Mannitol-Coated Hydroxypropyl Methylcellulose as a Directly Compressible Controlled Release Excipient for Moisture-Sensitive Drugs: A Stability Perspective
Abstract
1. Introduction
2. Results
2.1. Particle Size Distribution of Neat and Co-Processed Excipients
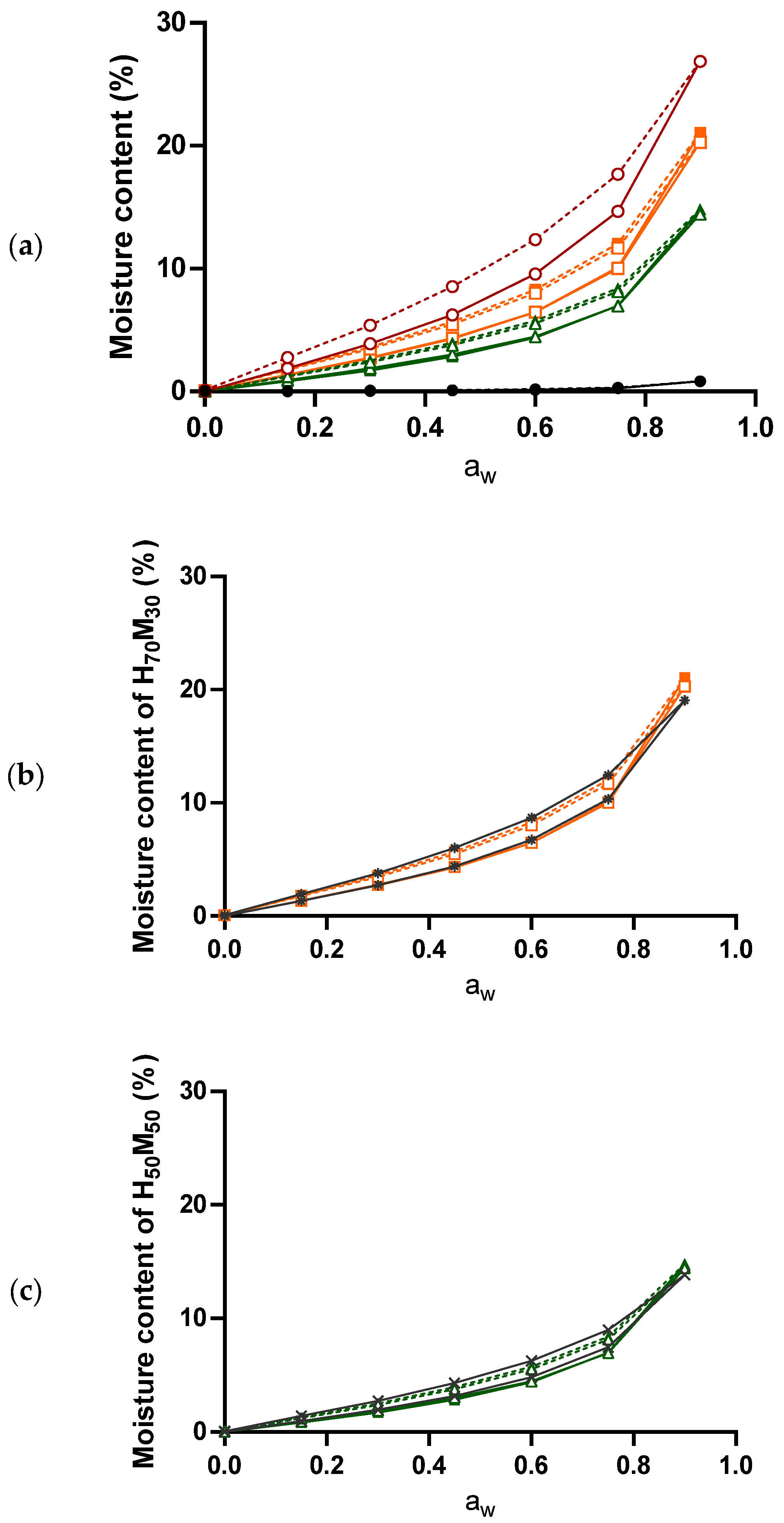
2.2. The Relationship between Moisture Content and Water Activity
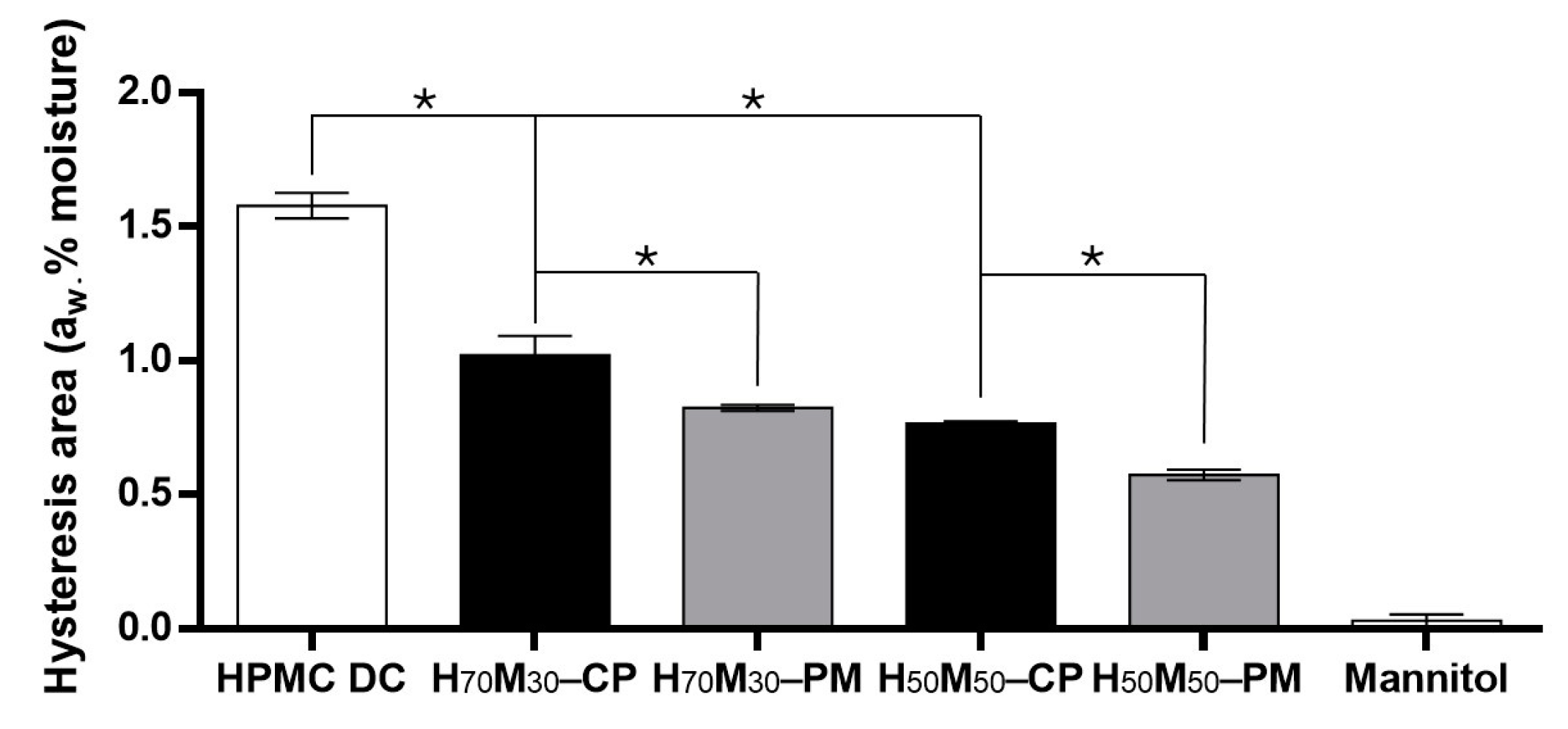
2.3. Moisture Sorption–Desorption Isotherm
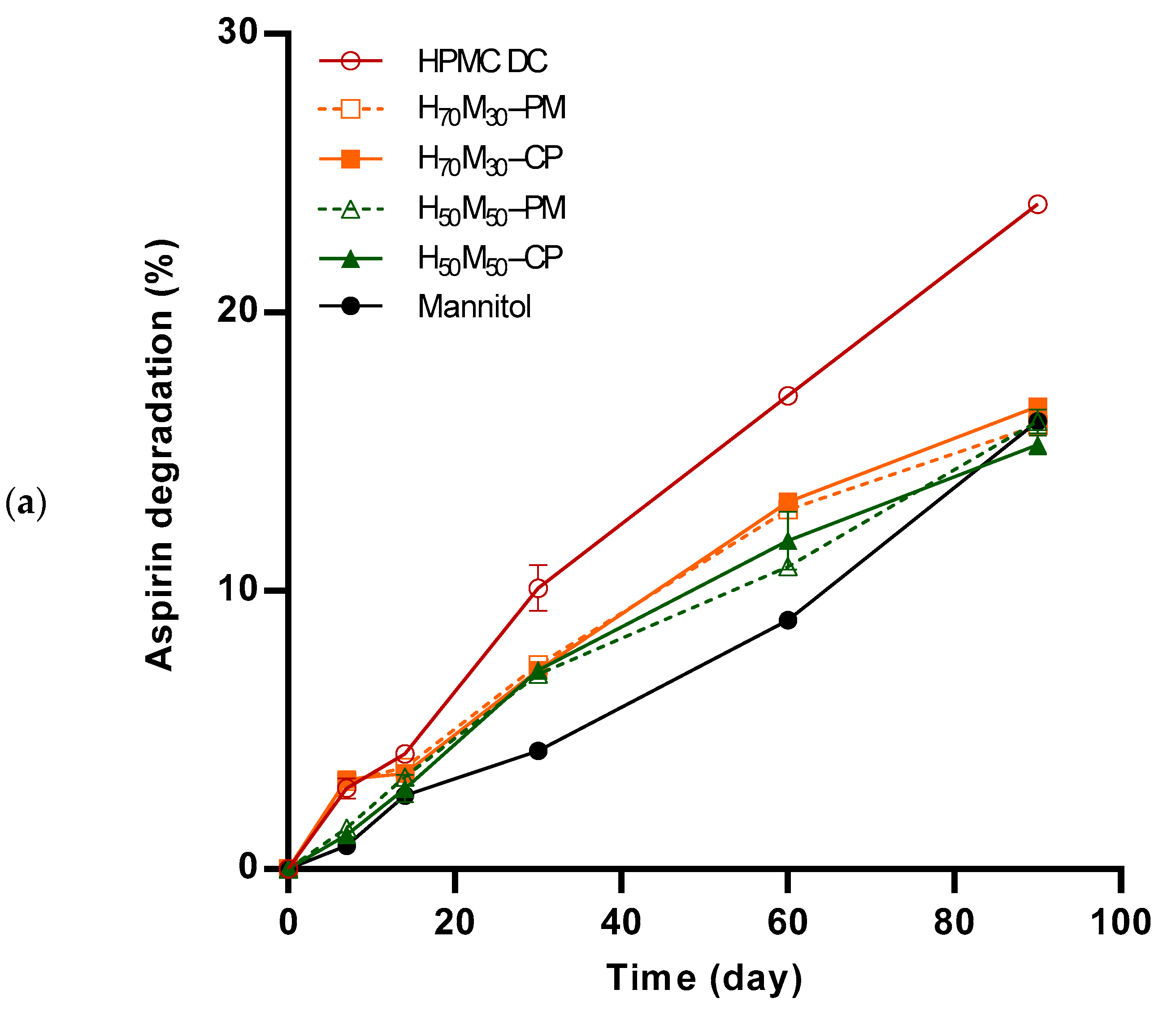
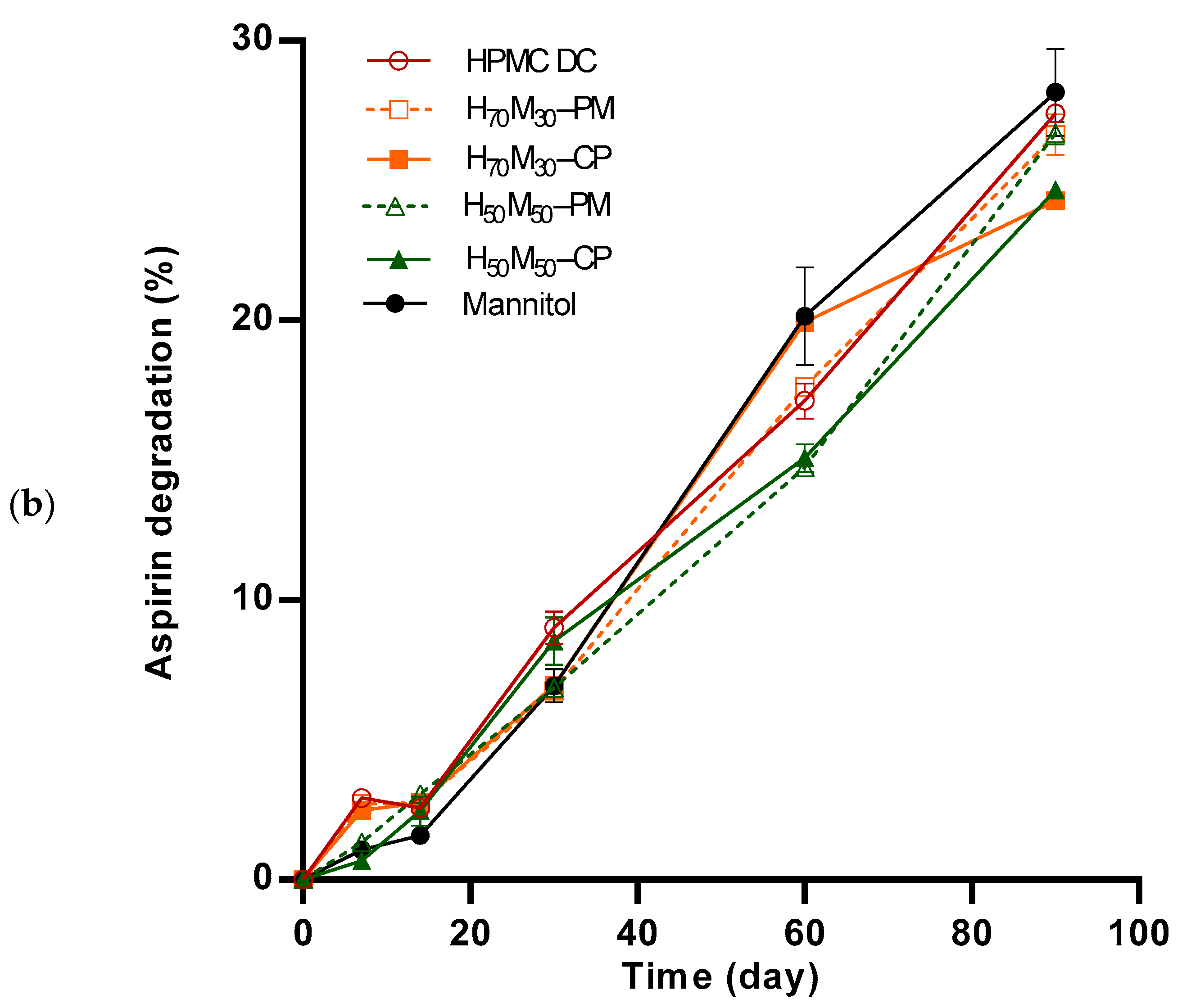
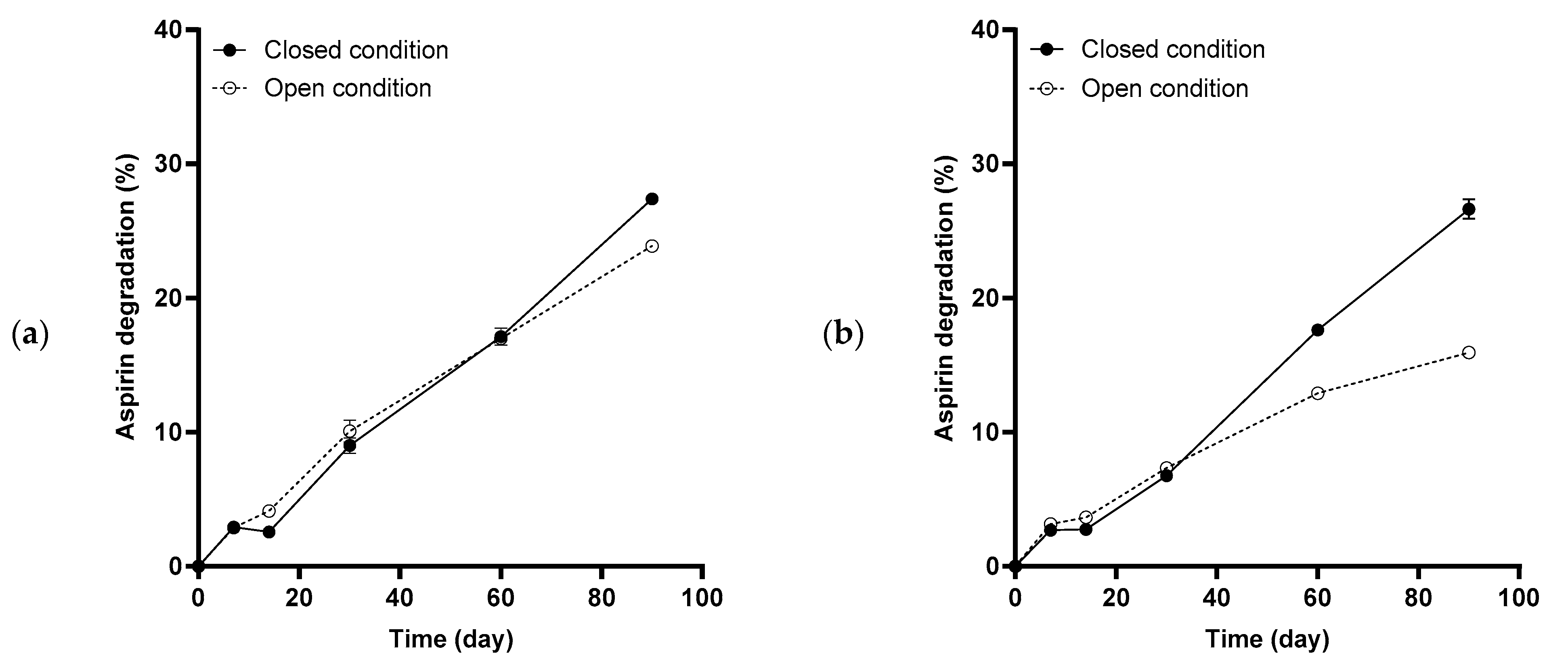
2.4. Aspirin Degradation under Accelerated Stability Conditions
2.5. Aspirin Degradation Rate
3. Discussion
3.1. Mannitol as a Moisture Protective Coating to Enhance Formulation Stability
3.2. Relationship between Water Activity and Drug Stability
