Sustained Long-Term Decline in Anti-HCV Neutralizing Antibodies in HIV/HCV-Coinfected Patients Five Years after HCV Therapy: A Retrospective Study
Abstract
1. Introduction
2. Objective
3. Results
3.1. Patient Characteristics
3.2. Anti-HCV Antibody Titers
3.3. Anti-HCV Nonresponse Rate
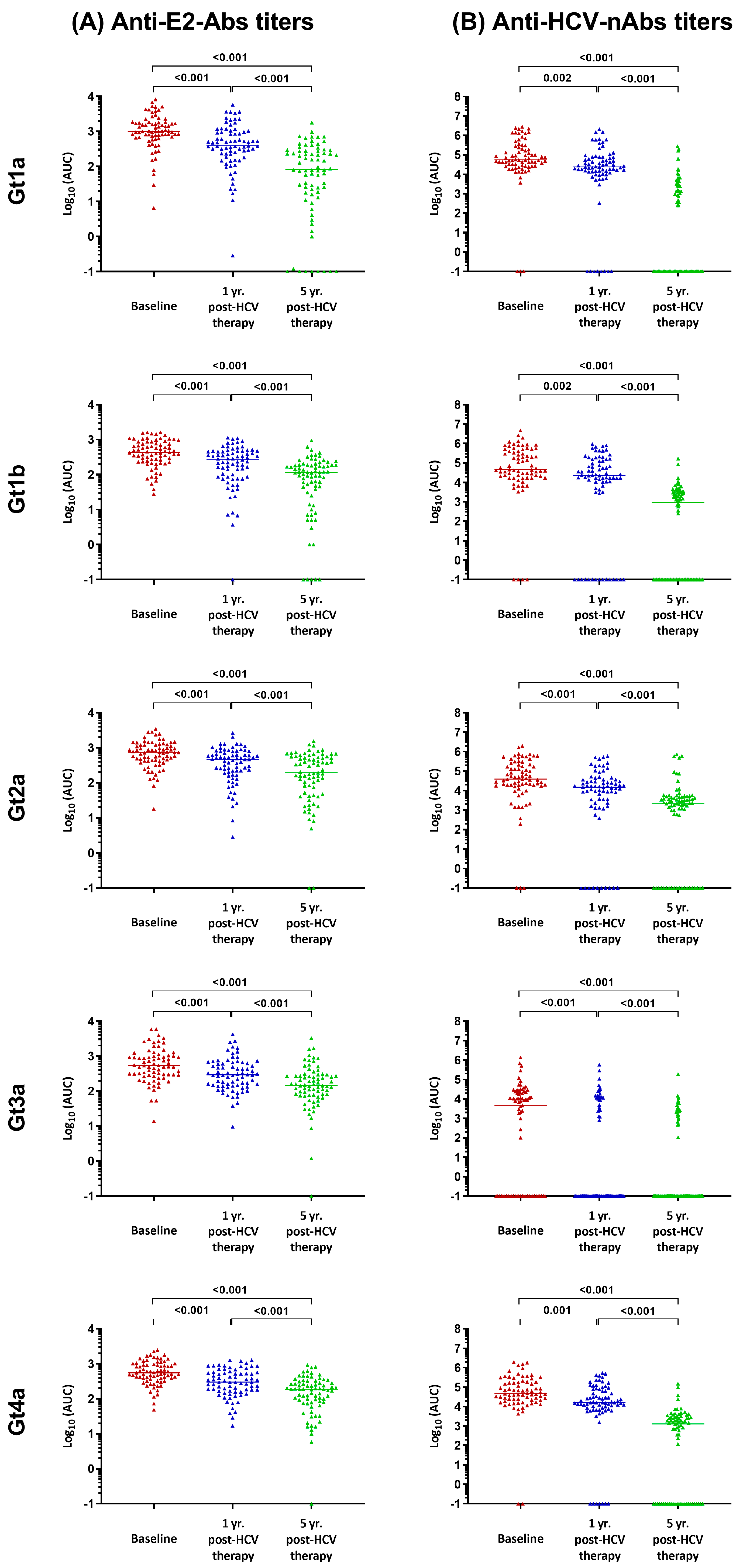
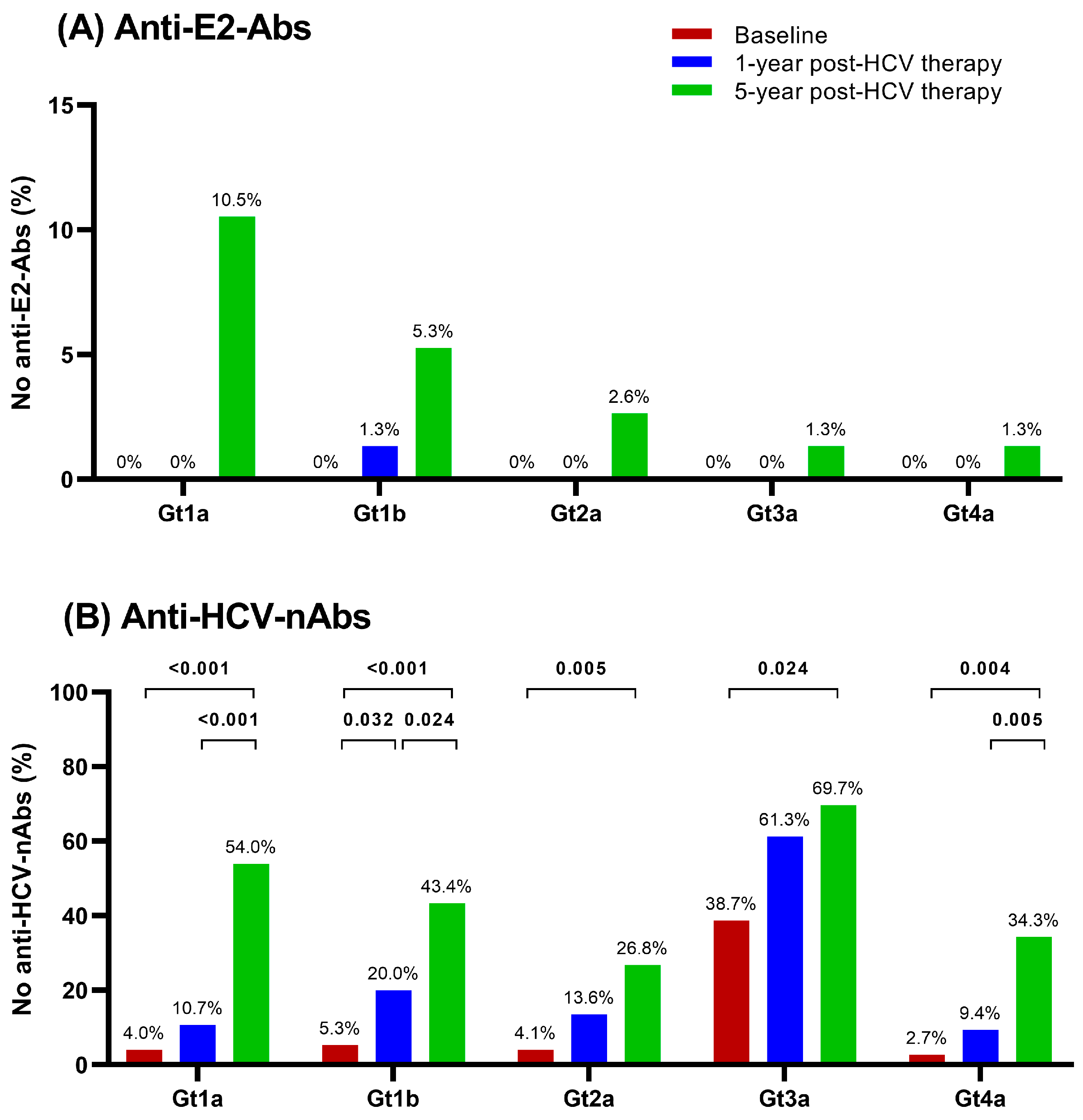
4. Discussion
Limitations of This Study
5. Materials and Methods
5.1. Design and Patients
5.2. Clinical Data and Samples
5.3. Laboratory Assays
5.3.1. Cell Culture
5.3.2. Cloning, Expression, and Purification of E2 Glycoproteins
5.3.3. Chimeric Viruses
5.3.4. Quantitative ELISA Antibody Titration and HCV Neutralization Assays
5.4. Outcomes
5.5. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| AMR | arithmetic mean ratio |
| Anti-E2-Abs | anti-E2 antibodies |
| Anti-HCV-nAbs | neutralizing antibodies against hepatitis C virus |
| cART | combination antiretroviral therapy |
| DAA | direct-acting antiviral |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ELISA | enzyme-linked immunosorbent assay |
| FBS | fetal bovine serum |
| FDR | false discovery rate |
| GLMM | generalized linear mixed model |
| Gt | genotype |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| HCVcc | cell-culture-derived infectious HCV |
| HIV | human immunodeficiency virus |
| IFN | interferon |
| IQR | interquartile range |
| LSM | liver stiffness measurement |
| OR | odds ratio |
| PEG-IFNα | pegylated interferon alpha |
| RBV | ribavirin |
| RNA | ribonucleic acid |
| SVR | sustained virologic response |
| WHO | World Health Organization |
Appendix A
References
- Polaris Observatory, H.C.V.C. Global change in hepatitis c virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Dengue—Global Situation. 2024. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON518 (accessed on 10 July 2024).
- Martinello, M.; Solomon, S.S.; Terrault, N.A.; Dore, G.J. Hepatitis c. Lancet 2023, 402, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Available online: https://www.who.int/publications/i/item/9789241550345 (accessed on 21 August 2024).
- Falade-Nwulia, O.; Sulkowski, M.S.; Merkow, A.; Latkin, C.; Mehta, S.H. Understanding and addressing hepatitis c reinfection in the oral direct-acting antiviral era. J. Viral Hepat. 2018, 25, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Rockstroh, J.K.; Boesecke, C. Hepatitis c virus treatment as prevention: Challenges and opportunities in men who have sex with men. J. Infect. Dis. 2020, 222, S782–S788. [Google Scholar] [CrossRef]
