Effectiveness of Tri-Kaysorn-Mas Extract in Ameliorating Cognitive-like Behavior Deficits in Ovariectomized Mice via Activation of Multiple Mechanisms
Abstract
1. Introduction
2. Results
2.1. Effects of TKM Extract on In Vitro Studies for Antioxidant Activities
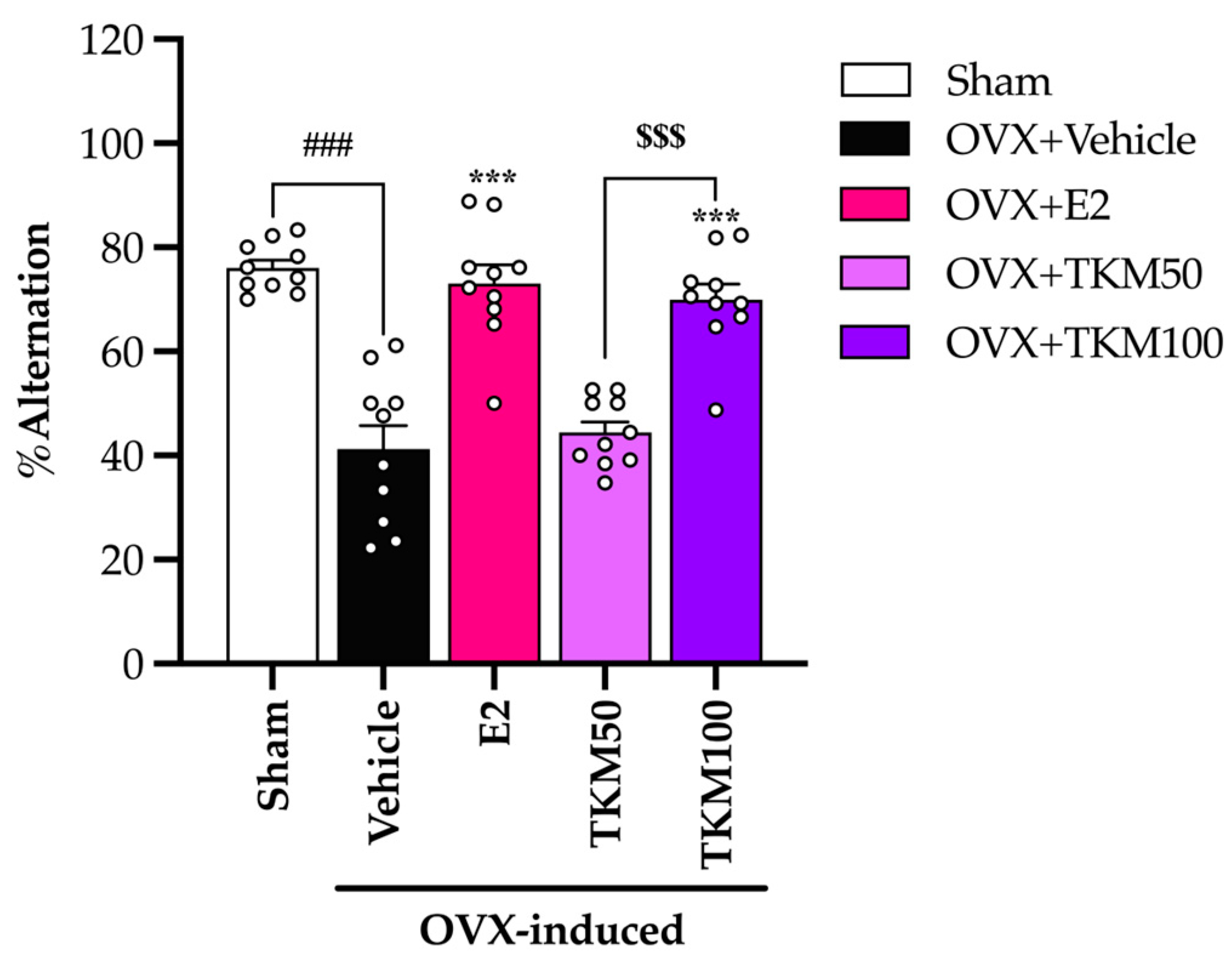
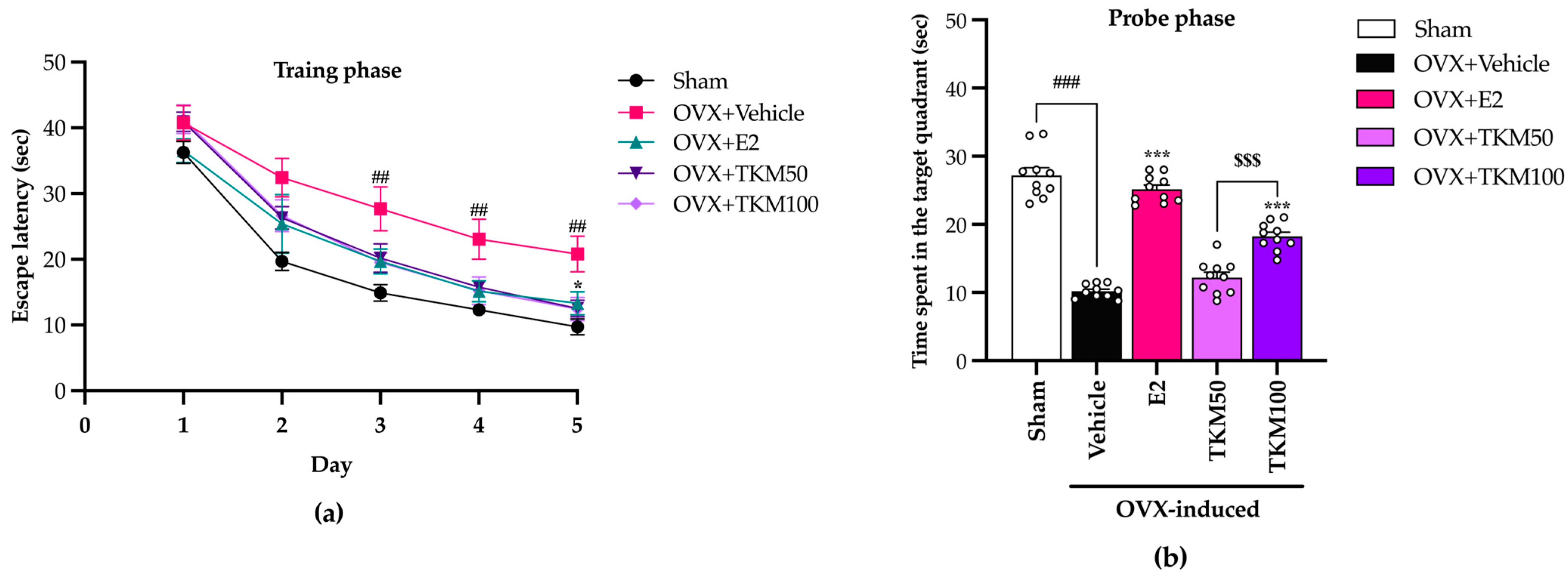
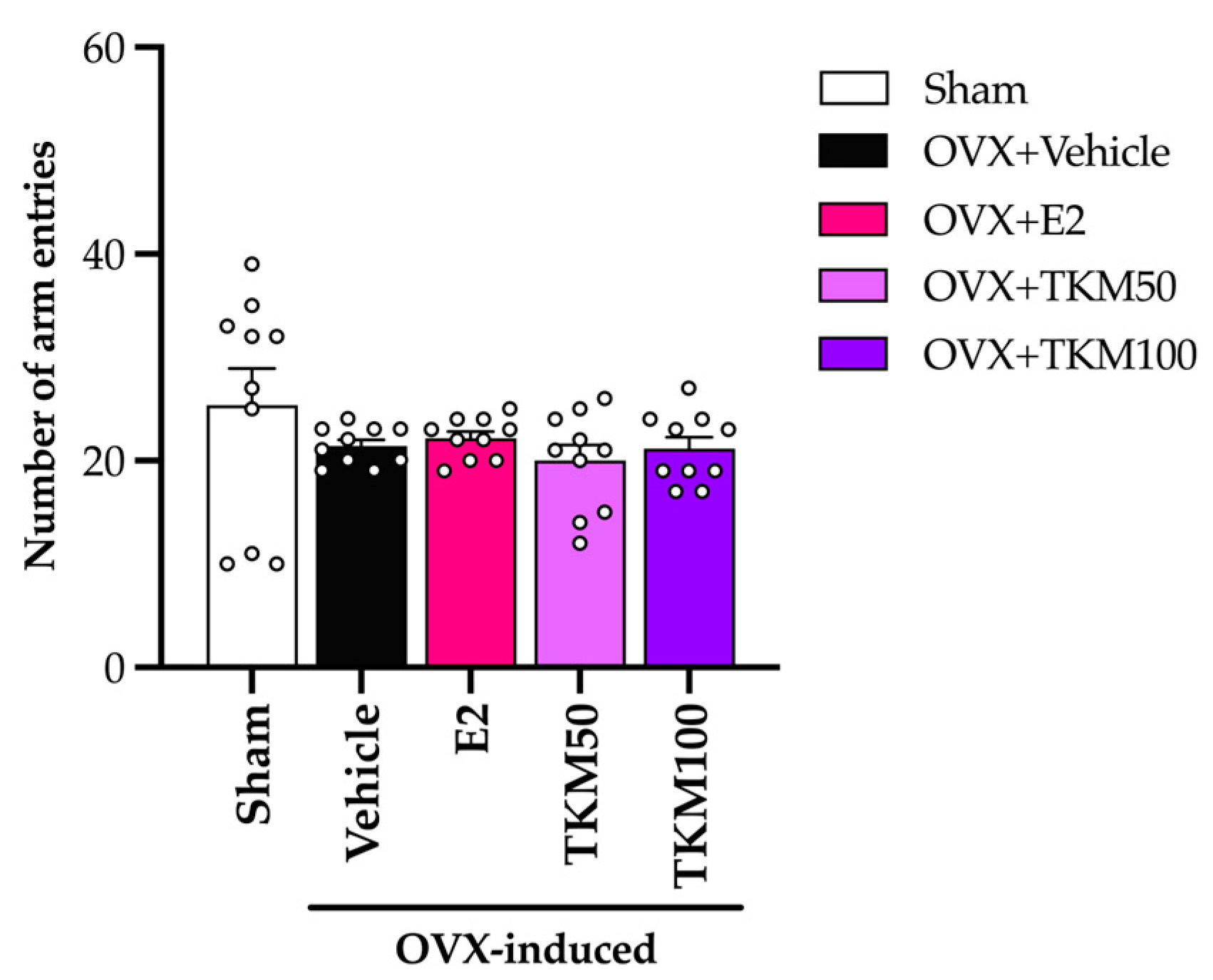
2.2. Effects of TKM Extract on OVX-Induced Cognitive-like Behavior
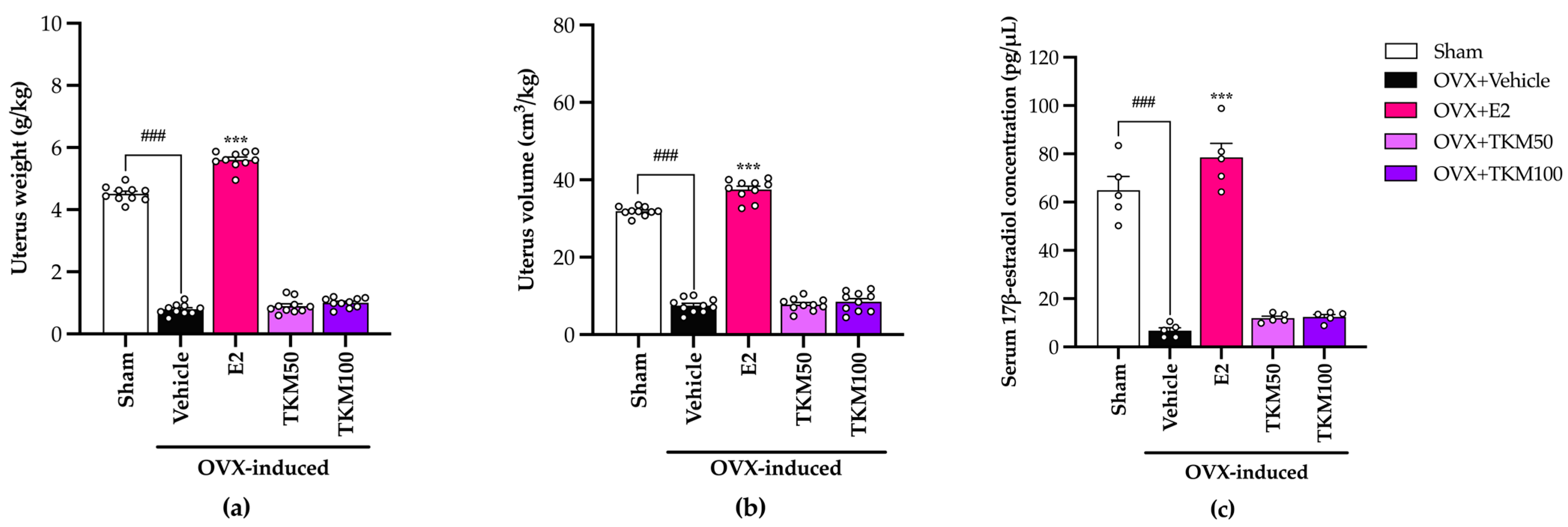
2.3. Effects of TKM Extract on Uterus Weight and Volume and Serum 17β-Estradiol (E2) Levels
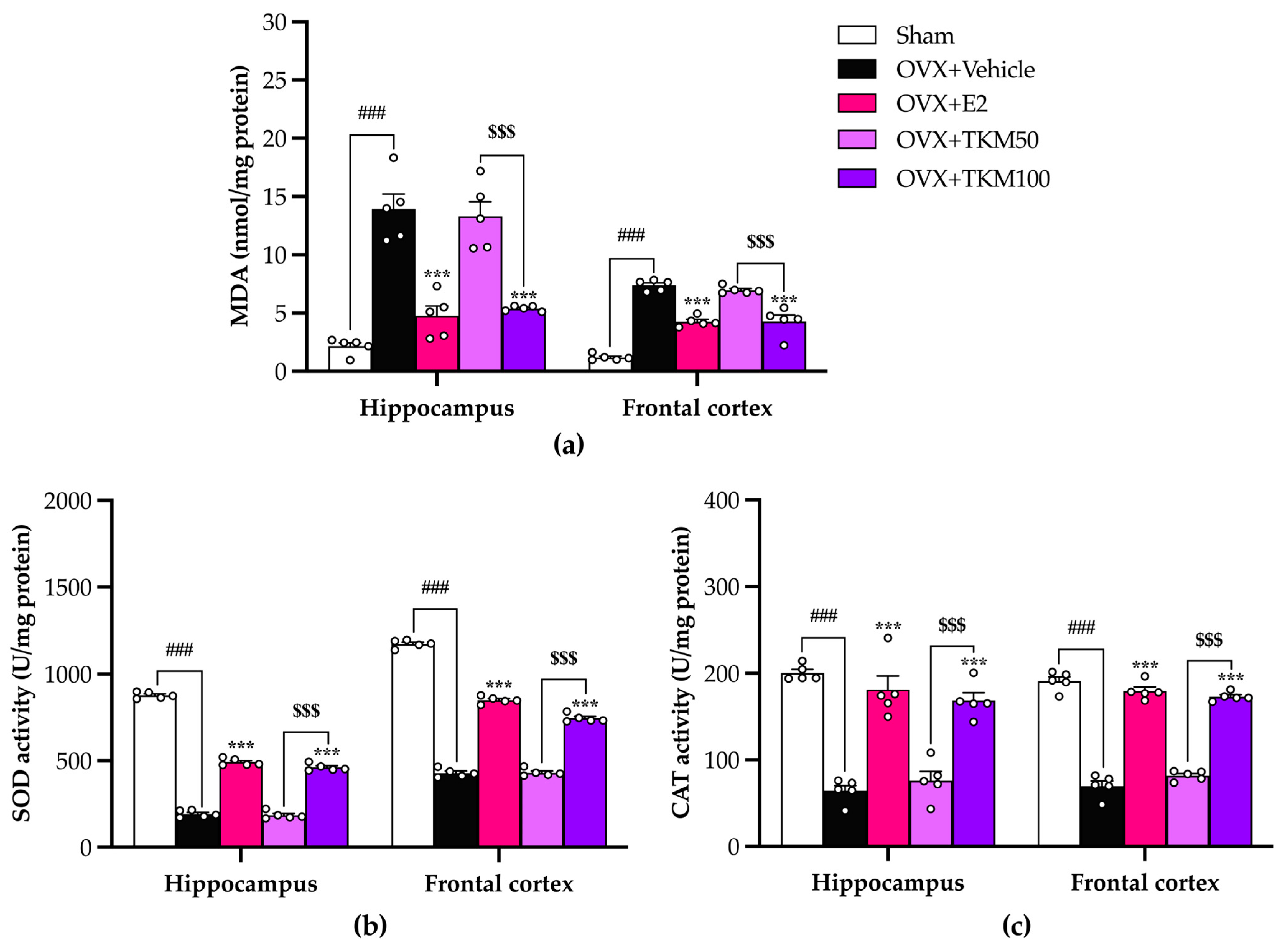
2.4. Effects of TKM Extract on Oxidative Stress and Antioxidant Enzyme Activities in the Brains
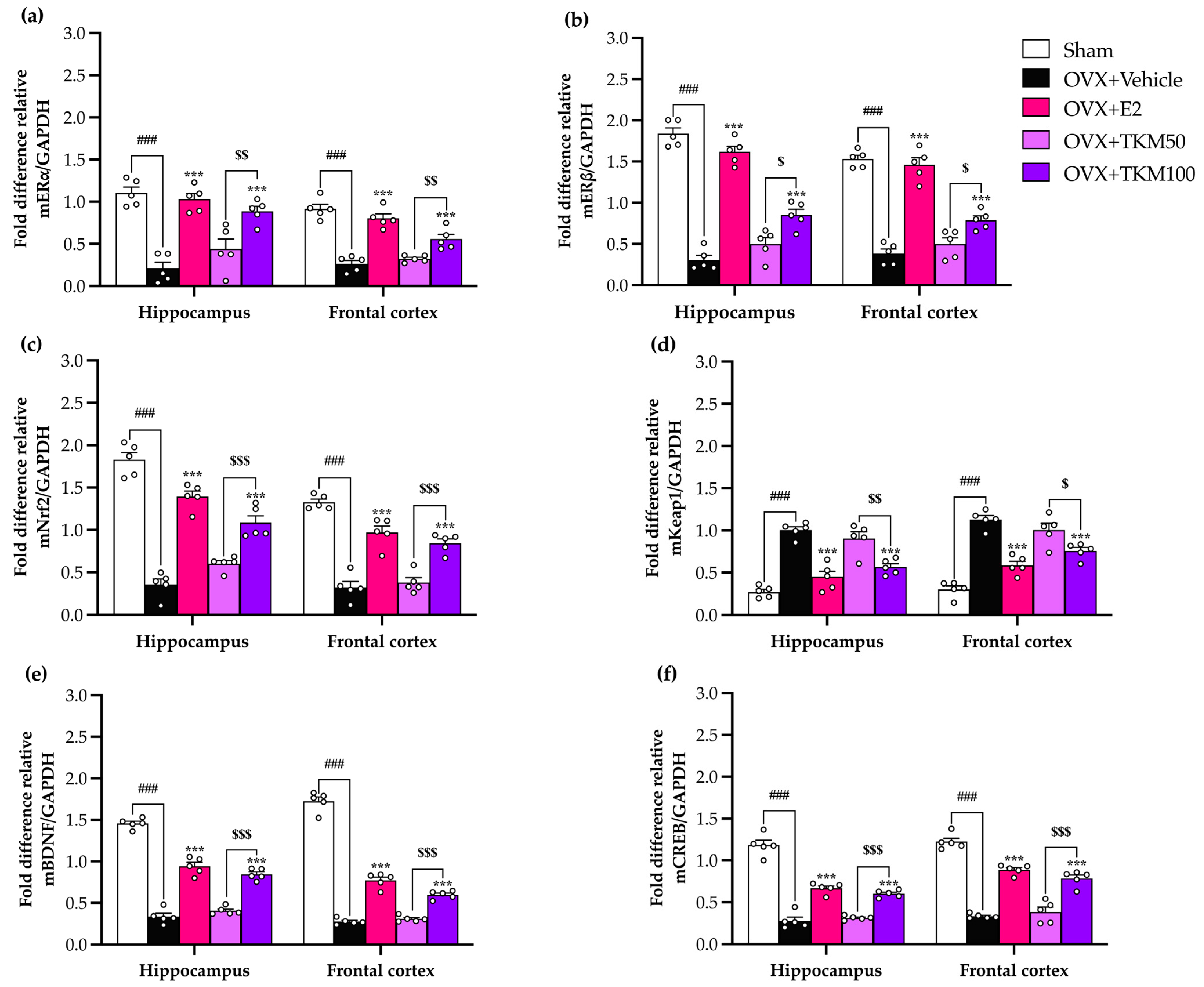
2.5. Effect of TKM Extract on Ovariectomized-Induced Changes in the Hippocampus and Frontal Cortex of Gene Encoding ERα, ERβ, Nrf2, Keap1, BDNF, and CREB
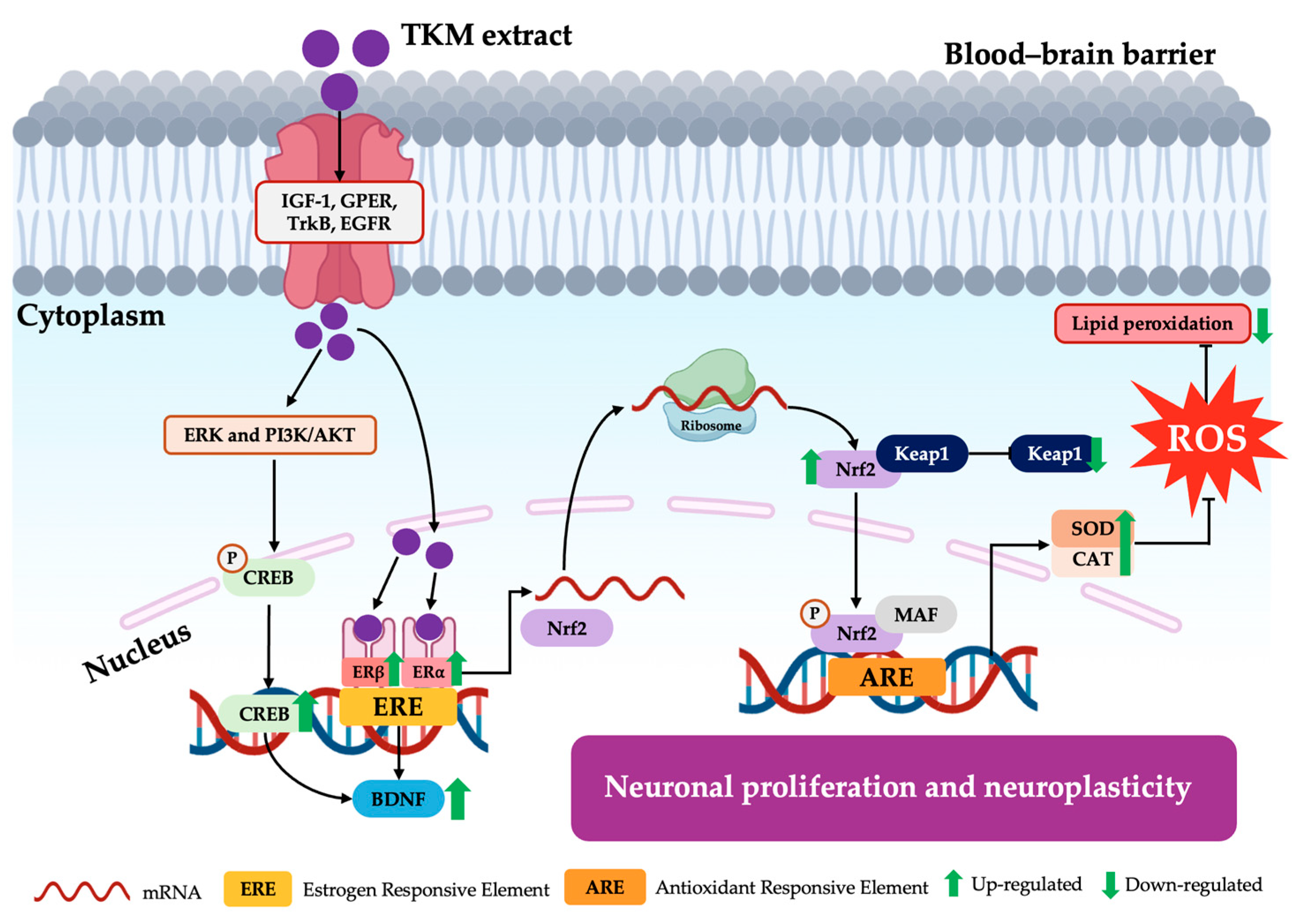
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Extractions
4.2. Determination of Antioxidant Activity by Oxygen Radical Absorbance Capacity (ORAC)
4.3. Determination of Superoxide Anion (O2•−) Radicals Scavenging Activity
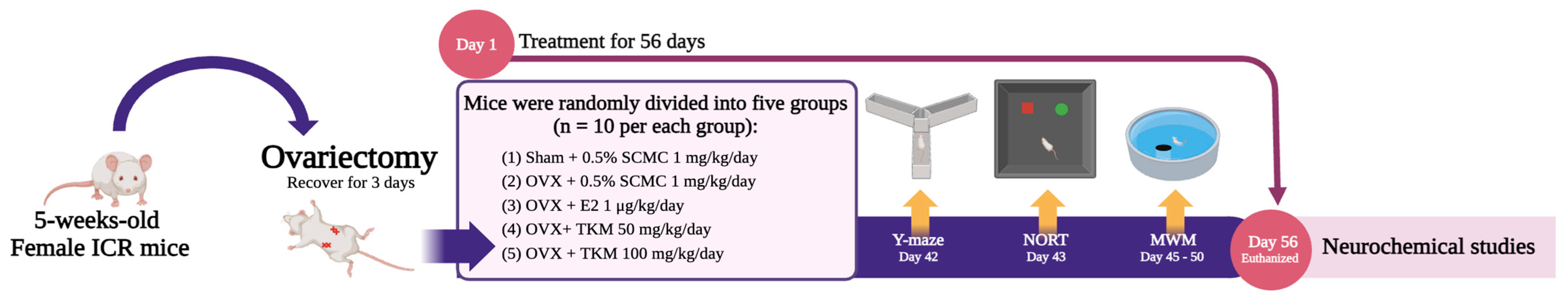
4.4. Animals
4.5. Surgical Procedure and Drug Administration
4.6. Y-Maze Test
4.7. Novel Object Recognition Test (NORT)
4.8. Morris Water Maze Test (MWM)
4.9. Locomotor Test (LT)
4.10. Measurement of Serum 17β-Estradiol Levels
4.11. Dissection of Uterus and Brain Tissue
4.12. Measurement of Lipid Peroxidation Activity in the Brain
4.13. Measurement of Enzymatic Antioxidant Activities
4.14. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conde, D.M.; Verdade, R.C.; Valadares, A.L.R.; Mella, L.F.B.; Pedro, A.O.; Costa-Paiva, L. Menopause and Cognitive Impairment: A Narrative Review of Current Knowledge. WJP 2021, 11, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Wardle-Pinkston, S.; Slavish, D.C.; Taylor, D.J. Insomnia and Cognitive Performance: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2019, 48, 101205. [Google Scholar] [CrossRef] [PubMed]
- Stute, P.; Wienges, J.; Koller, A.-S.; Giese, C.; Wesemüller, W.; Janka, H.; Baumgartner, S. Cognitive Health after Menopause: Does Menopausal Hormone Therapy Affect It? Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101565. [Google Scholar] [CrossRef]
