A Phytochemical Analysis and the Pharmacological Implications of the Seagrass Halodule uninervis: An Overview
Abstract
1. Introduction
2. Taxonomic Classification of Halodule uninervis (WoRMS)
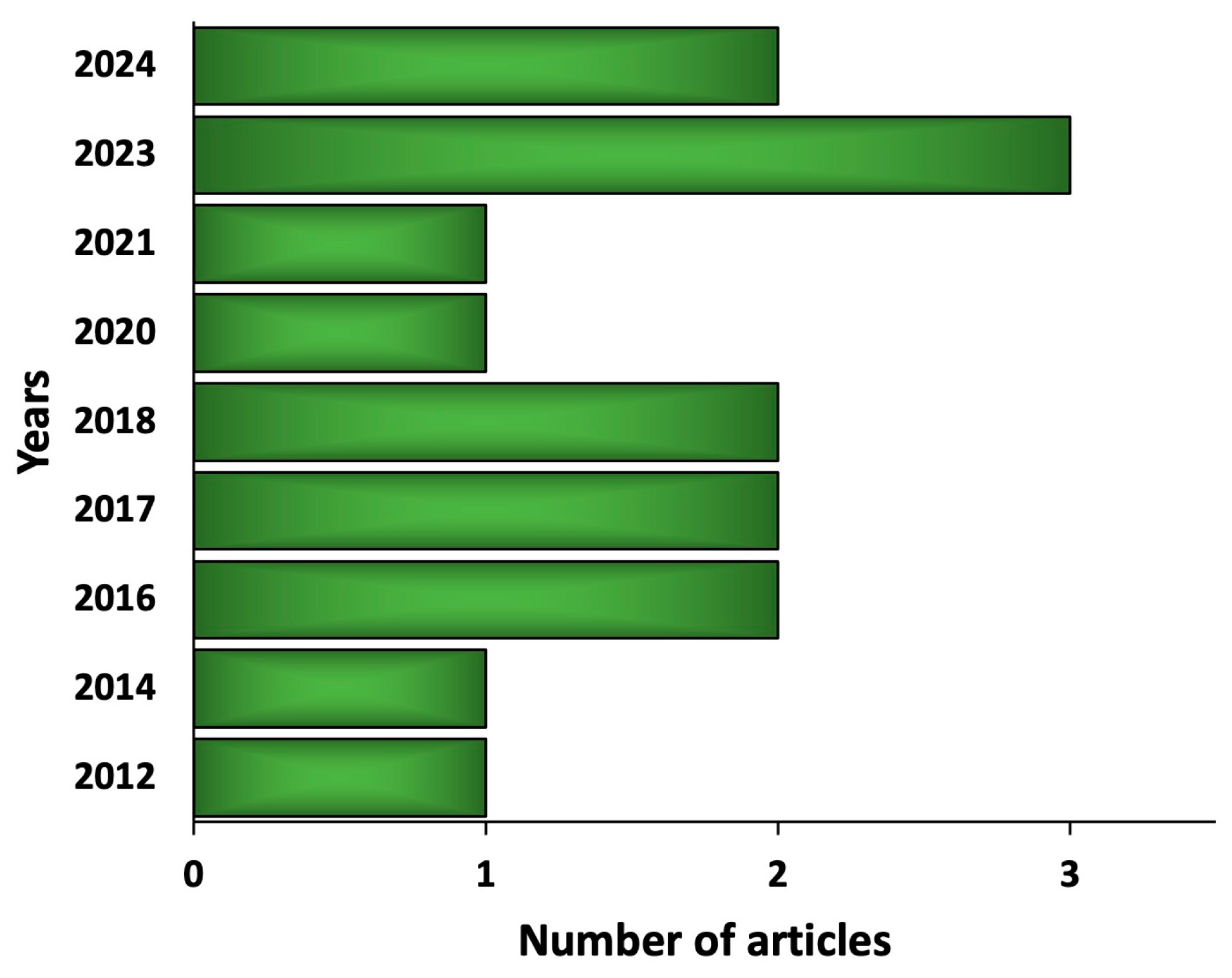
3. Methods
4. Botanical and Ecological Characteristics of Halodule uninervis
4.1. Botanical Characteristics
4.2. Ecological Characteristics
5. Phytochemical Constituents of Halodule uninervis
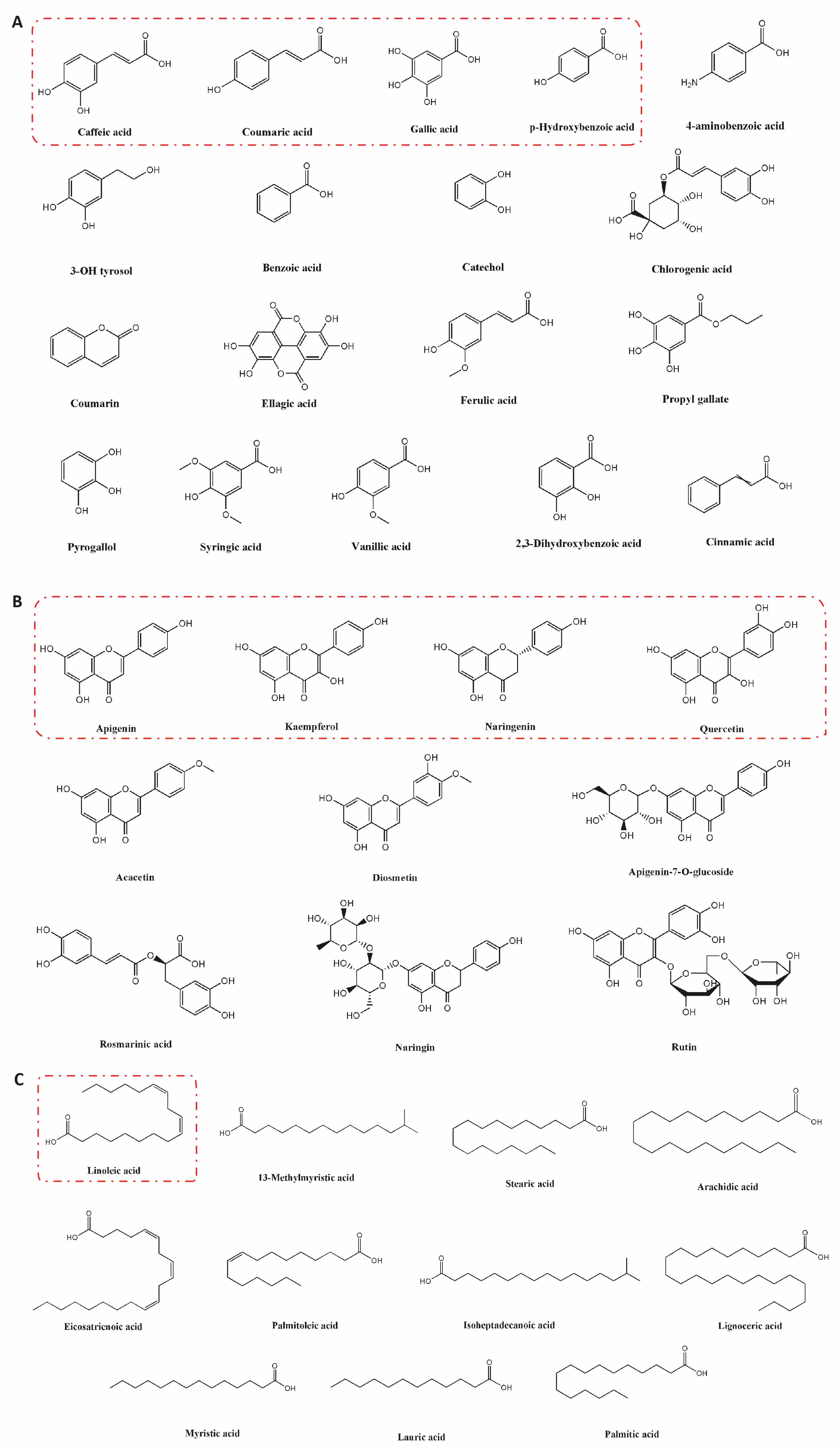
5.1. Bioactive Metabolites
5.2. Macro- and Micronutrients
6. Pharmacological Activities of Halodule uninervis
6.1. Antioxidant Activities
6.2. Antimicrobial Activities
6.3. Larvicidal Effect
6.4. Anticancer Activities
6.5. Antidiabetic Effect
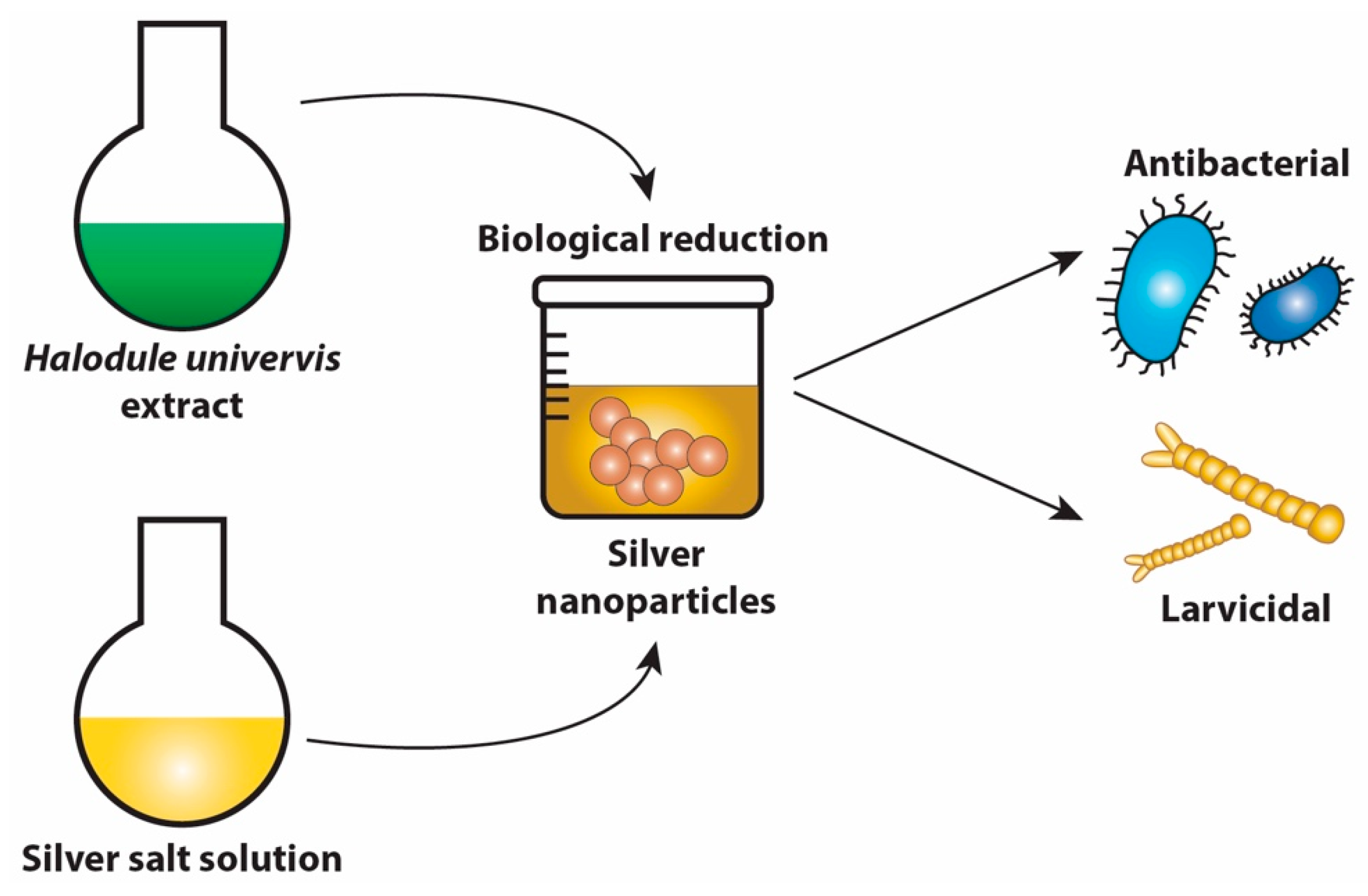
6.6. Green Nanotechnology
7. Safety Profile
8. Conclusions and Future Implications
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019. Available online: https://iris.who.int/handle/10665/312342 (accessed on 30 June 2024).
- Rathore, S.S.; Curtis, J.P.; Wang, Y.; Bristow, M.R.; Krumholz, H.M. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA 2003, 289, 871–878. [Google Scholar] [CrossRef]
