In Silico and In Vivo Studies of β-Sitosterol Nanoparticles as a Potential Therapy for Isoprenaline-Induced Cognitive Impairment in Myocardial Infarction, Targeting Myeloperoxidase
Abstract
1. Introduction
2. Results
2.1. Molecular Docking
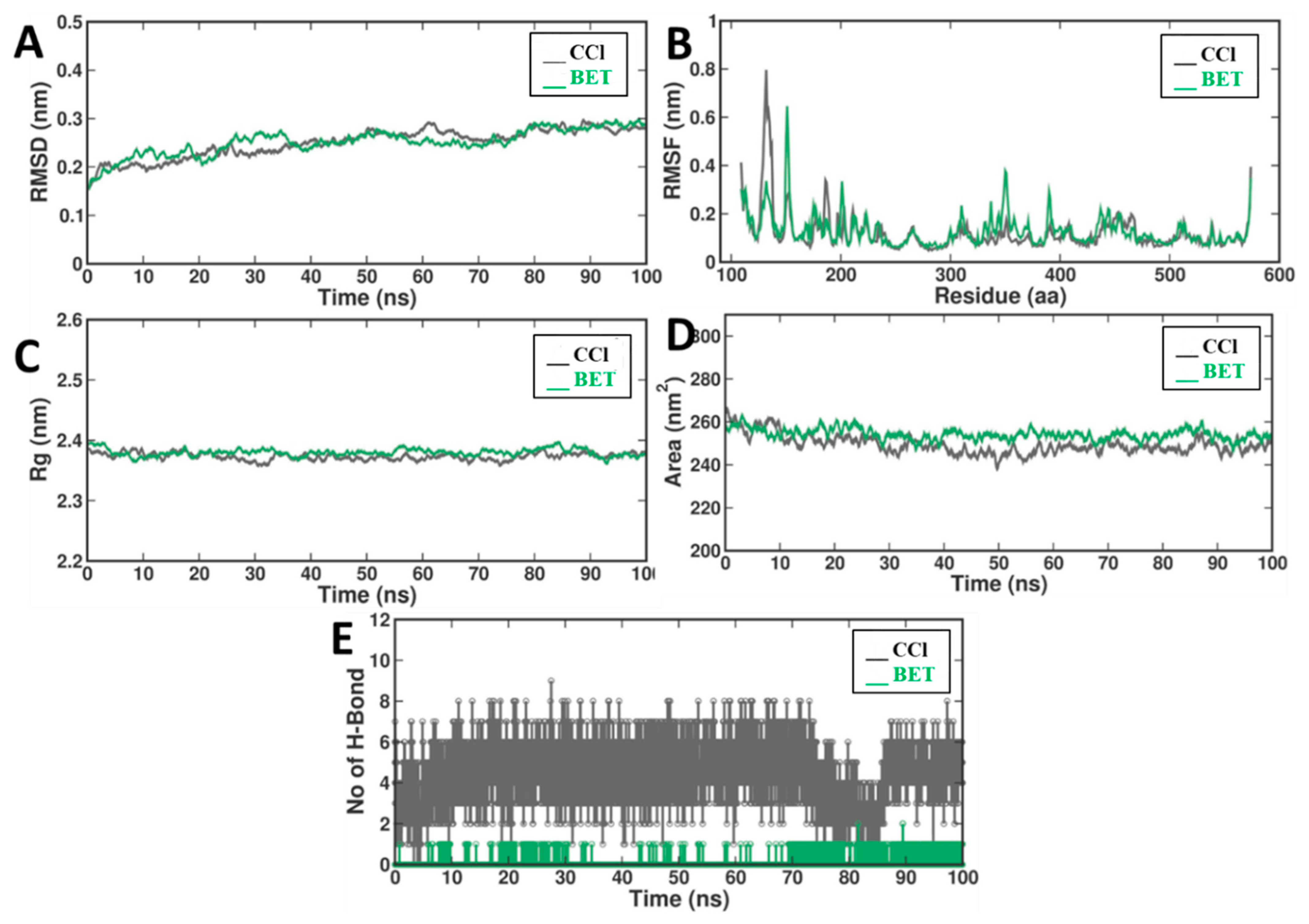
2.2. Molecular Dynamics
2.2.1. Root Mean Square Deviation (RMSD)
2.2.2. Root Mean Square Fluctuation (RMSF)
2.2.3. Radius of Gyration (Rg)
2.2.4. Solvent Accessible Surface Area (SASA)
2.2.5. Interhydrogen Bonds
2.2.6. MM-PBSA
2.3. Characterization of BETNs
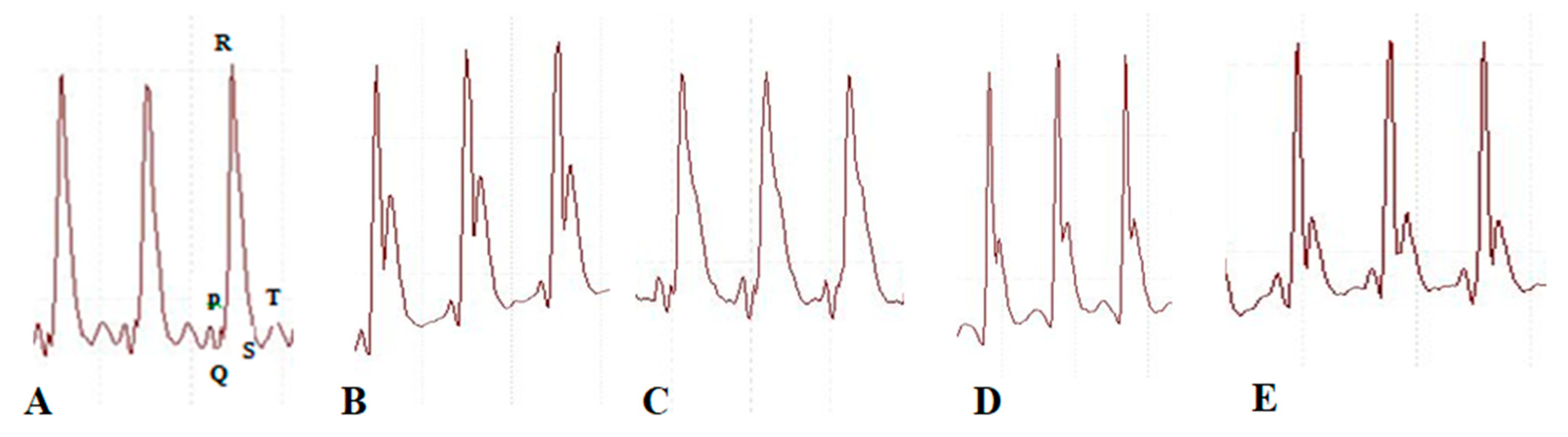
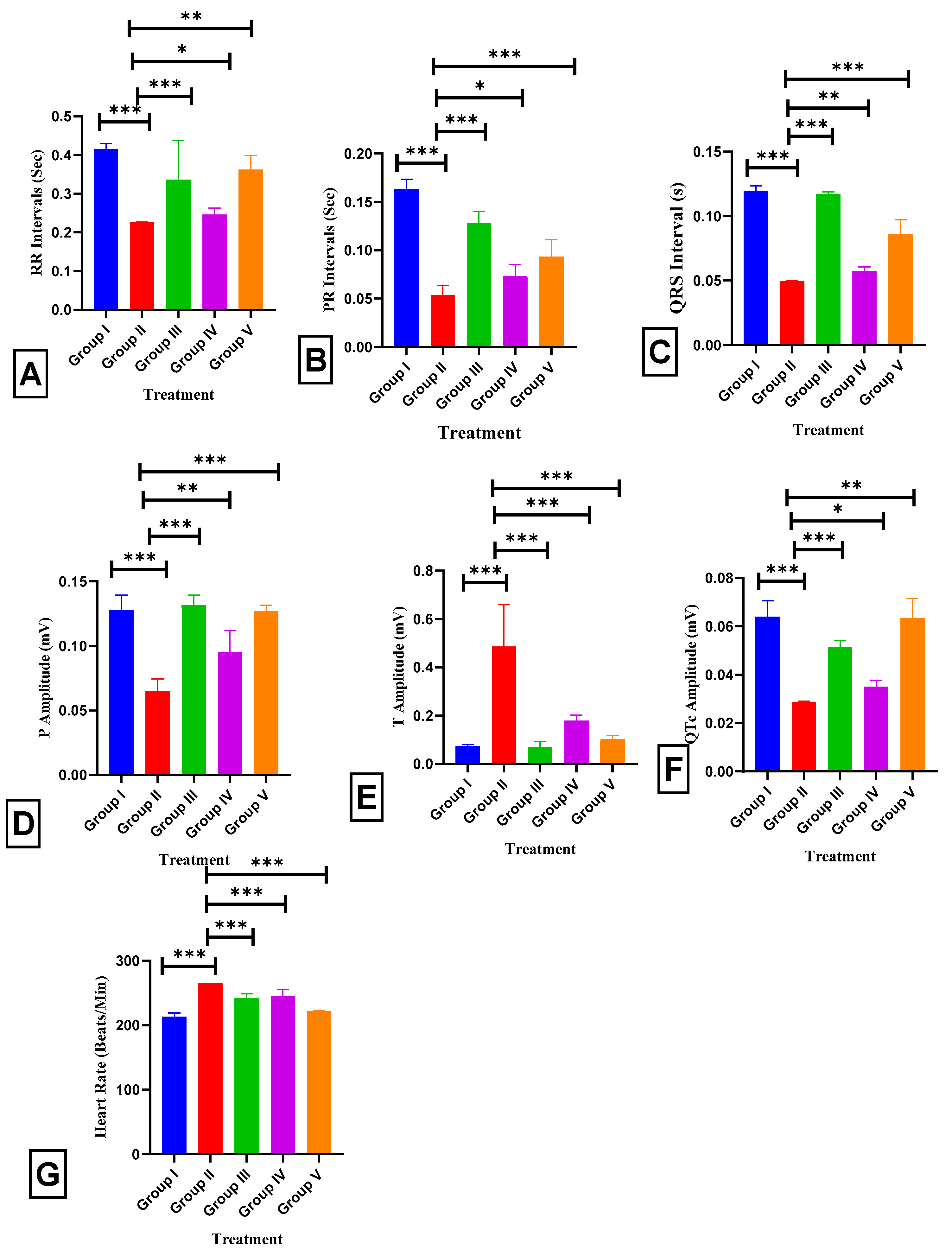
2.4. Effect of BETNs on ECG in ISO-Treated Rats
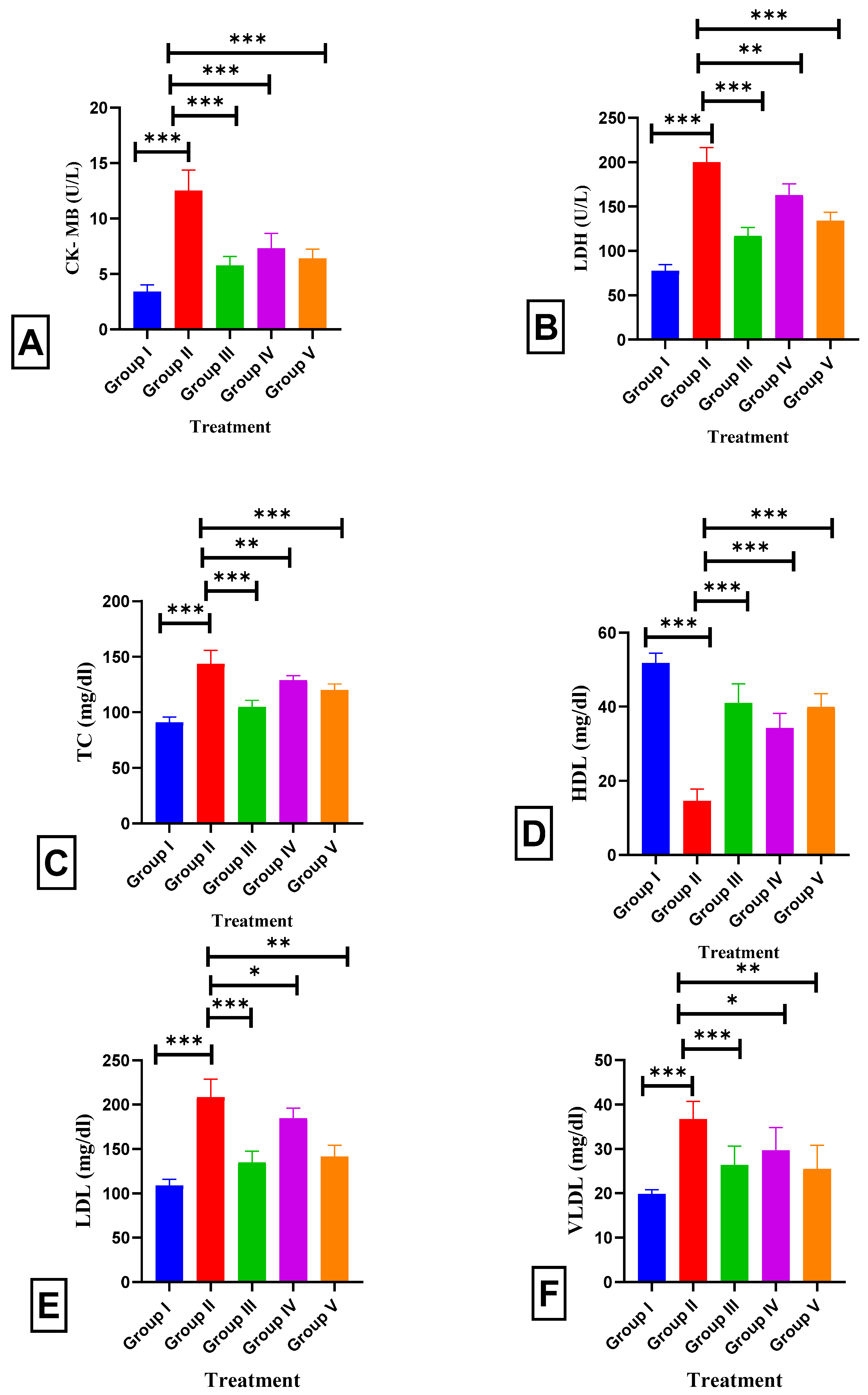
2.5. Effect of BETNs on Cardiac Parameters and Lipid Profiles in ISO-Treated Rats
2.6. Effect of BETNs on Cognitive Function
2.6.1. MWM Test
2.6.2. Pole Climb Test
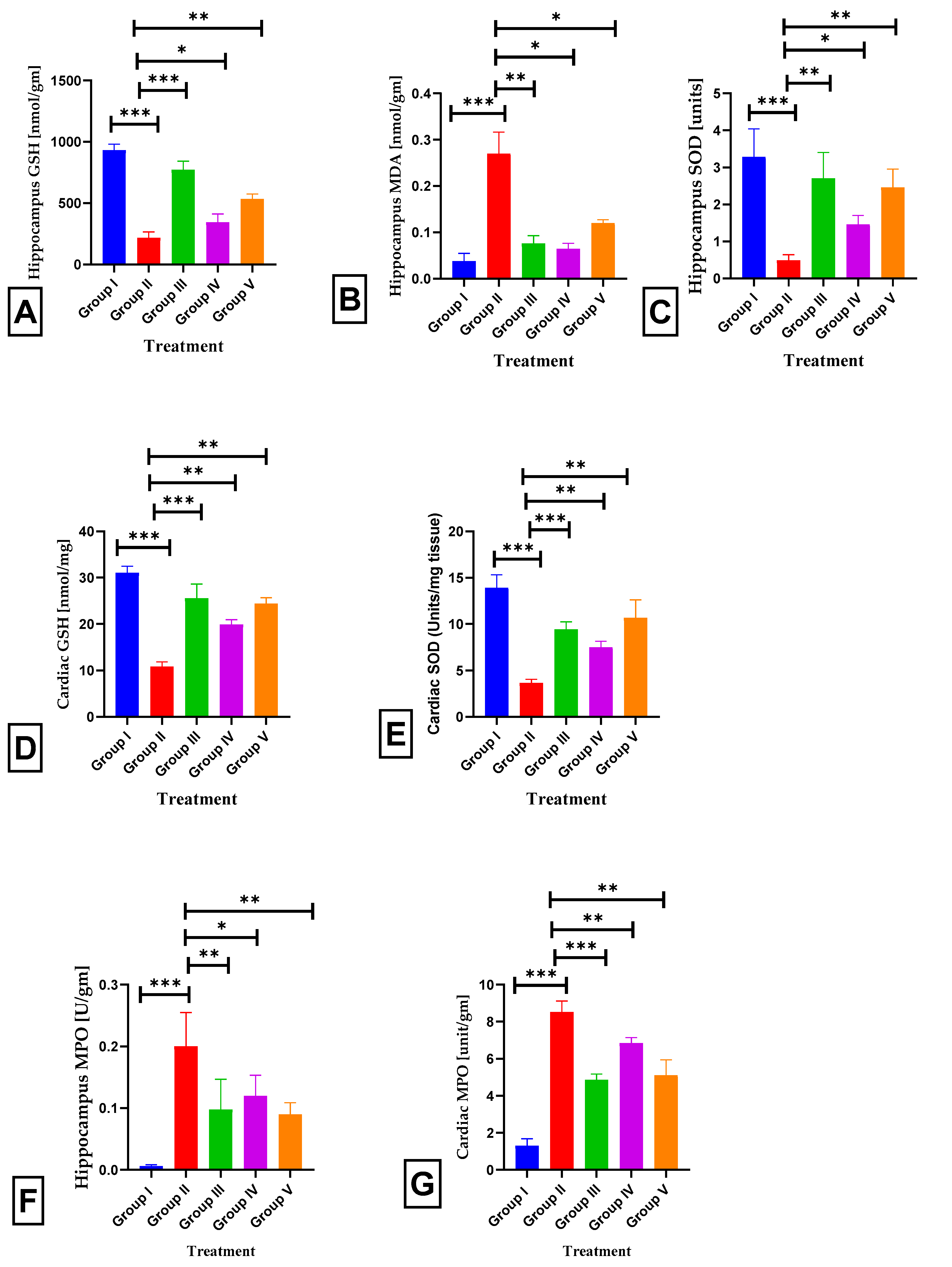
2.7. Effects of BETNs on Hippocampal and Cardiac Biochemical Parameters in ISO-Treated Rats
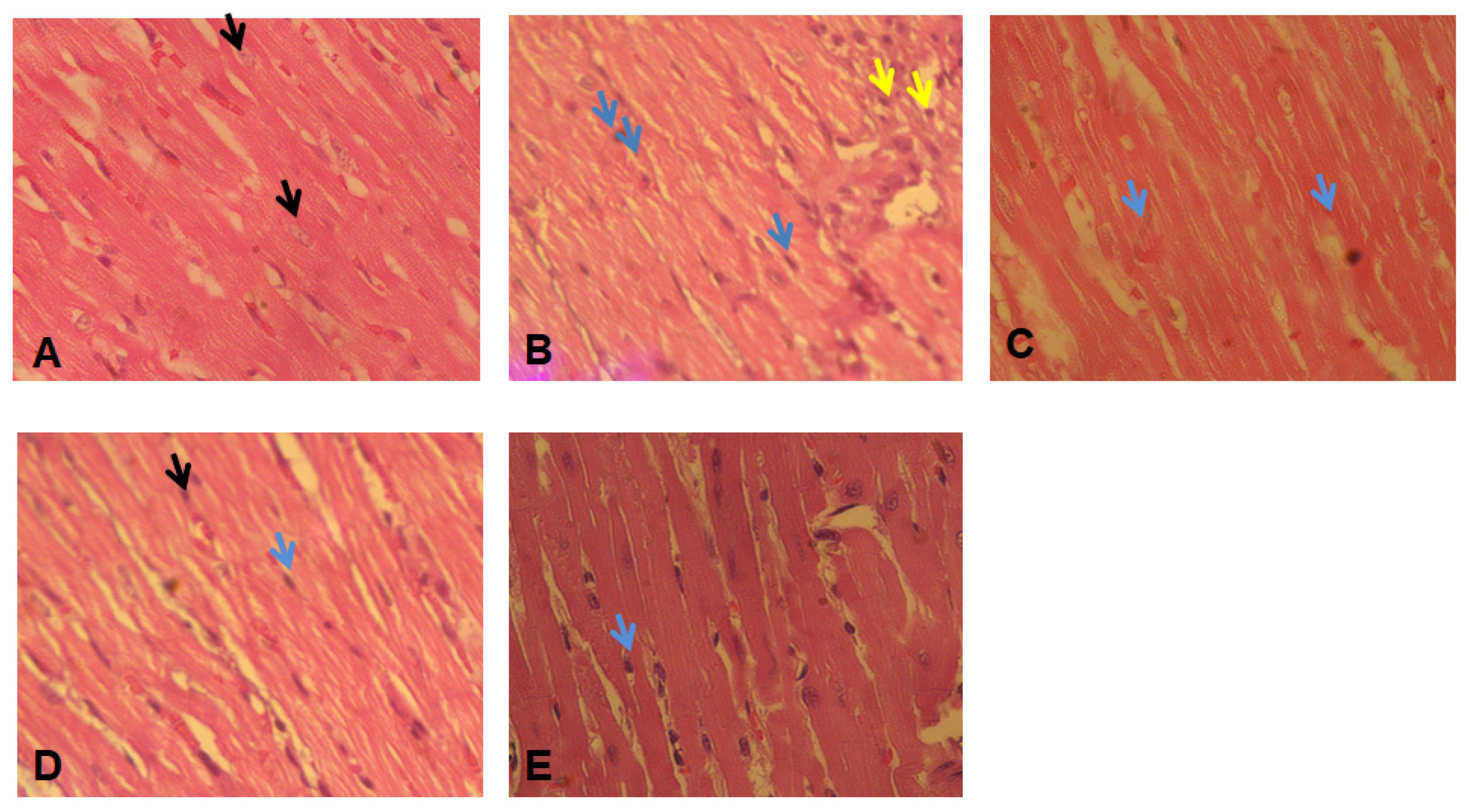
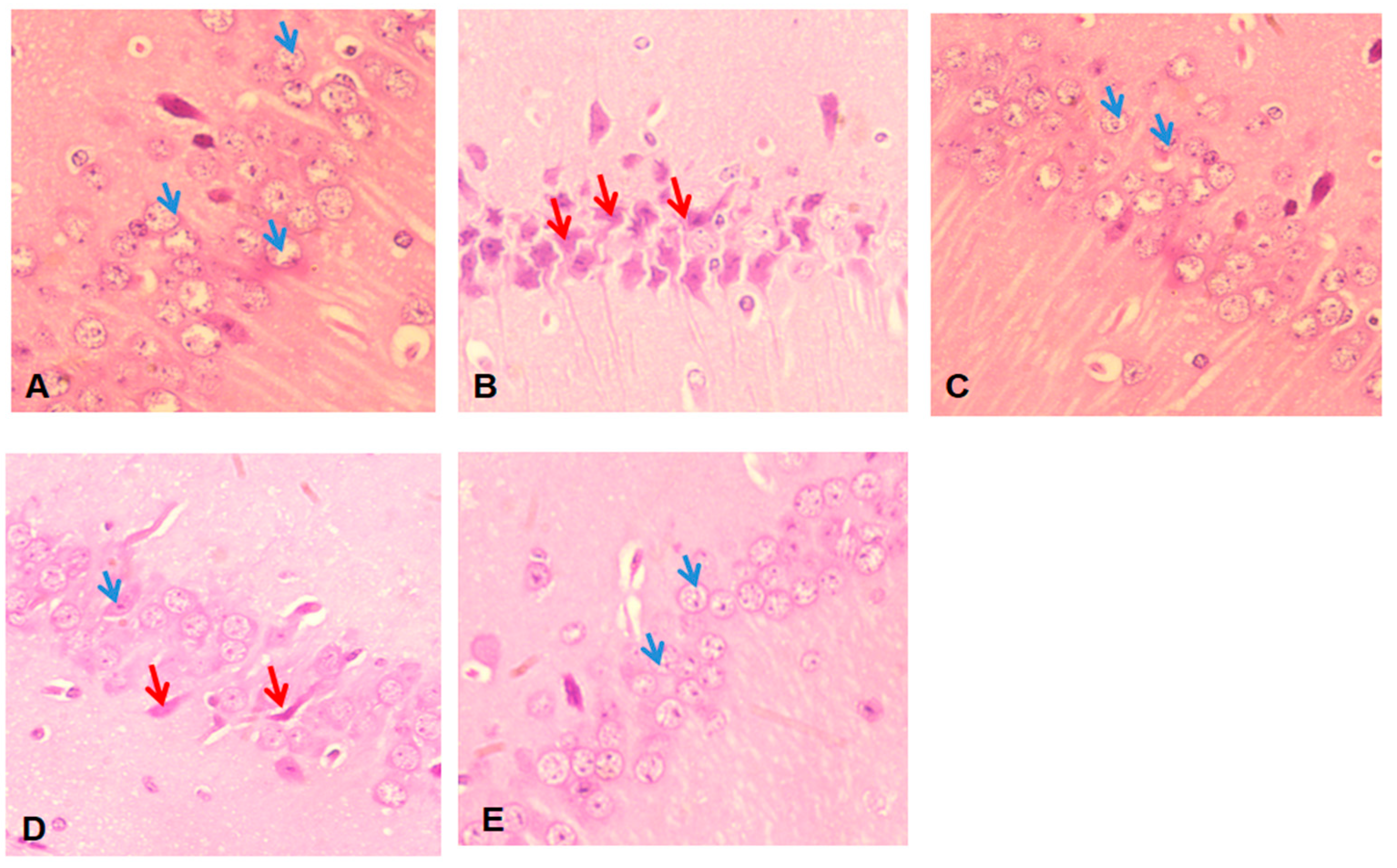
2.8. Histopathological Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. In Silico Studies
4.2.1. Molecular Docking
4.2.2. Molecular Dynamics
4.3. Preparation of BETNs
4.4. Animals
4.5. Experimental Procedure
4.6. Lipid Profile Analysis
4.7. Electrocardiogram Analysis
4.8. Estimation of Tissue Parameters
4.9. Histological Studies
4.10. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.Z.; Chong, C.L.; Chuah, S.C.; Huang, S.H.; Nai, H.S.; Tong, H.T.; Whiteman, M.; Moore, P.K. Cardioprotective effects of nitroparacetamol and paracetamol in acute phase of myocardial infarction in experimental rats. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H517–H524. [Google Scholar] [CrossRef] [PubMed]
- Bagatini, M.D.; Martins, C.C.; Battisti, V.; Gasparetto, D.; da Rosa, C.S.; Spanevello, R.M.; Ahmed, M.; Schmatz, R.; Schetinger, M.R.; Morsch, V.M. Oxidative stress versus antioxidant defenses in patients with acute myocardial infarction. Heart Vessel 2011, 26, 55–63. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.L.; Liu, H.R.; Rose, P.; Zhu, Y.Z. Protective effects of cysteine analogues on acute myocardial ischemia: Novel modulators of endogenous H(2)S production. Antioxid. Redox Signal 2010, 12, 1155–1165. [Google Scholar] [CrossRef]