- Thompson, K.A.; Blank, G.; Toy, J.; Moore, D.M.; Lachowsky, N.; Bacani, N.; Zhang, W.; Sereda, P.; Lima, V.D.; Barrios, R.; et al. Prevalence and incidence of hepatitis c infection amongst men who have sex with men in a population-based pre-exposure prophylaxis program in British Columbia, Canada. Liver Int. 2022, 42, 1528–1535. [Google Scholar] [CrossRef]
- Berenguer, J.; Gil-Martin, A.; Jarrin, I.; Montes, M.L.; Dominguez, L.; Aldamiz-Echevarria, T.; Tellez, M.J.; Santos, I.; Troya, J.; Losa, J.E.; et al. Reinfection by hepatitis c virus following effective all-oral direct-acting antiviral drug therapy in HIV/hepatitis c virus coinfected individuals. AIDS 2019, 33, 685–689. [Google Scholar] [CrossRef]
- Osburn, W.O.; Fisher, B.E.; Dowd, K.A.; Urban, G.; Liu, L.; Ray, S.C.; Thomas, D.L.; Cox, A.L. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 2010, 138, 315–324. [Google Scholar] [CrossRef]
- Osburn, W.O.; Snider, A.E.; Wells, B.L.; Latanich, R.; Bailey, J.R.; Thomas, D.L.; Cox, A.L.; Ray, S.C. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 2014, 59, 2140–2151. [Google Scholar] [CrossRef]
- Mazouz, S.; Salinas, E.; Bedard, N.; Filali, A.; Khedr, O.; Swadling, L.; Abdel-Hakeem, M.S.; Siddique, A.; Barnes, E.; Bruneau, J.; et al. Differential immune transcriptomic profiles between vaccinated and resolved HCV reinfected subjects. PLoS Pathog. 2022, 18, e1010968. [Google Scholar] [CrossRef]
- Sepulveda-Crespo, D.; Yelamos, M.B.; Diez, C.; Gomez, J.; Hontanon, V.; Torresano-Felipe, F.; Berenguer, J.; Gonzalez-Garcia, J.; Ibanez-Samaniego, L.; Llop, E.; et al. Negative impact of HIV infection on broad-spectrum anti-HCV neutralizing antibody titers in HCV-infected patients with advanced HCV-related cirrhosis. Biomed. Pharmacother. 2022, 150, 113024. [Google Scholar] [CrossRef]
- Vigon, L.; Vazquez-Moron, S.; Berenguer, J.; Gonzalez-Garcia, J.; Jimenez-Sousa, M.A.; Guardiola, J.M.; Crespo, M.; de Los Santos, I.; Von Wichmann, M.A.; Carrero, A.; et al. Rapid decrease in titer and breadth of neutralizing anti-HCV antibodies in HIV/HCV-coinfected patients who achieved SVR. Sci. Rep. 2019, 9, 12163. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Crespo, D.; Resino, S.; Martinez, I. Hepatitis C virus vaccine design: Focus on the humoral immune response. J. Biomed. Sci. 2020, 27, 78. [Google Scholar] [CrossRef]