- Behar, M.; Olshwang, D.; Magora, F.; Davidson, J.T. Epidural morphine in treatment of pain. Lancet 1979, 313, 527–529. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Markman, M.; Mekhail, T.M. Paclitaxel in cancer therapy. Expert Opin. Pharmacother. 2002, 3, 755–766. [Google Scholar] [CrossRef]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.M.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M. Exploring marine as a rich source of bioactive peptides: Challenges and opportunities from marine pharmacology. Mar. Drugs 2022, 20, 208. [Google Scholar] [CrossRef]
- Schmidtko, A.; Lötsch, J.; Freynhagen, R.; Geisslinger, G. Ziconotide for treatment of severe chronic pain. Lancet 2010, 375, 1569–1577. [Google Scholar] [CrossRef]
- Gordon, E.M.; Sankhala, K.K.; Chawla, N.; Chawla, S.P. Trabectedin for soft tissue sarcoma: Current status and future perspectives. Adv. Ther. 2016, 33, 1055–1071. [Google Scholar] [CrossRef]
- de la Torre-Castro, M.; Rönnbäck, P. Links between humans and seagrasses—An example from tropical East Africa. Ocean Coast. Manag. 2004, 47, 361–387. [Google Scholar] [CrossRef]
- Milchakova, N. Ecosystem Services of Seagrasses. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.-N., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 837–856. [Google Scholar]
- Short, F.T.; Short, C.A.; Novak, A.B. Seagrasses. In The Wetland Book: II: Distribution, Description and Conservation; Finlayson, C.M., Milton, G.R., Prentice, R.C., Davidson, N.C., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–19. [Google Scholar]
- Supriadi, A.; Baehaki, A.; Pratama, M.C. Antibacterial activity of methanol extract from seagrass of Halodule uninervis in the coastal of Lampung. Pharm. Lett. 2016, 8, 77–79. Available online: https://www.researchgate.net/profile/Ace-Baehaki/publication/306499588_Antibacterial_activity_of_methanol_extract_from_seagrass_of_Halodule_Uninervis_in_the_coastal_of_Lampung/links/5976cf78aca2728d02706f14/Antibacterial-activity-of-methanol-extract-from-seagrass-of-Halodule-Uninervis-in-the-coastal-of-Lampung.pdf (accessed on 20 June 2024).
