Pharmacological Inhibition of Endogenous Hydrogen Sulfide Production Slows Bladder Cancer Progression in an Intravesical Murine Model
Abstract
1. Introduction
2. Results
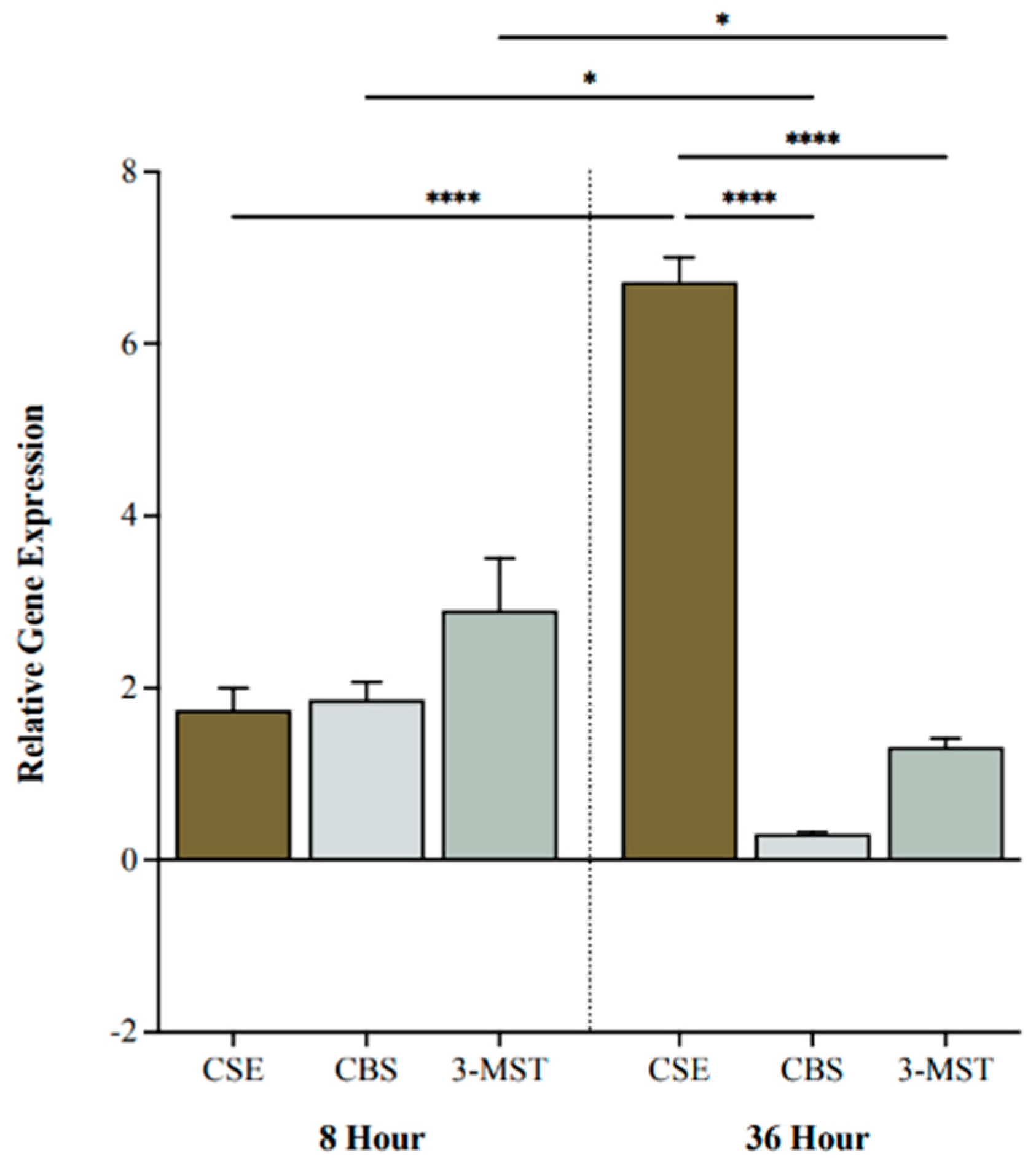
2.1. CSE Gene Expression Is Upregulated under Hypoxic Conditions
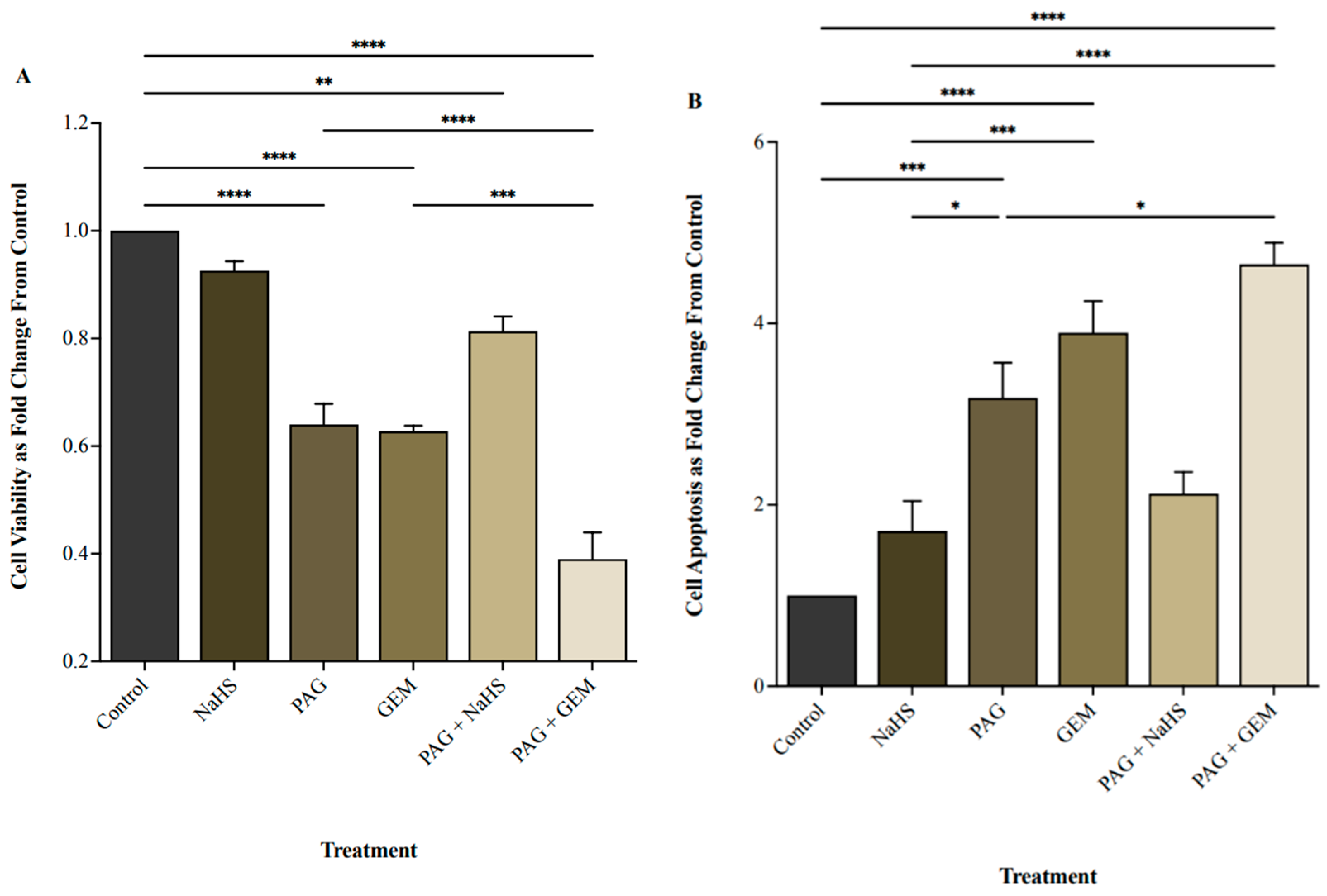
2.2. Inhibiting CSE Activity Attenuates BC Cell Viability in the Presence of Chemotherapy
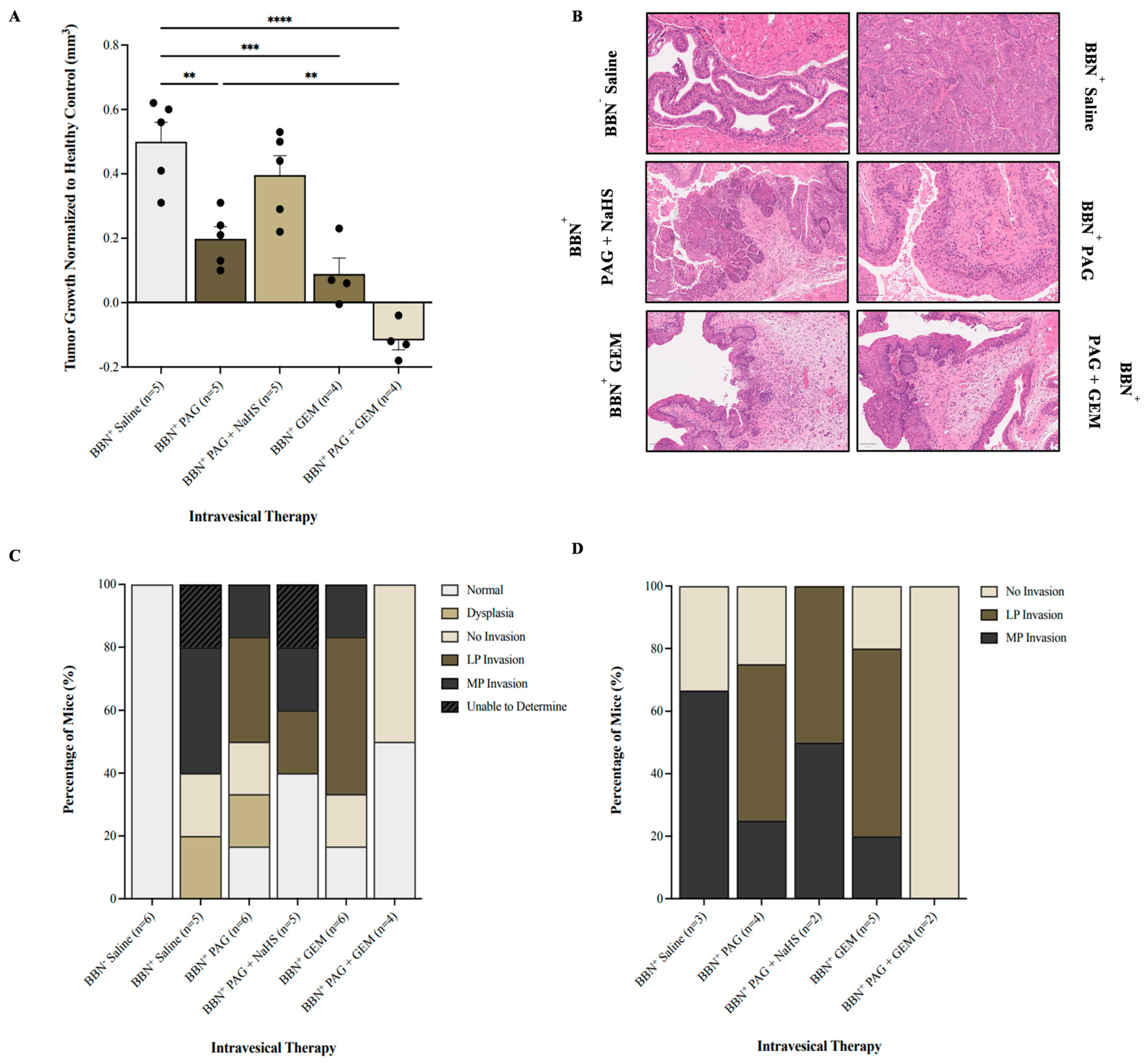
2.3. Inhibiting CSE Activity Promotes Tumor Regression and Abrogates Invasion in the Presence of Chemotherapy
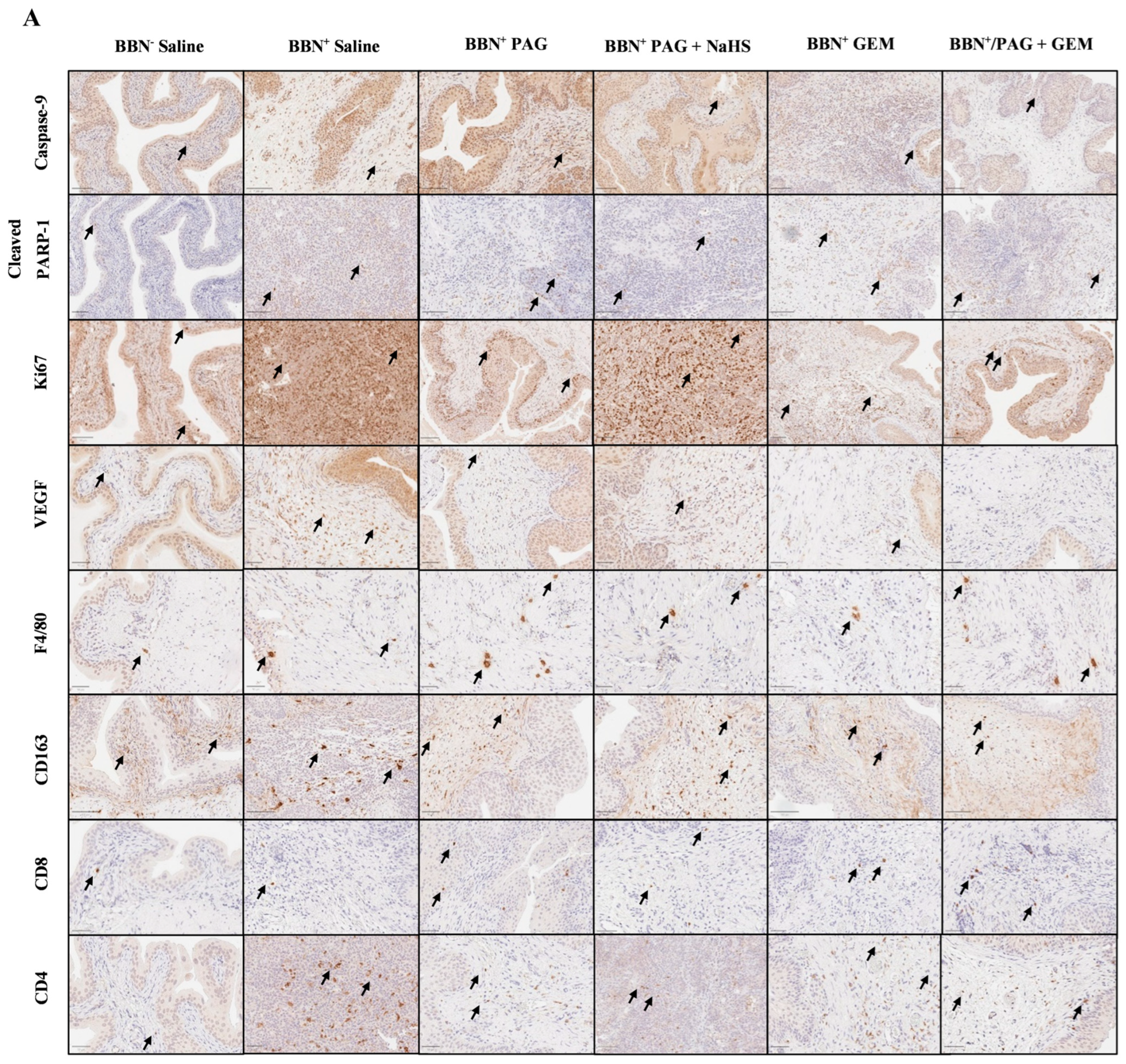
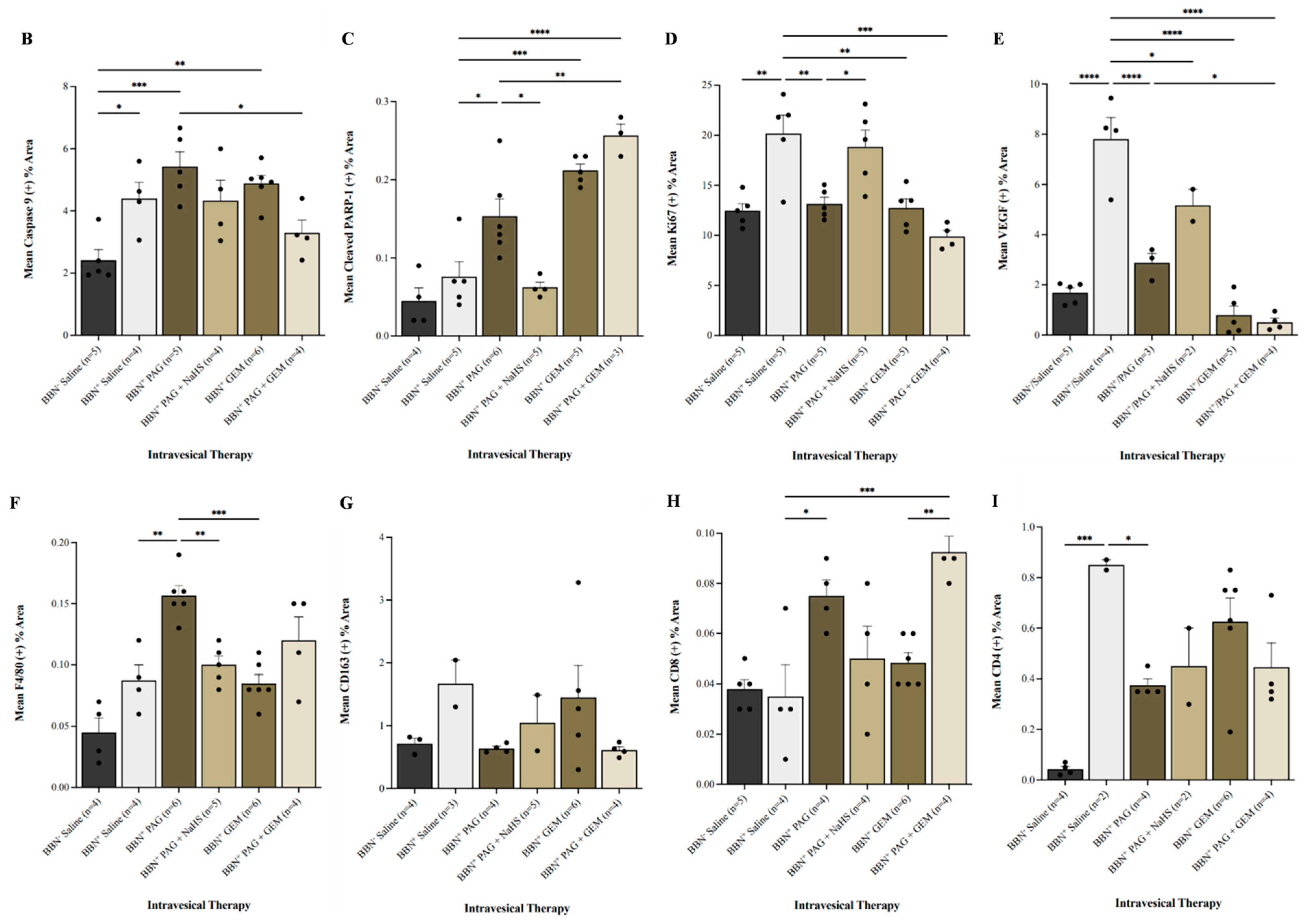
2.4. Inhibiting CSE Activity Induces Bladder Tumor Apoptosis, Attenuates Neovascularization and Proliferation, Alters Bladder Tumor Immune Response, and Enhances Pro-Apoptotic and Anti-Neovascularization Effects of Chemotherapy
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Quantitative PCR (qPCR) Analysis
4.3. Flow Cytometry
4.4. Experimental Animals and Reagents
4.5. Murine BC Model
4.6. Magnetic Resonance Imaging
4.7. Intravesical Therapy
4.8. Histological Staining
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, G.J.; Relouw, S.; McFarlane, L.; Sener, A. Redox System and Oxidative Stress-Targeted Therapeutic Approaches in Bladder Cancer. Antioxidants 2024, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Relouw, S.; Dugbartey, G.J.; Sener, A. Non-Invasive Imaging Modalities in Intravesical Murine Models of Bladder Cancer. Cancers 2023, 15, 2381. [Google Scholar] [CrossRef]
