Characterization and Evaluation of the Cytotoxicity of Pregabalin Gels for Oral Application
Abstract
1. Introduction
2. Results
2.1. Obtaining the Gel Formulation
2.2. Characterization of the Drug and Gel Formulation
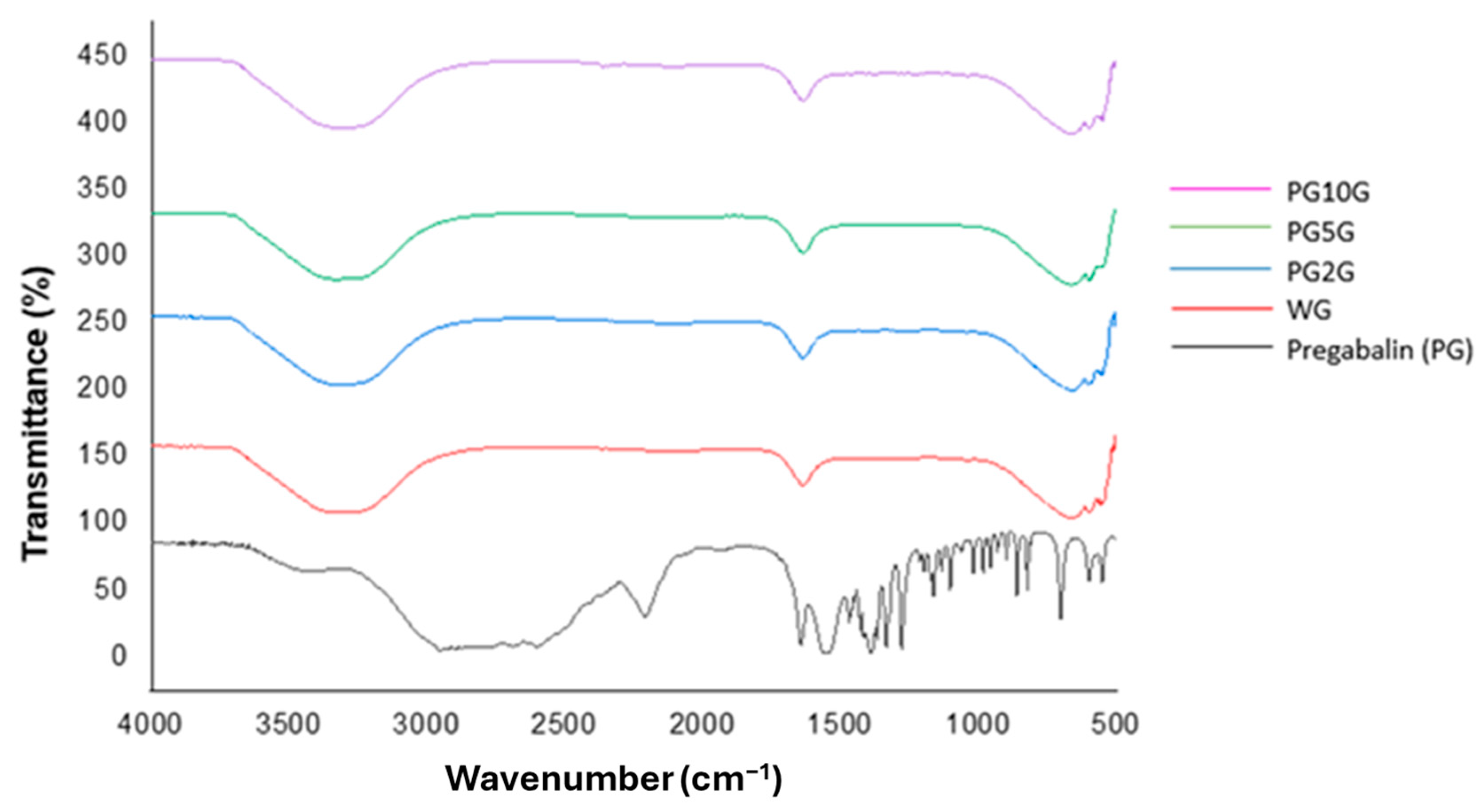
2.2.1. Fourier-Transform Infrared Spectrometry (FTIR)
2.2.2. Thermogravimetric Analysis (TG/DTG)
2.2.3. Differential Scanning Calorimetry (DSC)
2.3. Evaluation of Gel Formulation Stability
2.4. Rheological Properties
2.5. Evaluation of Cytotoxicity of Gel Formulations
Cell Viability Assessment
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Obtaining the Gel Formulation
4.3. Preparation of Pregabalin Solutions
4.4. Characterization of the Drug and Gel Formulation
4.4.1. Fourier-Transform Infrared Spectrometry (FTIR)
4.4.2. Thermogravimetric Analysis (TG/DTG)
4.4.3. Differential Scanning Calorimetry (DSC)
4.5. Evaluation of Gel Formulation Stability
4.5.1. Centrifugation Test
4.5.2. Preliminary Stability Study
4.5.3. Accelerated Stability Study
4.6. pH Value Determination
4.7. Rheological Properties
4.8. Evaluation of Cytotoxicity of Gel Formulations
4.8.1. Preparation of Gel Formulations
4.8.2. Cell Culture and Maintenance
4.8.3. Cell Viability Assessment
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fornasari, D. Pharmacotherapy for Neuropathic Pain: A Review. Pain Ther. 2017, 6 (Suppl. S1), 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Wang, H.; Nie, H.Y.; Bu, G.; Shen, X.D.; Wang, H. The efficacy of pregabalin for acute pain control in herpetic neuralgia patients: A meta-analysis. Medicine 2017, 96, e9167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Peng, L.; Liu, D. Pregabalin alleviates neuropathic pain via inhibition of the PKCε/TRPV1 pathway. Neurosci. Lett. 2022, 766, 136348. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Bell, R.F.; Straube, S.; Wiffen, P.J.; Aldington, D.; Moore, R.A. Pregabalin for neuropathic pain in adults. Cochrane Database Syst. Rev. 2019, 1, CD007076. [Google Scholar] [CrossRef] [PubMed]
- Boudieu, L.; Mountadem, S.; Lashermes, A.; Meleine, M.; Ulmann, L.; Rassendren, F.; Aissouni, Y.; Sion, B.; Carvalho, F.A.; Ardid, D. Blocking α 2 δ-1 Subunit Reduces Bladder Hypersensitivity and Inflammation in a Cystitis Mouse Model by Decreasing NF-kB Pathway Activation. Front. Pharmacol. 2019, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Wu, Z.; Zhang, L.C.; Zhang, Z.; Chen, R.P.; Huang, Y.H.; Chen, H. Efficacy and safety of pregabalin for treating painful diabetic peripheral neuropathy: A meta-analysis. Acta Anaesthesiol. Scand. 2015, 59, 147–159. [Google Scholar] [CrossRef]
- Luo, Y.; Suttle, A.; Zhang, Q.; Wang, P.; Chen, Y. Transient Receptor Potential (TRP) Ion Channels in Orofacial Pain. Mol. Neurobiol. 2021, 58, 2836–2850. [Google Scholar] [CrossRef]