- Kim, Y.H.; Chun, Y.S.; Park, J.W.; Kim, C.H.; Kim, M.S. Involvement of adrenergic pathways in activation of catalase by myocardial ischemia-reperfusion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R1450–R1458. [Google Scholar] [CrossRef][Green Version]
- Jefferson, A.L.; Himali, J.J.; Au, R.; Seshadri, S.; Decarli, C.; O’Donnell, C.J.; Wolf, P.A.; Manning, W.J.; Beiser, A.S.; Benjamin, E.J. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study). Am. J. Cardiol. 2011, 108, 1346–1351. [Google Scholar] [CrossRef]
- Laudisio, A.; Marzetti, E.; Pagano, F.; Cocchi, A.; Bernabei, R.; Zuccala, G. Digoxin and cognitive performance in patients with heart failure: A cohort, pharmacoepidemiological survey. Drugs Aging 2009, 26, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Dikic, A.; Radmilo, L.; Zivanovic, Z.; Kekovic, G.; Sekulic, S.; Kovacic, Z.; Radmilo, R. Cognitive impairment and depression after acute myocardial infarction: Associations with ejection fraction and demographic characteristics. Acta Neurol. Belg. 2021, 121, 1615–1622. [Google Scholar] [CrossRef]
- Sokoreli, I.; Pauws, S.C.; Steyerberg, E.W.; de Vries, G.J.; Riistama, J.M.; Tesanovic, A.; Kazmi, S.; Pellicori, P.; Cleland, J.G.; Clark, A.L. Prognostic value of psychosocial factors for first and recurrent hospitalizations and mortality in heart failure patients: Insights from the OPERA-HF study. Eur. J. Heart Fail. 2018, 20, 689–696. [Google Scholar] [CrossRef]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef]
- Tang, W.H.; Shrestha, K.; Troughton, R.W.; Borowski, A.G.; Klein, A.L. Integrating plasma high-sensitivity C-reactive protein and myeloperoxidase for risk prediction in chronic systolic heart failure. Congest. Heart Fail. 2011, 17, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Rafaqat, S.; Afzal, S.; Khurshid, H.; Safdar, S.; Rafaqat, S.; Rafaqat, S. The Role of Major Inflammatory Biomarkers in the Pathogenesis of Atrial Fibrillation. J. Innov. Card. Rhythm Manag. 2022, 13, 5265–5277. [Google Scholar] [CrossRef] [PubMed]
- Zammit, A.R.; Katz, M.J.; Bitzer, M.; Lipton, R.B. Cognitive Impairment and Dementia in Older Adults with Chronic Kidney Disease: A Review. Alzheimer Dis. Assoc. Disord. 2016, 30, 357–366. [Google Scholar] [CrossRef]
