Novel Therapeutic Effects of Euphorbia heterophylla L. Methanol Extracts in Macular Degeneration Caused by Blue Light in A2E-Laden ARPE-19 Cells and Retina of BALB/c Mice
Abstract
1. Introduction
2. Results
2.1. Identification of Bioactive Compounds in the MEE
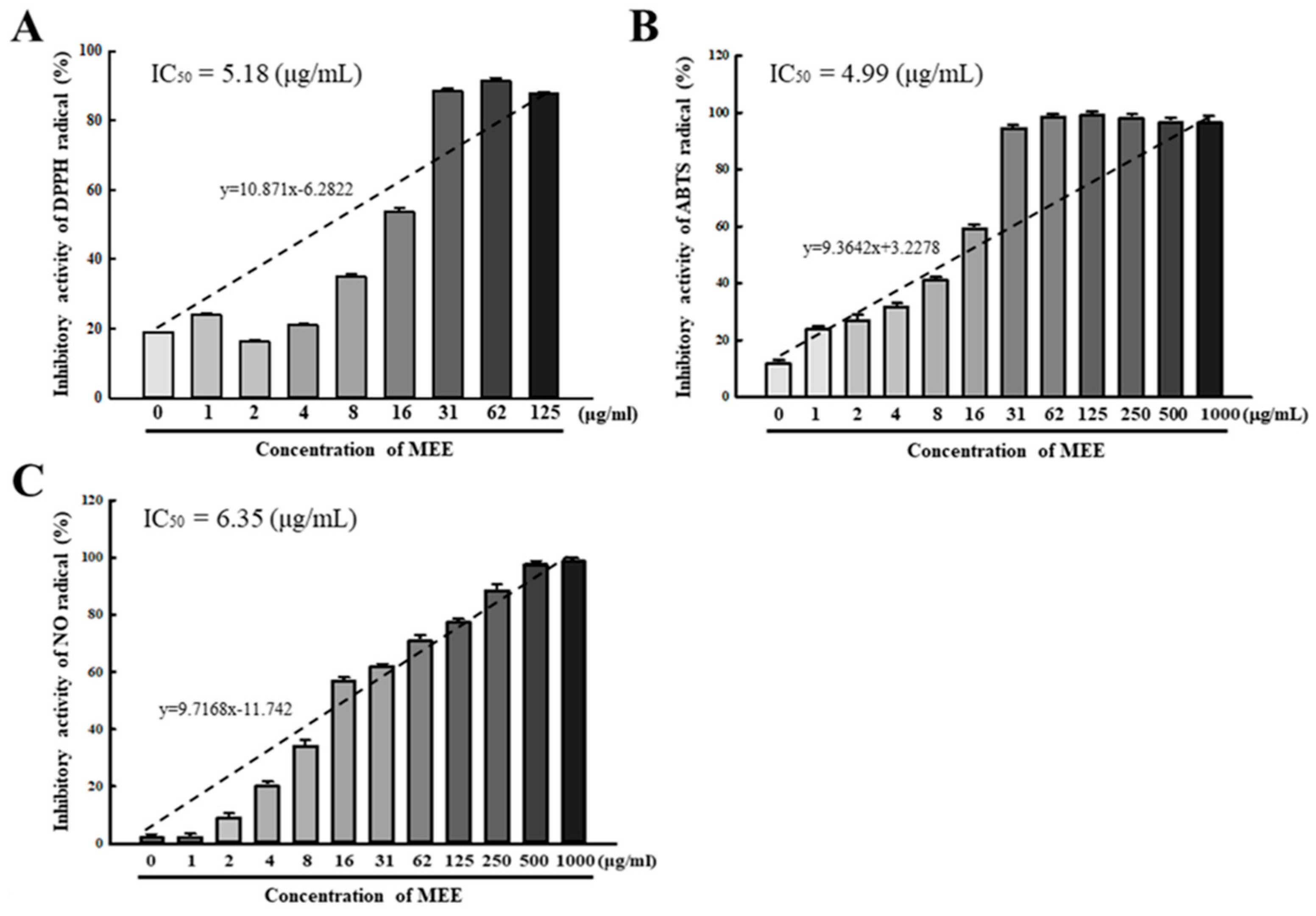
2.2. Antioxidative Activity of the MEE against Free Radicals
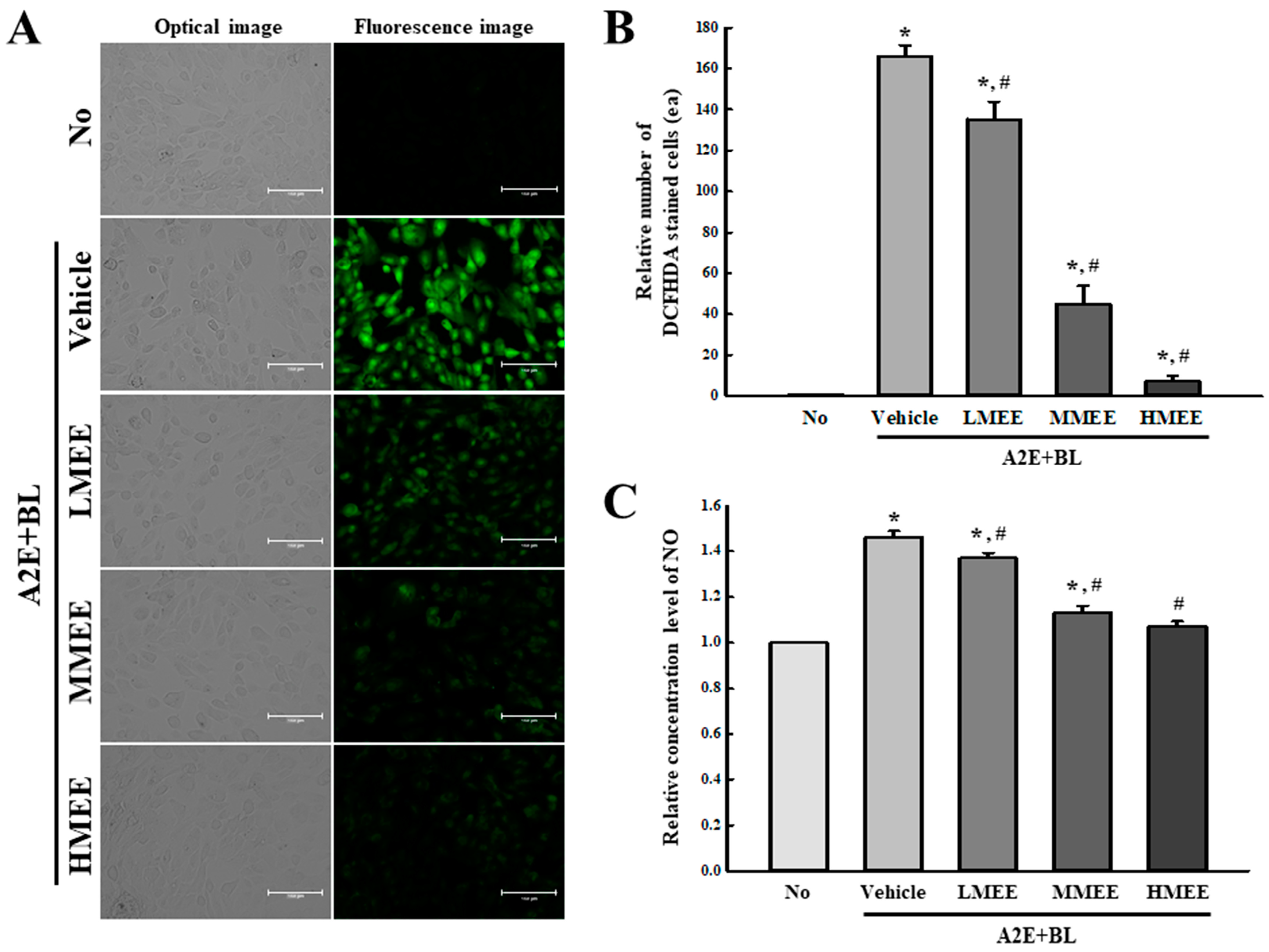
2.3. Suppression of Oxidative Stress by the MEE in A2E + BL-Exposed ARPE-19 Cells
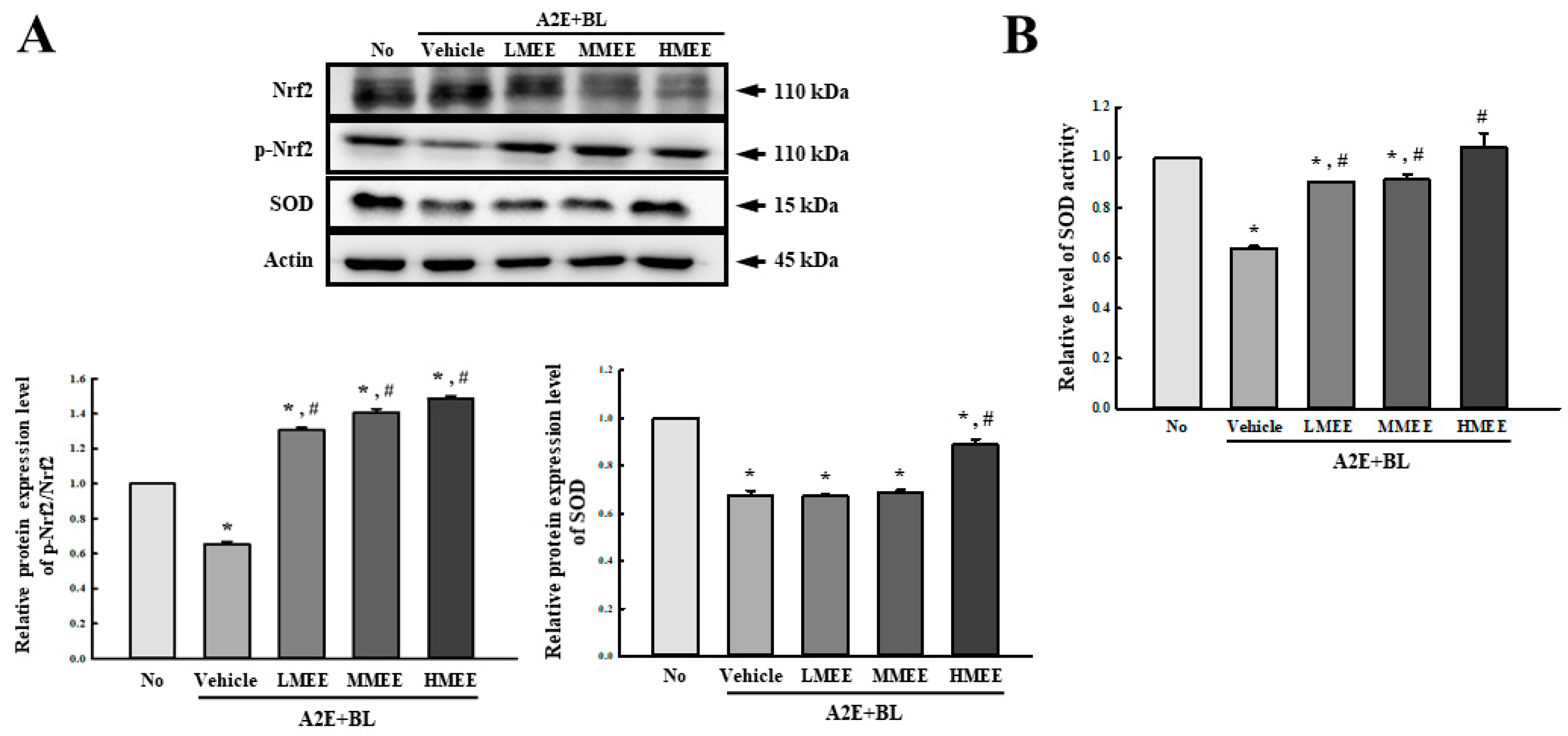
2.4. Antioxidant Activity of the MEE in A2E + BL-Exposed ARPE-19 Cells
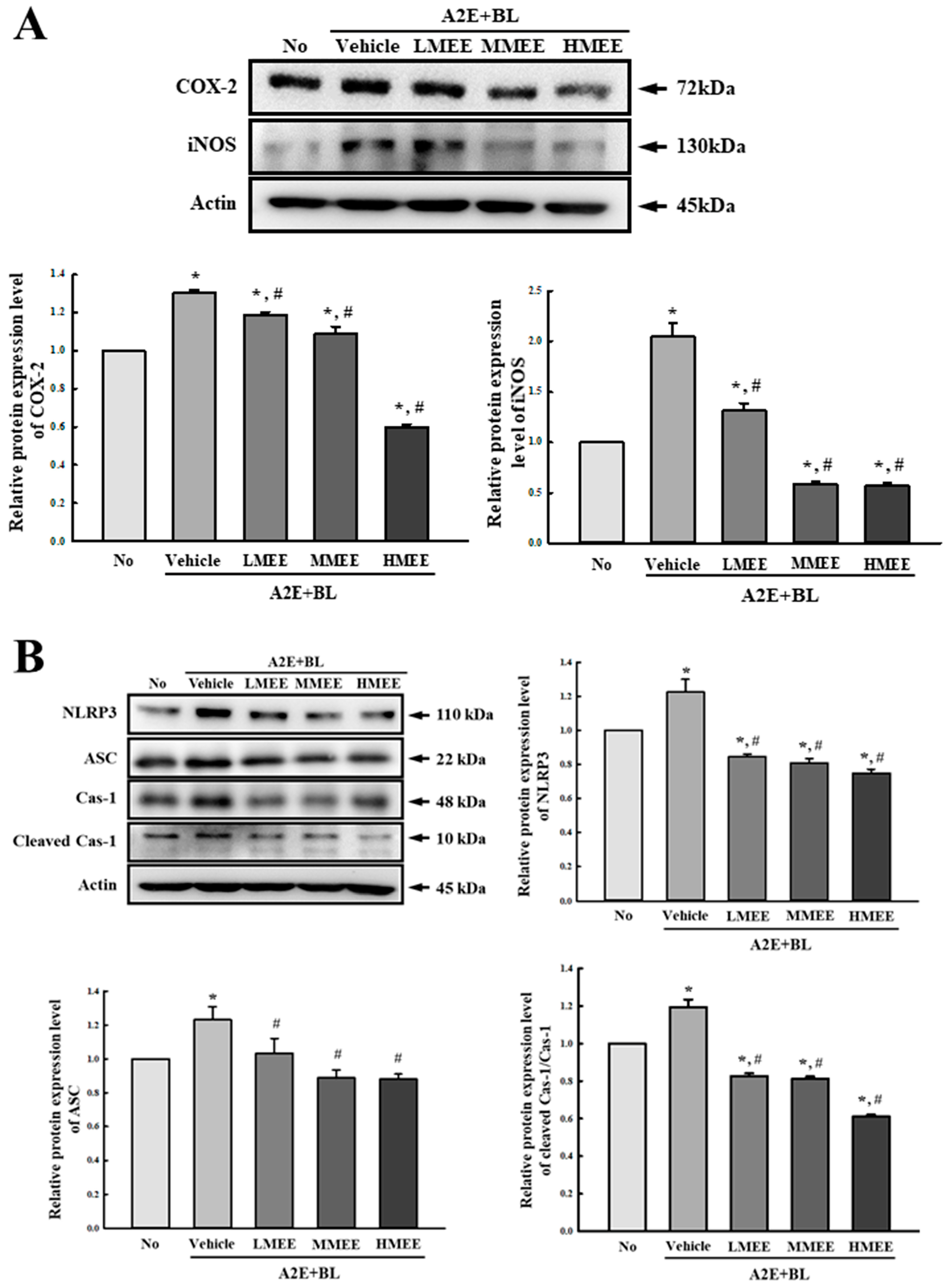
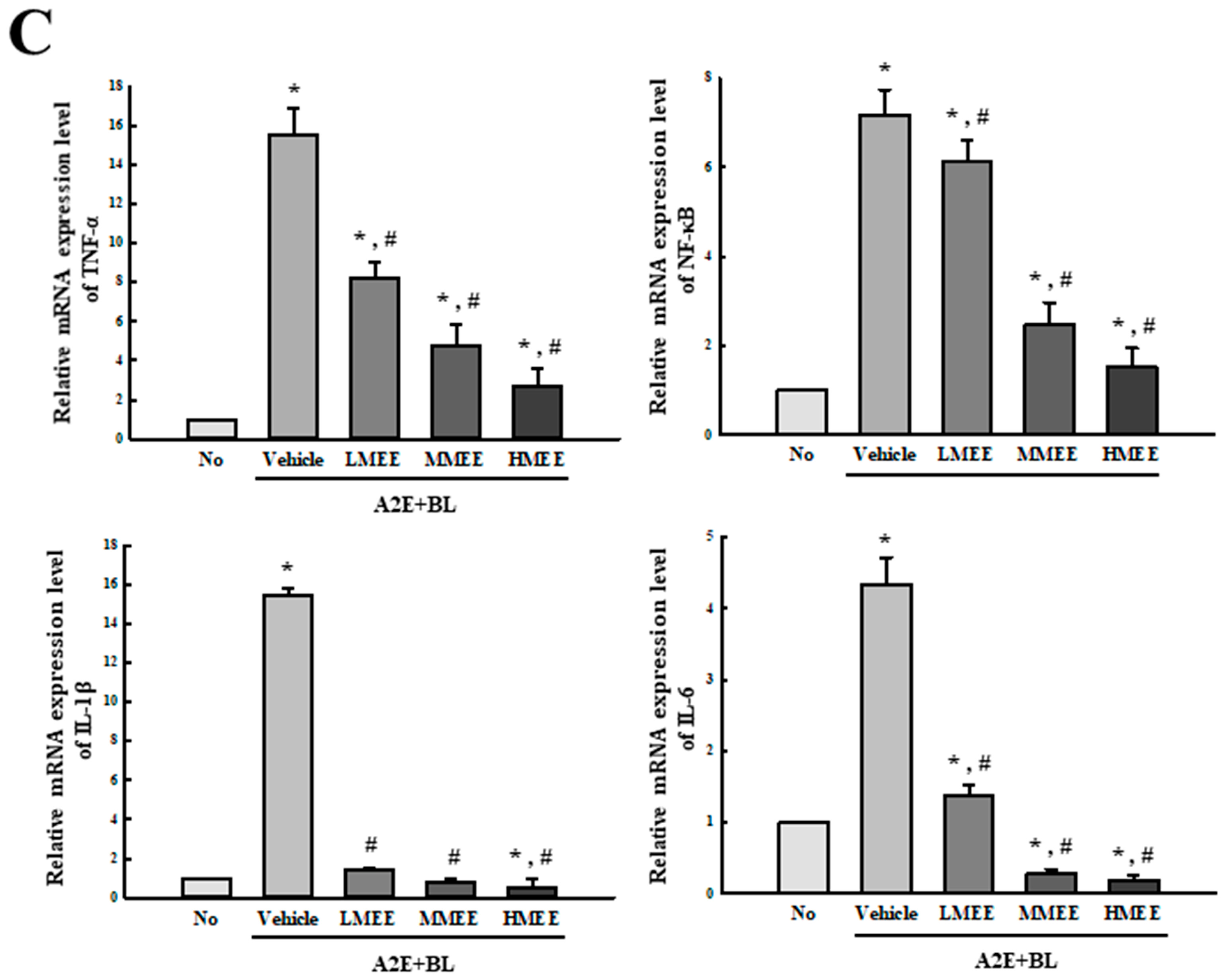
2.5. Suppression of Inflammatory Response in A2E + BL-Exposed ARPE-19 Cells by the MEE
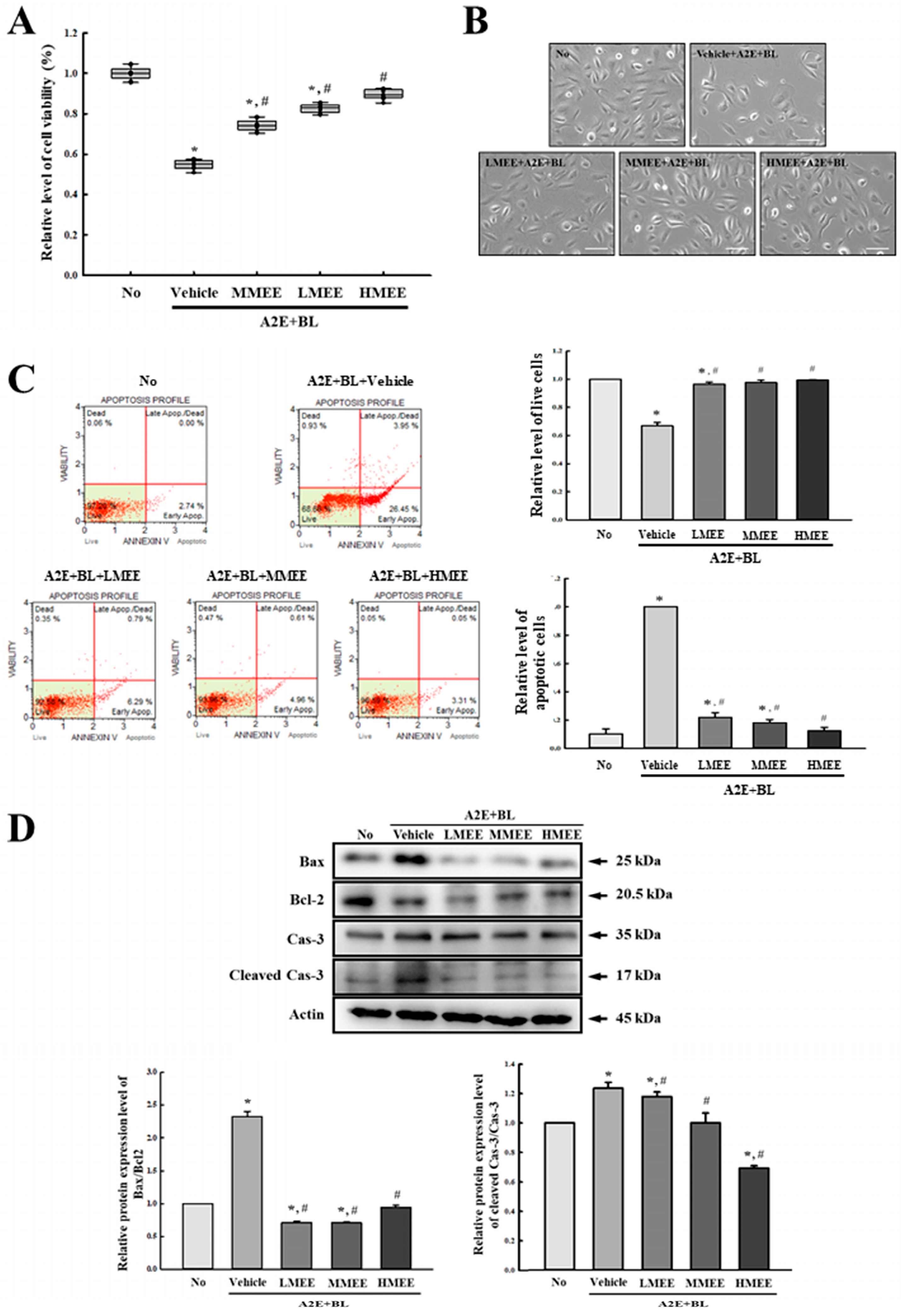
2.6. Suppression of Cell Death by the MEE in A2E + BL-Exposed ARPE-19 Cells
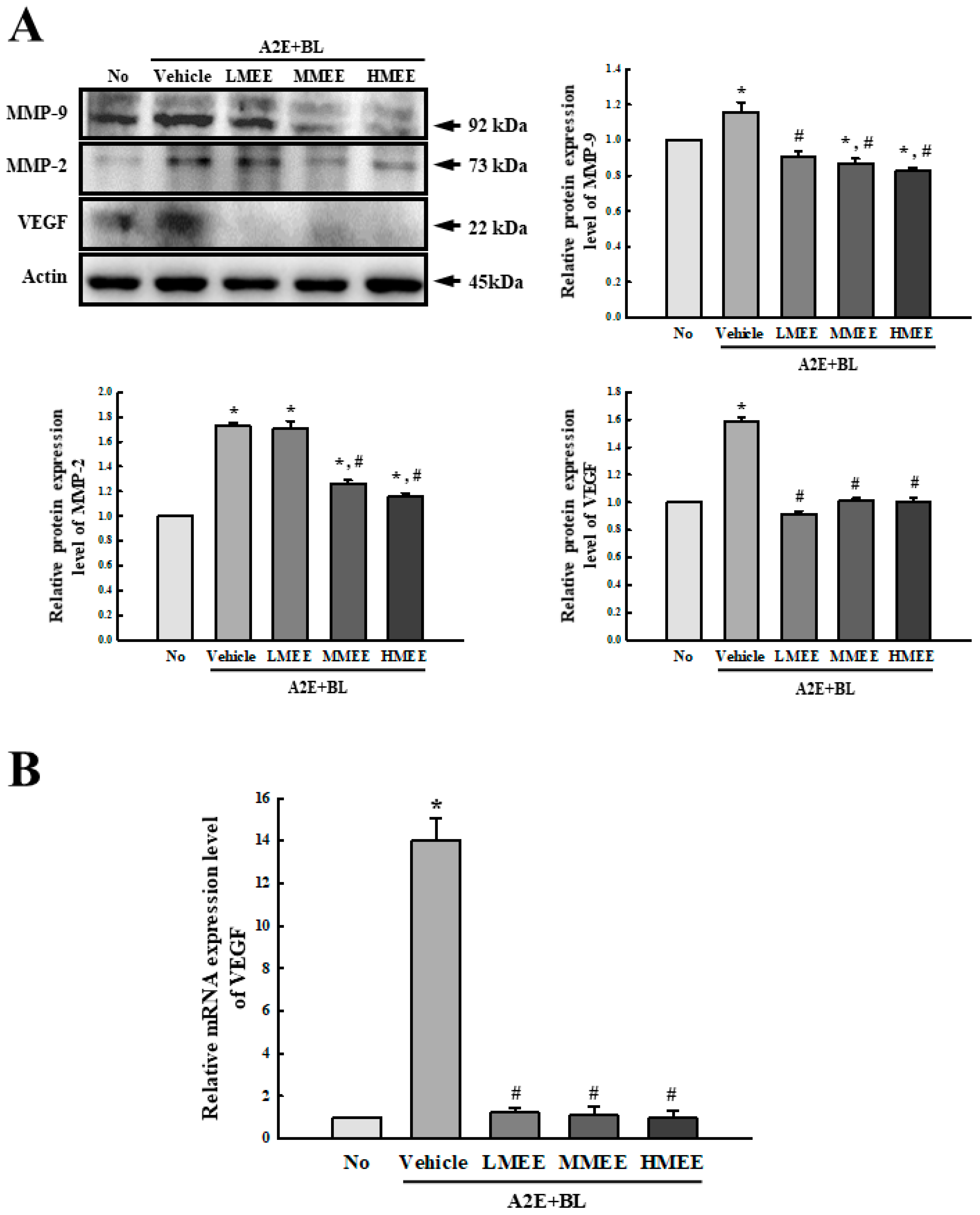
2.7. Improvement in the Regulation of Angiogenesis by the MEE in A2E + BL-Exposed ARPE-19 Cells
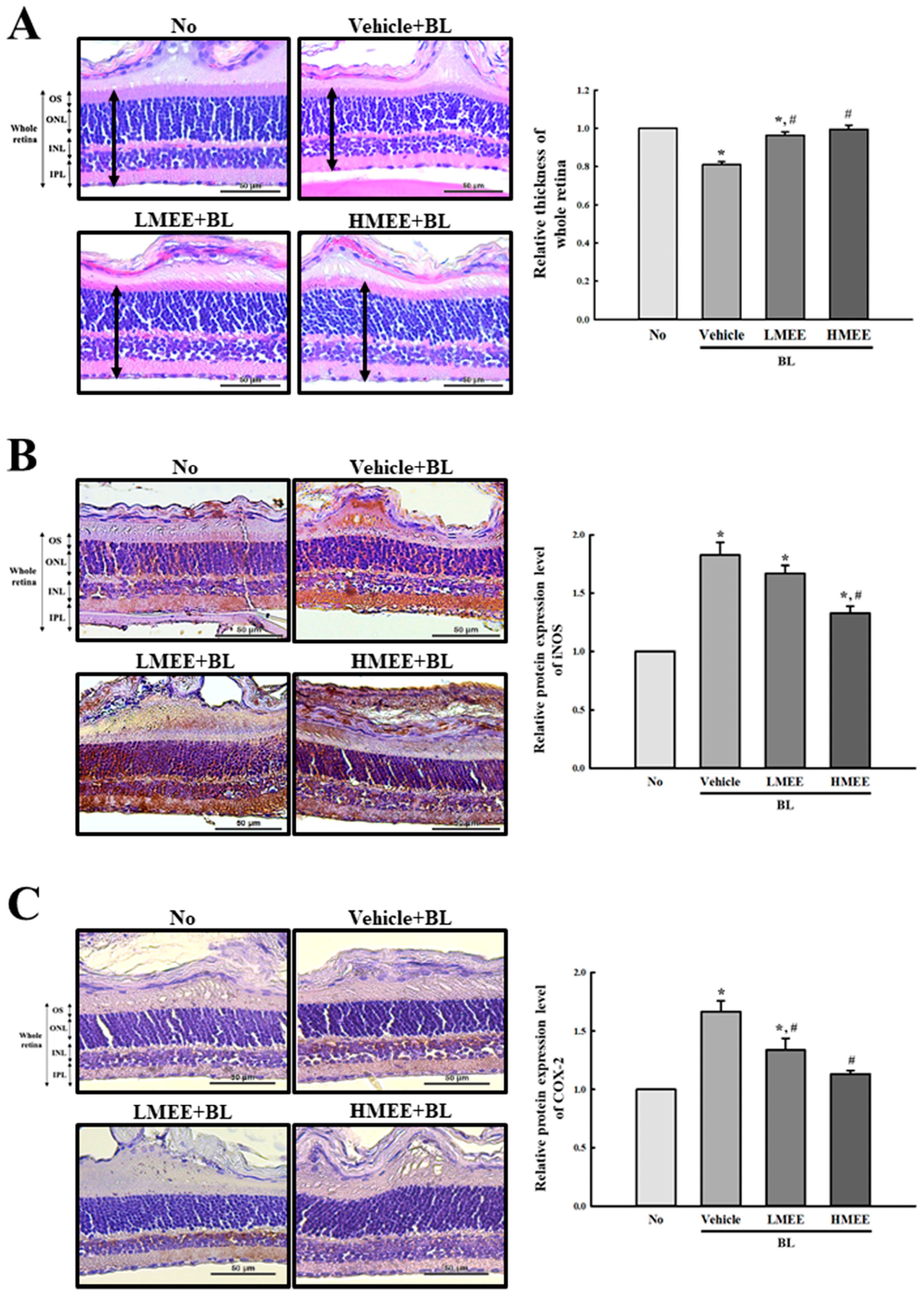
2.8. Verification of the In Vitro Effects of the MEE on the BL-Induced Photoreceptor Degranulation in the Retina of BALB/c Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of MEE
4.2. Identification of Bioactive Compounds in MEE
4.3. Free Radical Scavenging Activity MEE
4.4. Synthesis and Purification of A2E
4.5. In Vitro Study
4.5.1. Cell Culture and Cell Viabilities Assay
4.5.2. Analysis of the Apoptotic Cell Population
4.5.3. Intracellular ROS
4.5.4. Analysis of NO
4.5.5. Analysis of SOD Activity
4.5.6. Western Blot Analysis
4.5.7. Real-Time Quantitative PCR Analysis
4.6. In Vivo Study
4.6.1. Experimental Design for Animal Study
4.6.2. Histopathological Analysis