- Shah, R.; Ahovegbe, L.; Niebel, M.; Shepherd, J.; Thomson, E.C. Non-epidemic HCV genotypes in low- and middle-income countries and the risk of resistance to current direct-acting antiviral regimens. J. Hepatol. 2021, 75, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Yechezkel, I.; Law, M.; Tzarum, N. From structural studies to HCV vaccine design. Viruses 2021, 13, 833. [Google Scholar] [CrossRef] [PubMed]
- Stroh, L.J.; Krey, T. HCV Glycoprotein Structure and Implications for b-cell vaccine development. Int. J. Mol. Sci. 2020, 21, 6781. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Lavillette, D.; Li, Q.; Zhong, J. Role of hepatitis c virus envelope glycoprotein e1 in virus entry and assembly. Front. Immunol. 2018, 9, 1411. [Google Scholar] [CrossRef]
- McCaffrey, K.; Boo, I.; Owczarek, C.M.; Hardy, M.P.; Perugini, M.A.; Fabri, L.; Scotney, P.; Poumbourios, P.; Drummer, H.E. An optimized hepatitis c virus e2 glycoprotein core adopts a functional homodimer that efficiently blocks virus entry. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef]
- Kinchen, V.J.; Zahid, M.N.; Flyak, A.I.; Soliman, M.G.; Learn, G.H.; Wang, S.; Davidson, E.; Doranz, B.J.; Ray, S.C.; Cox, A.L.; et al. Broadly neutralizing antibody mediated clearance of human hepatitis c virus infection. Cell Host Microbe 2018, 24, 717–730.e715. [Google Scholar] [CrossRef]
- Bankwitz, D.; Bahai, A.; Labuhn, M.; Doepke, M.; Ginkel, C.; Khera, T.; Todt, D.; Stroh, L.J.; Dold, L.; Klein, F.; et al. Hepatitis C reference viruses highlight potent antibody responses and diverse viral functional interactions with neutralising antibodies. Gut 2021, 70, 1734–1745. [Google Scholar] [CrossRef]
- Dowd, K.A.; Netski, D.M.; Wang, X.H.; Cox, A.L.; Ray, S.C. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology 2009, 136, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Del Moral-Sanchez, I.; Sliepen, K. Strategies for inducing effective neutralizing antibody responses against HIV-1. Expert Rev. Vaccines 2019, 18, 1127–1143. [Google Scholar] [CrossRef]