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Van Der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 3596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–744. [Google Scholar] [CrossRef]
- Sylvester, R.J. Bacillus calmette-guerin treatment of non-muscle invasive bladder cancer. Int. J. Urol. 2011, 18, 113–120. [Google Scholar] [CrossRef]
- Bhindi, B.; Kool, R.; Kilkarni, G.S.; Siemens, R.; Aprikian, A.G.; Breau, R.H.; Brimo, F.; Fairey, A.; French, C.; Hanna, N.; et al. Canadian urological association guideline on the management of non-muscle-invasive bladder cancer. Can. Urol. Assoc. J. 2021, 14, 424–457. [Google Scholar] [CrossRef]
- Meng, M.V.; Gschwend, J.E.; Shore, N.; Grossfeld, G.D.; Mostafid, H.; Black, P.C. Emerging immunotherapy options for bacillus calmette-guerin unresponsive non-muscle invasive bladder cancer. Urol. J. 2019, 202, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Shabsigh, A.; Korets, R.; Vora, K.C.; Brooks, C.M.; Cronin, A.M.; Savage, C.; Raj, G.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur. Urol. 2009, 55, 164–176. [Google Scholar] [CrossRef]
- Joyce, D.D.; Sharma, V.; Williams, S.B. Cost-effectiveness and economic impact of bladder cancer management: An updated review of the literature. Pharm. Econ. 2023, 41, 751–769. [Google Scholar] [CrossRef]