3.3. Entrapment of Volatile Acidic Degradation By-Products Autocatalyzed Aspirin Degradation
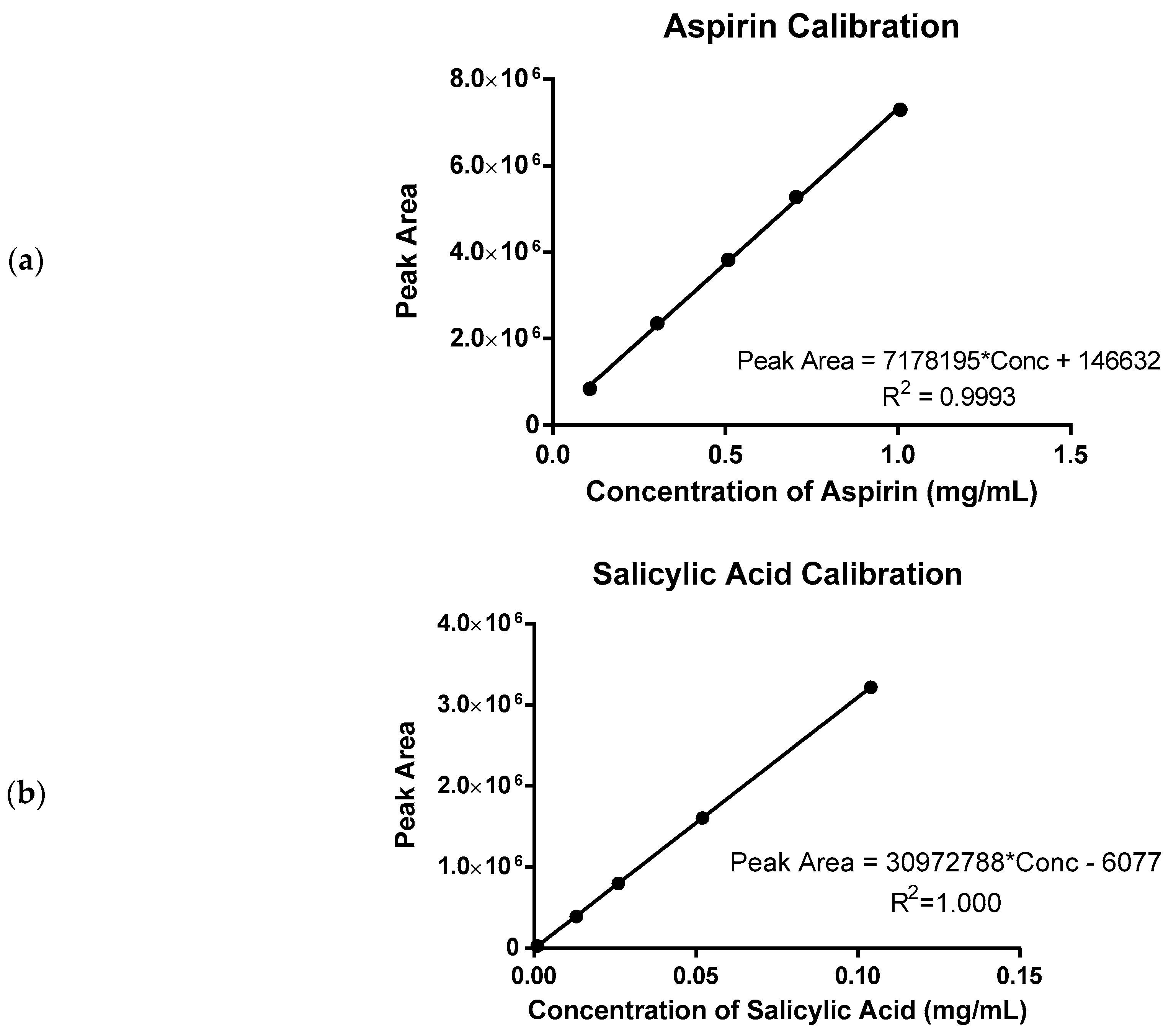
4. Materials and Methods
4.1. Materials
4.2. Particle Size Analysis
4.3. Determination of Moisture Content
4.4. Determination of Water Activity
4.5. Generation of Moisture Sorption Isotherm
4.6. Preparation of Tablets
4.7. Stability Studies
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maderuelo, C.; Zarzuelo, A.; Lanao, J.M. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control. Release 2011, 154, 2–19. [Google Scholar] [CrossRef]
- Sun, W.-J.; Chen, H.; Aburub, A.; Sun, C.C. A platform direct compression formulation for low dose sustained-release tablets enabled by a dual particle engineering approach. Powder Technol. 2019, 342, 856–863. [Google Scholar] [CrossRef]