4.6.3. Immunohistochemical Staining Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Garcia, J.; Usategui-Martin, R.; Sanabria, M.R.; Fernandez-Perez, E.; Telleria, J.J.; Coco-Martin, R.M. Pathophysiology of age-related macular degeneration: Implications for treatment. Ophthalmic Res. 2022, 65, 615–636. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; McGinnis, J.F. Nanoceria: A potential therapeutic for dry AMD. Adv. Exp. Med. Biol. 2016, 854, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Travis, G.H.; Golczak, M.; Moise, A.R.; Palczewski, K. Diseases caused by defects in the visual cycle: Retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 469–512. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Golczak, M.; Chou, S.; Desai, A.; Hoppel, C.L.; Matsuyama, S.; Palczewski, K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 2009, 284, 15173–15183. [Google Scholar] [CrossRef] [PubMed]
- Boyer, N.P.; Higbee, D.; Currin, M.B.; Blakeley, L.R.; Chen, C.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: Their origin is 11-cis-retinal. J. Biol. Chem. 2012, 287, 22276–22286. [Google Scholar] [CrossRef]
- Roberts, J.E.; Kukielczak, B.M.; Hu, D.N.; Miller, D.S.; Bilski, P.; Sik, R.H.; Motten, A.G.; Chignell, C.F. The role of A2E in prevention or enhancement of light damage in human retinal pigment epithelial cells. Photochem. Photobiol. 2002, 75, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Wu, Y.; Kim, C.Y.; Zhou, J. Phospholipid meets all-trans-retinal: The making of RPE bisretinoids. J. Lipid Res. 2010, 51, 247–261. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Vollmer-Snarr, H.R.; Zhou, J.; Jang, Y.P.; Jockusch, S.; Itagaki, Y.; Nakanishi, K. A2E-epoxides damage DNA in retinal pigment epithelial cells. Vitamin E and other antioxidants inhibit A2E-epoxide formation. J. Biol. Chem. 2003, 278, 18207–18213. [Google Scholar] [CrossRef]