- Krammer, F. Strategies to induce broadly protective antibody responses to viral glycoproteins. Expert Rev. Vaccines 2017, 16, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Lee, D.E.; Kadam, R.U.; Liu, T.; Giang, E.; Nieusma, T.; Garces, F.; Tzarum, N.; Woods, V.L., Jr.; Ward, A.B.; et al. Structural flexibility at a major conserved antibody target on hepatitis C virus E2 antigen. Proc. Natl. Acad. Sci. USA 2016, 113, 12768–12773. [Google Scholar] [CrossRef] [PubMed]
- Scheel, T.K.; Gottwein, J.M.; Jensen, T.B.; Prentoe, J.C.; Hoegh, A.M.; Alter, H.J.; Eugen-Olsen, J.; Bukh, J. Development of JFH1-based cell culture systems for hepatitis C virus genotype 4a and evidence for cross-genotype neutralization. Proc. Natl. Acad. Sci. USA 2008, 105, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.R.; Flyak, A.I.; Cohen, V.J.; Li, H.; Wasilewski, L.N.; Snider, A.E.; Wang, S.; Learn, G.H.; Kose, N.; Loerinc, L.; et al. Broadly neutralizing antibodies with few somatic mutations and hepatitis C virus clearance. JCI Insight 2017, 2, e92872. [Google Scholar] [CrossRef]
- Salas, J.H.; Urbanowicz, R.A.; Guest, J.D.; Frumento, N.; Figueroa, A.; Clark, K.E.; Keck, Z.; Cowton, V.M.; Cole, S.J.; Patel, A.H.; et al. An antigenically diverse, representative panel of envelope glycoproteins for hepatitis c virus vaccine development. Gastroenterology 2022, 162, 562–574. [Google Scholar] [CrossRef]
- Kinchen, V.J.; Bailey, J.R. Defining breadth of hepatitis c virus neutralization. Front. Immunol. 2018, 9, 1703. [Google Scholar] [CrossRef]
- Blanco, J.R.; Rivero-Juarez, A. HCV genotype 3: A wolf in sheep’s clothing. Expert Rev. Anti Infect. Ther. 2016, 14, 149–152. [Google Scholar] [CrossRef][Green Version]
- Buti, M.; Esteban, R. Hepatitis C virus genotype 3: A genotype that is not ‘easy-to-treat’. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Goossens, N.; Negro, F. Is genotype 3 of the hepatitis C virus the new villain? Hepatology 2014, 59, 2403–2412. [Google Scholar] [CrossRef]
- Chan, A.; Patel, K.; Naggie, S. Genotype 3 infection: The last stand of hepatitis c virus. Drugs 2017, 77, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Beq, S.; Rozlan, S.; Pelletier, S.; Willems, B.; Bruneau, J.; Lelievre, J.D.; Levy, Y.; Shoukry, N.H.; Cheynier, R. Altered thymic function during interferon therapy in HCV-infected patients. PLoS ONE 2012, 7, e34326. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Yu, M.L. Evolution of interferon-based therapy for chronic hepatitis C. Hepat. Res. Treat. 2010, 2010, 140953. [Google Scholar] [CrossRef]