- Braunstein, A.; Goryachenkova, E.; Tolosa, E.; Willhardt, I.; Yefremova, L. Specificity and some other properties of liver serine sulphhydrase: Evidence for its identity with cystathionine β-synthase. Biochim. Biophys. Acta 1971, 242, 247–260. [Google Scholar] [CrossRef]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.K.; Yamada, K.; Chiku, T.; Koutmos, M.; Banerjee, R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J. Biol. Chem. 2013, 288, 20002–20013. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, M.R.; Coletta, C.; Chao, C.; Szabo, C. The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer. Antioxid. Redox Signal. 2015, 22, 424–448. [Google Scholar] [CrossRef] [PubMed]
- Sonke, E.; Verrydt, M.; Postenka, C.O.; Pardhan, S.; Willie, C.J.; Mazzola, C.R.; Hammers, M.D.; Pluth, M.D.; Lobb, I.; Power, N.E.; et al. Inhibition of endogenous hydrogen sulfide production in clear-cell renal cell carcinoma cell lines and xenografts restricts their growth, survival and angiogenic potential. Nitric Oxide 2015, 49, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, Z.; Chen, J.; Zhuang, X.; Feng, J.; Li, J. Exogenous hydrogen sulfide exerts proliferation, anti-apoptosis, migration effects and accelerates cell cycle progression in multiple myeloma cells via activating the Akt pathway. Oncol. Rep. 2021, 36, 1909–1916. [Google Scholar] [CrossRef]