- Wu, Y.; Yanase, E.; Feng, X.; Siegel, M.M.; Sparrow, J.R. Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 7275–7280. [Google Scholar] [CrossRef]
- Jang, Y.P.; Zhou, J.; Nakanishi, K.; Sparrow, J.R. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem. Photobiol. 2005, 81, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Abdouh, M.; Lu, M.; Chen, Y.; Goyeneche, A.; Burnier, J.V.; Burnier, M.N., Jr. Filtering blue light mitigates the deleterious effects induced by the oxidative stress in human retinal pigment epithelial cells. Exp. Eye Res. 2022, 217, 108978. [Google Scholar] [CrossRef] [PubMed]
- Nordgaard, C.L.; Karunadharma, P.P.; Feng, X.; Olsen, T.W.; Ferrington, D.A. Mitochondrial proteomics of the retinal pigment epithelium at progressive stages of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2848–2855. [Google Scholar] [CrossRef] [PubMed]
- Krogh Nielsen, M.; Subhi, Y.; Rue Molbech, C.; Nilsson, L.L.; Nissen, M.H.; Sørensen, T.L. Imbalances in tissue inhibitors of metalloproteinases differentiate choroidal neovascularization from geographic atrophy. Acta Ophthalmol. 2019, 97, 84–90. [Google Scholar] [CrossRef]
- Blasiak, J.; Pawlowska, E.; Szczepanska, J.; Kaarniranta, K. Interplay between autophagy and the ubiquitin-proteasome system and its role in the pathogenesis of age-related macular degeneration. Int. J. Mol. Sci. 2019, 20, 210. [Google Scholar] [CrossRef]
- Shaban, H.; Richter, C. A2E and blue light in the retina: The paradigm of age-related macular degeneration. Biol. Chem. 2002, 383, 537–545. [Google Scholar] [CrossRef]
- Xie, T.; Cai, J.; Yao, Y.; Sun, C.; Yang, Q.; Wu, M.; Xu, Z.; Sun, X.; Wang, X. LXA4 protects against blue-light induced retinal degeneration in human A2E-laden RPE cells and Balb/c mice. Ann. Transl. Med. 2021, 9, 1249. [Google Scholar] [CrossRef]