- Maughan, A.; Ogbuagu, O. Pegylated interferon alpha 2a for the treatment of hepatitis C virus infection. Expert Opin. Drug Metab. Toxicol. 2018, 14, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, A.; Zagozdzon, R.; Ciszek, M. Modulation of the immune system in chronic hepatitis c and during antiviral interferon-free therapy. Arch. Immunol. Ther. Exp. 2019, 67, 79–88. [Google Scholar] [CrossRef]
- Vanhommerig, J.W.; Thomas, X.V.; van der Meer, J.T.; Geskus, R.B.; Bruisten, S.M.; Molenkamp, R.; Prins, M.; Schinkel, J.; Group, M.S. Hepatitis C virus (HCV) antibody dynamics following acute HCV infection and reinfection among HIV-infected men who have sex with men. Clin. Infect. Dis. 2014, 59, 1678–1685. [Google Scholar] [CrossRef]
- Aebi-Popp, K.; Wandeler, G.; Salazar-Vizcaya, L.; Metzner, K.; Stockle, M.; Cavassini, M.; Hoffmann, M.; Luthi, A.; Suter, F.; Bernasconi, E.; et al. Rapid decline of anti-hepatitis C virus (HCV) antibodies following early treatment of incident HCV infections in HIV-infected men who have sex with men. HIV Med. 2018, 19, 420–425. [Google Scholar] [CrossRef]
- Wiegand, J.; Jackel, E.; Cornberg, M.; Hinrichsen, H.; Dietrich, M.; Kroeger, J.; Fritsch, W.P.; Kubitschke, A.; Aslan, N.; Tillmann, H.L.; et al. Long-term follow-up after successful interferon therapy of acute hepatitis C. Hepatology 2004, 40, 98–107. [Google Scholar] [CrossRef]
- Lo Re, V., 3rd; Kallan, M.J.; Tate, J.P.; Localio, A.R.; Lim, J.K.; Goetz, M.B.; Klein, M.B.; Rimland, D.; Rodriguez-Barradas, M.C.; Butt, A.A.; et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: A cohort study. Ann. Intern. Med. 2014, 160, 369–379. [Google Scholar] [CrossRef]
- Dong, Y.; Zhi, X.; Lei, G. Changes of body immunity and inflammatory response in HIV/HCV co-infected patients. Exp. Ther. Med. 2019, 17, 403–407. [Google Scholar] [CrossRef]
- Xu, W.; Santini, P.A.; Sullivan, J.S.; He, B.; Shan, M.; Ball, S.C.; Dyer, W.B.; Ketas, T.J.; Chadburn, A.; Cohen-Gould, L.; et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat. Immunol. 2009, 10, 1008–1017. [Google Scholar] [CrossRef]