- Ataizi, Z.S.; Ertilav, K. Pregabalin reduces oxaliplatin-induced oxidative neurotoxicity through modulation of TRPV1 channels in DBTRG neuronal cell line. Anticancer Drugs 2020, 31, 728–736. [Google Scholar] [CrossRef]
- Yajima, M.; Takahashi, Y.; Sugimura, Y.K.; Kato, F. Pregabalin attenuates long-lasting post-inflammatory nociplastic mechanical sensitization in mice. Neurobiol. Pain 2023, 13, 100131. [Google Scholar] [CrossRef]
- Cheng, E.T.L.; Cheik-Hussein, M.; Lin, N.; Lewin, A.M.; Mcauley, J.H.; Harrisid, I.A. A meta-epidemiological study on the reported treatment effect of pregabalin in neuropathic pain trials over time. PLoS ONE 2023, 18, e0280593. [Google Scholar] [CrossRef]
- Kimura, S.Y.; Takahiro, S.J.; Kato, S.C.; Naomi, H.K.; Yamaguchi, Y.T.; Hiroshi, T.Y.; Kenyoshi, K.S.; Miyoshi, S.M.; Iseki, Y. Switching from Pregabalin to Mirogabalin in Patients with Peripheral Neuropathic Pain: A Multi-Center, Prospective, Single-Arm, Open-Label Study. Pain Ther. 2021, 10, 711–727. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Luo, R.; Jiang, J.; Xiang, Z. The preemptive effects of oral pregabalin on perioperative pain management in lower limb orthopedic surgery: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 237. [Google Scholar] [CrossRef]
- Moore, R.A.; Straube, S.; Wiffen, P.J.; Derry, S.; Mcquay, H.J. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst. Rev. 2019, 1, CD007076. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Aamir, M.N.; Sheikh, F.A.; Ahmad, N.; Alotaibi, N.F.; Bukhari, S.N.A. Preparation, Characterization of Pregabalin and Withania coagulans Extract-Loaded Topical Gel, and Their Comparative Effect on Burn Injury. Gels 2022, 8, 402. [Google Scholar] [CrossRef] [PubMed]
- Özakar, E.; Sevinç-Özakar, R.; Yılmaz, B. Preparation, Characterization, and Evaluation of Cytotoxicity of Fast Dissolving Hydrogel Based Oral Thin Films Containing Pregabalin and Methylcobalamin. Gels 2023, 9, 147. [Google Scholar] [CrossRef]
- Arafa, M.G.; Ayoub, B.M. DOE Optimization of Nano-based Carrier of Pregabalin as Hydrogel: New Therapeutic & Chemometric Approaches for Controlled Drug Delivery Systems. Sci. Rep. 2017, 7, 41503. [Google Scholar]
- Nagao, M.; Masataka, T.; Sugiyama, E.; Shinouchi, R.; Shibata, K.; Yoshikawa, M.; Yamamoto, T.; Sato, V.H.; Nobe, K.; Sato, H. Evaluation of in vitro transdermal permeation, mass spectrometric imaging, and in vivo analgesic effects of pregabalin using a pluronic lecithin organogel formulation in mice. Pharmacol. Res. Perspect. 2022, 10, e00919. [Google Scholar] [CrossRef]
- Fukasawa, H.; Muratake, H.; Nagae, M.; Sugiyama, K.; Shudo, K. Transdermal Administration of Aqueous Pregabalin Solution as a Potential Treatment Option for Patients with Neuropathic Pain to Avoid Central Nervous System-Mediated Side Effects. Biol. Pharm. Bull. 2014, 37, 1816–1819. [Google Scholar] [CrossRef]