- Gasbarri, A.; Tavares, M.C.H.; Rodrigues, R.C.; Tomaz, C.; Pompili, A. Estrogen, Cognitive Functions and Emotion: An Overview on Humans, Non-Human Primates and Rodents in Reproductive Years. Rev. Neurosci. 2012, 23, 587–606. [Google Scholar] [CrossRef]
- Luine, V.; Frankfurt, M. Estrogenic Regulation of Memory: The First 50 Years. Horm. Behav. 2020, 121, 104711. [Google Scholar] [CrossRef]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef]
- McCarthy, M.; Raval, A.P. The Peri-Menopause in a Woman’s Life: A Systemic Inflammatory Phase That Enables Later Neurodegenerative Disease. J. Neuroinflamm. 2020, 17, 317. [Google Scholar] [CrossRef]
- Song, Y.; Li, S.; Li, X.; Chen, X.; Wei, Z.; Liu, Q.; Cheng, Y. The Effect of Estrogen Replacement Therapy on Alzheimer’s Disease and Parkinson’s Disease in Postmenopausal Women: A Meta-Analysis. Front. Neurosci. 2020, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Kwakowsky, A.; Milne, M.; Waldvogel, H.; Faull, R. Effect of Estradiol on Neurotrophin Receptors in Basal Forebrain Cholinergic Neurons: Relevance for Alzheimer’s Disease. Int. J. Mol. Sci. 2016, 17, 2122. [Google Scholar] [CrossRef]
- Monthakantirat, O.; Sukano, W.; Umehara, K.; Noguchi, H.; Chulikhit, Y.; Matsumoto, K. Effect of Miroestrol on Ovariectomy-Induced Cognitive Impairment and Lipid Peroxidation in Mouse Brain. Phytomedicine 2014, 21, 1249–1255. [Google Scholar] [CrossRef]
- Kishida, K.T.; Klann, E. Sources and Targets of Reactive Oxygen Species in Synaptic Plasticity and Memory. Antioxid. Redox Signal. 2007, 9, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Balu, M.; Sangeetha, P.; Murali, G.; Panneerselvam, C. Age-related Oxidative Protein Damages in Central Nervous System of Rats: Modulatory Role of Grape Seed Extract. Int. J. Dev. Neurosci. 2005, 23, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Lejri, I.; Grimm, A.; Eckert, A. Mitochondria, Estrogen and Female Brain Aging. Front. Aging Neurosci. 2018, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Venier, M. The Controversial History of Hormone Replacement Therapy. Medicina 2019, 55, 602. [Google Scholar] [CrossRef] [PubMed]