- Košir, D.; Vrečer, F. The performance of HPMC matrix tablets using various agglomeration manufacturing processes. Drug Dev. Ind. Pharm. 2017, 43, 329–337. [Google Scholar] [CrossRef]
- Tajarobi, F.; Abrahmsén-Alami, S.; Hansen, M.; Larsson, A. The impact of dose and solubility of additives on the release from HPMC matrix tablets—Identifying critical conditions. Pharm. Res. 2009, 26, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Schaller, B.E.; Moroney, K.M.; Castro-Dominguez, B.; Cronin, P.; Belen-Girona, J.; Ruane, P.; Croker, D.M.; Walker, G.M. Systematic development of a high dosage formulation to enable direct compression of a poorly flowing API: A case study. Int. J. Pharm. 2019, 566, 615–630. [Google Scholar] [CrossRef]
- Van Snick, B.; Holman, J.; Cunningham, C.; Kumar, A.; Vercruysse, J.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous direct compression as manufacturing platform for sustained release tablets. Int. J. Pharm. 2017, 519, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, C.; Timmins, P.; Sharif, S.; Minko, T. Characterization of a novel hydroxypropyl methylcellulose (HPMC) direct compression grade excipient for pharmaceutical tablets. Int. J. Pharm. 2020, 583, 119343. [Google Scholar] [CrossRef]
- Moussa, E.; Siepmann, F.; Flament, M.; Benzine, Y.; Penz, F.; Siepmann, J.; Karrout, Y. Controlled release tablets based on HPMC:lactose blends. J. Drug Deliv. Sci. Technol. 2019, 52, 607–617. [Google Scholar] [CrossRef]
- Umprayn, K.; Mendes, R.W. Hygroscopicity and moisture adsorption kinetics of pharmaceutical solids: A review. Drug Dev. Ind. Pharm. 1987, 13, 653–693. [Google Scholar] [CrossRef]
- Zilker, M.; Sörgel, F.; Holzgrabe, U. A systematic review of the stability of finished pharmaceutical products and drug substances beyond their labeled expiry dates. J. Pharm. Biomed. Anal. 2019, 166, 222–235. [Google Scholar] [CrossRef]
- Waterman, K.C.; Adami, R.C. Accelerated aging: Prediction of chemical stability of pharmaceuticals. Int. J. Pharm. 2005, 293, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Waterman, K.C.; Adami, R.C.; Alsante, K.M.; Antipas, A.S.; Arenson, D.R.; Carrier, R.; Hong, J.; Landis, M.S.; Lombardo, F.; Shah, J.C. Hydrolysis in pharmaceutical formulations. Pharm. Dev. Technol. 2002, 7, 113–146. [Google Scholar] [CrossRef] [PubMed]
- ICHQ1A. Stability Testing of New Drug Substances and Products. Available online: https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf (accessed on 28 August 2024).