- Steele, G. Preformulation as an Aid to Product Design in Early Drug Development. In Pharmaceutical Preformulation and Formulation; CRC Press: Boca Raton, FL, USA, 2001; pp. 175–237. [Google Scholar]
- Singh, G.R.; Manirul, H.S.; Shanker, P. Development and Validation of Pregabalin in Bulk, Pharmaceutical Formulations and in Human Urine Samples by UV Spectrophotometry. Int. J. Biomed. Sci. 2009, 5, 175–178. [Google Scholar]
- Parreiras, S.O.; Szesz, A.L.; Coppla, F.M.; Martini, E.C.; Farago, P.V.; Loguercio, A.D.; Reis, A. Effect of an experimental desensitizing agent on reduction of bleaching-induced tooth sensitivity: A triple-blind randomized clinical trial. J. Am. Dent. Assoc. 2018, 149, 281–290. [Google Scholar] [CrossRef]
- Vochikovski, L.; Michael, F.W.; Rezende, M.; Renata, T.M.O.; da Silva, K.L.; Farago, P.V.; Loguercio, A.D.; Reis, A. Effect of an experimental desensitizing gel on bleaching-induced tooth sensitivity after in-office bleaching-a double-blind, randomized controlled trial. Clin. Oral Investig. 2023, 27, 1567–1576. [Google Scholar] [CrossRef]
- Poubel, L.A.C.; Gouvea, V.D.G.; Calazans, F.S.; Castro, D.E.; Alves, W.V.; Marins, S.S.; Barcelos, R.; Barceleiro, M.O. Pre-operative use of dexamethasone does not reduce incidence or intensity of bleaching-induced tooth sensitivity. A triple-blind, parallel-design, randomized clinical trial. Clin. Oral Investig. 2019, 23, 435–444. [Google Scholar] [CrossRef]
- da Silva, K.L.; Sutil, E.; Hortkoff, D.; Terra, R.M.O.; Rezende, M.; Reis, A.; Loguercio, A.D.; Vilela, A.P.; Farago, P.V. Coadministration of ibuprofen/caffeine on bleaching-induced tooth sensitivity: A randomized clinical trial. Braz. Dent. J. 2021, 32, 105–115. [Google Scholar] [CrossRef]
- Rezende, M.; Bonafé, E.; Vochikovski, L.; Farago, P.V.; Loguercio, A.D.; Reis, A.; Kossatz, S. Pre- and postoperative dexamethasone does not reduce bleaching-induced tooth sensitivity: A randomized, triple-masked clinical trial. J. Am. Dent. Assoc. 2016, 147, 41–49. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Kirkpatrick, P. Pregabalin. Nat. Rev. Drug Discov. 2005, 4, 455–456. [Google Scholar]
- Prnjavorac, B.; Kunic, S.; Pejanovic-Skobic, N.; Gorana, N.P.; Zirojevic, D.; Vukas, S.K.; Campara, M.T.; Skopljak, A. Pregabalin in the Treatment of Peripheral and Central Chronic Neuropathic Pain. Mater. Sociomed. 2023, 35, 42–47. [Google Scholar]
- Asghar, A.; Aamir, M.N.; Sheikh, F.A.; Ahmad, N.; Elsherif, M.A.; Nasir, S.; Bukhari, S.N.A. Co-Combination of Pregabalin and Withania coagulans-Extract-Loaded Topical Gel Alleviates Allodynia and Hyperalgesia in the Chronic Sciatic Nerve Constriction Injury for Neuropathic Pain in Animal Model. Molecules 2022, 27, 4433. [Google Scholar] [CrossRef]
- Kheirabadi, N.R.; Chiolerio, A.; Szaciłowski, K.; Adamatzky, A. Neuromorphic Liquids, Colloids, and Gels: A Review. ChemPhysChem 2023, 24, e202200390. [Google Scholar] [CrossRef]