- Carozza, G.; Zerti, D.; Tisi, A.; Ciancaglini, M.; Maccarrone, M.; Maccarone, R. An overview of retinal light damage models for preclinical studies on age-related macular degeneration: Identifying molecular hallmarks and therapeutic targets. Rev. Neurosci. 2023, 35, 303–330. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef]
- Galindo-Camacho, R.M.; Blanco-Llamero, C.; da Ana, R.; Fuertes, M.A.; Señoráns, F.J.; Silva, A.M.; García, M.L.; Souto, E.B. Therapeutic approaches for age-related macular degeneration. Int. J. Mol. Sci. 2022, 23, 11769. [Google Scholar] [CrossRef]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-related macular degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Dong, N.; Yang, M.; Wang, J.; Feng, X.; Wang, Y. Complement inhibitors in age-related macular degeneration: A potential therapeutic option. J. Immunol. Res. 2021, 2021, 9945725. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Kijlstra, A.; Webers, C.A.B.; Berendschot, T.T.J.M. Lutein and factor D: Two intriguing players in the field of age-related macular degeneration. Arch. Biochem. Biophys. 2015, 572, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Leung, I.Y. Macular pigment: New clinical methods of detection and the role of carotenoids in age-related macular degeneration. Optometry 2008, 79, 266–272. [Google Scholar] [CrossRef]
- Rezende, F.A.; Lapalme, E.; Qian, C.X.; Smith, L.E.; SanGiovanni, J.P.; Sapieha, P. Omega-3 supplementation combined with anti-vascular endothelial growth factor lowers vitreal levels of vascular endothelial growth factor in wet age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 1071–1078. [Google Scholar] [CrossRef]