- Szabo, C.; Coletta, C.; Chao, C.; Módis, K.; Szczesny, B.; Papapetropoulos, A.; Hellmich, M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12474–12479. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, H.; Liu, Y.; Zhang, W.; Duan, X.; Li, M.; Shi, X.; Wang, T. Cystathionine γ-lyase promotes the metastasis of breast cancer via the VEGF signaling pathway. Int. J. Oncol. 2019, 55, 473–487. [Google Scholar] [CrossRef]
- Panza, E.; De Cicco, P.; Armogida, C.; Scognamiglio, G.; Gigantino, V.; Botti, G.; Germano, D.; Napolitano, M.; Papapetropoulos, A.; Bucci, M.; et al. Role of the cystathionine gamma lyase/hydrogen sulfide pathway in human melanoma progression. Pigment. Cell Melanoma Res. 2015, 28, 61–72. [Google Scholar] [CrossRef]
- Khattak, S.; Rauf, M.A.; Khan, N.H.; Zhang, Q.Q.; Chen, H.J.; Muhammad, P.; Ansari, M.A.; Alomary, M.N.; Jahangir, M.; Zhang, C.Y.; et al. Hydrogen Sulfide Biology and Its Role in Cancer. Molecules 2022, 27, 3389. [Google Scholar] [CrossRef]
- Gai, J.W.; Qin, W.; Liu, M.; Wang, H.F.; Zhang, M.; Li, M.; Zhou, W.H.; Ma, Q.T.; Liu, G.M.; Song, W.H.; et al. Expression profile of hydrogen sulfide and its synthases correlates with tumor stage and grade in urothelial cell carcinoma of bladder. Urol. Oncol. 2016, 34, 166.e15–166.e20. [Google Scholar] [CrossRef] [PubMed]
- Panza, E.; Bello, I.; Smimmo, M.; Brancaleone, V.; Mitidieri, E.; Bucci, M.; Cirino, G.; Sorrentino, R. Endogenous and exogenous hydrogen sulfide modulates urothelial bladder carcinoma development in human cell lines. Biomed. Pharmacother. 2022, 151, 113137. [Google Scholar] [CrossRef] [PubMed]