- Alemasi, A.; Cao, N.; An, X.; Wu, J.; Gu, H.; Yu, H.; Song, Y.; Wang, H.; Zhang, Y.; Xiao, H.; et al. Exercise Attenuates Acute beta-Adrenergic Overactivation-Induced Cardiac Fibrosis by Modulating Cytokines. J. Cardiovasc. Transl. Res. 2019, 12, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Nichtova, Z.; Novotova, M.; Kralova, E.; Stankovicova, T. Morphological and functional characteristics of models of experimental myocardial injury induced by isoproterenol. Gen. Physiol. Biophys. 2012, 31, 141–151. [Google Scholar] [CrossRef]
- Tkachenko, V.; Kovalchuk, Y.; Bondarenko, N.; Bondarenko, O.; Ushakova, G.; Shevtsova, A. The Cardio- and Neuroprotective Effects of Corvitin and 2-Oxoglutarate in Rats with Pituitrin-Isoproterenol-Induced Myocardial Damage. Biochem. Res. Int. 2018, 2018, 9302414. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, X.; Zhang, T.; Chen, C.; Dong, X.; Can, Y.; Liu, P. Behavioral and Biochemical Effects of KXS on Postmyocardial Infarction Depression. Front. Pharmacol. 2020, 11, 561817. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Han, B.; Li, Z.; Wang, B.; Jiang, P.; Zhang, J.; Ma, W.; Zhou, D.; Li, X.; et al. Anti-breast-Cancer Activity Exerted by beta-Sitosterol-d-glucoside from Sweet Potato via Upregulation of MicroRNA-10a and via the PI3K-Akt Signaling Pathway. J. Agric. Food Chem. 2018, 66, 9704–9718. [Google Scholar] [CrossRef]
- Amanat, M.; Daula, A.; Singh, R. Potential Antidiabetic Activity of beta-sitosterol from Zingiber roseum Rosc. via Modulation of Peroxisome Proliferator-activated Receptor Gamma (PPARgamma). Comb. Chem. High. Throughput Screen. 2024, 27, 1676–1699. [Google Scholar] [CrossRef]
- Shokry, S.; Hegazy, A.; Abbas, A.M.; Mostafa, I.; Eissa, I.H.; Metwaly, A.M.; Yahya, G.; El-Shazly, A.M.; Aboshanab, K.M.; Mostafa, A. Phytoestrogen beta-Sitosterol Exhibits Potent In Vitro Antiviral Activity against Influenza A Viruses. Vaccines 2023, 11, 228. [Google Scholar] [CrossRef]
- Anwar, R.; Sukmasari, S.; Siti Aisyah, L.; Puspita Lestari, F.; Ilfani, D.; Febriani Yun, Y.; Diki Prestya, P. Antimicrobial Activity of beta-Sitosterol Isolated from Kalanchoe tomentosa Leaves Against Staphylococcus aureus and Klebsiella pneumonia. Pak. J. Biol. Sci. 2022, 25, 602–607. [Google Scholar] [CrossRef]