- van Leeuwen, E.M.; Emri, E.; Merle, B.M.J.; Colijn, J.M.; Kersten, E.; Cougnard-Gregoire, A.; Dammeier, S.; Meester-Smoor, M.; Pool, F.M.; de Jong, E.K.; et al. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res. 2018, 67, 56–86. [Google Scholar] [CrossRef]
- Xing, Y.; Liang, S.; Zhao, Y.; Yang, S.; Ni, H.; Li, H. Protection of Aronia melanocarpa fruit extract from sodium-iodate-induced damages in rat retina. Nutrients 2021, 13, 4411. [Google Scholar] [CrossRef]
- Osada, H.; Okamoto, T.; Kawashima, H.; Toda, E.; Miyake, S.; Nagai, N.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Neuroprotective effect of bilberry extract in a murine model of photo-stressed retina. PLoS ONE 2017, 12, e0178627. [Google Scholar] [CrossRef]
- Pham, T.N.M.; Shin, C.Y.; Park, S.H.; Lee, T.H.; Ryu, H.Y.; Kim, S.B.; Auh, K.; Jeong, K.W. Solanum melongena L. extract protects retinal pigment epithelial cells from blue light-induced phototoxicity in in vitro and in vivo models. Nutrients 2021, 13, 359. [Google Scholar] [CrossRef]
- Cho, H.M.; Jo, Y.D.; Choung, S.Y. Protective effects of Spirulina maxima against blue light-induced retinal damages in A2E-laden ARPE-19 cells and Balb/c mice. Nutrients 2022, 14, 401. [Google Scholar] [CrossRef]
- Lee, S.J.; Roh, Y.J.; Kim, J.E.; Jin, Y.J.; Song, H.J.; Seol, A.; Park, S.H.; Douangdeuane, B.; Souliya, O.; Choi, S.I.; et al. Protective effects of Dipterocarpus tuberculatus in blue light-induced macular degeneration in A2E-laden ARPE19 cells and retina of Balb/c mice. Antioxidants 2023, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Mwine, T.J.; Damme, V.P. Why do Euphorbiaceae tick as medicinal plants? A review of Euphorbiaceae family and its medicinal features. J. Med. Plants Res. 2011, 5, 652–662. Available online: https://www.researchgate.net/publication/228478254 (accessed on 1 April 2011).