- Ghandourah, M.; Hawas, U.W.; Abou El-Kassem, L.T.; Shaher, F.M. Fatty Acids and Other Chemical Compositions of Some Seagrasses Collected from the Saudi Red Sea with Potential of Antioxidant and Anticancer Agents. Thalass. Int. J. Mar. Sci. 2021, 37, 13–22. [Google Scholar] [CrossRef]
- Parthasarathi, P.; Umamaheswari, A.; Banupriya, R.; Elumalai, S. Phytochemical screening and in-vitro anticancer activity of ethyl acetate fraction of Seagrass Halodule uninervis from Mandapam Coastal Region Rameswaram Gulf of Mannar India. Int. J. Pharm. Sci. Drug Res. 2021, 13, 677–684. [Google Scholar] [CrossRef]
- Gono, C.M.P.; Ahmadi, P.; Hertiani, T.; Septiana, E.; Putra, M.Y.; Chianese, G. A comprehensive update on the bioactive compounds from seagrasses. Mar. Drugs 2022, 20, 406. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Mahomoodally, M.F.; Sadeer, N.B.; Seok, P.G.; Zengin, G.; Palaniveloo, K.; Khalil, A.A.; Rauf, A.; Rengasamy, K.R. Nutritional and bioactive potential of seagrasses: A review. S. Afr. J. Bot. 2021, 137, 216–227. [Google Scholar] [CrossRef]
- Guiry, M.; Guiry, G. AlgaeBase. World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2013; Available online: https://www.algaebase.org (accessed on 30 April 2024).
- Jawad, L.A. The Arabian Seas: Biodiversity, Environmental Challenges and Conservation Measures; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Isaac, F.M. Marine botany of the Kenya coast: 4 Angiosperms. J. East Afr. Nat. Hist. 1968, 1968, 29–47. [Google Scholar]
- Kendrick, G.A.; Waycott, M.; Carruthers, T.J.; Cambridge, M.L.; Hovey, R.; Krauss, S.L.; Lavery, P.S.; Les, D.H.; Lowe, R.J.; Vidal, O.M.I. The central role of dispersal in the maintenance and persistence of seagrass populations. Bioscience 2012, 62, 56–65. [Google Scholar] [CrossRef]
- Kuo, J.; Den Hartog, C. Seagrass taxonomy and identification key. Glob. Seagrass Res. Methods 2001, 33, 31–58. [Google Scholar] [CrossRef]
- Lanyon, J. Guide to the Identification of Seagrasses in the Great Barrier Reef Region; Great Barrier Reef Marine Park Authority: Townsville, Australia, 1986.
- Bujang, J.S.; Nazri, N.A.; Zakaria, M.H.; Arshad, A.; Ogawa, H. Morphological plasticity of Halodule species in response to different environments. Mar. Res. Indones. 2008, 33, 2–16. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Van der Velde, G.; Gorissen, M.; Meijer, G.; Van’t Hof, T.; Den Hartog, C. Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes, using a visual census technique. Estuar. Coast. Shelf Sci. 2000, 51, 31–44. [Google Scholar] [CrossRef]
- Ondiviela, B.; Losada, I.J.; Lara, J.L.; Maza, M.; Galván, C.; Bouma, T.J.; van Belzen, J. The role of seagrasses in coastal protection in a changing climate. Coast. Eng. 2014, 87, 158–168. [Google Scholar] [CrossRef]
- Potouroglou, M.; Bull, J.C.; Krauss, K.W.; Kennedy, H.A.; Fusi, M.; Daffonchio, D.; Mangora, M.M.; Githaiga, M.N.; Diele, K.; Huxham, M. Measuring the role of seagrasses in regulating sediment surface elevation. Sci. Rep. 2017, 7, 11917. [Google Scholar] [CrossRef] [PubMed]
- Short, F.; Carruthers, T.; Waycott, M.; Kendrick, G.; Fourqurean, J.; Callabine, A.; Kenworthy, W.; Dennison, W. Halodule Uninervis; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Mahdy, A.; Ghallab, A.; Madkour, H.; Osman, A. Status of Seagrass community in Northern Protected Islands, Hurghada, Red Sea, Egypt. Aquat. Sci. Fish Resour. (ASFR) 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Al-Rousan, S.; Al-Horani, F.; Eid, E.; Khalaf, M. Assessment of seagrass communities along the Jordanian coast of the Gulf of Aqaba, Red Sea. Mar. Biol. Res. 2011, 7, 93–99. [Google Scholar] [CrossRef]
- Jupp, B.; Durako, M.J.; Kenworthy, W.; Thayer, G.; Schillak, L. Distribution, abundance, and species composition of seagrasses at several sites in Oman. Aquat. Bot. 1996, 53, 199–213. [Google Scholar] [CrossRef]