- Waterman, K.C.; MacDonald, B.C. Package selection for moisture protection for solid, oral drug products. J. Pharm. Sci. 2010, 99, 4437–4452. [Google Scholar] [CrossRef]
- Pearnchob, N.; Siepmann, J.; Bodmeier, R. Pharmaceutical applications of shellac: Moisture-protective and taste-masking coatings and extended-release matrix tablets. Drug Dev. Ind. Pharm. 2003, 29, 925–938. [Google Scholar] [CrossRef]
- Delwiche, S.R.; Pitt, R.E.; Norris, K.H. Examination of starch-water and cellulose-water interactions with near infrared (NIR) diffuse reflectance spectrospocy. Starch-Starke 1991, 43, 85–92. [Google Scholar] [CrossRef]
- Veronica, N.; Hiew, T.N.; Liew, C.V.; Heng, P.W.S. Insights into the moisture scavenging properties of different types of starch in tablets containing a moisture-sensitive drug. Mol. Pharm. 2020, 17, 4616–4628. [Google Scholar] [CrossRef]
- Ahlneck, C.; Zografi, G. The molecular basis of moisture effects on the physical and chemical stability of drugs in the solid state. Int. J. Pharm. 1990, 62, 87–95. [Google Scholar] [CrossRef]
- Li, L.; Sun, S.; Parumasivam, T.; Denman, J.A.; Gengenbach, T.; Tang, P.; Mao, S.; Chan, H.-K. l-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur. J. Pharm. Biopharm. 2016, 102, 132–141. [Google Scholar] [CrossRef]
- Zhou, Q.T.; Loh, Z.H.; Yu, J.; Sun, S.-p.; Gengenbach, T.; Denman, J.A.; Li, J.; Chan, H.-K. How much surface coating of hydrophobic azithromycin is sufficient to prevent moisture-induced decrease in aerosolisation of hygroscopic amorphous colistin powder? AAPS J. 2016, 18, 1213–1224. [Google Scholar] [CrossRef]
- Parmar, P.K.; Rao, S.G.; Bansal, A.K. Co-processing of small molecule excipients with polymers to improve functionality. Expert Opin. Drug Deliv. 2021, 18, 907–928. [Google Scholar] [CrossRef]
- Ohrem, H.L.; Schornick, E.; Kalivoda, A.; Ognibene, R. Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharm. Dev. Technol. 2014, 19, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Frigaard, N.-U. Sugar and sugar alcohol production in genetically modified cyanobacteria. In Genetically Engineered Foods; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 31–47. [Google Scholar]
- Wisselink, H.W.; Weusthuis, R.A.; Eggink, G.; Hugenholtz, J.; Grobben, G.J. Mannitol production by lactic acid bacteria: A review. Int. Dairy J. 2002, 12, 151–161. [Google Scholar] [CrossRef]
- Deis, R.C.; Kearsley, M.W. Sorbitol and mannitol. In Sweeteners and Sugar Alternatives in Food Technology; O’Donnell, K., Kearsley, M.W., Eds.; Wiley Online Books: New York, NY, USA, 2012; pp. 331–346. [Google Scholar]
- Lakio, S.; Tajarobi, P.; Wikström, H.; Fransson, M.; Arnehed, J.; Ervasti, T.; Simonaho, S.-P.; Ketolainen, J.; Folestad, S.; Abrahmsén-Alami, S. Achieving a robust drug release from extended release tablets using an integrated continuous mixing and direct compression line. Int. J. Pharm. 2016, 511, 659–668. [Google Scholar] [CrossRef]
- Katsuno, E.; Tahara, K.; Takeuchi, Y.; Takeuchi, H. Orally disintegrating tablets prepared by a co-processed mixture of micronized crospovidone and mannitol using a ball mill to improve compactibility and tablet stability. Powder Technol. 2013, 241, 60–66. [Google Scholar] [CrossRef]
- Higuchi, M.; Tanaka, S.; Tamura, K.; Sakata, Y. Hygroscopicity of a sugarless coating layer formed by the interaction between mannitol and poly (vinyl alcohol)(PVA). Colloids Surf. B Biointerfaces 2014, 123, 557–565. [Google Scholar] [CrossRef]
- Kang, C.Y.X.; Foo, W.C.; Lam, K.H.; Chow, K.T.; Lui, Y.S.; Goh, H.P.; Salome, A.; Boit, B.; Lefevre, P.; Hiew, T.N.; et al. Mannitol-coated hydroxypropyl methylcellulose as an alternative directly compressible controlled release excipient. Int. J. Pharm. 2024, 660, 124298. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Costantino, H.R.; Curley, J.G.; Wu, S.; Hsu, C.C. Water sorption behavior of lyophilized protein–sugar systems and implications for solid-state interactions. Int. J. Pharm. 1998, 166, 211–221. [Google Scholar] [CrossRef]