- Wilson, A.K. Euphorbia heterophylla: A review of distribution, importance and control. Int. J. Pest Manag. 1981, 27, 32–38. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Abd-ElGawad, A.M.; El Gendy, A.E.G.; Assaeed, A.M. Chemical characterization of Euphorbia heterophylla L. essential oils and their antioxidant activity and allelopathic potential on Cenchrus echinatus L. Chem. Biodivers. 2019, 16, e1900051. [Google Scholar] [CrossRef] [PubMed]
- Lingaraju, K.; Naika, H.R.; Nagaraju, G.; Nagabhushana, H. Biocompatible synthesis of reduced graphene oxide from Euphorbia heterophylla (L.) and their in-vitro cytotoxicity against human cancer cell lines. Biotechnol. Rep. 2019, 24, e00376. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Dall’Acqua, S.; Sinan, K.I.; Sut, S.; Ferrarese, I.; Etienne, O.K.; Sadeer, N.B.; Ak, G.; Zengin, G. Phenolic compounds analysis of three Euphorbia species by LC-DAD-MSn and their biological properties. J. Pharm. Biomed. Anal. 2020, 189, 113477. [Google Scholar] [CrossRef]
- Kook, D.; Wolf, A.H.; Alice, L.Y.; Neubauer, A.S.; Priglinger, S.G.; Kampik, A.; Welge-Lüssen, U.C. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1712–1720. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Nowak, J.Z. Age-related macular degeneration (AMD): Pathogenesis and therapy. Pharmacol. Rep. 2006, 58, 353–363. [Google Scholar]
- Ramkumar, H.L.; Tuo, J.; Shen, D.F.; Zhang, J.; Cao, X.; Chew, E.Y.; Chan, C.C. Nutrient supplementation with n3 polyunsaturated fatty acids, lutein, and zeaxanthin decrease A2E accumulation and VEGF expression in the retinas of Ccl2/Cx3cr1-deficient mice on Crb1rd8 background. J. Nutr. 2013, 143, 1129–1135. [Google Scholar] [CrossRef]
- Arnault, E.; Barrau, C.; Nanteau, C.; Gondouin, P.; Bigot, K.; Viénot, F.; Gutman, E.; Fontaine, V.; Villette, T.; Cohen-Tannoudji, D.; et al. Phototoxic action spectrum on a retinal pigment epithelium model of age-related macular degeneration exposed to sunlight normalized conditions. PLoS ONE 2013, 8, e71398. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, M.A.; Gulin, A.A.; Feldman, T.B.; Bel’skich, Y.C.; Arbukhanova, P.M.; Astaf’ev, A.A.; Nadtochenko, V.A.; Borzenok, S.A.; Ostrovsky, M.A. Time-of-flight secondary ion mass spectrometry to assess spatial distribution of A2E and its oxidized forms within lipofuscin granules isolated from human retinal pigment epithelium. Anal. Bioanal. Chem. 2016, 408, 7521–7528. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.M.; Parmar, T.; Arai, E.; Perusek, L.; Maeda, A. A2E-associated cell death and inflammation in retinal pigmented epithelial cells from human induced pluripotent stem cells. Stem Cell Res. 2018, 27, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, T.; Jiang, Y.; Wu, L.; Cai, X.; Sun, X.; Sun, X. Photooxidative damage in retinal pigment epithelial cells via GRP78 and the protective role of grape skin polyphenols. Food Chem. Toxicol. 2014, 74, 216–224. [Google Scholar] [CrossRef]
- Yoon, S.M.; Lee, B.L.; Guo, Y.R.; Choung, S.Y. Preventive effect of Vaccinium uliginosum L. extract and its fractions on age-related macular degeneration and its action mechanisms. Arch. Pharm. Res. 2016, 39, 21–32. [Google Scholar] [CrossRef]
- Lee, B.L.; Kang, J.H.; Kim, H.M.; Jeong, S.H.; Jang, D.S.; Jang, Y.P.; Choung, S.Y. Polyphenol-enriched Vaccinium uliginosum L. fractions reduce retinal damage induced by blue light in A2E-laden ARPE-19 cell cultures and mice. Nutr. Res. 2016, 36, 1402–1414. [Google Scholar] [CrossRef]
- Park, S.I.; Lee, E.H.; Kim, S.R.; Jang, Y.P. Anti-apoptotic effects of Curcuma longa L. extract and its curcuminoids against blue light-induced cytotoxicity in A2E-laden human retinal pigment epithelial cells. J. Pharm. Pharmacol. 2017, 69, 334–340. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, Y.R.; Shim, J.; Choi, Y.S.; Kim, Y.T.; Kim, M.K.; Kim, M.J. Suppressive effect of Arctium lappa L. leaves on retinal damage against A2E-induced ARPE-19 cells and mice. Molecules 2020, 25, 1737. [Google Scholar] [CrossRef]
- Kim, J.; Cho, K.; Choung, S.Y. Protective effect of Prunella vulgaris var. L extract against blue light induced damages in ARPE-19 cells and mouse retina. Free Radic. Biol. Med. 2020, 152, 622–631. [Google Scholar] [CrossRef]