- West, A.P., Jr.; Scharf, L.; Scheid, J.F.; Klein, F.; Bjorkman, P.J.; Nussenzweig, M.C. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell 2014, 156, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Titanji, K.; De Milito, A.; Cagigi, A.; Thorstensson, R.; Grutzmeier, S.; Atlas, A.; Hejdeman, B.; Kroon, F.P.; Lopalco, L.; Nilsson, A.; et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 2006, 108, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.; Steel, A.; Clark, S.A.; Moyle, G.; Nelson, M.; Henderson, D.C.; Wilson, R.; Gotch, F.; Gazzard, B.; Kelleher, P. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J. Immunol. 2007, 178, 8212–8220. [Google Scholar] [CrossRef] [PubMed]
- Dorner, M.; Horwitz, J.A.; Robbins, J.B.; Barry, W.T.; Feng, Q.; Mu, K.; Jones, C.T.; Schoggins, J.W.; Catanese, M.T.; Burton, D.R.; et al. A genetically humanized mouse model for hepatitis C virus infection. Nature 2011, 474, 208–211. [Google Scholar] [CrossRef]
- Bukh, J.; Engle, R.E.; Faulk, K.; Wang, R.Y.; Farci, P.; Alter, H.J.; Purcell, R.H. Immunoglobulin with high-titer in vitro cross-neutralizing hepatitis c virus antibodies passively protects chimpanzees from homologous, but not heterologous, challenge. J. Virol. 2015, 89, 9128–9132. [Google Scholar] [CrossRef] [PubMed]
- Medrano, L.M.; Garcia-Broncano, P.; Berenguer, J.; Gonzalez-Garcia, J.; Jimenez-Sousa, M.A.; Guardiola, J.M.; Crespo, M.; Quereda, C.; Sanz, J.; Canorea, I.; et al. Elevated liver stiffness is linked to increased biomarkers of inflammation and immune activation in HIV/hepatitis C virus-coinfected patients. Aids 2018, 32, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Vetter, D.; Friedman, S.L. Chapter 7—Liver fibrogenesis: Mechanisms and clinical relevance. In Blumgart’s Surgery of the Liver, Biliary Tract and Pancreas, 2-Volume Set, 6th ed.; Jarnagin, W.R., Ed.; Elsevier: Philadelphia, PA, USA, 2017; pp. 110–122.e115. [Google Scholar]
- Berenguer, J.; Rodriguez-Castellano, E.; Carrero, A.; Von Wichmann, M.A.; Montero, M.; Galindo, M.J.; Mallolas, J.; Crespo, M.; Tellez, M.J.; Quereda, C.; et al. Eradication of hepatitis C virus and non-liver-related non-acquired immune deficiency syndrome-related events in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology 2017, 66, 344–356. [Google Scholar] [CrossRef]