- Asghar, Z.; Jamshaid, T.; Sajid-Ur-Rehman, M.; Jamshaid, U.; Gad, H.A. Novel Transethosomal Gel Containing Miconazole Nitrate; Development, Characterization, and Enhanced Antifungal Activity. Pharmaceutics 2023, 15, 2537. [Google Scholar] [CrossRef]
- Schönherr, H.; Goonoo, N.; Dupin, D.; Pus, C.; Zanfirescu, A.; Negres, S. Recent Progress in Gels for Neuropathic Pain. Gels 2023, 9, 417. [Google Scholar] [CrossRef]
- Cringoli, M.C.; Marchesan, S.; Melchionna, M.; Fornasiero, P.; Pellegrino, F.; Cesano, F. Nanostructured Gels for Energy and Environmental Applications. Molecules 2020, 25, 5620. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.; Rocha, A.I.; Leal, P.; Estanqueiro, M.; Lobo, J.M.S. Development and characterization of mucoadhesive buccal gels containing lipid nanoparticles of ibuprofen. Int. J. Pharm. 2017, 533, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2016, 52, 456–506. [Google Scholar] [CrossRef]
- Song, Y.; Cong, Y.; Wang, B.; Zhang, N. Applications of Fourier transform infrared spectroscopy to pharmaceutical preparations. Expert. Opin. Drug Deliv. 2020, 17, 551–571. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Diaconu, A.; Nita, L.E.; Tudorachi, N.; Mititelu-Tartau, L.; Creteanu, A.; Dragostin, O.; Rusu, D.; Popa, G. The influence of excipients on physical and pharmaceutical properties of oral lyophilisates containing a pregabalin-acetaminophen combination. Expert. Opin. Drug Deliv. 2017, 14, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Ghumman, S.A.; Bashir, S.; Noreen, S.; Khan, A.M.; Malik, M.Z. Taro-corms mucilage-alginate microspheres for the sustained release of pregabalin: In vitro & in vivo evaluation. Int. J. Biol. Macromol. 2019, 139, 1191–1202. [Google Scholar] [PubMed]
- Lamichhane, S.; Park, J.B.; Sohn, D.H.; Lee, S. Customized Novel Design of 3D Printed Pregabalin Tablets for Intra-Gastric Floating and Controlled Release Using Fused Deposition Modeling. Pharmaceutics 2019, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, G. A review on pharmaceutical preformulation studies in formulation and development of new drug molecules. Int. J. Pharm. Sci. Res. 2016, 7, 2313. [Google Scholar]
- Giron, D. Applications of Thermal Analysis and Coupled Techniques in Pharmaceutical Industry. J. Therm. Anal. Calorim. 2004, 68, 335–357. [Google Scholar] [CrossRef]
- Steendam, R.R.; Khandavilli, U.R.; Keshavarz, L.; Frawley, P.J. Solution versus Crystal Hydration: The Case of γ-Amino Acid Pregabalin. Cryst. Growth Des. 2019, 19, 4483–4488. [Google Scholar] [CrossRef]
- Ubaid, M.; Ilyas, S.; Mir, S.; Khan, A.K.; Rashid, R.; Khan, M.Z.U.; Kanwal, Z.G.; Nawaz, A.; Shah, A.; Murtaza, G. Formulation and in vitro evaluation of carbopol 934-based modified clotrimazole gel for topical application. An. Acad. Bras. Ciências 2016, 88, 2303–2317. [Google Scholar] [CrossRef]