- Phromswat, D.; Mahawijit, N.; Nilborworn, P.; Dej-adisai, S. Anti-Acetylcholinesterase Activity of Traditional Thai Herbal Combination Medicines. ThaksinJ 2012, 15, 34–43. [Google Scholar]
- Sihanat, A.; Rittaisong, N.; Chaichamnong, N.; Prommee, N.; Watcharaput, N.; Noppakao, P.; Yamprasert, R. In Vitro Antioxidants Activities of Extract of Tri-Kasorn-Mas Remedy and Its Plant Ingredients. Creat. Sci. 2023, 15, 249899. [Google Scholar] [CrossRef]
- Manandhar, B.; Paudel, K.R.; Sharma, B.; Karki, R. Phytochemical Profile and Pharmacological Activity of Aegle Marmelos Linn. J. Integr. Med. 2018, 16, 153–163. [Google Scholar] [CrossRef]
- Li, D.; Cai, C.; Liao, Y.; Wu, Q.; Ke, H.; Guo, P.; Wang, Q.; Ding, B.; Fang, J.; Fang, S. Systems Pharmacology Approach Uncovers the Therapeutic Mechanism of Medicarpin against Scopolamine-Induced Memory Loss. Phytomedicine 2021, 91, 153662. [Google Scholar] [CrossRef]
- Min, J.I.A.; Yuan, L.I.; Hai-Liang, X.I.N.; Ting-Ting, H.O.U.; Zhang, N.-D.; Hong-Tao, X.U.; Zhang, Q.-Y.; Lu-Ping, Q.I.N. Estrogenic Activity of Osthole and Imperatorin in MCF-7 Cells and Their Osteoblastic Effects in Saos-2 Cells. Chin. J. Nat. Med. 2016, 14, 413–420. [Google Scholar]
- Chen, G.; Zhu, M.; Guo, M. Research Advances in Traditional and Modern Use of Nelumbo Nucifera: Phytochemicals, Health Promoting Activities and Beyond. Crit. Rev. Food Sci. Nutr. 2019, 59, S189–S209. [Google Scholar] [CrossRef]
- Kouhestani, S.; Jafari, A.; Babaei, P. Kaempferol Attenuates Cognitive Deficit via Regulating Oxidative Stress and Neuroinflammation in an Ovariectomized Rat Model of Sporadic Dementia. Neural Regen. Res. 2018, 13, 1827. [Google Scholar] [PubMed]
- Oh, S.M.; Kim, Y.P.; Chung, K.H. Biphasic Effects of Kaempferol on the Estrogenicity in Human Breast Cancer Cells. Arch. Pharmacal Res. 2006, 29, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, R.; Calani, L.; Brighenti, F.; Crozier, A.; Ottonello, S.; Del Rio, D. Glucuronidation Does Not Suppress the Estrogenic Activity of Quercetin in Yeast and Human Breast Cancer Cell Model Systems. Arch. Biochem. Biophys. 2014, 559, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Munoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The Flavonoid Quercetin Ameliorates Alzheimer’s Disease Pathology and Protects Cognitive and Emotional Function in Aged Triple Transgenic Alzheimer’s Disease Model Mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef]
