Applications of Capillary Electrophoresis for the Detection of Adulterants in Dietary Supplements
Abstract
1. Introduction
2. Overview of Capillary Electrophoresis
3. Applications of CE in Detecting Adulterants
4. Challenges and Considerations
5. Review Methodology
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Rautiainen, S.; Manson, J.E.; Lichtenstein, A.H.; Sesso, H.D. Dietary supplements and disease prevention—A global overview. Nat. Rev. Endocrinol. 2016, 12, 407–420. [Google Scholar] [CrossRef]
- Binns, C.W.; Lee, M.K.; Lee, A.H. Problems and prospects: Public health regulation of dietary supplements. Annu. Rev. Public Health 2018, 39, 403–420. [Google Scholar] [CrossRef]
- Rocha, T.; Amaral, J.S.; Oliveira, M.B.P. Adulteration of dietary supplements by the illegal addition of synthetic drugs: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 43–62. [Google Scholar] [CrossRef] [PubMed]
- White, C.M. Continued risk of dietary supplements adulterated with approved and unapproved drugs: Assessment of the US Food and Drug Administration’s tainted supplements database 2007 through 2021. J. Clin. Pharmacol. 2022, 62, 928–934. [Google Scholar] [CrossRef]
- Marcus, D.M. Dietary supplements: What’s in a name? What’s in the bottle? Drug Test. Anal. 2016, 8, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Senol, F.S.; Skalicka-Wozniak, K.; Georgiev, M.; Sener, B. Adulteration and Safety Issues in Nutraceuticals and Dietary Supplements: Innocent or Risky; Nutraceuticals, Nanotechnology in the Agri-Food Industry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–182. [Google Scholar]
- Czepielewska, E.; Makarewicz-Wujec, M.; Różewski, F.; Wojtasik, E.; Kozłowska-Wojciechowska, M. Drug adulteration of food supplements: A threat to public health in the European Union? Regul. Toxicol. Pharmacol. 2018, 97, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, L.; Krynitsky, A.J.; Rader, J.I. Mass spectrometric analysis of pharmaceutical adulterants in products labeled as botanical dietary supplements or herbal remedies: A review. Anal. Bioanal. Chem. 2014, 406, 6767–6790. [Google Scholar] [CrossRef]
- Jagim, A.R.; Harty, P.S.; Erickson, J.L.; Tinsley, G.M.; Garner, D.; Galpin, A.J. Prevalence of adulteration in dietary supplements and recommendations for safe supplement practices in sport. Front. Sports Act. Living 2023, 5, 1239121. [Google Scholar]
- Walker, M.J.; Naughton, D.P.; Deshmukh, N.; Burns, D.T. A review of methods for the simultaneous detection of illegal ingredients in food supplements. J. Appl. Phycol. A 2016, 44, 51–56. [Google Scholar]
- Liu, Y.; Lu, F. Adulterated pharmaceutical chemicals in botanical dietary supplements: Novel screening approaches. Rev. Anal. Chem. 2017, 36, 20160032. [Google Scholar]
- Voeten, R.L.; Ventouri, I.K.; Haselberg, R.; Somsen, G.W. Capillary electrophoresis: Trends and recent advances. Anal. Chem. 2018, 90, 1464–1481. [Google Scholar] [CrossRef] [PubMed]