- Pineda-Alegria, J.A.; Sanchez, J.E.; Gonzalez-Cortazar, M.; von Son-de Fernex, E.; Gonzalez-Garduno, R.; Mendoza-de Gives, P.; Zamilpa, A.; Aguilar-Marcelino, L. In vitro nematocidal activity of commercial fatty acids and beta-sitosterol against Haemonchus contortus. J. Helminthol. 2020, 94, e135. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J.Y.; Park, J.H.; Jung, H.S.; Kim, J.S.; Kang, S.S.; Kim, Y.S.; Han, Y. Immunoregulatory activity by daucosterol, a beta-sitosterol glycoside, induces protective Th1 immune response against disseminated Candidiasis in mice. Vaccine 2007, 25, 3834–3840. [Google Scholar] [CrossRef]
- Day, C.E. Hypocholesterolemic activity of beta-sitosterol in cholesterol fed sea quail. Artery 1991, 18, 125–132. [Google Scholar]
- Chauhan, S.; Sharma, A.; Upadhyay, N.K.; Singh, G.; Lal, U.R.; Goyal, R. In-vitro osteoblast proliferation and in-vivo anti-osteoporotic activity of Bombax ceiba with quantification of Lupeol, gallic acid and beta-sitosterol by HPTLC and HPLC. BMC Complement. Altern. Med. 2018, 18, 233. [Google Scholar] [CrossRef]
- Shi, J.; Vakoc, C.R. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol. Cell 2014, 54, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Duchateau, G.; Cochrane, B.; Windebank, S.; Herudzinska, J.; Sanghera, D.; Burian, A.; Muller, M.; Zeitlinger, M.; Lappin, G. Absolute oral bioavailability and metabolic turnover of beta-sitosterol in healthy subjects. Drug Metab. Dispos. 2012, 40, 2026–2030. [Google Scholar] [CrossRef]
- Praveen Kumar, P.; Sunil Kumar, K.T.; Kavya Nainita, M.; Sai Tarun, A.; Raghu Ramudu, B.G.; Deepika, K.; Pramoda, A.; Yasmeen, C. Cerebroprotective Potential of Hesperidin Nanoparticles Against Bilateral Common Carotid Artery Occlusion Reperfusion Injury in Rats and In silico Approaches. Neurotox. Res. 2020, 37, 264–274. [Google Scholar] [CrossRef] [PubMed]
- K, P.; Prasanth, D.S.N.B.K.; Shadakshara, M.K.R.; Ahmad, S.F.; Seemaladinne, R.; Rudrapal, M.; Pasala, P.K. Citronellal as a Promising Candidate for Alzheimer’s Disease Treatment: A Comprehensive Study on In Silico and In Vivo Anti-Acetylcholine Esterase Activity. Metabolites 2023, 13, 1133. [Google Scholar] [CrossRef]
- Saikia, S.; Bordoloi, M. Molecular Docking: Challenges, Advances and its Use in Drug Discovery Perspective. Curr. Drug Targets 2019, 20, 501–521. [Google Scholar] [CrossRef]
- Fan, J.; Fu, A.; Zhang, L. Progress in molecular docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar]