- Khan, S.; Khan, H.U.; Khan, F.A.; Shah, A.; Wadood, A.; Ahmad, S.; Almehmadi, M.; Alsaiari, A.A.; Shah, F.U.; Kamran, N. Anti-Alzheimer and Antioxidant Effects of Nelumbo nucifera L. Alkaloids, Nuciferine and Norcoclaurine in Alloxan-Induced Diabetic Albino Rats. Pharmaceuticals 2022, 15, 1205. [Google Scholar] [CrossRef]
- Can, Ö.D.; Özkay, Ü.D.; Üçel, U.İ. Anti-Depressant-like Effect of Vitexin in BALB/c Mice and Evidence for the Involvement of Monoaminergic Mechanisms. Eur. J. Pharmacol. 2013, 699, 250–257. [Google Scholar] [CrossRef]
- Nurdiana, S.; Goh, Y.M.; Hafandi, A.; Dom, S.M.; Syimal’ain, A.N.; Syaffinaz, N.N.; Ebrahimi, M. Improvement of Spatial Learning and Memory, Cortical Gyrification Patterns and Brain Oxidative Stress Markers in Diabetic Rats Treated with Ficus Deltoidea Leaf Extract and Vitexin. J. Tradit. Complement. Med. 2018, 8, 190–202. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.; Nguyen, Q.N.; Phung, H.M.; Shin, M.-S.; Kim, J.-Y.; Choi, H.; Shim, S.H.; Kang, K.S. Identification of the Active Ingredient and Beneficial Effects of Vitex Rotundifolia Fruits on Menopausal Symptoms in Ovariectomized Rats. Biomolecules 2021, 11, 1033. [Google Scholar] [CrossRef]
- de Oliveira, D.R.; Zamberlam, C.R.; Gaiardo, R.B.; Rêgo, G.M.; Cerutti, J.M.; Cavalheiro, A.J.; Cerutti, S.M. Flavones from Erythrina Falcata Are Modulators of Fear Memory. BMC Complement. Altern. Med. 2014, 14, 288. [Google Scholar] [CrossRef]
- Luine, V.; Frankfurt, M. Interactions between Estradiol, BDNF and Dendritic Spines in Promoting Memory. Neuroscience 2013, 239, 34–45. [Google Scholar] [CrossRef]
- Chulikhit, Y.; Sukhano, W.; Daodee, S.; Putalun, W.; Wongpradit, R.; Khamphukdee, C.; Umehara, K.; Noguchi, H.; Matsumoto, K.; Monthakantirat, O. Effects of Pueraria Candollei Var Mirifica (Airy Shaw and Suvat.) Niyomdham on Ovariectomy-Induced Cognitive Impairment and Oxidative Stress in the Mouse Brain. Molecules 2021, 26, 3442. [Google Scholar] [CrossRef] [PubMed]