- Toraño, J.S.; Ramautar, R.; de Jong, G. Advances in capillary electrophoresis for the life sciences. J. Chromatogr. B 2019, 1118, 116–136. [Google Scholar] [CrossRef]
- Silva, M. Micellar electrokinetic chromatography: A review of methodological and instrumental innovations focusing on practical aspects. Electrophoresis 2013, 34, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Klepárník, K. Recent advances in combination of capillary electrophoresis with mass spectrometry: Methodology and theory. Electrophoresis 2015, 36, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Ganzera, M. Quality control of herbal medicines by capillary electrophoresis: Potential, requirements, and applications. Electrophoresis 2008, 29, 3489–3503. [Google Scholar] [CrossRef] [PubMed]
- Rabanes, H.R.; Guidote, A.M., Jr.; Quirino, J.P. Capillary electrophoresis of natural products: Highlights of the last five years (2006–2010). Electrophoresis 2012, 33, 180–195. [Google Scholar] [CrossRef]
- Gackowski, M.; Przybylska, A.; Kruszewski, S.; Koba, M.; Mądra-Gackowska, K.; Bogacz, A. Recent applications of capillary electrophoresis in the determination of active compounds in medicinal plants and pharmaceutical formulations. Molecules 2021, 26, 4141. [Google Scholar] [CrossRef]
- Przybylska, A.; Gackowski, M.; Koba, M. Application of capillary electrophoresis to the analysis of bioactive compounds in herbal raw materials. Molecules 2021, 26, 2135. [Google Scholar] [CrossRef]
- Muschietti, L.; Redko, F.; Ulloa, J. Adulterants in selected dietary supplements and their detection methods. Drug Test. Anal. 2020, 12, 861–886. [Google Scholar] [CrossRef]
- Ku, Y.R.; Chang, Y.S.; Wen, K.C.; Ho, L.K. Analysis and confirmation of synthetic anorexics in adulterated traditional Chinese medicines by high-performance capillary electrophoresis. J. Chromatogr. A 1999, 848, 537–543. [Google Scholar] [CrossRef]
- Ku, Y.R.; Tsai, M.J.; Lin, J.H.; Wen, K.C. Micellar Electrokinetic Capillary Chromatography of Clobenzorex HCI and Diazepam Adulterated in Anorexiant Traditional Chinese Medicine. Chin. Pharm. J. 1996, 32–40. [Google Scholar]
- Cheng, H.L.; Tseng, M.C.; Tsai, P.L.; Her, G.R. Analysis of synthetic chemical drugs in adulterated Chinese medicines by capillary electrophoresis/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 1473–1480. [Google Scholar] [CrossRef]
- Ku, Y.R.; Chag, L.Y.; Ho, L.K.; Lin, J.H. Analysis of synthetic anti-diabetic drugs in adulterated traditional Chinese medicines by high-performance capillary electrophoresis. J. Pharm. Biomed. Anal. 2003, 33, 329–334. [Google Scholar] [CrossRef]
- Avula, B.; Khan, I.A. Separation and determination of ephedrine enantiomers and synephrine by high performance capillary electrophoresis in dietary supplements. Chromatographia 2004, 59, 71–77. [Google Scholar] [CrossRef]