- Abdelbary, E.M.; Al Ashwal, A.A. Distribution and Abundance of Seagrasses in Qatar Marine Zone. In The Arabian Seas: Biodiversity, Environmental Challenges and Conservation Measures; Springer: Berlin/Heidelberg, Germany, 2021; pp. 327–362. [Google Scholar] [CrossRef]
- Qurban, M.A.B.; Karuppasamy, M.; Krishnakumar, P.K.; Garcias-Bonet, N.; Duarte, C.M. Seagrass distribution, composition and abundance along the Saudi Arabian coast of Red Sea. In Oceanographic and Biological Aspects of the Red Sea; Springer: Berlin/Heidelberg, Germany, 2019; pp. 367–385. [Google Scholar] [CrossRef]
- Bandeira, S. Diversity and distribution of seagrasses around Inhaca Island, southern Mozambique. S. Afr. J. Bot. 2002, 68, 191–198. [Google Scholar] [CrossRef]
- Mcmillan, C. Flowering under controlled conditions by Cymodocea serrulata, Halophila stipulacea, Syringodium isoetifolium, Zostera capensis and Thalassia hemprichii from Kenya. Aquat. Bot. 1980, 8, 323–336. [Google Scholar] [CrossRef]
- Ragavan, P.; Jayaraj, R.; Muruganantham, M.; Jeeva, C.; Ubare, V.V.; Saxena, A.; Mohan, P. Species composition and Distribution of Seagrasses of the Andaman and Nicobar Islands. Vegetos 2016, 29, 78–87. Available online: https://www.researchgate.net/profile/Sivaperuman-Chandrakasan/publication/377267675_Species_Abundance_and_Distribution_of_Coastal_and_Marine_Bird_of_India/links/659e03e12468df72d3063d30/Species-Abundance-and-Distribution-of-Coastal-and-Marine-Bird-of-India.pdf#page=83 (accessed on 24 June 2024).
- Daby, D. Some quantitative aspects of seagrass ecology in a coastal lagoon of Mauritius. Mar. Biol. 2003, 142, 193–203. [Google Scholar] [CrossRef]
- Kalugina-Gutnik, A.; Perestenko, L.; Titlyanova, T. Species composition, distribution and abundance of algae and seagrasses of the Seychelles Islands. Atoll Res. Bull. 1992, 369, 1–67. [Google Scholar] [CrossRef]
- Adharini, R.I.; Yuniarga, T.R.; Prasetya, N.L.; Rachman, F. Community Structure of Seagrass in Harapan Island, Seribu Islands, Indonesia. Ilmu Kelaut. Indones. J. Mar. Sci. 2022, 27, 20–28. [Google Scholar] [CrossRef]
- Sidik, B.J.; Harah, Z.M.; Pauzi, A.M.; Madhavan, S. Halodule species from Malaysia—Distribution and morphological variation. Aquat. Bot. 1999, 65, 33–45. [Google Scholar] [CrossRef]
- Castillejos, J.; Collantes, C.A.; Trinchera, H.; Morata-Fuentes, J. Community Structure of Seagrasses in Cuatro Islas, Philippines. Int. Proc. Chem. Biol. Environ. Eng. 2018, 103, 55. [Google Scholar] [CrossRef]
- Prathep, A.; Rattanachot, E.; Tuntiprapas, P. Seasonal variations in seagrass percentage cover and biomass at Koh Tha Rai, Nakhon Si Thammarat Province, Gulf of Thailand. Sonklanakarin J. Sci. Technol. 2010, 32, 497. Available online: https://www.thaiscience.info/journals/Article/SONG/10660119.pdf (accessed on 24 June 2024).
- Nguyen, H.D.; Nguyen, X.H.; Pham, H.T.; Nguyen, T.L. Seagrass beds along the southern coast of Vietnam and their significance for associated flora and fauna. Collect. Mar. Res. Work. 2000, 10, 149–190. [Google Scholar]
- Huang, X.; Huang, L.; Li, Y.; Xu, Z.; Fong, C.; Huang, D.; Han, Q.; Huang, H.; Tan, Y.; Liu, S. Main seagrass beds and threats to their habitats in the coastal sea of South China. Chin. Sci. Bull. 2006, 51, 136–142. [Google Scholar] [CrossRef]
- McKenzie, L.J.; Yoshida, R.L.; Aini, J.W.; Andréfouet, S.; Colin, P.L.; Cullen-Unsworth, L.C.; Hughes, A.T.; Payri, C.E.; Rota, M.; Shaw, C. Seagrass ecosystems of the Pacific Island Countries and Territories: A global bright spot. Mar. Pollut. Bull. 2021, 167, 112308. [Google Scholar] [CrossRef] [PubMed]
- Khalafallah, A.A.; Geneid, Y.A.; Shaetaey, S.A.; Shaaban, B. Responses of the seagrass Halodule uninervis (Forssk.) Aschers. to hypersaline conditions. Egypt. J. Aquat. Res. 2013, 39, 167–176. [Google Scholar] [CrossRef]
- Short, F.T. World Atlas of Seagrasses; Univ of California Press: Berkeley, CA, USA, 2003. [Google Scholar]
- Den Hartog, C. The Sea-Grasses of the World; North-Holland: Amsterdam, The Netherlands, 1970. Available online: https://pdf.usaid.gov/pdf_docs/PNAAM467.pdf (accessed on 30 June 2024).