- Daodee, S.; Monthakantirat, O.; Tantipongpiradet, A.; Maneenet, J.; Chotritthirong, Y.; Boonyarat, C.; Khamphukdee, C.; Kwankhao, P.; Pitiporn, S.; Awale, S.; et al. Effect of Yakae-Prajamduen-Jamod Traditional Thai Remedy on Cognitive Impairment in an Ovariectomized Mouse Model and Its Mechanism of Action. Molecules 2022, 27, 4310. [Google Scholar] [CrossRef]
- Adekoya, A.E.; Chusri, S.; Beng, E.O.B.; Idowu, A.T. In Vitro Inhibitory Potentials of Thai Formulated Polyherbal Tea against Oxidative Stress Promoters. Isr. J. Plant Sci. 2021, 68, 166–173. [Google Scholar] [CrossRef]
- Kumar, G.; Khanum, F. Neuroprotective Potential of Phytochemicals. Pharmacogn. Rev. 2012, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, G.; Morón-Oset, J.; Díaz-Castroverde, S.; García-Font, N.; Roncero, C.; López-Muñoz, F.; Marco Contelles, J.L.; Oset-Gasque, M.J. Neuroprotection by Phytoestrogens in the Model of Deprivation and Resupply of Oxygen and Glucose In Vitro: The Contribution of Autophagy and Related Signaling Mechanisms. Antioxidants 2020, 9, 545. [Google Scholar] [CrossRef]
- Gorzkiewicz, J.; Bartosz, G.; Sadowska-Bartosz, I. The Potential Effects of Phytoestrogens: The Role in Neuroprotection. Molecules 2021, 26, 2954. [Google Scholar] [CrossRef]
- Tamir, S.; Izrael, S.; Vaya, J. The Effect of Oxidative Stress on ERα and ERβ Expression. J. Steroid Biochem. Mol. Biol. 2002, 81, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez De La Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Machado, M.M.F.; Banin, R.M.; Thomaz, F.M.; De Andrade, I.S.; Boldarine, V.T.; De Souza Figueiredo, J.; Hirata, B.K.S.; Oyama, L.M.; Lago, J.H.G.; Ribeiro, E.B.; et al. Ginkgo Biloba Extract (GbE) Restores Serotonin and Leptin Receptor Levels and Plays an Antioxidative Role in the Hippocampus of Ovariectomized Rats. Mol. Neurobiol. 2021, 58, 2692–2703. [Google Scholar] [CrossRef]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef]