- Sitton, A.; Schmid, M.G.; Gübitz, G.; Aboul-Enein, H.Y. Determination of lipoic acid in dietary supplement preparations by capillary electrophoresis. J. Biochem. Biophys. Methods 2004, 61, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Sombra, L.L.; Gómez, M.R.; Olsina, R.; Martínez, L.D.; Silva, M.F. Comparative study between capillary electrophoresis and high performance liquid chromatography in ‘guarana’ based phytopharmaceuticals. J. Pharm. Biomed. Anal. 2005, 36, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Phinney, K.W.; Ihara, T.; Sander, L.C. Determination of ephedrine alkaloid stereoisomers in dietary supplements by capillary electrophoresis. J. Chromatogr. A 2005, 1077, 90–97. [Google Scholar] [CrossRef]
- Prokorátová, V.; Kvasnička, F.; Ševčík, R.; Voldřich, M. Capillary electrophoresis determination of carnitine in food supplements. J. Chromatogr. A 2005, 1081, 60–64. [Google Scholar] [CrossRef]
- Weiss, D.J.; Austria, E.J.; Anderton, C.R.; Hompesch, R.; Jander, A. Analysis of green tea extract dietary supplements by micellar electrokinetic chromatography. J. Chromatogr. A 2006, 1117, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Cianchino, V.; Acosta, G.; Ortega, C.; Martínez, L.D.; Gomez, M.R. Analysis of potential adulteration in herbal medicines and dietary supplements for weight control by capillary electrophoresis. Food Chem. 2008, 108, 1075–1081. [Google Scholar] [CrossRef]
- Malavaki, C.J.; Asimakopoulou, A.P.; Lamari, F.N.; Theocharis, A.D.; Tzanakakis, G.N.; Karamanos, N.K. Capillary electrophoresis for the quality control of chondroitin sulfates in raw materials and formulations. Anal. Biochem. 2008, 374, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Orlandini, S.; Giannini, I.; Pinzauti, S.; Furlanetto, S. Multivariate optimisation and validation of a capillary electrophoresis method for the analysis of resveratrol in a nutraceutical. Talanta 2008, 74, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, L.; Castro-Puyana, M.; García-Ruiz, C.; Crego, A.L.; Marina, M.L. Determination of L-and D-carnitine in dietary food supplements using capillary electrophoresis–tandem mass spectrometry. Food Chem. 2010, 120, 921–928. [Google Scholar] [CrossRef]
- de Carvalho, L.M.; Martini, M.; Moreira, A.P.; Garcia, S.C.; do Nascimento, P.C.; Bohrer, D. Determination of synthetic pharmaceuticals in phytotherapeutics by capillary zone electrophoresis with contactless conductivity detection (CZE-C4D). Microchem. J. 2010, 96, 114–119. [Google Scholar] [CrossRef]
- Carvalho, L.D.; Cohen, P.A.; Silva, C.V.; Moreira, A.P.L.; Falcão, T.M.; Dal Molin, T.R.; Martini, M. A new approach to determining pharmacologic adulteration of herbal weight loss products. Food Addit. Contam. Part A 2012, 29, 1661–1667. [Google Scholar] [CrossRef]
- Akamatsu, S.; Mitsuhashi, T. Development of a simple capillary electrophoretic determination of glucosamine in nutritional supplements using in-capillary derivatization with o-phthalaldehyde. Food Chem. 2012, 130, 1137–1141. [Google Scholar] [CrossRef]