- Diez, C.; Berenguer, J.; Ibanez-Samaniego, L.; Llop, E.; Perez-Latorre, L.; Catalina, M.V.; Hontanon, V.; Jimenez-Sousa, M.A.; Aldamiz-Echevarria, T.; Martinez, J.; et al. Persistence of clinically significant portal hypertension after eradication of hepatitis c virus in patients with advanced cirrhosis. Clin. Infect. Dis. 2020, 71, 2726–2729. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, M.; Tello, D.; Yelamos, B.; Gomez-Gutierrez, J.; Pacheco, B.; Ortega, S.; Serrano, A.G.; Peterson, D.L.; Gavilanes, F. Structural properties of the ectodomain of hepatitis C virus E2 envelope protein. Virus Res. 2009, 139, 91–99. [Google Scholar] [CrossRef][Green Version]
- Wakita, T.; Pietschmann, T.; Kato, T.; Date, T.; Miyamoto, M.; Zhao, Z.; Murthy, K.; Habermann, A.; Krausslich, H.G.; Mizokami, M.; et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 2005, 11, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Gottwein, J.M.; Scheel, T.K.; Jensen, T.B.; Lademann, J.B.; Prentoe, J.C.; Knudsen, M.L.; Hoegh, A.M.; Bukh, J. Development and characterization of hepatitis C virus genotype 1-7 cell culture systems: Role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology 2009, 49, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Gottwein, J.M.; Scheel, T.K.; Hoegh, A.M.; Lademann, J.B.; Eugen-Olsen, J.; Lisby, G.; Bukh, J. Robust hepatitis C genotype 3a cell culture releasing adapted intergenotypic 3a/2a (S52/JFH1) viruses. Gastroenterology 2007, 133, 1614–1626. [Google Scholar] [CrossRef]
- Frey, A.; Di Canzio, J.; Zurakowski, D. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 1998, 221, 35–41. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Data |
|---|---|
| No. | 76 |
| Epidemiological data | |
| Age (years), median [IQR] | 51 [47–54] |
| Gender (male), n (%) | 62 (81.6) |
| BMI (kg/m2), median [IQR] | 24.7 [21.7–28.7] |
| Smoker, n (%) | |
| - Never | 5 (6.6) |
| - Previous (>6 months) | 21 (27.6) |
| - Current | 50 (65.8) |
| Alcohol intake (>50 g/day), n (%) | |
| - Never | 40 (52.6) |
| - Previous (>6 months) | 33 (43.4) |
| - Current | 3 (3.9) |
| Intravenous drug user, n (%) | |
| - Never | 18 (23.7) |
| - Previous (>6 months) | 58 (76.3) |
| - Current | 0 (0) |
| HIV markers | |
| Prior AIDS, n (%) | 3 (3.9) |
| Nadir CD4+/mm3, median [IQR] | 145.0 [95.0–238.5] |
| Nadir < 200 CD4+/mm3, n (%) | 24 (32.4) |
| Baseline CD4+/mm3, median [IQR] | 518.0 [294.0–721.8] |
| Baseline > 500 CD4+/mm3, n (%) | 40 (52.6) |
| HIV antiretroviral therapy, n (%) | |
| NRTI + NNRTI | 19 (27.5) |
| NRTI + II | 30 (43.5) |