- Maioli, S.; Leander, K.; Nilsson, P.; Nalvarte, I. Estrogen Receptors and the Aging Brain. Essays Biochem. 2021, 65, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Warabi, E. Mechanism of Rapid Nuclear Factor-E2-Related Factor 2 (Nrf2) Activation via Membrane-Associated Estrogen Receptors: Roles of NADPH Oxidase 1, Neutral Sphingomyelinase 2 and Epidermal Growth Factor Receptor (EGFR). Antioxidants 2019, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Li, A.; Liu, R.; Yang, H.; Xia, X. The Phytochemical Potential for Brain Disease Therapy and the Possible Nanodelivery Solutions for Brain Access. Front. Oncol. 2022, 12, 936054. [Google Scholar] [CrossRef]
- Cersosimo, M.G.; Benarroch, E.E. Estrogen Actions in the Nervous System: Complexity and Clinical Implications. Neurology 2015, 85, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E.; MacLusky, N.J. Estrogen and Brain-Derived Neurotrophic Factor (BDNF) in Hippocampus: Complexity of Steroid Hormone-Growth Factor Interactions in the Adult CNS. Front. Neuroendocrinol. 2006, 27, 415–435. [Google Scholar] [CrossRef]
- Ren, H.; Han, R.; Liu, X.; Wang, L.; Koehler, R.C.; Wang, J. Nrf2-BDNF-TrkB Pathway Contributes to Cortical Hemorrhage-Induced Depression, but Not Sex Differences. J. Cereb. Blood Flow Metab. 2021, 41, 3288–3301. [Google Scholar] [CrossRef]
- Tian, L.; Peng, Y.; Yang, K.; Cao, J.; Du, X.; Liang, Z.; Shi, J.; Zhang, J. The ERα-NRF2 Signalling Axis Promotes Bicalutamide Resistance in Prostate Cancer. Cell Commun. Signal. 2022, 20, 178. [Google Scholar] [CrossRef]
- Aleshina, Y.A.; Aleshin, V.A. Evolutionary Changes in Primate Glutamate Dehydrogenases 1 and 2 Influence the Protein Regulation by Ligands, Targeting and Posttranslational Modifications. Int. J. Mol. Sci. 2024, 25, 4341. [Google Scholar] [CrossRef]
- Li, C.; Li, M.; Chen, P.; Narayan, S.; Matschinsky, F.M.; Bennett, M.J.; Stanley, C.A.; Smith, T.J. Green Tea Polyphenols Control Dysregulated Glutamate Dehydrogenase in Transgenic Mice by Hijacking the ADP Activation Site. J. Biol. Chem. 2011, 286, 34164–34174. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Pirunkaset, E.; Boonyarat, C.; Maneenet, J.; Khamphukdee, C.; Daodee, S.; Monthakantirat, O.; Awale, S.; Kijjoa, A.; Chulikhit, Y. Effect of Diacetylcurcumin Manganese Complex on Rotenone-Induced Oxidative Stress, Mitochondria Dysfunction, and Inflammation in the SH-SY5Y Parkinson’s Disease Cell Model. Molecules 2024, 29, 957. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar] [PubMed]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-Peptide (1–42)-Induced Oxidative Stress in Alzheimer Disease: Importance in Disease Pathogenesis and Progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Tantipongpiradet, A.; Monthakantirat, O.; Daodee, S.; Boonyarat, C.; Matsumoto, K.; Pitiporn, S.; Chulikhit, Y. Yakae-Prajamduen-Jamod Recipe Reduced Anxiety Behavior and Brain Oxidative Damage in Ovariectomized Mice. Songklanakarin J. Sci. Technol. 2020, 42, 172179. [Google Scholar] [CrossRef]
- Maneenet, J.; Daodee, S.; Monthakantirat, O.; Boonyarat, C.; Khamphukdee, C.; Kwankhao, P.; Pitiporn, S.; Awale, S.; Chulikhit, Y.; Kijjoa, A. Kleeb Bua Daeng, a Thai Traditional Herbal Formula, Ameliorated Unpredictable Chronic Mild Stress-Induced Cognitive Impairment in ICR Mice. Molecules 2019, 24, 4587. [Google Scholar] [CrossRef] [PubMed]
- Tantipongpiradet, A.; Monthakantirat, O.; Vipatpakpaiboon, O.; Khampukdee, C.; Umehara, K.; Noguchi, H.; Fujiwara, H.; Matsumoto, K.; Sekeroglu, N.; Kijjoa, A.; et al. Effects of Puerarin on the Ovariectomy-Induced Depressive-like Behavior in ICR Mice and Its Possible Mechanism of Action. Molecules 2019, 24, 4569. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Y.; Piao, Y.; Nagaoka, K.; Watanabe, G.; Taya, K.; Li, C. Protective Effects of Nuclear Factor Erythroid 2-Related Factor 2 on Whole Body Heat Stress-Induced Oxidative Damage in the Mouse Testis. Reprod. Biol. Endocrinol. 2013, 11, 23. [Google Scholar] [CrossRef]
- Zhao, S.; Ghosh, A.; Lo, C.-S.; Chenier, I.; Scholey, J.W.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.-L.; Chan, J.S.D. Nrf2 Deficiency Upregulates Intrarenal Angiotensin-Converting Enzyme-2 and Angiotensin 1–7 Receptor Expression and Attenuates Hypertension and Nephropathy in Diabetic Mice. Endocrinology 2018, 159, 836–852. [Google Scholar] [CrossRef]

| Extract | Tri-Kaysorn-Mas (TKM) |
|---|---|
| ORAC assay (μM Trolox equivalent/g extract) | 1.83 ± 0.02 |
| Superoxide radical scavenging (IC50) mg/mL | 0.59 ± 0.01 |
| Genes | Primer Sequences | Product Length | References | |
|---|---|---|---|---|
| GAPDH | sense | 5′-AACACAGTCCATGCCATCAC-3′ | 452 bp | [32] |
| antisense | 5′-TCCACCACCCTGTTGCTGTA-3′ | |||
| ERα | sense | 5′-GACCAGATGGTCAGTGCCTT-3′ | 166 bp | [56] |