- Kodama, S.; Taga, A.; Aizawa, S.I.; Kemmei, T.; Honda, Y.; Suzuki, K.; Yamamoto, A. Direct enantioseparation of lipoic acid in dietary supplements by capillary electrophoresis using trimethyl-β-cyclodextrin as a chiral selector. Electrophoresis 2012, 33, 2441–2445. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.P.L.; Motta, M.J.; Dal Molin, T.R.; Viana, C.; de Carvalho, L.M. Determination of diuretics and laxatives as adulterants in herbal formulations for weight loss. Food Addit. Contam. Part A 2013, 30, 1230–1237. [Google Scholar] [CrossRef]
- Viana, C.; Ferreira, M.; Romero, C.S.; Bortoluzzi, M.R.; Lima, F.O.; Rolim, C.M.; de Carvalho, L.M. A capillary zone electrophoretic method for the determination of hypoglycemics as adulterants in herbal formulations used for the treatment of diabetes. Anal. Methods 2013, 5, 2126–2133. [Google Scholar] [CrossRef]
- Akamatsu, S.; Mitsuhashi, T. Development of a simple analytical method using capillary electrophoresis-tandem mass spectrometry for product identification and simultaneous determination of free amino acids in dietary supplements containing royal jelly. J. Food Compos. Anal. 2013, 30, 47–51. [Google Scholar] [CrossRef]
- Akamatsu, S.; Mitsuhashi, T. Simultaneous determination of pharmaceutical components in dietary supplements for weight loss by capillary electrophoresis tandem mass spectrometry. Drug Test. Anal. 2014, 6, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.G.; Aguiar, F.P.; Jesus, D.P.D. A rapid and simple method for determination of 5-hydroxytryptophan in dietary supplements by capillary electrophoresis. J. Braz. Chem. Soc. 2014, 25, 783–787. [Google Scholar] [CrossRef]
- Václavíková, E.; Kvasnička, F. Quality control of chondroitin sulphate used in dietary supplements. Czech J. Food Sci. 2015, 33, 165–173. [Google Scholar] [CrossRef]
- dos Santos, V.B.; Daniel, D.; Singh, M.; do Lago, C.L. Amphetamine and derivatives in natural weight loss pills and dietary supplements by capillary electrophoresis-tandem mass spectrometry. J. Chromatogr. B 2016, 1038, 19–25. [Google Scholar] [CrossRef]
- Tero-Vescan, A.; Vari, C.E.; Imre, S.; Ősz, B.E.; Filip, C.; Hancu, G. Comparative analysis by HPLC-UV and capillary electrophoresis of dietary supplements for weight loss. Farmacia 2016, 64, 699–705. [Google Scholar]
- Wang, D.; Man, R.; Shu, M.; Liu, H.; Gao, Y.; Luan, F. Detection of sibutramine and phenolphthalein in functional foods using capillary electrophoresis. Anal. Methods 2016, 8, 621–626. [Google Scholar] [CrossRef]
- Müller, L.S.; Muratt, D.T.; Molin, T.R.D.; Urquhart, C.G.; Viana, C.; de Carvalho, L.M. Analysis of pharmacologic adulteration in dietary supplements by capillary zone electrophoresis using simultaneous contactless conductivity and UV detection. Chromatographia 2018, 81, 689–698. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, M.H.; Vu, M.T.; Duong, H.A.; Pham, H.V.; Mai, T.D. Dual-channeled capillary electrophoresis coupled with contactless conductivity detection for rapid determination of choline and taurine in energy drinks and dietary supplements. Talanta 2019, 193, 168–175. [Google Scholar] [CrossRef]