- Lefcheck, J.S.; Hughes, B.B.; Johnson, A.J.; Pfirrmann, B.W.; Rasher, D.B.; Smyth, A.R.; Williams, B.L.; Beck, M.W.; Orth, R.J. Are coastal habitats important nurseries? A meta-analysis. Conserv. Lett. 2019, 12, e12645. [Google Scholar] [CrossRef]
- Heck Jr, K.; Hays, G.; Orth, R.J. Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar. Ecol. Prog. Ser. 2003, 253, 123–136. [Google Scholar] [CrossRef]
- Singh, S.; Lal, M.M.; Southgate, P.C.; Wairiu, M.; Singh, A. Trace metal content in sediment cores and seagrass biomass from a tropical southwest Pacific Island. Mar. Pollut. Bull. 2021, 171, 112745. [Google Scholar] [CrossRef] [PubMed]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- De Boer, W. Seagrass–sediment interactions, positive feedbacks and critical thresholds for occurrence: A review. Hydrobiologia 2007, 591, 5–24. [Google Scholar] [CrossRef]
- Ahmed, F.S.; Mahmoud, A.-B.S.E.-D.; EL-Swaify, Z.A.; Salah El-Din, R.A. A comparative Evaluation of Phytochemical and Antimicrobial Properties of Selected Aquatic and Terrestrial Halophyte Plants Growing in Egypt. Int. J. Theor. Appl. Res. 2023, 2, 169–182. [Google Scholar] [CrossRef]
- Wehbe, N.; Badran, A.; Baydoun, S.; Al-Sawalmih, A.; Maresca, M.; Baydoun, E.; Mesmar, J.E. The Antioxidant Potential and Anticancer Activity of Halodule uninervis Ethanolic Extract against Triple-Negative Breast Cancer Cells. Antioxidants 2024, 13, 726. [Google Scholar] [CrossRef]
- Ahmed, F.S.; El-Saied, A.-B.S.E.-D.; Salah El Din, R.A.E.-l.; El Swaify, Z.A. Phytochemical screening and anticancer activities of some terrestrial and aquatic plants growing in saline habitat. Al-Azhar J. Agric. Res. 2023, 48, 89–106. [Google Scholar] [CrossRef]
- Wan Hazma, W.; Muta Harah, Z.; Japar Sidik, B.; Natrah, F. Macro and micro nutrients of tropical seagrasses, Halophila ovalis, H. spinulosa and Halodule uninervis in Johore, Malaysia. Iran. J. Fish. Sci. 2015, 14, 246–261. [Google Scholar]
- Immaculate, J.; Lilly, T.; Patterson, J. Macro and micro nutrients of seagrass species from Gulf of Mannar, India. MOJ Food Process Technol. 2018, 6, 391–398. [Google Scholar] [CrossRef]
- Pradheeba, M.; Dilipan, E.; Nobi, E.; Thangaradjou, T.; Sivakumar, K. Evaluation of Seagrasses for Their Nutritional Value. 2011. Available online: https://api.semanticscholar.org/CorpusID:56039382 (accessed on 25 June 2024).
- Baehaki, A.; Supriadi, A.; Pratama, M.C. Antioxidant Activity of Methanol Extract of Halodule uninervis Seagrass from the Coastal of Lampung, Indonesia. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1173–1177. Available online: http://repository.unsri.ac.id/id/eprint/105203 (accessed on 24 June 2024).
- Ramah, S.; Etwarysing, L.; Auckloo, N.; Gopeechund, A.; Bhagooli, R.; Bahorun, T. Prophylactic antioxidants and phenolics of seagrass and seaweed species: A seasonal variation study in a Southern Indian Ocean Island, Mauritius. Internet J. Med. Update-EJOURNAL 2014, 9, 27–37. Available online: https://www.ajol.info/index.php/ijmu/article/view/101361 (accessed on 30 June 2024).
- Gumgumjee, N.M.; Bukhari, D.A.; Alshehri, W.A.; Hajar, A. Antibacterial activity of Halodule uninervis leaves extracts against some bacterial pathogens strains. Pharmacophore 2018, 9, 52–59. Available online: https://pharmacophorejournal.com/lujsxTa (accessed on 24 June 2024).
- Hamisi, M.I.; Mbusi, L.D.; Lyimo, T.J. Antibacterial activity against Salmonella typhi and phytochemical screening of seven seagrass species from the coast of Tanzania. West. Indian Ocean J. Mar. Sci. 2023, 22, 83–93. Available online: https://www.ajol.info/index.php/wiojms/article/view/241179 (accessed on 24 June 2024).