- solaiman, a.; Abd-el-Moneim, R. Therapeutic effect of bone marrow derived mesenchymal stem cells versus their exosomes on isoproterenol induced ventricular myocardium changes in adult rats: A histological, immunohistochemical and biochemical study. Egypt. J. Histol. 2022, 46, 641–666. [Google Scholar] [CrossRef]
- Kumar, R.N.; Prasanth, D.; Midthuri, P.G.; Ahmad, S.F.; Badarinath, A.V.; Karumanchi, S.K.; Seemaladinne, R.; Nalluri, R.; Pasala, P.K. Unveiling the Cardioprotective Power: Liquid Chromatography–Mass Spectrometry (LC–MS)-Analyzed Neolamarckia cadamba (Roxb.) Bosser Leaf Ethanolic Extract against Myocardial Infarction in Rats and In Silico Support Analysis. Plants 2023, 12, 3722. [Google Scholar] [CrossRef]
- El Husseini, N.; Katzan, I.L.; Rost, N.S.; Blake, M.L.; Byun, E.; Pendlebury, S.T.; Aparicio, H.J.; Marquine, M.J.; Gottesman, R.F.; Smith, E.E.; et al. Cognitive Impairment After Ischemic and Hemorrhagic Stroke: A Scientific Statement from the American Heart Association/American Stroke Association. Stroke 2023, 54, e272–e291. [Google Scholar] [CrossRef]
- Pasala, P.K.; Raghupathi, N.K.; Yaraguppi, D.A.; Challa, R.R.; Vallamkonda, B.; Ahmad, S.F.; Chennamsetty, Y.; Kumari, P.V.K.; Dsnbk, P. Potential preventative impact of aloe-emodin nanoparticles on cerebral stroke-associated myocardial injury by targeting myeloperoxidase: In supporting with In silico and In vivo studies. Heliyon 2024, 10, e33154. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Roux, B. Absolute binding free energy calculations using molecular dynamics simulations with restraining potentials. Biophys. J. 2006, 91, 2798–2814. [Google Scholar] [CrossRef]
- Jangwan, N.S.; Khan, M.; Das, R.; Altwaijry, N.; Sultan, A.M.; Khan, R.; Saleem, S.; Singh, M.F. From petals to healing: Consolidated network pharmacology and molecular docking investigations of the mechanisms underpinning Rhododendron arboreum flower’s anti-NAFLD effects. Front. Pharmacol. 2024, 15, 1366279. [Google Scholar] [CrossRef]
- Bharatham, N.; Finch, K.E.; Min, J.; Mayasundari, A.; Dyer, M.A.; Guy, R.K.; Bashford, D. Performance of a docking/molecular dynamics protocol for virtual screening of nutlin-class inhibitors of Mdmx. J. Mol. Graph. Model. 2017, 74, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Vamshi, G.; D, S.N.B.K.P.; Sampath, A.; Dammalli, M.; Kumar, P.; B, S.G.; Pasala, P.K.; Somasekhar, G.; Challa, M.C.; Alluril, R.; et al. Possible cerebroprotective effect of citronellal: Molecular docking, MD simulation and in vivo investigations. J. Biomol. Struct. Dyn. 2024, 42, 1208–1219. [Google Scholar] [CrossRef]