- Duong, H.A.; Vu, M.T.; Nguyen, T.D.; Nguyen, M.H.; Mai, T.D. Determination of 10-hydroxy-2-decenoic acid and free amino acids in royal jelly supplements with purpose-made capillary electrophoresis coupled with contactless conductivity detection. J. Food Compos. Anal. 2020, 87, 103422. [Google Scholar] [CrossRef]
- Restaino, O.F.; De Rosa, M.; Schiraldi, C. High-performance capillary electrophoresis to determine intact keratan sulfate and hyaluronic acid in animal origin chondroitin sulfate samples and food supplements. Electrophoresis 2020, 41, 1740–1748. [Google Scholar] [CrossRef]
- Kvasnička, F.; Rajchl, A. Electrophoretic determination of taurine. J. Chromatogr. A 2021, 1645, 462075. [Google Scholar] [CrossRef] [PubMed]
- Riasová, P.; Jenčo, J.; Moreno-González, D.; Vander Heyden, Y.; Mangelings, D.; Polášek, M.; Jáč, P. Development of a capillary electrophoresis method for the separation of flavonolignans in silymarin complex. Electrophoresis 2022, 43, 930–938. [Google Scholar] [CrossRef]
- Cizmarova, I.; Matuskova, M.; Stefanik, O.; Horniakova, A.; Mikus, P.; Piestansky, J. Determination of thiamine and pyridoxine in food supplements by a green ultrasensitive two-dimensional capillary electrophoresis hyphenated with mass spectrometry. Chem. Papers 2022, 76, 6235–6245. [Google Scholar] [CrossRef]
- Amorim, T.L.; Duarte, L.M.; de la Fuente, M.A.; de Oliveira, M.A.L.; Gómez-Cortés, P. Fast capillary electrophoresis method for determination of docosahexaenoic and eicosapentaenoic acids in marine oils omega-3 supplements. J. Chromatogr. A 2020, 1613, 460641. [Google Scholar] [CrossRef]
- Do, Y.N.; Kieu, T.L.P.; Dang, T.H.M.; Nguyen, Q.H.; Dang, T.H.; Tran, C.S.; Vu, A.P.; Do, T.T.; Nguyen, T.N.; Pham, T.N.M.; et al. Green analytical method for simultaneous determination of glucosamine and calcium in dietary supplements by capillary electrophoresis with capacitively coupled contactless conductivity detection. J. Anal. Methods Chem. 2023, 2023, 2765508. [Google Scholar] [CrossRef]
- Pukleš, I.; Páger, C.; Sakač, N.; Šarkanj, B.; Matasović, B.; Samardžić, M.; Budetić, M.; Marković, D.; Jozanović, M. Electrophoretic determination of L-carnosine in health supplements using an integrated lab-on-a-chip platform with contactless conductivity detection. Int. J. Mol. Sci. 2023, 24, 14705. [Google Scholar] [CrossRef]
- Pukleš, I.; Páger, C.; Sakač, N.; Matasović, B.; Kovač-Andrić, E.; Šarkanj, B.; Samardžić, M.; Budetić, M.; Molnárová, K.; Marković, D.; et al. A new green approach to L-histidine and β-alanine analysis in dietary supplements using rapid and simple contactless conductivity detection integrated with high-resolution glass-microchip electrophoresis. Anal. Bioanal. Chem. 2024, 416, 3605–3617. [Google Scholar] [CrossRef]
- Chien, H.J.; Zheng, Y.F.; Wang, W.C.; Kuo, C.Y.; Hsu, Y.M.; Lai, C.C. Determination of adulteration, geographical origins, and species of food by mass spectrometry. Mass Spectrom. Rev. 2023, 42, 2273–2323. [Google Scholar] [CrossRef]
- Rapaka, R.S.; Coates, P.M. Dietary supplements and related products: A brief summary. Life Sci. 2006, 78, 2026–2032. [Google Scholar] [CrossRef]