- Crouter, A.; Briens, L. The effect of moisture on the flowability of pharmaceutical excipients. AAPS PharmSciTech 2014, 15, 65–74. [Google Scholar] [CrossRef]
- Zhang, J.; Zografi, G. Water vapor absorption into amorphous sucrose-poly(vinyl pyrrolidone) and trehalose–poly (vinyl pyrrolidone) mixtures. J. Pharm. Sci. 2001, 90, 1375–1385. [Google Scholar] [CrossRef]
- Veronica, N.; Heng, P.W.S.; Liew, C.V. Understanding the roles of excipients in moisture management in solid dosage forms. Mol. Pharm. 2024, 21, 2484–2500. [Google Scholar] [CrossRef]
- Leeson, L.J.; Mattocks, A.M. Decomposition of aspirin in the solid state. J. Am. Pharm. Assoc. 1958, 47, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.W.; Reutzel-Edens, S.M.; Zografi, G. Characterization of the “hygroscopic” properties of active pharmaceutical ingredients. J. Pharm. Sci. 2008, 97, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.K.; Patel, I.J.; Cutie, A.J.; Wadke, D.A.; Monkhouse, D.C.; Reier, G.E. The effect of selected direct compression excipients on the stability of aspirin as a model hydrolyzable drug. Drug Dev. Ind. Pharm. 1988, 14, 77–98. [Google Scholar] [CrossRef]
- Jeromenok, J.; Weber, J. Restricted access: On the nature of adsorption/desorption hysteresis in amorphous, microporous polymeric materials. Langmuir 2013, 29, 12982–12989. [Google Scholar] [CrossRef]
- Szakonyi, G.; Zelkó, R. The effect of water on the solid state characteristics of pharmaceutical excipients: Molecular mechanisms, measurement techniques, and quality aspects of final dosage form. Int. J. Pharm. Investig. 2012, 2, 18–25. [Google Scholar]
- Tarlier, N.; Soulairol, I.; Sanchez-Ballester, N.; Baylac, G.; Aubert, A.; Lefevre, P.; Bataille, B.; Sharkawi, T. Deformation behavior of crystallized mannitol during compression using a rotary tablet press simulator. Int. J. Pharm. 2018, 547, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Rajabi-Siahboomi, A.R.; Levina, M.; Upadhye, S.B.; Teckoe, J. Excipient selection in oral solid dosage formulations containing moisture sensitive drugs. In Excipient Applications in Formulation Design and Drug Delivery; Narang, A.S., Boddu, S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 385–421. [Google Scholar]
- Snider, B.; Liang, P.; Pearson, N. Implementation of water-activity testing to replace Karl Fischer water testing. Pharm. Technol. 2007, 31, 56. [Google Scholar]
- Hiew, T.N. Preformulation Study of Pharmaceutical Excipients: A Water Activity Perspective. Ph.D. Thesis, National University of Singapore, Singapore, 2017. [Google Scholar]
- Hiew, T.N.; Huang, R.; Popov, I.; Feldman, Y.; Heng, P.W.S. A study of moisture sorption and dielectric processes of starch and sodium starch glycolate. Pharm. Res. 2017, 34, 2675–2688. [Google Scholar] [CrossRef]
- Ougi, K.; Okada, K.; Leong, K.H.; Hayashi, Y.; Kumada, S.; Onuki, Y. Effect of the molecular mobility of water adsorbed by disintegrants on storage-induced hydrolytic degradation of acetylsalicylic acid incorporated into tablets under humid conditions. Eur. J. Pharm. Sci. 2020, 154, 105502. [Google Scholar] [CrossRef]
- Bell, L.N.; Labuza, T.P. Aspartame degradation kinetics as affected by pH in intermediate and low moisture food systems. J. Food Sci. 1991, 56, 17–20. [Google Scholar] [CrossRef]