- Mahyoub, J.A.; Aziz, A.T.; Panneerselvam, C.; Murugan, K.; Roni, M.; Trivedi, S.; Nicoletti, M.; Hawas, U.W.; Shaher, F.M.; Bamakhrama, M.A. Seagrasses as sources of mosquito nano-larvicides? Toxicity and uptake of Halodule uninervis-biofabricated silver nanoparticles in dengue and Zika virus vector Aedes aegypti. J. Clust. Sci. 2017, 28, 565–580. [Google Scholar] [CrossRef]
- Khattab, R.; Gaballa, A.; Zakaria, S.; Sallam, I.; Ali, A. Larvicidal effect of crude extracts of some marine plants (mangrove and seagrasses) on mosquitoes of Culex pipiens. Egypt. J. Aquat. Biol. Fish. 2012, 16, 99–105. [Google Scholar] [CrossRef][Green Version]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, M.; Rahman, P. Medicinal plants: Their use in anticancer treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103. [Google Scholar] [CrossRef] [PubMed]
- Baehaki, A.; Lestari, S.; Hendri, M.; Ariska, F. Antidiabetic Activity with N-Hexane, Ethyl-Acetate and Ethanol Extract of Halodule uninervis Seagrass. Pharmacogn. J. 2020, 12, 805–808. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Sundarapandian, M. Antidiabetic activity of methanolic extract of Halodule uninervis in streptozotocin-induced diabetic mice. J. Pharm. Sci. Res. 2017, 9, 1864–1868. Available online: https://www.proquest.com/scholarly-journals/antidiabetic-activity-methanolic-extract-halodule/docview/1967755443/se-2?accountid=8555 (accessed on 24 June 2024).
- Mazari, S.A.; Ali, E.; Abro, R.; Khan, F.S.A.; Ahmed, I.; Ahmed, M.; Nizamuddin, S.; Siddiqui, T.H.; Hossain, N.; Mubarak, N.M. Nanomaterials: Applications, waste-handling, environmental toxicities, and future challenges–A review. J. Environ. Chem. Eng. 2021, 9, 105028. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of silver nanoparticles by chemical reduction method. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Alhamid, M.Z.; Hadi, B.S.; Khumaeni, A. Synthesis of silver nanoparticles using laser ablation method utilizing Nd: YAG laser. In Proceedings of the AIP Conference Proceedings, Surakarta, Indonesia, 20 July 2019. [Google Scholar]
- Mwenze, N.M.; Juma, M.; Maaza, M.; Birech, Z.; Dhlamini, M. Laser liquid ablation for silver nanoparticles synthesis and conjugation with hydroxychloroquine for COVID-19 treatment. In Materials Today: Proceedings; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Ali, I.; Qiang, T.Y.; Ilahi, N.; Adnan, M.; Sajjad, W. Green synthesis of silver nanoparticles by using bacterial extract and its antimicrobial activity against pathogens. Int. J. Biosci. 2018, 13, 1–5. [Google Scholar] [CrossRef]
- Feroze, N.; Arshad, B.; Younas, M.; Afridi, M.I.; Saqib, S.; Ayaz, A. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc. Res. Tech. 2020, 83, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef]
- Saggar, S.; Mir, P.A.; Kumar, N.; Chawla, A.; Uppal, J.; Kaur, A. Traditional and herbal medicines: Opportunities and challenges. Pharmacogn. Res. 2022, 14, 107–114. [Google Scholar] [CrossRef]
- Hossain, C.M.; Gera, M.; Ali, K.A. Current status and challenges of herbal drug development and regulatory aspect: A global perspective. Asian J. Pharm. Clin. Res 2022, 15, 31–41. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Amutha, V.; Aiswarya, D.; Deepak, P.; Selvaraj, R.; Tamilselvan, C.; Perumal, P.; Balasubramani, G. Toxicity potential evaluation of ethyl acetate extract of Cymodocea serrulata against the mosquito vectors vis-a-vis zebrafish embryos and Artemia salina cysts. S. Afr. J. Bot. 2023, 152, 230–239. [Google Scholar] [CrossRef]
- Kavitha, D.; Padmini, R.; Alekhya, V.; Chandravadivelu, G.; Dhanaraju, M.D. Comparative acute toxicity study of Syringodium isoetifolium on aquatic and rodent experimental animals. Hacet. Univ. J. Fac. Pharm. 2023, 43, 221–231. [Google Scholar] [CrossRef]
- Orno, T.G.; Rantesalu, A. Invitro citotoxicity assays of seagrass (Enhalus acoroides) methanol extract from Soropia Coastal waters Southeast Sulawesi Regency. Indones. J. Med. Lab. Sci. Technol. 2020, 2, 27–33. [Google Scholar] [CrossRef][Green Version]
- Amudha, P.; Vanitha, V. Toxicological, biochemical and histopathological evaluation of the ethanolic extract of seagrass-Enhalus acoroides in albino wistar rats. Biocatal. Agric. Biotechnol. 2019, 18, 101082. [Google Scholar] [CrossRef]