- Bolshakov, D.S.; Amelin, V.G. Capillary electrophoresis in assessing the quality and safety of foods. J. Anal. Chem. 2023, 78, 815–855. [Google Scholar] [CrossRef]
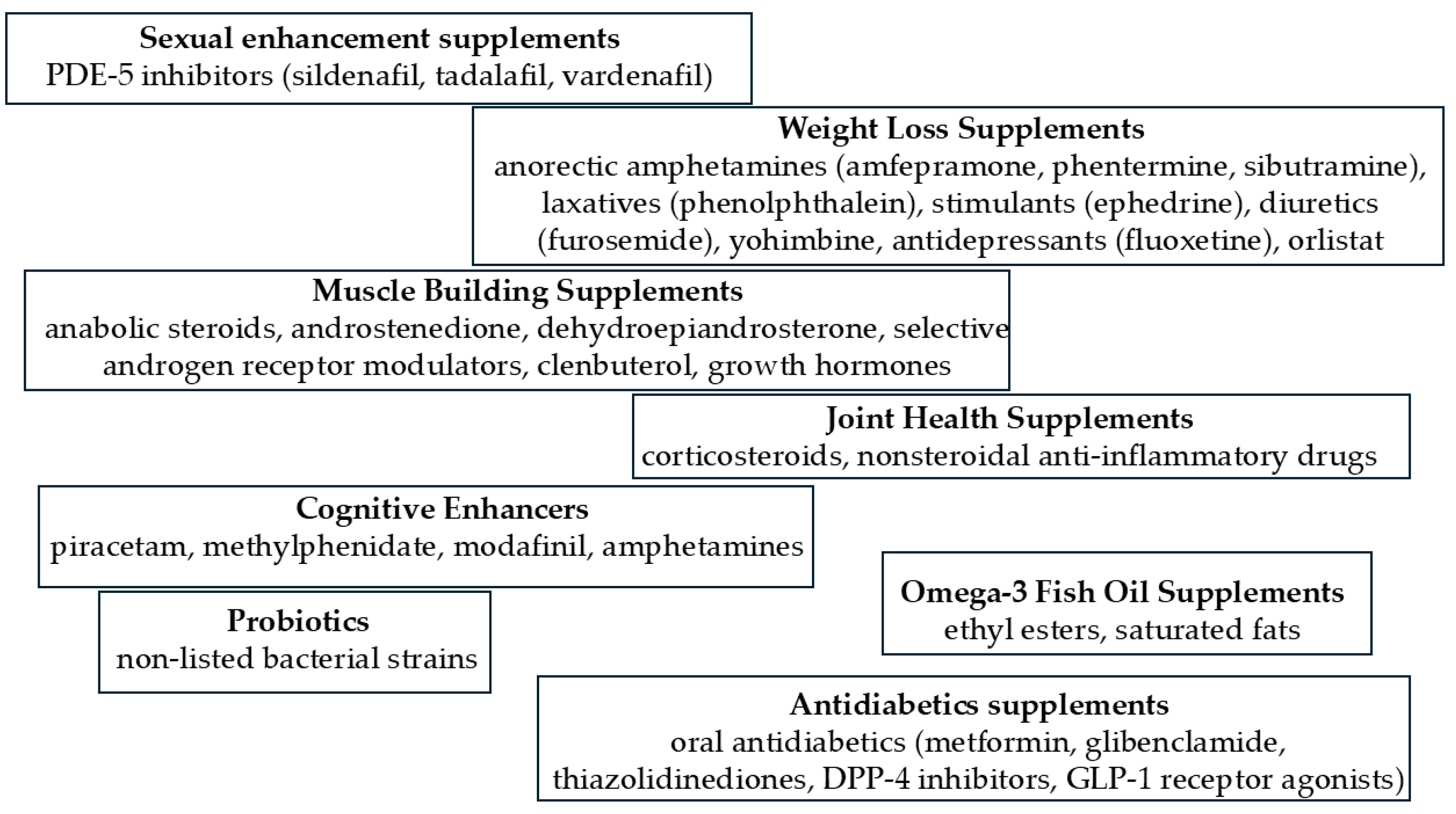
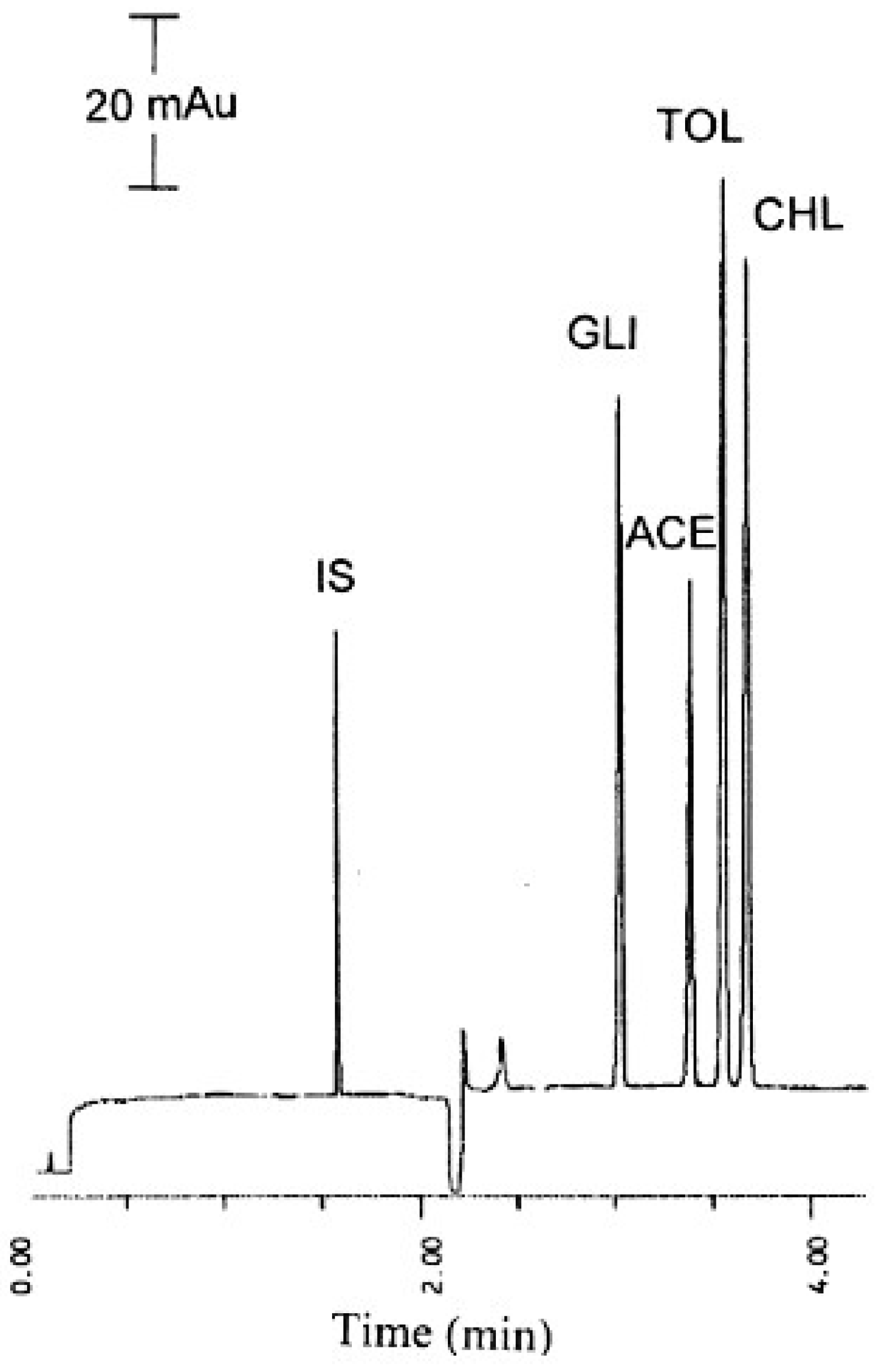
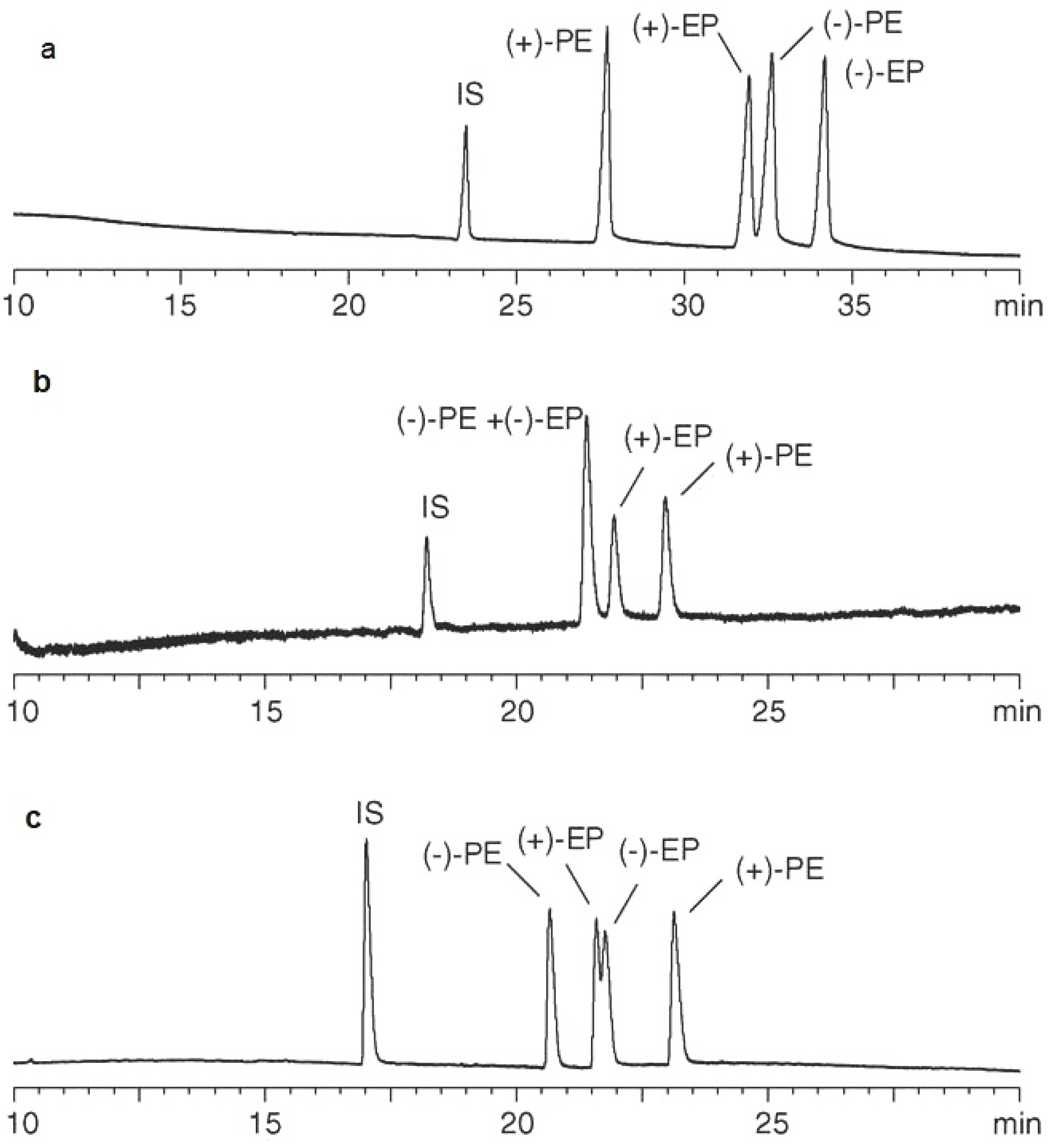
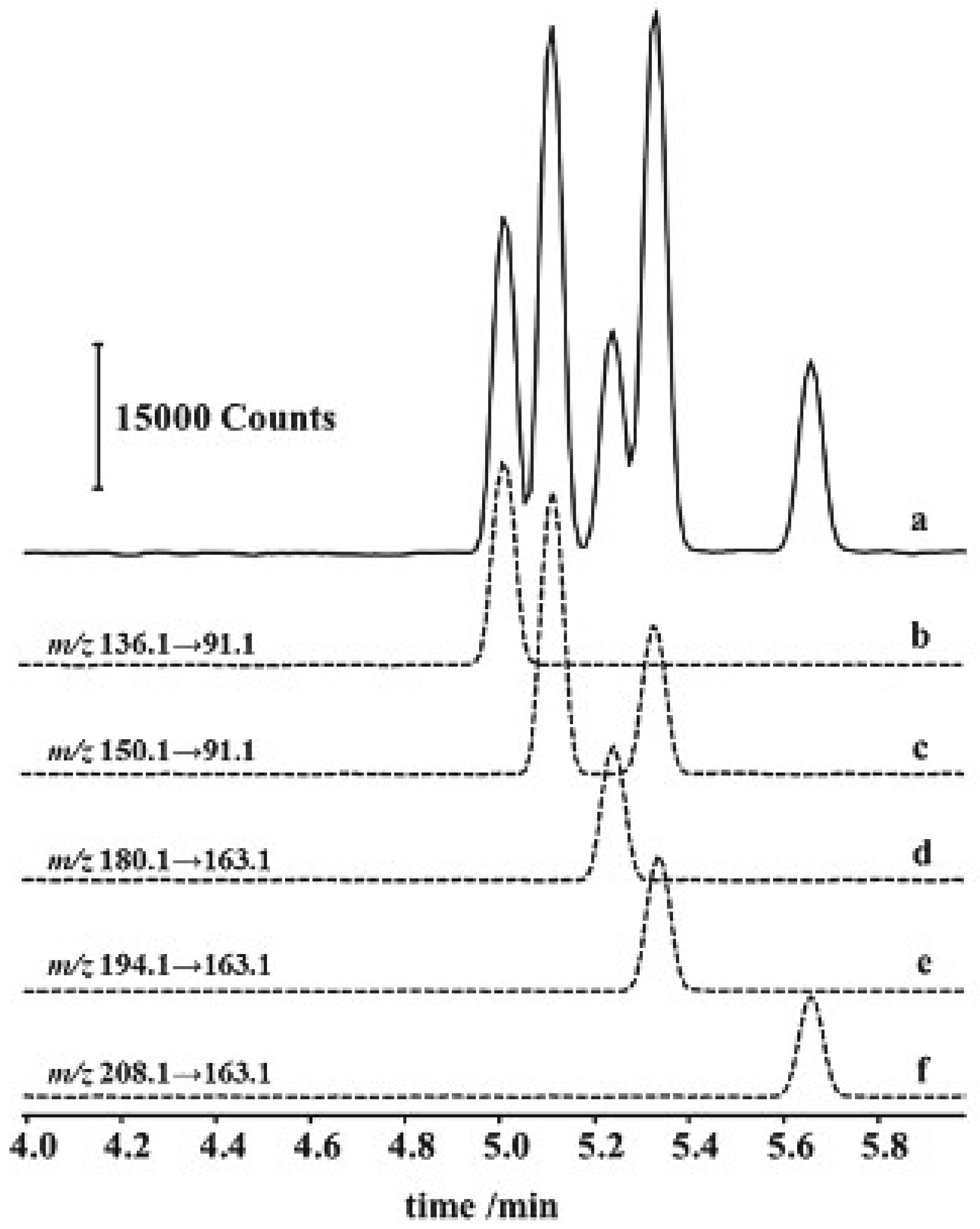
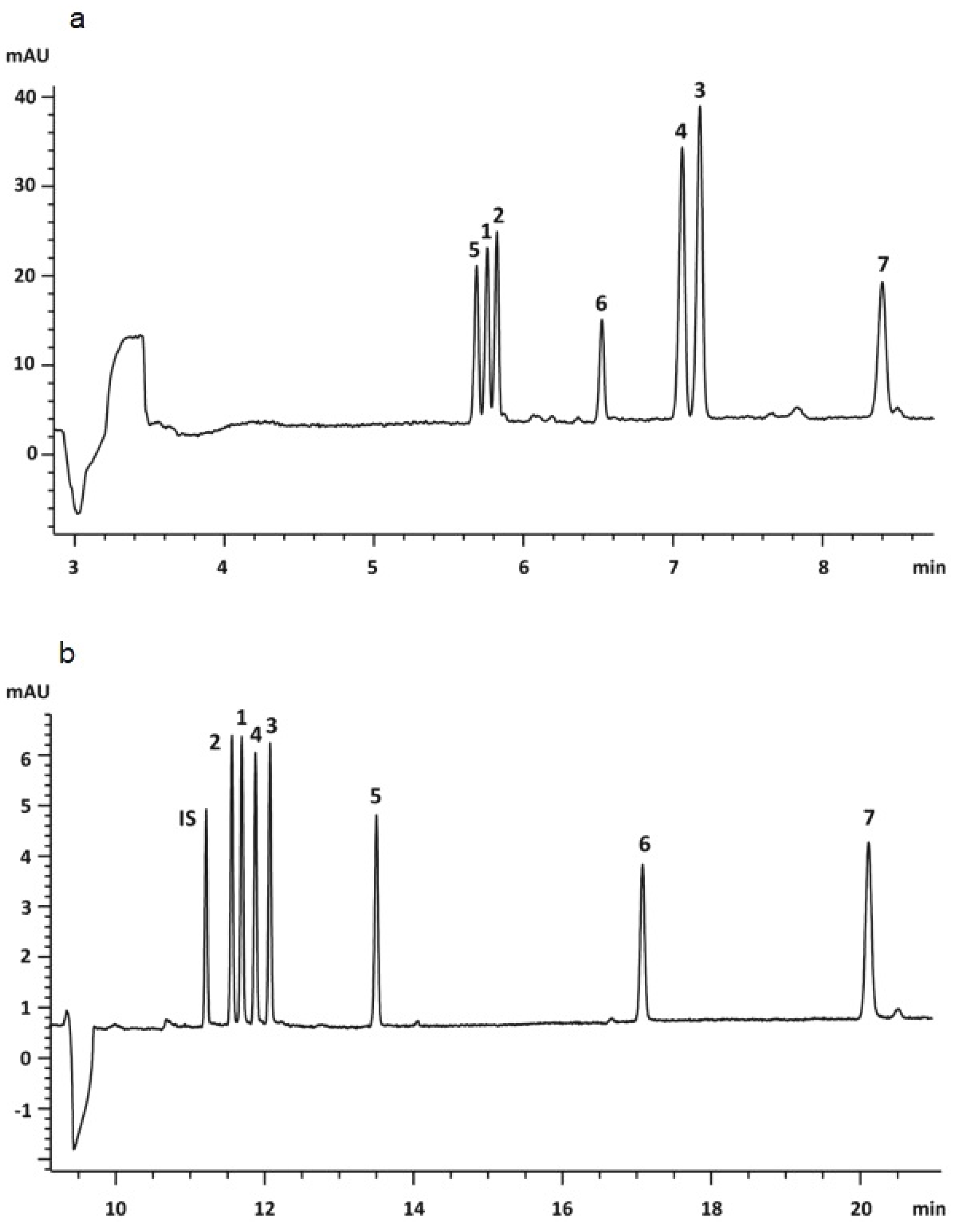
| CE Technique | Analytical Conditions | Product | Substances | Reference |
|---|---|---|---|---|
| CZE | 120 mM of phosphate, 15% acetonitrile, pH 2.0, 16 kV, 30 °C UV detection 200 nm | traditional Chinese medicinal powders | clobenzorex, diethylpropion, fenfluramine, methamphetamine, phenylpropanolamine, phentermine | [21] |
| CZE MEKC CE-ESI-MS | CZE: 40 mM of ammonium acetate, pH 9.0, 20 kV MEKC: 40 mM of ammonium acetate, 20 mM of SDS, pH 9.0, 20 kV UV detection 254 nm CE-ESI-MS: sheath liquid composition methanol/water (70:30) containing 0.2% formic acid, ESI voltage +4 kV | traditional Chinese medicinal powders | acetaminophen, bucetin, caffeine, diazepam, ethoxybenzamide, fenbufen, flufenamic acid, indomethacin, ketoprofen, mefenamic acid, niflumic acid, oxyphenbutazone, phenylbutazone, prednisolone, salicylamide, sulindac | [23] |
| CZE | 100 mM of phosphate–borate, pH 7.50, 15 kV, 30 °C UV detection 200 nm | traditional Chinese medicines | acetohexamide, chlorpropamide, glibenclamide, tolbutamide | [24] |
| CE | 55 mM of phosphate, 10 mM of borate, 20 mM of HP-β-CD, 7 mM of β-CD, 30% methanol, pH 8.6, 30 kV, 37 °C UV detection 210 nm | tablets, capsules | ephedrine, pseudoephedrine stereoisomers | [25] |
| CZE | 50 mM of phosphate, 20% methanol, pH 7.0, 15 kV, 25 °C UV detection 208 nm | tablets | alpha-lipoic acid | [26] |
| CZE | 20 mm of sodium tetraborate, pH 9.2, 25 kV, 25 °C UV detection 212 nm | guarana powder and tablets | caffeine, theobromine, theophylline | [27] |
| CZE | Method I: 25 mM of phosphate, pH 2.5, 2.8% sulfated-β-CD + 1.2% heptakis(2,6-di-O-methyl)-β-CD, −15 kV, 25 °C Method II: 25 mM of phosphate, pH 2.5, 4% 4% heptakis(2,6-di-O-methyl)-β-CD, 30 kV, 25 °C Method III: 25 mM of phosphate, pH 2.5, 4% hydroxypropyl-β-CD, 25 kV, 25 °C UV detection 210 nm | ephedra products | ephedrine, pseudoephedrine stereoisomers | [28] |
| cITP CZE | cITP: terminating electrolyte 10 mM of acetic acid, leading electrolyte 40 mM of acetic acid, 20 mM of ammonium hydroxide CZE: 15 mM of Tris, 25 mM of phosphate, 15 kV UV detection 254 nm | food supplements | L-carnitine | [29] |
| MEKC | 5 mM of borate, 60 mM of phosphate, 50 mM of SDS, pH 7.00, 27 kV, 25 °C UV detection 210 nm | dietary supplements containing green tea extracts | caffeine, catechins ((−)-epigallocatechin, (+)-catechin, (−)-epigallocatechin-3-gallate, (−)-epicatechin, or (−)-epicatechin gallate) | [30] |
| CZE | 50 mM of phosphate, pH 3.0, 30 kV, 25 °C UV detection 200 nm | tablets, hard capsules, liquid formulations | chondroitin sulfate (glycosaminoglycan or DNA impurities, hyaluronan impurities) | [32] |