- Prasanth, D.; Murahari, M.; Chandramohan, V.; Panda, S.P.; Atmakuri, L.R.; Guntupalli, C. In silico identification of potential inhibitors from Cinnamon against main protease and spike glycoprotein of SARS CoV-2. J. Biomol. Struct. Dyn. 2021, 39, 4618–4632. [Google Scholar] [CrossRef]
- Guntupalli, C.; Ponugoti, M.; Prasanth, D.S.N.B.K.; Malothu, N. Cerebroprotective effects of khellin: Validation through computational studies in a bilateral common carotid artery occlusion/reperfusion (BCCAO/R) model. Mol. Simul. 2024, 50, 322–331. [Google Scholar] [CrossRef]
- Adediwura, V.A.; Koirala, K.; Do, H.N.; Wang, J.; Miao, Y. Understanding the impact of binding free energy and kinetics calculations in modern drug discovery. Expert Opin. Drug Discov. 2024, 19, 671–682. [Google Scholar] [CrossRef]
- Hammond, C.A.; Blades, N.J.; Chaudhry, S.I.; Dodson, J.A.; Longstreth, W.T., Jr.; Heckbert, S.R.; Psaty, B.M.; Arnold, A.M.; Dublin, S.; Sitlani, C.M.; et al. Long-Term Cognitive Decline After Newly Diagnosed Heart Failure: Longitudinal Analysis in the CHS (Cardiovascular Health Study). Circ. Heart Fail. 2018, 11, e004476. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pasala, P.; Donakonda, M.; Dintakurthi, P.S.; Rudrapal, M.; Gouru, S.A.; Ruksana, K. Investigation of Cardioprotective Activity of Silybin: Network Pharmacology, Molecular Docking, and In Vivo Studies. ChemistrySelect 2023, 8, e202300148. [Google Scholar]
- Pasala, P.K.; Dsnbk, P.; Rudrapal, M.; Challa, R.R.; Ahmad, S.F.; Vallamkonda, B.; R, R.B. Anti-Parkinson potential of hesperetin nanoparticles: In vivo and in silico investigations. Nat. Prod. Res. 2024, 1–10. [Google Scholar] [CrossRef]
- Mishra, A.; Das, S.; Kumari, S. Potential role of herbal plants and beta sitosterol as a bioactive constituent in circumventing Alzheimer’s Disease. Plant Sci. Today 2024, 11, 454–465. [Google Scholar]
- Adebiyi, O.E.; Olayemi, F.O.; Olopade, J.O.; Tan, N.-H. Βeta-sitosterol enhances motor coordination, attenuates memory loss and demyelination in a vanadium-induced model of experimental neurotoxicity. Pathophysiology 2019, 26, 21–29. [Google Scholar] [CrossRef]