- Wahafu, W.; Gai, J.; Song, L.; Ping, H.; Wang, M.; Yang, F.; Niu, Y.; Xing, N. Increased H2S and its synthases in urothelial cell carcinoma of the bladder, and enhanced cisplatin-induced apoptosis following H2S inhibition in EJ cells. Oncol. Lett. 2018, 15, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Raven, P.A.; D’Costa, N.M.; Moskalev, I.; Tan, Z.; Frees, S.; Chavez-Munoz, C.; So, A.I. Development of murine intravesical orthotopic human bladder cancer (mio-hBC) model. Am. J. Clin. Exp. Urol. 2018, 6, 245–259. [Google Scholar]
- Glaser, A.P.; Procissi, D.; Yu, Y.; Meeks, J.J. Magnetic resonance imaging assessment of carcinogen-induced murine bladder tumors. J. Vis. Exp. 2019, 29, e59101. [Google Scholar]
- Liu, Z.; Tang, Q.; Qi, T.; Othmane, B.; Yang, Z.; Chen, J.; Hu, J.; Zu, X. A robust hypoxia risk score predicts the clinical outcomes and microenvironment immune characters in bladder cancer. Front. Immunol. 2021, 12, 725223. [Google Scholar] [CrossRef]
- Khan, N.H.; Wang, D.; Wang, W.; Shahid, M.; Khattak, S.; Ngowi, E.E.; Sarfraz, M.; Ji, X.Y.; Zhang, C.Y.; Wu, D.D. Pharmacological inhibition of endogenous hydrogen sulfide attenuates breast cancer progression. Molecules 2022, 27, 4049. [Google Scholar] [CrossRef]