| CZE | 23 mM of borate, 7% acetonitrile (v/v), pH 10.0, 26 kV, 20 °C UV detection 280 nm | effervescent tablets | resveratrol | [33] |
| CE-ESI-MS | 50 mM of ammonium formate, pH 2.5, 0.2% (m/v) succinyl-γ-CD (4 succinyl groups/CD ring), 25 kV, 25 °C MS: sheath liquid, isopropanol/water (50/50 v/v) with 0.1% formic acid | tablets, capsules, biscuits, drinks | carnitine enantiomers | [34] |
| CZE | 50 mM of phosphate in a mixture of water/acetonitrile 50/50 (v/v), 15 kV, 25 °C C4D detection at 600 kHz and 2 Vpp | slimming capsules | amfepramone, fenproporex, fluoxetine, sibutramine | [35] |
| CZE | 20 mM of borate, 5 mM of o-phthalaldehyde (derivatization agent), 5 mM of 3-mercaptopropionic acid, pH 9.20, 25 kV, 30 °C UV detection 340 nm | dietary supplements | glucosamine | [36] |
| CE | 100 mM of phosphate, pH 7.0, 8 mM of TM-β-CD, 18 kV, 20 °C UV detection 200 nm | dietary supplements | alpha-lipoic acid (chiral purity) | [38] |
| CZE | 20 mM of phosphate + 30% methanol, pH 9.2, 15 kV, 25 °C C4D detection at 400 kHz and 2 Vpp | slimming capsules | amiloride, chlorthalidone, furosemide, hydrochlorothiazide phenolphthalein amfepramone fluoxetine, paroxetine | [39] |
| CZE | 20 mM of sodium acetate, pH 10.0, −15 kV, 25 °C C4D detection at 400 kHz and 2 Vpp | antidiabetic capsules | chlorpropamide, glibenclamide, gliclazide, metformin | [40] |
| CE-MS-MS | 20 mM of ammonium formate in 20% acetonitrile, pH 8.0, 30 kV MS: sheath liquid, 5 mM of ammonium formate and 0.1% (v/v) formic acid in 50% v/v methanol/water | slimming tablets, capsules, powders | acetazolamide, furosemide, hydrochlorothiazide, spironolactone, triamterene, trichloromethiazide bisacodyl, dioctyl sulfosuccinate, picosulfate, phenolphthalein, sennoside A, B fenfluramine, mazindol, N-didemethylsibutramine, N-nitrosofenfluramine, phentermine, sibutamine fluoxetine diazepam | [42] |
| CZE | 20 mM of phosphate, pH 10.0, 0.2 mM of CTAB, −20 kV, 25 °C UV detection 214 nm | tablets and capsules | 5-hydroxytryptophan (5-HTP) | [43] |
| cITP CZE | cITP: leading electrolyte: 5 mM of hydrochloric acid + 10 mM of glycine + 0.01% of 2-hydroxyethylcellulose, pH 2.8; terminating electrolyte: 10 mM of citric acid UV detection 254 nm CZE: 25 mM of phosphate + 21 mM of Tris, pH 3.0, −20 kV UV detection 232 nm | raw material used for dietary supplements production | chondroitin sulphate | [44] |
| CE-MS-MS | 100 mM of formic acid, pH 2.4, 25 kV, 20 °C MS: sheath liquid 20 mM of formic acid, pH 2.7, in methanol/water 50:50 (v/v) | tablets, capsules, tea | amphetamine, methamphetamine, methylenedioxyamphetamine, methylenedioxymethamphetamine, methylenedioxyethylamphetamine and phentermine | [45] |
| CZE MEKC | 50 mM of phosphate, pH 4.8, 25 kV, 20 °C 50 mM of sodium tetraborate + 50 mM of SDS, pH 9.8, 25 kV, 20 °C UV detection 210 nm | slimming tablets, capsules | caffeine, ephedrine, sibutramine, yohimbine | [46] |
| MEKC | 20 mM of phosphate + 20 mM of SDS, pH 11.0, 25 kV, 20 °C UV detection 223 nm | slimming capsules, tea | phenolphthalein, sibutramine | [47] |