- Menczel, J.D.; Prime, R.B. Thermal Analysis of Polymers: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–688. [Google Scholar]
- Menczel, J.D.; Judovits, L.; Prime, R.B.; Bair, H.E.; Reading, M.; Swier, S. Differential Scanning Calorimetry (DSC). In Thermal Analysis of Polymers: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 7–239. [Google Scholar]
- Yasin, H.; Al-Taani, B.; Salem, M. Preparation and characterization of ethylcellulose microspheres for sustained-release of pregabalin. Res. Pharm. Sci. 2020, 6, 1–15. [Google Scholar] [CrossRef]
- Mourtas, S.; Fotopoulou, S.; Duraj, S.; Sfika, V.; Tsakiroglou, C.; Antimisiaris, S.G. Liposomal drugs dispersed in hydrogels: Effect of liposome, drug and gel properties on drug release kinetics. Colloids Surf. B Biointerfaces 2007, 55, 212–221. [Google Scholar] [CrossRef]
- Haddad, N.; Hasian, J. Effect of combination of some Polymers with Carbopol 940 on Pregabalin Release Rate from Emulgels. Res. J. Pharm. Technol. 2022, 15, 2003–2009. [Google Scholar] [CrossRef]
- Kolman, M.; Smith, C.; Chakrabarty, D.; Amin, S. Rheological stability of carbomer in hydroalcoholic gels: Influence of alcohol type. Int. J. Cosmet. Sci. 2021, 43, 748–763. [Google Scholar] [CrossRef]
- Calienni, M.N.; Martínez, L.M.; Izquierdo, M.C.; Alonso, S.D.V.; Montanari, J. Rheological and Viscoelastic Analysis of Hybrid Formulations for Topical Application. Pharmaceutics 2023, 15, 2392. [Google Scholar] [CrossRef]
- Saez, V.; de Menezes, F.D.; dos Santos, C.C.; Alencar, L.M.R.; Ricci-Junior, E.; Mansur, C.R.E.; Santos-Oliveira, R. Graphene quantum dots nanoparticles changed the rheological properties of hydrophilic gels (carbopol). J. Mol. Liq. 2019, 287, 110949. [Google Scholar] [CrossRef]
- Nagam, S.P.; Naga Jyothi, A.; Poojitha, J.; Aruna, S.; Nadendla, R.R. A Comprehensive review on hydrogels. Int. J. Curr. Pharm. Rev. Res. 2016, 8, 19–23. [Google Scholar]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; da Silva Almeida, J.R.G. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef]
- Shen, X.; Chen, X.; He, Y.; Xu, H.; Zhu, J. Efficacy and safety of pregabalin in eye pain: A systematic review. Medicine 2023, 102, e32875. [Google Scholar] [CrossRef] [PubMed]
- Alles, S.R.; Cain, S.M.; Snutch, T.P. Pregabalin as a Pain Therapeutic: Beyond Calcium Channels. Front. Cell Neurosci. 2020, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rish, E.Y.; Mansour, A.T.; Mansour, H.T.; Dahabiyeh, L.A.; Aleidi, S.M.; Bustanji, Y. Pregabalin inhibits in vivo and in vitro cytokine secretion and attenuates spleen inflammation in Lipopolysaccharide/concanavalin A -induced murine models of inflammation. Sci. Rep. 2020, 10, 4007. [Google Scholar] [CrossRef]
- Mandroli, P.; Prabhakar, A.; Bhat, K.; Krishnamurthy, S.; Bogar, C. An in vitro evaluation of cytotoxicity of curcumin against human periodontal ligament fibroblasts. AYU 2019, 40, 192. [Google Scholar] [CrossRef]