- Oh, D.Y.; Kwek, S.S.; Raju, S.S.; Li, T.; McCarthy, E.; Chow, E.; Aran, D.; Ilano, A.; Pai, C.C.S.; Rancan, C.; et al. Intratumoral CD4+ T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell 2020, 181, 1612–1625. [Google Scholar] [CrossRef]
- Zhao, K.; Ju, Y.J.; Li, S.; Altaany, Z.; Wang, R.; Yang, G. S-sulfhydration of MEK1 Leads to PARP-1 activation and DNA damage repair. EMBO Rep. 2014, 15, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Zhang, J.; Li, H.X.; Zhang, Y.X.; Jing, M.R.; Cai, C.B.; Wang, D.; Qi, H.W.; Wang, Y.Z.; Chen, H.J.; et al. Inhibition of endogenous hydrogen sulfide production suppresses the growth of nasopharyngeal carcinoma cells. Mol. Carcinog. 2023, 62, 652–664. [Google Scholar] [CrossRef]
- Gazzaniga, P.; Silvestri, I.; Gradilone, A.; Scarpa, S.; Morrone, S.; Gandini, O.; Gianni, W.; Frati, L.; Aglianò, A.M. Gemcitabine-induced apoptosis in 5637 cell line: An in vitro model for high-risk superficial bladder cancer. Anticancer Drugs. 2007, 18, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kuramitsu, Y.; Tokuda, K.; Baron, B.; Kitagawa, T.; Akada, J.; Maehara, S.I.; Maehara, Y.; Nakamura, K. Gemcitabine induces poly (ADP-ribose) polymerase-1 (PARP-1) degradation through autophagy in pancreatic cancer. PLoS ONE 2014, 9, e109076. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Significance of Ki-67 in non-muscle invasive bladder cancer patients: A systemic review and meta-analysis. Oncotarget 2017, 8, 100614–100630. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Ding, S. The crosstalk between tumor-associated macrophages (TAMs) and tumor cells and the corresponding targeted therapy. Front. Oncol. 2020, 10, 590941. [Google Scholar] [CrossRef] [PubMed]
- Rhee, I. Diverse Macrophages Polarization in Tumor Microenvironment. Arch. Pharm. Res. 2016, 39, 1588–1596. [Google Scholar] [CrossRef]
- Hori, S.; Miyake, M.; Tatsumi, Y.; Onishi, S.; Morizawa, Y.; Nakai, Y.; Tanaka, N.; Fujimoto, K. Topical and systemic immunoreaction triggered by intravesical chemotherapy in N-butyl-N-(4-hydroxybutyl) nitorosamine induced bladder cancer mouse model. PLoS ONE 2017, 12, e0175494. [Google Scholar] [CrossRef]
- Yue, T.; Li, J.; Zhu, J.; Zuo, S.; Wang, X.; Liu, Y.; Liu, J.; Liu, X.; Wang, P.; Chen, S. Hydrogen sulfide creates a favorable immune microenvironment for colon cancer. Cancer Res. 2023, 83, 595–612. [Google Scholar] [CrossRef]
| Primer | Forward Sequence (5′→3′) | Reverse Sequence (5′→3′) |
|---|---|---|
| -actin | AGCACAGAGCCTCGCCTTT | ATCATCATCCATGGTGAGCTGG |
| CSE | AGGTTTAGCAGCCACTGTAAC | GGGGTTTCGATCCAAACAAGC |
| CBS | GGCCAAGTGTGAGTTCTTCAA | GGCTCGATAATCGTGTCCCC |
| 3-MST | CATTTCGCGGAGTACGCAG | GCTGGCGTCGTAGATCACG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Relouw, S.; Dugbartey, G.J.; McLeod, P.; Knier, N.N.; Santiesteban, F.M.; Foster, P.J.; Cadieux-Pitre, H.-A.; Hague, N.M.; Caine, J.; Belletti, K.; et al. Pharmacological Inhibition of Endogenous Hydrogen Sulfide Production Slows Bladder Cancer Progression in an Intravesical Murine Model. Pharmaceuticals 2024, 17, 1212. https://doi.org/10.3390/ph17091212