- Park, D.W.; Lee, Y.G.; Jeong, Y.J.; Jeon, H.; Kang, S.C. Preventive effects against retinal degeneration by Centella asiatica extract (CA-HE50) and asiaticoside through apoptosis suppression by the Nrf2/HO-1 signaling pathway. Antioxidants 2021, 10, 613. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Y.; Sun, T.; Yao, X.; Sun, X. Supplementation of procyanidins B2 attenuates photooxidation-induced apoptosis in ARPE-19 cells. Int. J. Food Sci. Nutr. 2016, 67, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, V.; Monteiro, E.; Brazhnikova, E.; Lesage, L.; Balducci, C.; Guibout, L.; Feraille, L.; Elena, P.P.; Sahel, J.A.; Veillet, S.; et al. Norbixin protects retinal pigmented epithelium cells and photoreceptors against A2E-mediated phototoxicity in vitro and in vivo. PLoS ONE 2016, 11, e0167793. [Google Scholar] [CrossRef] [PubMed]
- García-Onrubia, L.; Valentín-Bravo, F.J.; Coco-Martin, R.M.; González-Sarmiento, R.; Pastor, J.C.; Usategui-Martín, R.; Pastor-Idoate, S. Matrix metalloproteinases in age-related macular degeneration (AMD). Int. J. Mol. Sci. 2020, 21, 5934. [Google Scholar] [CrossRef] [PubMed]
- Hollborn, M.; Stathopoulos, C.; Steffen, A.; Wiedemann, P.; Kohen, L.; Bringmann, A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal models of age related macular degeneration. Mol. Asp. Med. 2012, 33, 487–509. [Google Scholar] [CrossRef]
- Edwards, A.O.; Malek, G. Molecular genetics of AMD and current animal models. Angiogenesis 2007, 10, 119–132. [Google Scholar] [CrossRef]
- Coffey, P.J.; Gias, C.; McDermott, C.J.; Lundh, P.; Pickering, M.C.; Sethi, C.; Bird, A.; Fitzke, F.W.; Maass, A.; Chen, L.L.; et al. Complement factor H deficiency in aged mice causes retinal abnormalities and visual dysfunction. Proc. Natl. Acad. Sci. USA 2007, 104, 16651–16656. [Google Scholar] [CrossRef]
- Combadière, C.; Feumi, C.; Raoul, W.; Keller, N.; Rodéro, M.; Pézard, A.; Lavalette, S.; Houssier, M.; Jonet, L.; Picard, E.; et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Investig. 2007, 117, 2920–2928. [Google Scholar] [CrossRef]
- Hashizume, K.; Hirasawa, M.; Imamura, Y.; Noda, S.; Shimizu, T.; Shinoda, K.; Kurihara, T.; Noda, K.; Ozawa, Y.; Ishida, S.; et al. Retinal dysfunction and progressive retinal cell death in SOD1-deficient mice. Am. J. Pathol. 2008, 172, 1325–1331. [Google Scholar] [CrossRef]
- Cousins, S.W.; Espinosa-Heidmann, D.G.; Alexandridou, A.; Sall, J.; Dubovy, S.; Csaky, K. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp. Eye Res. 2002, 75, 543–553. [Google Scholar] [CrossRef]
- Wolfender, J.L.; Eugster, P.J.; Bohni, N.; Cuendet, M. Advanced methods for natural product drug discovery in the field of nutraceuticals. Chimia 2011, 65, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; Ibrahim, W.H.; Schneider-Stock, R.; Hassan, H.M. Camel milk lactoferrin reduces the proliferation of colorectal cancer cells and exerts antioxidant and DNA damage inhibitory activities. Food Chem. 2013, 141, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, A.; Liñares, G.G.; Bujjamer, J.M.; Gorojod, R.M.; Alcon, S.P.; Martínez, J.H.; Baldessari, A.; Grecco, H.E.; Kotler, M.L. Toxicity of blue led light and A2E is associated to mitochondrial dynamics impairment in ARPE-19 cells: Implications for age-related macular degeneration. Arch. Toxicol. 2019, 93, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, A.; Kubina, R.; Kabała-Dzik, A.; Tanasiewicz, M. Induction of cell cycle arrest and apoptotic response of head and neck squamous carcinoma cells (Detroit 562) by caffeic acid and caffeic acid phenethyl ester derivative. Evid. Based Complement. Alternat. Med. 2017, 2017, 6793456. [Google Scholar] [CrossRef]
- Hans, C.; Saini, R.; Sachdeva, M.U.S.; Sharma, P. 2′,7′-Dichlorofluorescein (DCF) or 2′,7′-dichlorodihydrofluorescein diacetate (DCFH2-DA) to measure reactive oxygen species in erythrocytes. Biomed. Pharmacother. 2021, 138, 111512. [Google Scholar] [CrossRef]
- Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Nitrite and nitrate measurement by Griess reagent in human plasma: Evaluation of interferences and standardization. Methods Enzymol. 2008, 440, 361–380. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin. Chim. Acta. 2000, 293, 157–166. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. Assay of superoxide dismutase activity in a plate assay using WST-1. Free Radic. Biol. Med. 2017, 103, 188–191. [Google Scholar] [CrossRef]