| antisense | 5′-ACTCGAGAAGGTGGACCTGA-3′ | |||
| ERβ | sense | 5′-CAGTAACAAGGGCATGGAAC-3′ | 204 bp | [56] |
| antisense | 5′-GTACATGTCCCACTTCTGAC-3′ | |||
| Nrf2 | sense | 5′-CAGTGCTCCTATGCGTGAA-3′ | 109 bp | [57] |
| antisense | 5′-GCGGCTTGAATGTTTGTC-3′ | |||
| Keap1 | sense | 5′-CATCCACCCTAAGGTCATGGA-3′ | 291 bp | [58] |
| antisense | 5′-GACAGGTTGAAGAACTCCTCC-3′ | |||
| BDNF | sense | 5′-GACAAGGCAACTTGGCCTAC-3′ | 334 bp | [10] |
| antisense | 5′-CCTGTCACACACGCTCAGCTC-3′ | |||
| CREB | sense | 5′-TACCCAGGGAGGAGCAATAC-3′ | 183 bp | [10] |
| antisense | 5′-GAGGCAGCTTGAACAACAAC-3′ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mading, A.; Chotritthirong, Y.; Chulikhit, Y.; Daodee, S.; Boonyarat, C.; Khamphukdee, C.; Sukketsiri, W.; Kwankhao, P.; Pitiporn, S.; Monthakantirat, O. Effectiveness of Tri-Kaysorn-Mas Extract in Ameliorating Cognitive-like Behavior Deficits in Ovariectomized Mice via Activation of Multiple Mechanisms. Pharmaceuticals 2024, 17, 1182. https://doi.org/10.3390/ph17091182
Mading A, Chotritthirong Y, Chulikhit Y, Daodee S, Boonyarat C, Khamphukdee C, Sukketsiri W, Kwankhao P, Pitiporn S, Monthakantirat O. Effectiveness of Tri-Kaysorn-Mas Extract in Ameliorating Cognitive-like Behavior Deficits in Ovariectomized Mice via Activation of Multiple Mechanisms. Pharmaceuticals. 2024; 17(9):1182. https://doi.org/10.3390/ph17091182
Chicago/Turabian StyleMading, Abdulwaris, Yutthana Chotritthirong, Yaowared Chulikhit, Supawadee Daodee, Chantana Boonyarat, Charinya Khamphukdee, Wanida Sukketsiri, Pakakrong Kwankhao, Supaporn Pitiporn, and Orawan Monthakantirat. 2024. "Effectiveness of Tri-Kaysorn-Mas Extract in Ameliorating Cognitive-like Behavior Deficits in Ovariectomized Mice via Activation of Multiple Mechanisms" Pharmaceuticals 17, no. 9: 1182. https://doi.org/10.3390/ph17091182
APA StyleMading, A., Chotritthirong, Y., Chulikhit, Y., Daodee, S., Boonyarat, C., Khamphukdee, C., Sukketsiri, W., Kwankhao, P., Pitiporn, S., & Monthakantirat, O. (2024). Effectiveness of Tri-Kaysorn-Mas Extract in Ameliorating Cognitive-like Behavior Deficits in Ovariectomized Mice via Activation of Multiple Mechanisms. Pharmaceuticals, 17(9), 1182. https://doi.org/10.3390/ph17091182