Relouw S, Dugbartey GJ, McLeod P, Knier NN, Santiesteban FM, Foster PJ, Cadieux-Pitre H-A, Hague NM, Caine J, Belletti K, et al. Pharmacological Inhibition of Endogenous Hydrogen Sulfide Production Slows Bladder Cancer Progression in an Intravesical Murine Model. Pharmaceuticals. 2024; 17(9):1212. https://doi.org/10.3390/ph17091212
Chicago/Turabian StyleRelouw, Sydney, George J. Dugbartey, Patrick McLeod, Natasha N. Knier, Francisco Martinez Santiesteban, Paula J. Foster, Heather-Anne Cadieux-Pitre, Nicole M. Hague, Jenna Caine, Kaitlin Belletti, and et al. 2024. "Pharmacological Inhibition of Endogenous Hydrogen Sulfide Production Slows Bladder Cancer Progression in an Intravesical Murine Model" Pharmaceuticals 17, no. 9: 1212. https://doi.org/10.3390/ph17091212
APA StyleRelouw, S., Dugbartey, G. J., McLeod, P., Knier, N. N., Santiesteban, F. M., Foster, P. J., Cadieux-Pitre, H.-A., Hague, N. M., Caine, J., Belletti, K., Major, S., O’Neil, C., Gabril, M. Y., Moussa, M., Huynh, M. J., Haeryfar, S. M. M., & Sener, A. (2024). Pharmacological Inhibition of Endogenous Hydrogen Sulfide Production Slows Bladder Cancer Progression in an Intravesical Murine Model. Pharmaceuticals, 17(9), 1212. https://doi.org/10.3390/ph17091212