- Tan, A.A.; Azman, S.N.; Abdul Rani, N.R.; Kua, B.C.; Sasidharan, S.; Kiew, L.V.; Othman, N.; Noordin, R.; Chen, Y. Optimal protein extraction methods from diverse sample types for protein profiling by using Two-Dimensional Electrophoresis (2DE). Trop. Biomed. 2011, 28, 620–629. [Google Scholar]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nogawa, S.; Forster, C.; Zhang, F.; Nagayama, M.; Ross, M.E.; Iadecola, C. Interaction between inducible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia. Proc. Natl. Acad. Sci. USA 1998, 95, 10966–10971. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, J.E.; Lee, S.J.; Gong, J.E.; Jin, Y.J.; Lee, H.; Hwang, D.Y. Promotion of the inflammatory response in mid colon of complement component 3 knockout mice. Sci. Rep. 2022, 12, 1700. [Google Scholar] [CrossRef]
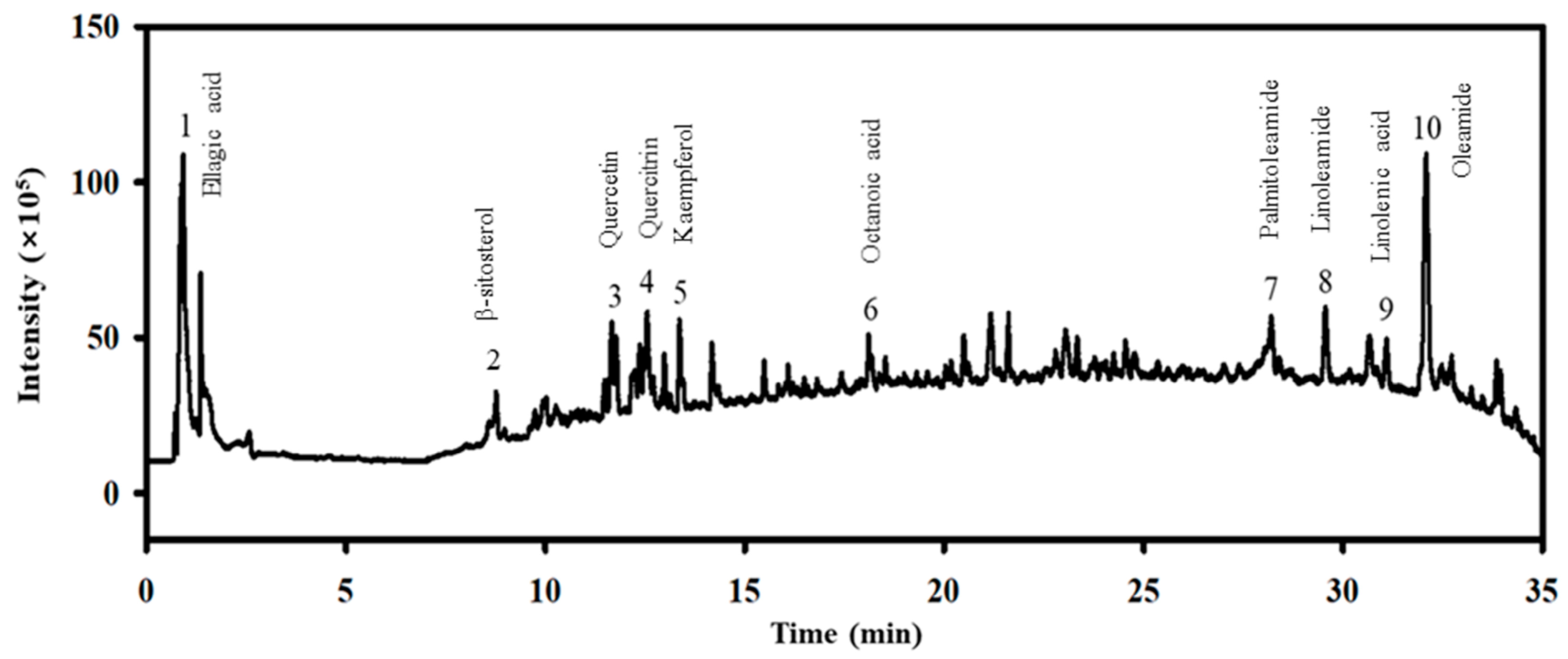
| No. | Compound Name | Molecular Formula | Molecular Weight (g/mol) | Time (min) | Expected m/z | Polarity | Observed m/z | Relative Content (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Ellagic acid | C14H6O8 | 302.19 | 0.92 | 303.2121 | [M + H]+ | 303.1991 | 2.81 |
| 2 | β-sitosterol | C29H50O | 414.71 | 10.62 | 415.3932 | [M + H]+ | 415.1879 | 0.03 |
| 3 | Quercetin | C15H10O7 | 302.24 | 11.67 | 303.0499 | [M + H]+ | 303.0506 | 8.46 |
| 4 | Quercetrin | C21H20O11 | 448.38 | 12.56 | 449.1078 | [M + H]+ | 449.1088 | 7.13 |
| 5 | Kaempferol | C15H10O6 | 286.23 | 13.36 | 287.055 | [M + H]+ | 287.0548 | 5.79 |
| 6 | Octanoic acid | C8H16O2 | 144.21 | 18.10 | 145.1223 | [M + H]+ | 144.9825 | 0.13 |
| 7 | Palmitoleamide | C16H31NO | 253.42 | 28.20 | 254.2478 | [M + H]+ | 254.2484 | 11.15 |
| 8 | Linoleamide | C18H33NO | 279.50 | 29.55 | 280.2635 | [M + H]+ | 280.264 | 14.20 |
| 9 | Linoleic acid | C18H30O2 | 278.43 | 31.12 | 279.2319 | [M + H]+ | 279.2359 | 0.06 |
| 10 | Oleamide | C18H35NO | 281.48 | 32.08 | 282.2791 | [M + H]+ | 282.2796 | 50.24 |
| Cell State | No | A2E + BL | |||
|---|---|---|---|---|---|
| Vehicle | LMEE | MMEE | HMEE | ||
| Live cells | 1.5 × 106 ± 8.71 × 103 | 2.4 × 105 ± 3.61 × 102 | 3.03 × 106 ± 4.37 × 103 | 2.9 × 106 ± 3.08 × 103 | 2.12 × 106 ± 1.39 × 103 |
| Early apoptotic cells | 1.51 × 103 ± 2.64 × 10 2 | 3.31 × 104 ± 64.6 | 1.1 × 104 ± 3.89 × 102 | 7.05 × 103 ± 2.92 × 102 | 2.97 × 103 ± 30.7 |
| Late apoptotic cells | 1.04 ± 2.07 | 6.88 × 102 ± 2.12 | 2.06 × 102 ± 5.93 | 9.07 × 10 ± 5.6 | 1.5 ± 0.43 |
| Dead cells | 4.35 × 10−2 ± 2.24 × 10−2 | 2.01 × 10 ± 2.06 | 15.9 ± 2.24 | 37.7 ± 1.13 | 0.27 ± 0.14 |
| Total apoptotic cells | 1.59 × 103 ± 2.66 × 102 | 4.34 × 104 ± 69.6 | 1.43 × 104 ± 2.99 × 102 | 8.71 × 103 ± 2.28 × 102 | 3.1 × 103 ± 36.2 |
| Layer | No | BL | ||
|---|---|---|---|---|
| Vehicle | LMEE | HMEE | ||
| OS (μm) | 10.89 ± 0.23 | 6.14 ± 0.22 * | 8.02 ± 0.44 *,# | 8.29 ± 0.47 *,# |
| ONL (μm) | 40.89 ± 2.43 | 38.85 ± 3.41 | 36.77 ± 1.12 *,# | 45.01 ± 4.38 *,# |
| INL (μm) | 16.83 ± 1.87 | 11.91 ± 1.15 * | 21.47 ± 0.92 *,# | 13.83 ± 0.85 * |
| IPL (μm) | 10.83 ± 0.79 | 10.56 ± 1.21 | 11.176 ± 0.81 | 11.59 ± 0.93 |
| Whole retina (μm) | 79.44 ± 5.32 | 67.46 ± 5.99 * | 77.436 ± 3.29 # | 78.72 ± 6.63 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seol, A.; Kim, J.-E.; Jin, Y.-J.; Song, H.-J.; Roh, Y.-J.; Kim, T.-R.; Park, E.-S.; Park, K.-H.; Park, S.-H.; Uddin, M.S.; et al. Novel Therapeutic Effects of Euphorbia heterophylla L. Methanol Extracts in Macular Degeneration Caused by Blue Light in A2E-Laden ARPE-19 Cells and Retina of BALB/c Mice. Pharmaceuticals 2024, 17, 1193. https://doi.org/10.3390/ph17091193
Seol A, Kim J-E, Jin Y-J, Song H-J, Roh Y-J, Kim T-R, Park E-S, Park K-H, Park S-H, Uddin MS, et al. Novel Therapeutic Effects of Euphorbia heterophylla L. Methanol Extracts in Macular Degeneration Caused by Blue Light in A2E-Laden ARPE-19 Cells and Retina of BALB/c Mice. Pharmaceuticals. 2024; 17(9):1193. https://doi.org/10.3390/ph17091193
Chicago/Turabian StyleSeol, Ayun, Ji-Eun Kim, You-Jeong Jin, Hee-Jin Song, Yu-Jeong Roh, Tae-Ryeol Kim, Eun-Seo Park, Ki-Ho Park, So-Hae Park, Muhammad Salah Uddin, and et al. 2024. "Novel Therapeutic Effects of Euphorbia heterophylla L. Methanol Extracts in Macular Degeneration Caused by Blue Light in A2E-Laden ARPE-19 Cells and Retina of BALB/c Mice" Pharmaceuticals 17, no. 9: 1193. https://doi.org/10.3390/ph17091193
APA StyleSeol, A., Kim, J.-E., Jin, Y.-J., Song, H.-J., Roh, Y.-J., Kim, T.-R., Park, E.-S., Park, K.-H., Park, S.-H., Uddin, M. S., Lee, S.-W., Choi, Y.-W., & Hwang, D.-Y. (2024). Novel Therapeutic Effects of Euphorbia heterophylla L. Methanol Extracts in Macular Degeneration Caused by Blue Light in A2E-Laden ARPE-19 Cells and Retina of BALB/c Mice. Pharmaceuticals, 17(9), 1193. https://doi.org/10.3390/ph17091193