| CZE | 20 mM of phosphate, 40 mM of sodium hydroxide + 30% methanol (v/v), pH 9.2, 15 kV, 25 °C UV detection 260 nm C4D detection at 400 kHz | weight loss, fat burning, appetite reduction supplements | amiloride, chlorthalidone, furosemide, hydrochlorothiazide fluoxetine, paroxetine phenolphthalein amfepramone | [48] |
| CZE | choline: 150 mM of Tris/lactic acid, pH 8.96, −10 kV taurine: 150 mM of Tris/acetic acid, pH 9.5, −10 kV C4D detection at 400 kHz | energy drinks, powdered infant formula, probiotic green rice samples | choline, taurine | [49] |
| CE | 10-hydroxy-2-decenoic acid: 20 mM of Tris(hydroxymethyl)aminomethane, pH8.5, −17 kV free amino acids: 2 M lactic acid C4D detection 400 kHz | royal jelly based dietary supplement | 10-hydroxy-2-decenoic acid, free amino acids | [50] |
| cITP-CZE | leading electrolyte: 5 mM of HCl, 10 mM of glycylglycine 0.05% 2-hydroxyethyl cellulose solution, pH 3.2 terminating electrolyte: 10 mM of citric acid BGE electrolyte: 50 mM of acetic acid, 20 mM of glycylglycine, and 0.1% 2-hydroxyethyl cellulose solution, pH 3.7 | dietary supplements, food samples | taurine | [52] |
| CZE | 100 mM of boric acid, 10% methanol, 5 mM of heptakis(2,3,6-tri-O-methyl)-β-CD, pH 9.0, 25 kV, 25 °C UV detection 200 nm | dietary supplements | flavonolignans (silybin A, silybin B, isosilybin A, isosilybin B, silychristin, silydianin) in silymarin | [53] |
| cITP-CE-ESI-MS | 10 mM of ammonium acetate + 20 mM of acetic acid, pH 4.5 spray liquid mixture of methanol + 0.1% acetic acid water solution (50:50, v/v) | tablets, effervescent tablets, drops | pyridoxine (vitamin B6), thiamine (vitamin B1) | [54] |
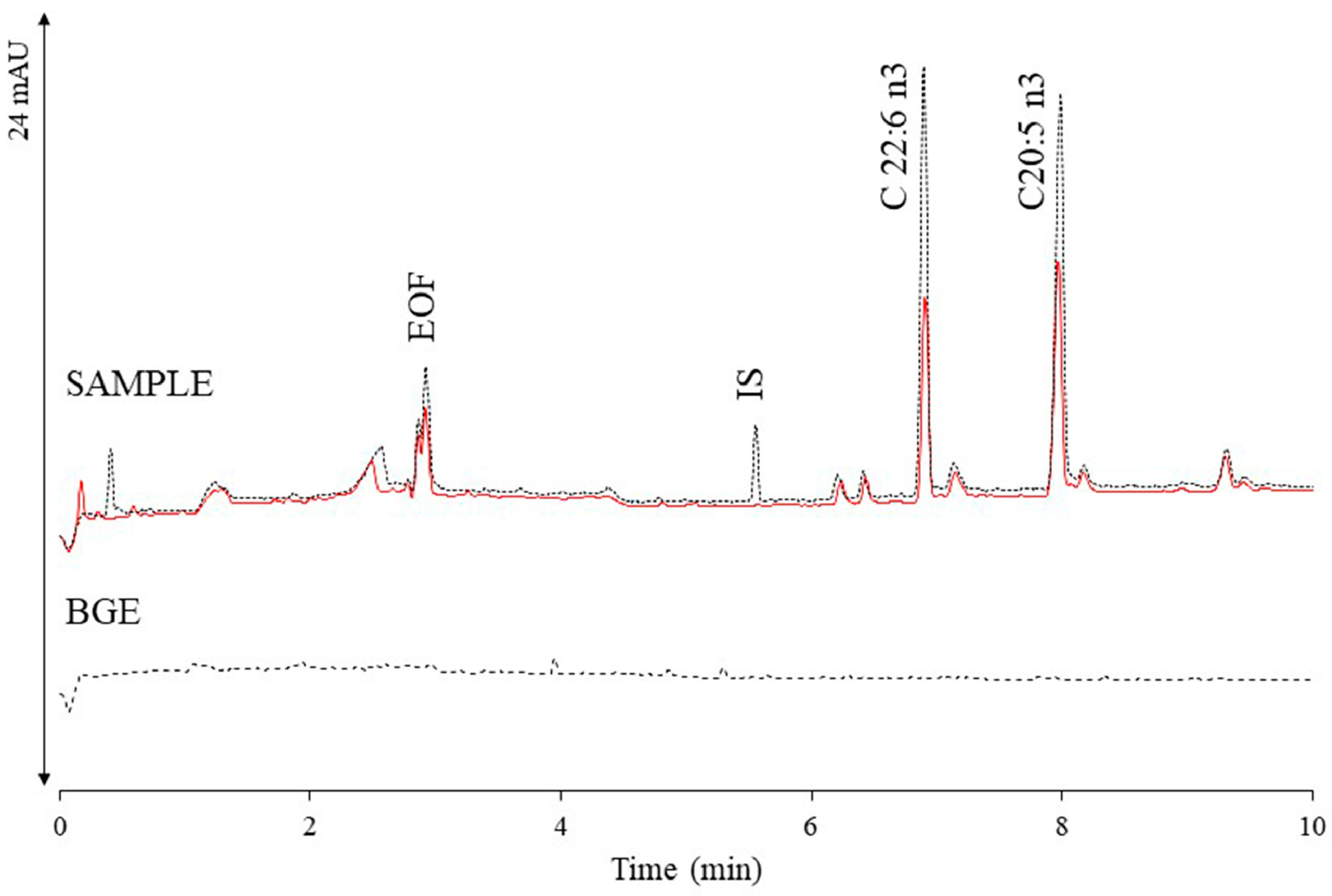
| CZE | 30 mM of sodium tetraborate, 12 mM of Brij 35, 33% methanol (v/v), 17% acetonitrile (v/v), 27 kV, 27 °C, UV detection 200 nm | marine oil gelulels | eicosapentaenoic acid, docosahexaenoic acid | [55] |
| CZE | 10 mM of Tris/acetic acid, pH 5.0, 20 kV, 20 °C C4D detection at 400 kHz | tablets, hard capsules | glucosamine, calcium | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hancu, G.; Székely-Szentmiklósi, B.; Stroia, D.G.; Kelemen, H. Applications of Capillary Electrophoresis for the Detection of Adulterants in Dietary Supplements. Pharmaceuticals 2024, 17, 1119. https://doi.org/10.3390/ph17091119
Hancu G, Székely-Szentmiklósi B, Stroia DG, Kelemen H. Applications of Capillary Electrophoresis for the Detection of Adulterants in Dietary Supplements. Pharmaceuticals. 2024; 17(9):1119. https://doi.org/10.3390/ph17091119
Chicago/Turabian StyleHancu, Gabriel, Blanka Székely-Szentmiklósi, Denisa Gabriela Stroia, and Hajnal Kelemen. 2024. "Applications of Capillary Electrophoresis for the Detection of Adulterants in Dietary Supplements" Pharmaceuticals 17, no. 9: 1119. https://doi.org/10.3390/ph17091119
APA StyleHancu, G., Székely-Szentmiklósi, B., Stroia, D. G., & Kelemen, H. (2024). Applications of Capillary Electrophoresis for the Detection of Adulterants in Dietary Supplements. Pharmaceuticals, 17(9), 1119. https://doi.org/10.3390/ph17091119