| NRTI + PI | 15 (21.7) |
| PI+II + NNRTI/MVC | 2 (2.9) |
| Others | 3 (4.3) |
| Liver disease markers | |
| LSM (kPa), median [IQR] | 20.3 [12.4–34.8] |
| - 9.5 to 12.4 kPa | 19 (25.0) |
| - 12.5 kPa to 19.9 kPa | 18 (23.7) |
| - ≥20 kPa | 39 (51.3) |
| Hepatic decompensation, n (%) | 8 (10.5) |
| HCV therapy, n (%) | |
| Previous HCV therapy | 43 (56.6) |
| Baseline HCV Therapy | |
| - IFN-based therapy | 50 (65.8) |
| - IFN-free DAAs therapy | 26 (34.2) |
| HCV markers | |
| HCV genotype, n (%) | |
| - 1 | 57 (75.0) |
| - 3 | 10 (13.2) |
| - 4 | 7 (9.2) |
| - Others/Unknown | 2 (2.6) |
| Log10 HCV-RNA (IU/mL), median [IQR] | 6.1 [5.8–6.6] |
| HCV-RNA ≥ 850,000 IU/mL, n (%) | 47 (62.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepúlveda-Crespo, D.; Volpi, C.; Amigot-Sánchez, R.; Yélamos, M.B.; Díez, C.; Gómez, J.; Hontañón, V.; Berenguer, J.; González-García, J.; Martín-Escolano, R.; et al. Sustained Long-Term Decline in Anti-HCV Neutralizing Antibodies in HIV/HCV-Coinfected Patients Five Years after HCV Therapy: A Retrospective Study. Pharmaceuticals 2024, 17, 1152. https://doi.org/10.3390/ph17091152
Sepúlveda-Crespo D, Volpi C, Amigot-Sánchez R, Yélamos MB, Díez C, Gómez J, Hontañón V, Berenguer J, González-García J, Martín-Escolano R, et al. Sustained Long-Term Decline in Anti-HCV Neutralizing Antibodies in HIV/HCV-Coinfected Patients Five Years after HCV Therapy: A Retrospective Study. Pharmaceuticals. 2024; 17(9):1152. https://doi.org/10.3390/ph17091152
Chicago/Turabian StyleSepúlveda-Crespo, Daniel, Camilla Volpi, Rafael Amigot-Sánchez, María Belén Yélamos, Cristina Díez, Julián Gómez, Víctor Hontañón, Juan Berenguer, Juan González-García, Rubén Martín-Escolano, and et al. 2024. "Sustained Long-Term Decline in Anti-HCV Neutralizing Antibodies in HIV/HCV-Coinfected Patients Five Years after HCV Therapy: A Retrospective Study" Pharmaceuticals 17, no. 9: 1152. https://doi.org/10.3390/ph17091152
APA StyleSepúlveda-Crespo, D., Volpi, C., Amigot-Sánchez, R., Yélamos, M. B., Díez, C., Gómez, J., Hontañón, V., Berenguer, J., González-García, J., Martín-Escolano, R., Resino, S., & Martínez, I. (2024). Sustained Long-Term Decline in Anti-HCV Neutralizing Antibodies in HIV/HCV-Coinfected Patients Five Years after HCV Therapy: A Retrospective Study. Pharmaceuticals, 17(9), 1152. https://doi.org/10.3390/ph17091152