- Jaiswal, S.; Anjum, M.M.; Arya, D.K.; Thakur, S.; Pandey, P.; Deepak, P.; Kanaujiya, S.; Anand, S.; Kaushik, A.S.; Mishra, V.; et al. Surface entrenched beta-sitosterol niosomes for enhanced cardioprotective activity against isoproterenol induced cardiotoxicity in rats. Int. J. Pharm. 2024, 653, 123872. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Al-Qattan, M.N.M.; Mordi, M.N. Development and application of fragment-based de novo inhibitor design approaches against Plasmodium falciparum GST. J. Mol. Model. 2023, 29, 281. [Google Scholar] [CrossRef]
- Almeida, V.M.; Dias, E.R.; Souza, B.C.; Cruz, J.N.; Santos, C.B.R.; Leite, F.H.A.; Queiroz, R.F.; Branco, A. Methoxylated flavonols from Vellozia dasypus Seub ethyl acetate active myeloperoxidase extract: In vitro and in silico assays. J. Biomol. Struct. Dyn. 2022, 40, 7574–7583. [Google Scholar] [CrossRef]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Gangadharappa, B.S.; Sharath, R.; Revanasiddappa, P.D.; Chandramohan, V.; Balasubramaniam, M.; Vardhineni, T.P. Structural insights of metallo-beta-lactamase revealed an effective way of inhibition of enzyme by natural inhibitors. J. Biomol. Struct. Dyn. 2020, 38, 3757–3771. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, D.; Murahari, M.; Chandramohan, V.; Guntupalli, C.; Atmakuri, L.R. Computational study for identifying promising therapeutic agents of hydroxychloroquine analogues against SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 40, 11822–11836. [Google Scholar] [CrossRef]
- Wei, D.; Wang, L.; Liu, C.; Wang, B. β-Sitosterol solubility in selected organic solvents. J. Chem. Eng. Data 2010, 55, 2917–2919. [Google Scholar]
- Haider, S.; Tabassum, S.; Perveen, T. Scopolamine-induced greater alterations in neurochemical profile and increased oxidative stress demonstrated a better model of dementia: A comparative study. Brain Res. Bull. 2016, 127, 234–247. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, L.H.; Jia, W.; Liu, X.M.; Dang, H.X.; Mai, W.L.; Wang, N.; Steinmetz, A.; Wang, Y.Q.; Xu, C.J. Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine-induced learning and memory impairment in mice. Phytother. Res. 2010, 24, 1748–1754. [Google Scholar] [CrossRef]
- Cook, L.; Weidley, E. Behavioral effects of some psychopharmacological agents. Ann. N. Y Acad. Sci. 1957, 66, 740–752. [Google Scholar] [CrossRef]
- Goverdhan, P.; Sravanthi, A.; Mamatha, T. Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int. J. Alzheimers Dis. 2012, 2012, 974013. [Google Scholar] [CrossRef]
