.879 | |
| Mobile phase ACN:H2O | 60:40 | 32.09 | 26.177 | 1.4041 | 10.23 | 0.564 | 1.78 | 0.854 |
| 0.8 * | 31.27 | 23.532 | 0.764 | 7.49 | 0.231 | 1.71 | 0.336 | |
| 70:30 | 39.61 | 32.068 | 0.185 | 5.86 | 0.984 | 2.63 | 4.055 | |
| pH | 4 | 34.89 | 28.3657 | 0.823 | 7.64 | 1.022 | 1.71 | 0.336 |
| 3 * | 28.72 | 23.532 | 0.764 | 7.49 | 0.231 | 1.71 | 0.336 |
| Ricinoleic Acid (RA) Concentration (µg/mL) | Recovery (%) | RSD (%) |
|---|---|---|
| 7.90 ± 0.14 | 100.10 ± 2.56 | 3.14 |
| 20.35 ± 0.28 | 96.90 ± 1.74 | |
| 32.10 ± 0.469 | 103.70 ± 1.92 |
| Sample | Theorical Concentration (mg/mL) | Experimental Concentration (mg/mL) | EE% |
|---|---|---|---|
| NCRA1 | 40.00 | 39.92 | 99 ± 0.14 |
| NCRA2 | 100.00 | 98.51 | 99 ± 0.49 |
| NCRA3 | 200.00 | 199.92 | 99 ± 0.06 |
| NCRA4 | 400.00 | 399.90 | 99 ± 0.39 |
| Samples | Aqueous Phase | Organic Phase | |||
|---|---|---|---|---|---|
| PVA 1% (mL) | PLGA (mg) | Span 60® (mg) | Ethyl Acetate (mL) | Ricinoleic Acid (mg) | |
| Blank NP | 10 | 50 | 10 | 2 | - |
| NCRA1 | 10 | 40 | 10 | 2 | 100 |
| NCRA2 | 10 | 50 | 10 | 2 | 100 |
| NCRA3 | 10 | 50 | 10 | 2 | 200 |
| NCRA4 | 10 | 50 | 10 | 2 | 400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Andrade, L.R.M.; dos Santos, L.F.; Pires, D.S.; Machado, É.P.; Martines, M.A.U.; Macedo, M.L.R.; Cardoso, T.F.M.; Severino, P.; Souto, E.B.; Kassab, N.M. A Newly Validated HPLC-DAD Method for the Determination of Ricinoleic Acid (RA) in PLGA Nanocapsules. Pharmaceuticals 2024, 17, 1220. https://doi.org/10.3390/ph17091220
de Andrade LRM, dos Santos LF, Pires DS, Machado ÉP, Martines MAU, Macedo MLR, Cardoso TFM, Severino P, Souto EB, Kassab NM. A Newly Validated HPLC-DAD Method for the Determination of Ricinoleic Acid (RA) in PLGA Nanocapsules. Pharmaceuticals. 2024; 17(9):1220. https://doi.org/10.3390/ph17091220
Chicago/Turabian Stylede Andrade, Lucas Rannier M., Larissa F. dos Santos, Débora S. Pires, Érika P. Machado, Marco Antonio U. Martines, Maria Ligia R. Macedo, Teófilo Fernando M. Cardoso, Patrícia Severino, Eliana B. Souto, and Najla M. Kassab. 2024. "A Newly Validated HPLC-DAD Method for the Determination of Ricinoleic Acid (RA) in PLGA Nanocapsules" Pharmaceuticals 17, no. 9: 1220. https://doi.org/10.3390/ph17091220
APA Stylede Andrade, L. R. M., dos Santos, L. F., Pires, D. S., Machado, É. P., Martines, M. A. U., Macedo, M. L. R., Cardoso, T. F. M., Severino, P., Souto, E. B., & Kassab, N. M. (2024). A Newly Validated HPLC-DAD Method for the Determination of Ricinoleic Acid (RA) in PLGA Nanocapsules. Pharmaceuticals, 17(9), 1220. https://doi.org/10.3390/ph17091220