- Pintor, A.V.B.; Queiroz, L.D.; Barcelos, R.; Primo, L.S.G.; Maia, L.C.; Alves, G.G. MTT versus other cell viability assays to evaluate the biocompatibility of root canal filling materials: A systematic review. Int. Endod. J. 2020, 53, 1348–1373. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.H.d.S.; Chemelo, G.P.; Alves, A.C.B.A.; Pinheiro, J.d.J.V.; Kataoka, M.S.d.S.; Silva, S.G.; de Oliveira, M.S.; de Aguiar Andrade, E.H.; Ribeiro, A.F.; de Alencar Menezes, T.O. Chemical Composition and Cytotoxicity Evaluation of Lippia origanoides Kunth (Verbenaceae) Leaves Essential Oil on Human Gingival Fibroblasts. J. Essent. Oil Bear. Plants 2021, 24, 704–713. [Google Scholar] [CrossRef]




| Characteristics | Formulation | |||
|---|---|---|---|---|
| WG | PG2G | PG5G | PG10G | |
| Aspect | Ho | Ho | Ho | Ho |
| Color | I | I | I | I |
| Odor | C | C | C | C |
| pH | 5.48 ± 0.10 | 6.07 ± 0.07 | 6.15 ± 0.09 | 6.33 ± 0.17 |
| Components | Quantity (%) | |||
|---|---|---|---|---|
| Gel W (Gel Base/White) | Gel PG2.0 | Gel PG5.0 | Gel PG10 | |
| Carbopol 940 | 1 | 1 | 1 | 1 |
| Methylparaben | 0.01 | 0.01 | 0.01 | 0.01 |
| Propylparaben | 0.05 | 0.05 | 0.05 | 0.05 |
| EDTA | 0.1 | 0.1 | 0.1 | 0.1 |
| Propylene glycol | 3 | 3 | 3 | 3 |
| Triethanolamine 50% (v/v) | qs | qs | qs | qs |
| Pregabalin | - | 2 | 5 | 10 |
| Distilled water | qsp 100 | qsp 100 | qsp 100 | qsp 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, G.M.B.; Ferreira, L.M.d.M.C.; Passos, M.F.; Rodrigues, A.P.D.; Franco, F.T.d.C.; Silva, C.M.; Silva Júnior, J.O.C.; Ribeiro-Costa, R.M.; Araújo, J.L.N. Characterization and Evaluation of the Cytotoxicity of Pregabalin Gels for Oral Application. Pharmaceuticals 2024, 17, 1168. https://doi.org/10.3390/ph17091168
Xavier GMB, Ferreira LMdMC, Passos MF, Rodrigues APD, Franco FTdC, Silva CM, Silva Júnior JOC, Ribeiro-Costa RM, Araújo JLN. Characterization and Evaluation of the Cytotoxicity of Pregabalin Gels for Oral Application. Pharmaceuticals. 2024; 17(9):1168. https://doi.org/10.3390/ph17091168
Chicago/Turabian StyleXavier, Gabriela Monteiro Barbosa, Lindalva Maria de Meneses Costa Ferreira, Marcele Fonseca Passos, Ana Paula Drummond Rodrigues, Felipe Tuji de Castro Franco, Cecy Martins Silva, José Otávio Carréra Silva Júnior, Roseane Maria Ribeiro-Costa, and Jesuína Lamartine Nogueira Araújo. 2024. "Characterization and Evaluation of the Cytotoxicity of Pregabalin Gels for Oral Application" Pharmaceuticals 17, no. 9: 1168. https://doi.org/10.3390/ph17091168
APA StyleXavier, G. M. B., Ferreira, L. M. d. M. C., Passos, M. F., Rodrigues, A. P. D., Franco, F. T. d. C., Silva, C. M., Silva Júnior, J. O. C., Ribeiro-Costa, R. M., & Araújo, J. L. N. (2024). Characterization and Evaluation of the Cytotoxicity of Pregabalin Gels for Oral Application. Pharmaceuticals, 17(9), 1168. https://doi.org/10.3390/ph17091168