- Unsworth, R.K.; McKenzie, L.J.; Collier, C.J.; Cullen-Unsworth, L.C.; Duarte, C.M.; Eklöf, J.S.; Jarvis, J.C.; Jones, B.L.; Nordlund, L.M. Global challenges for seagrass conservation. Ambio 2019, 48, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M. The future of seagrass meadows. Environ. Conserv. 2002, 29, 192–206. [Google Scholar] [CrossRef]




| Kingdom | Plantae |
|---|---|
| Phylum | Tracheophyta |
| Class | Magnoliopsida |
| Order | Alismatales |
| Family | Cymodoceaceae |
| Genus | Halodule |
| Species | Halodule uninervis |
| Binomial name | Halodule uninervis (Forsskål) Ascherson |
| Extract | Analytical Methods | Main Results | Compounds | Reference |
|---|---|---|---|---|
| Methanolic extract of the leaves | HPLC | 8 phenolic compounds, 3 flavonoids, and 12 fatty acids |
| [15] |
| Ethanolic extract of the root and shoot parts | HPLC | 10 phenolic compounds and 9 flavonoids |
| [57] |
| Ethanolic extract of the leaves | LC-MS/MS | 4 phenolic compounds and 12 flavonoids |
| [56] |
| Extract | Dose | Methods | Observations | References |
|---|---|---|---|---|
| Methanolic extract | 500, 1000, 1500, and 2000 ppm | DPPH radical scavenging assay and Ferric reducing power (FRAP) |
| [61] |
| Methanolic extract | N/A | FRAP and Trolox equivalent antioxidant capacity (TEAC) assays |
| [62] |
| Methanol/chloroform extract, USM 1 content, phenolic extract | 100–1000 mg/mL | DPPH radical scavenging assay |
| [15] |
| Ethanolic extract of the leaves | 5, 10, 25, 50, 100, 200, and 400 μg/mL | DPPH radical scavenging assay |
| [56] |
| Extract | Dose | Experimental Model | Main Results | Reference |
|---|---|---|---|---|
| Methanolic extract of the leaves | 500, 1000, 1500, and 2000 ppm |
|
| [14] |
| Aqueous extract of the leaves | N/A |
|
| [63] |
| Extract of the leaves in organic solvents (chloroform, ethanol, ethyl acetate, petroleum ether) | N/A |
|
| [63] |
| Ethanolic extract | 10 mg/mL |
|
| [55] |
| Extract of the leaves or roots in methanol, dichloromethane, and hexane | 100 mg/mL |
|
| [64] |
| H. uninervis-synthesized AgNPs 1 | 25, 50, 100 ppm |
|
| [65] |
| Extract | Dose | Methods | Main Results | Reference |
|---|---|---|---|---|
| Ethyl acetate extract | 0–8 ppm |
|
| [66] |
| Methanolic extract | 50, 100, 300, 500, 700 ppm |
|
| [65] |
| H. uninervis-synthesized AgNPs 1 | 5, 10, 15, 20, 25 ppm |
|
| [65] |
| Extract | Dose | Experimental Model | Observations | References |
|---|---|---|---|---|
| Ethyl acetate extract | 25, 50, and 100 mg/mL |
|
| [16] |
| Methanol/chloroform extract | 100–1000 mg/mL |
|
| [15] |
| Chloroform fraction | 100–1000 mg/mL |
|
| [15] |
| FAs content | 100–1000 mg/mL |
|
| [15] |
| USM 3 content | 100–1000 mg/mL |
|
| [15] |
| Ethanolic extract of the leaves | 100 and 200 μg/mL |
|
| [56] |
| Extract | Dose | Experimental Model | Observations | References |
|---|---|---|---|---|
| Ethanolic extract | 125, 250, 500, 1000, and 2000 ppm | In vitro inhibition of α-glucosidase enzyme assay |
| [69] |
| Ethyl acetate extract | 125, 250, 500, 1000, and 2000 ppm | In vitro inhibition of α-glucosidase enzyme assay |
| [69] |
| Methanolic extract | 150 and 250 mg/kg | Streptozotocin-induced diabetic mouse model |
| [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wehbe, N.; Bechelany, M.; Badran, A.; Al-Sawalmih, A.; Mesmar, J.E.; Baydoun, E. A Phytochemical Analysis and the Pharmacological Implications of the Seagrass Halodule uninervis: An Overview. Pharmaceuticals 2024, 17, 993. https://doi.org/10.3390/ph17080993
Wehbe N, Bechelany M, Badran A, Al-Sawalmih A, Mesmar JE, Baydoun E. A Phytochemical Analysis and the Pharmacological Implications of the Seagrass Halodule uninervis: An Overview. Pharmaceuticals. 2024; 17(8):993. https://doi.org/10.3390/ph17080993
Chicago/Turabian StyleWehbe, Nadine, Mikhael Bechelany, Adnan Badran, Ali Al-Sawalmih, Joelle Edward Mesmar, and Elias Baydoun. 2024. "A Phytochemical Analysis and the Pharmacological Implications of the Seagrass Halodule uninervis: An Overview" Pharmaceuticals 17, no. 8: 993. https://doi.org/10.3390/ph17080993
APA StyleWehbe, N., Bechelany, M., Badran, A., Al-Sawalmih, A., Mesmar, J. E., & Baydoun, E. (2024). A Phytochemical Analysis and the Pharmacological Implications of the Seagrass Halodule uninervis: An Overview. Pharmaceuticals, 17(8), 993. https://doi.org/10.3390/ph17080993