- Heidemann, D.R.; Jarosz, P.J. Preformulation studies involving moisture uptake in solid dosage forms. Pharm. Res. 1991, 8, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Kestur, U.; Desai, D.; Zong, Z.; Abraham, A.; Fiske, J. Effect of coating excipients on chemical stability of active coated tablets. Pharm. Dev. Technol. 2021, 26, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, J.T.; Attarchi, F. Decomposition of aspirin in the solid state in the presence of limited amounts of moisture III: Effect of temperature and a possible mechanism. J. Pharm. Sci. 1988, 77, 318–321. [Google Scholar] [CrossRef]
- Edwards, L.J. The hydrolysis of aspirin. Part 2. Trans. Faraday Soc. 1952, 48, 696–699. [Google Scholar] [CrossRef]
- Mwesigwa, E.; Basit, A.W. An investigation into moisture barrier film coating efficacy and its relevance to drug stability in solid dosage forms. Int. J. Pharm. 2016, 497, 70–77. [Google Scholar] [CrossRef]
- Siegel, S.; Reiner, R.H.; Zelinskie, J.A.; Hanus, E.J. Tablets of pyrilamine resin adsorbate with aspirin and vitamin C. J. Pharm. Sci. 1962, 51, 1068–1071. [Google Scholar] [CrossRef]
- Carstensen, J.; Attarchi, F.; Hou, X.-P. Decomposition of aspirin in the solid state in the presence of limited amounts of moisture. J. Pharm. Sci. 1985, 74, 741–745. [Google Scholar] [CrossRef]
- Ouyang, H.; Ang, S.J.; Lee, Z.Y.; Hiew, T.N.; Heng, P.W.S.; Chan, L.W. Effect of drug load and lipid–wax blends on drug release and stability from spray-congealed microparticles. Pharm. Dev. Technol. 2022, 27, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Veronica, N.; Liew, C.V.; Heng, P.W.S. Insights on the role of excipients and tablet matrix porosity on aspirin stability. Int. J. Pharm. 2020, 580, 119218. [Google Scholar] [CrossRef] [PubMed]



| Excipient | Storage Condition | |||
|---|---|---|---|---|
| Open | Closed | |||
| Degradation Rate (×10−3 day−1) | R2 | Degradation Rate (×10−3 day−1) | R2 | |
| HPMC DC | 6.36 | 0.99 | 5.58 | 0.97 |
| H70M30–PM | 5.22 | 0.95 | 4.72 | 0.97 |
| H70M30–CP | 5.10 | 0.95 | 4.76 | 0.96 |
| H50M50–PM | 4.79 | 1.00 | 4.65 | 1.00 |
| H50M50–CP | 4.70 | 1.00 | 5.08 | 0.98 |
| Mannitol | 3.33 | 0.98 | 4.26 | 0.98 |
| Degradation Rate | Mannitol (%, w/w) | Moisture Content (%) | Sorption AUC | Desorption AUC | Hysteresis Area | aw |
|---|---|---|---|---|---|---|
| Open conditions | −0.872 a | 0.981 c | 0.990 c | 0.991 c | 0.973 b | −0.837 a |
| Closed conditions | −0.943 a | 0.892 a | 0.826 a | 0.844 a | 0.910 a | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, C.Y.X.; Chow, K.T.; Lui, Y.S.; Salome, A.; Boit, B.; Lefevre, P.; Hiew, T.N.; Gokhale, R.; Heng, P.W.S. Mannitol-Coated Hydroxypropyl Methylcellulose as a Directly Compressible Controlled Release Excipient for Moisture-Sensitive Drugs: A Stability Perspective. Pharmaceuticals 2024, 17, 1167. https://doi.org/10.3390/ph17091167
Kang CYX, Chow KT, Lui YS, Salome A, Boit B, Lefevre P, Hiew TN, Gokhale R, Heng PWS. Mannitol-Coated Hydroxypropyl Methylcellulose as a Directly Compressible Controlled Release Excipient for Moisture-Sensitive Drugs: A Stability Perspective. Pharmaceuticals. 2024; 17(9):1167. https://doi.org/10.3390/ph17091167
Chicago/Turabian StyleKang, Christina Yong Xin, Keat Theng Chow, Yuan Siang Lui, Antoine Salome, Baptiste Boit, Philippe Lefevre, Tze Ning Hiew, Rajeev Gokhale, and Paul Wan Sia Heng. 2024. "Mannitol-Coated Hydroxypropyl Methylcellulose as a Directly Compressible Controlled Release Excipient for Moisture-Sensitive Drugs: A Stability Perspective" Pharmaceuticals 17, no. 9: 1167. https://doi.org/10.3390/ph17091167
APA StyleKang, C. Y. X., Chow, K. T., Lui, Y. S., Salome, A., Boit, B., Lefevre, P., Hiew, T. N., Gokhale, R., & Heng, P. W. S. (2024). Mannitol-Coated Hydroxypropyl Methylcellulose as a Directly Compressible Controlled Release Excipient for Moisture-Sensitive Drugs: A Stability Perspective. Pharmaceuticals, 17(9), 1167. https://doi.org/10.3390/ph17091167