- Bederson, J.B.; Pitts, L.H.; Tsuji, M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986, 17, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y. Reprint of: Generation of Superoxide Radical during Autoxidation of Hydroxylamine and an Assay for Superoxide Dismutase. Arch. Biochem. Biophys. 2022, 726, 109247. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Eiserich, J.P.; Hristova, M.; Cross, C.E.; Jones, A.D.; Freeman, B.A.; Halliwell, B.; van der Vliet, A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 1998, 391, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Nottingham, UK, 2008. [Google Scholar]
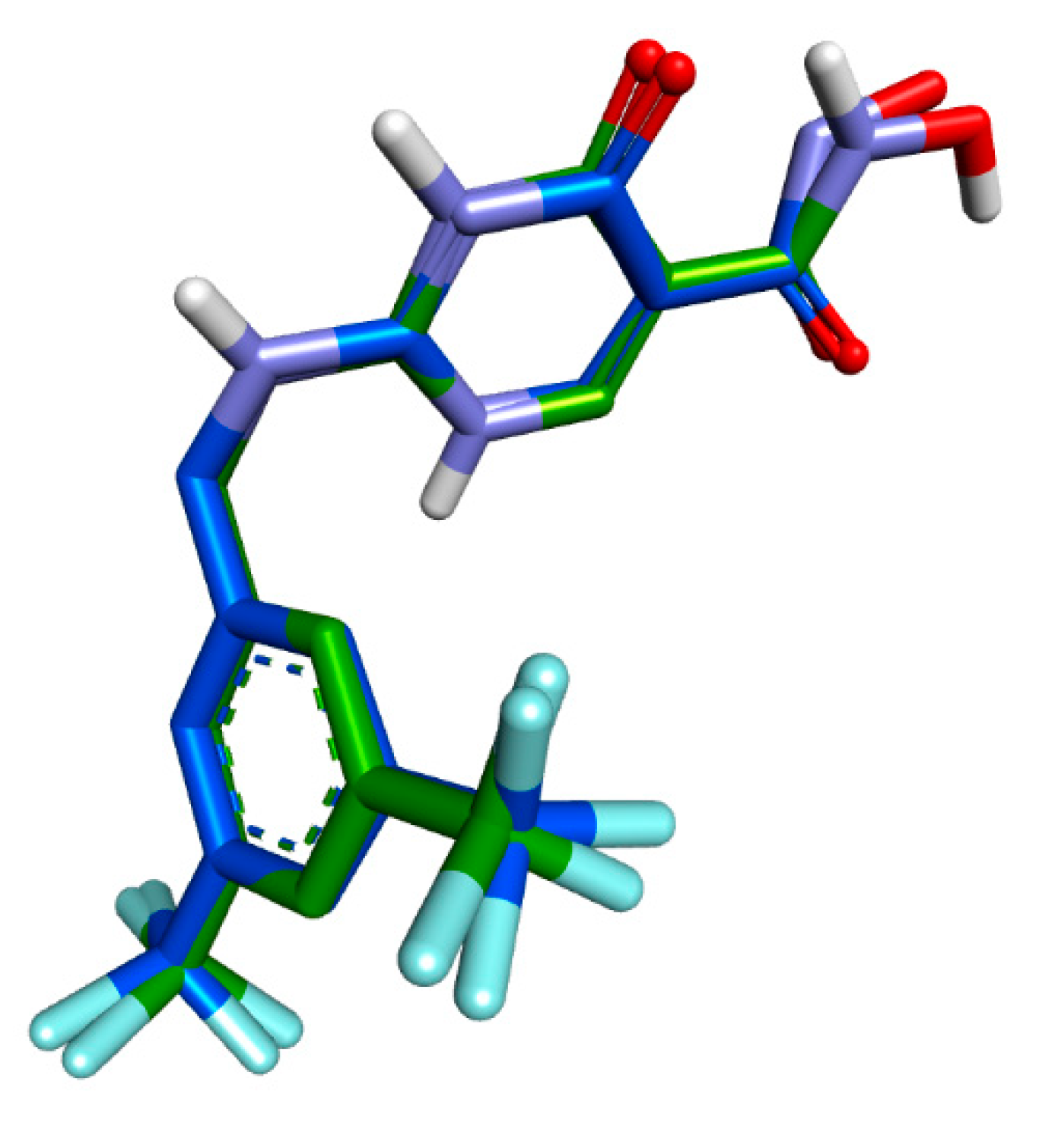
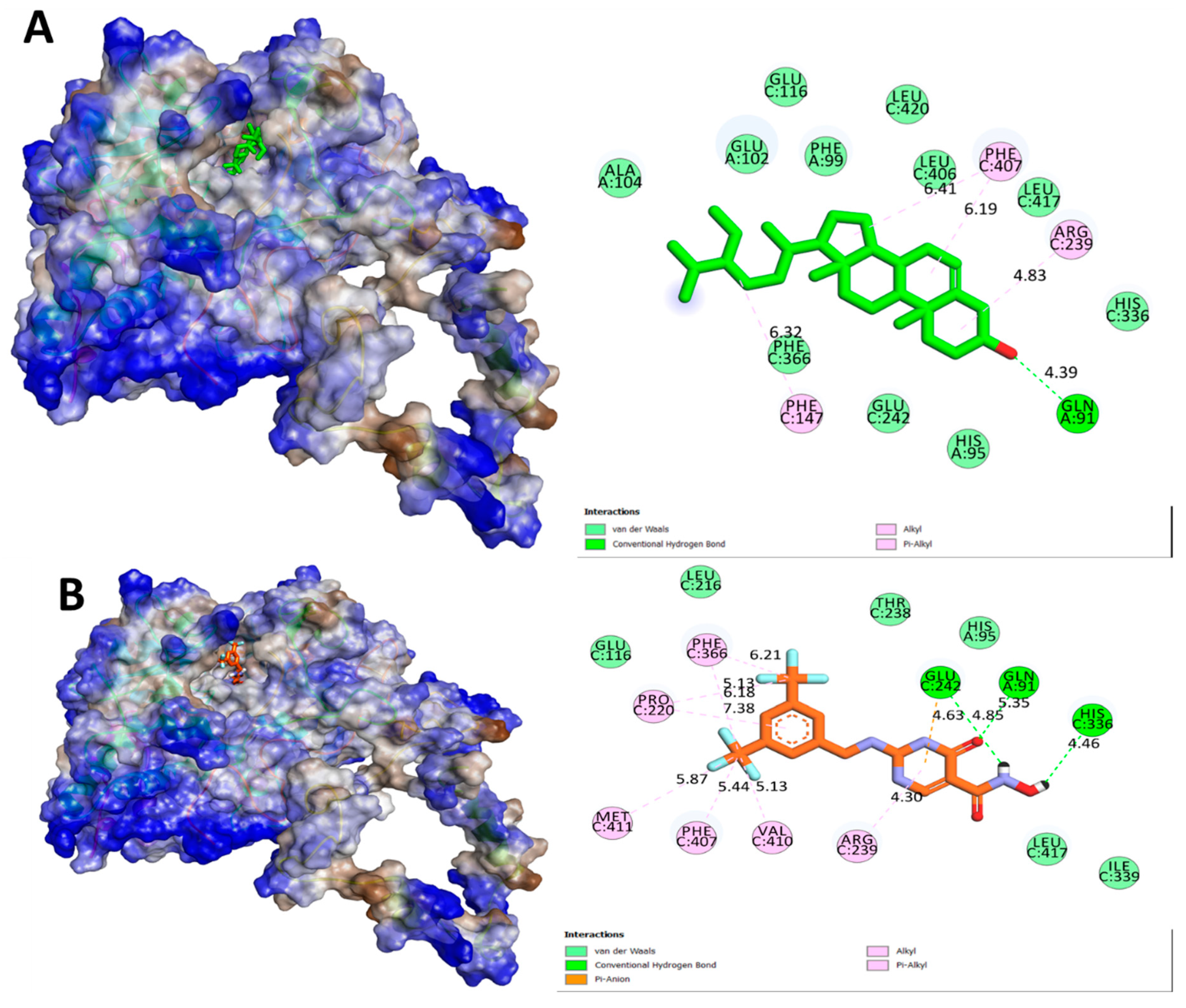
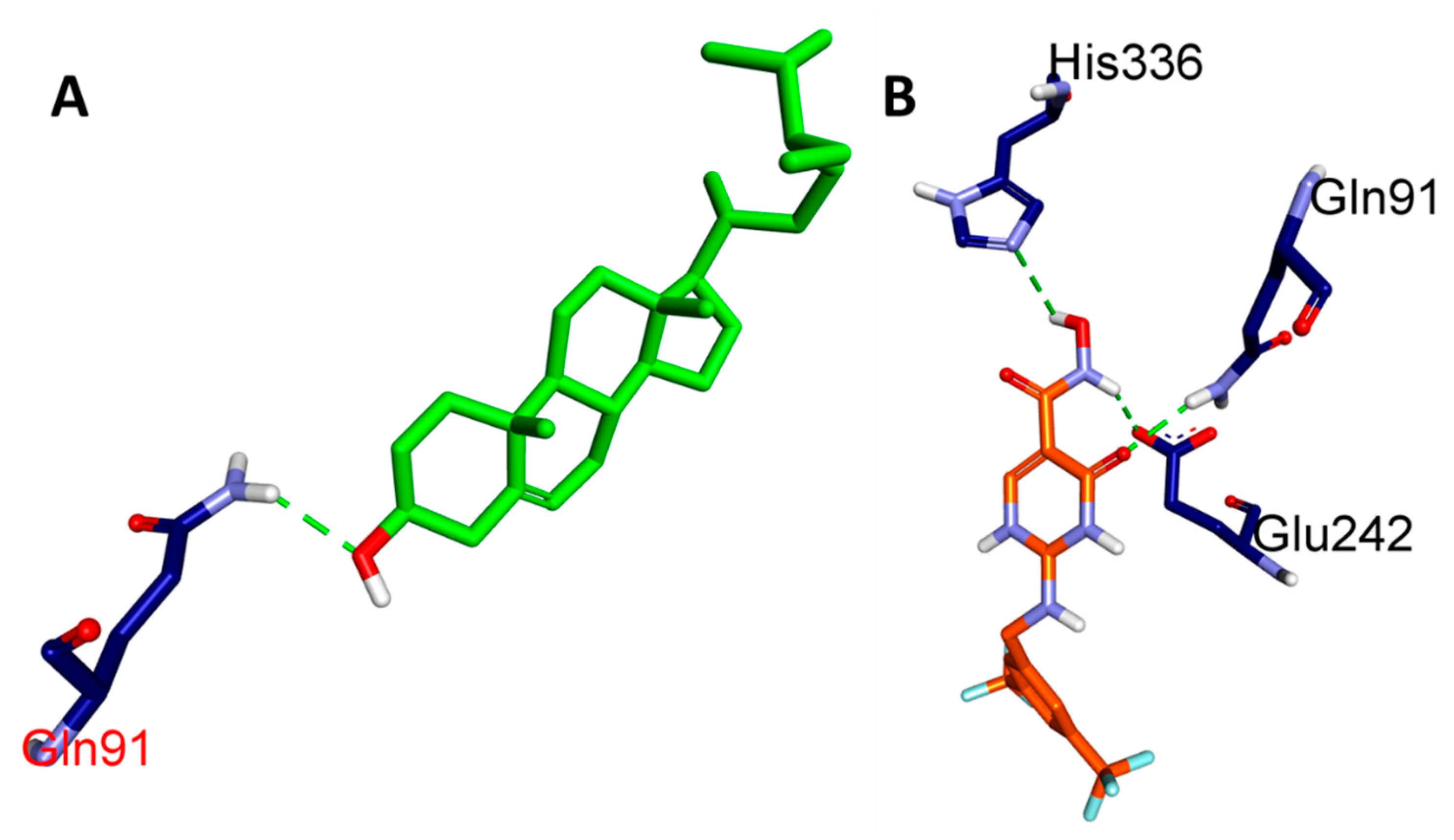
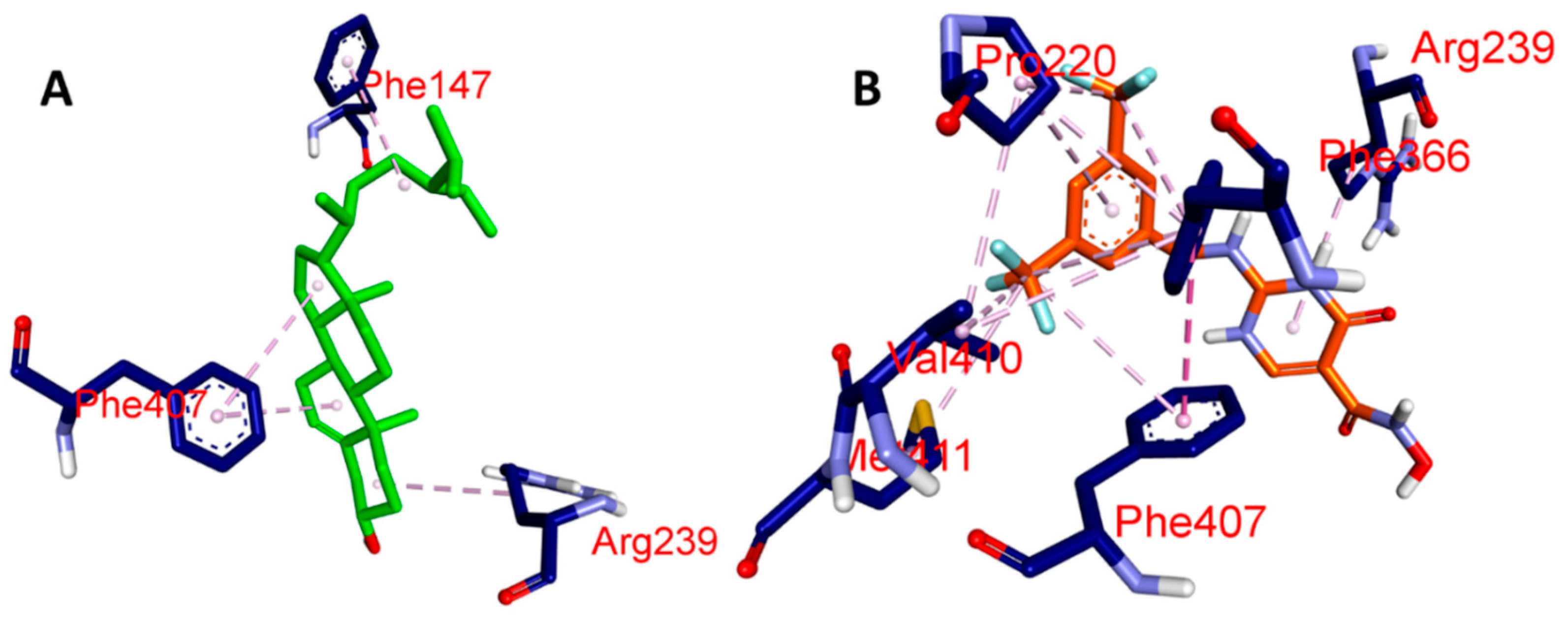
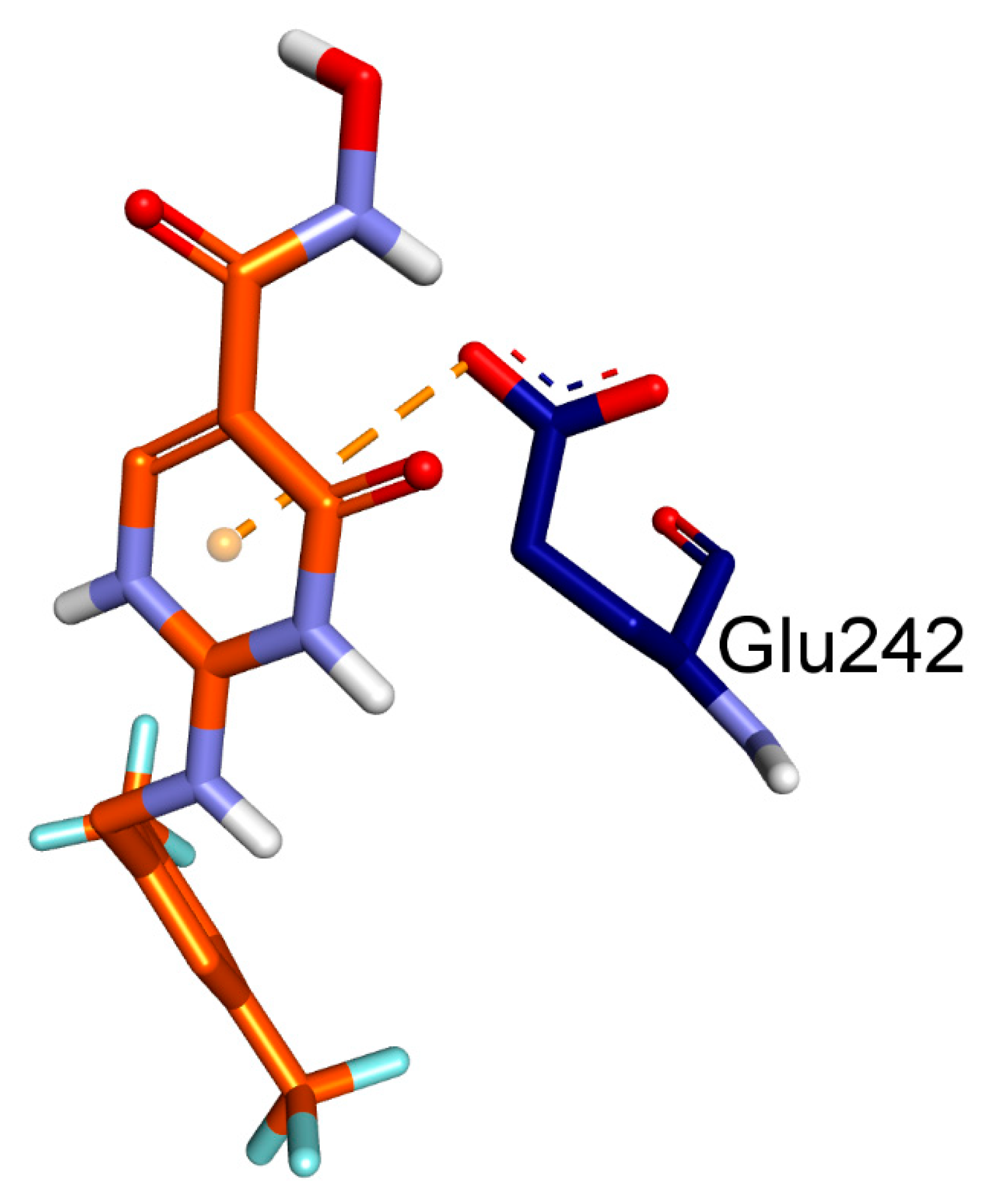


| Ligands | Protein | Binding Affinity, ΔG (Kcal/mol) | Amino Acids Involved and Distance (Å) | ||
|---|---|---|---|---|---|
| Hydrogen Bond Interactions | Hydrophobic Interactions | Electrostatic Interactions | |||
| BET | MPO (PDB: 4C1M) | −7.1 | GLN A:91 (4.39) | PHE A:147 (6.32), ARG A:239 (4.83), PHE A:407 (6.19, 6.41) | - |
| CCl | −7.7 | HIS A:91 (5.35), GLU A:242 (4.85), HIS A:336 (4.46) | ARG A:239 (4.30), VAL A:410 (5.13), PHE A:407 (5.44), MET A:411 (5.87), PRO A:220 (6.18 7.38), PHE A:366 (5.13, 6.21) | GLU A:242 (4.63) | |
| System | Van der Waal Energy | Electrostatic Energy | Polar Solvation Energy | Binding Energy |
|---|---|---|---|---|
| CCL | −112.771 ± 23.244 kJ/mol | −146.272 ± 27.497 kJ/mol | 253.003 ± 70.776 kJ/mol | −22.793 ± 30.727 kJ/mol |
| BET | −180.276 ± 9.119 kJ/mol | 1.010 ± 5.273 kJ/mol | 61.251 ± 15.562 kJ/mol | −140.699 ± 13.108 kJ/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tallapalli, P.S.; Reddy, Y.D.; Yaraguppi, D.A.; Matangi, S.P.; Challa, R.R.; Vallamkonda, B.; Ahmad, S.F.; Al-Mazroua, H.A.; Rudrapal, M.; Dintakurthi Sree Naga Bala Krishna, P.; et al. In Silico and In Vivo Studies of β-Sitosterol Nanoparticles as a Potential Therapy for Isoprenaline-Induced Cognitive Impairment in Myocardial Infarction, Targeting Myeloperoxidase. Pharmaceuticals 2024, 17, 1093. https://doi.org/10.3390/ph17081093
Tallapalli PS, Reddy YD, Yaraguppi DA, Matangi SP, Challa RR, Vallamkonda B, Ahmad SF, Al-Mazroua HA, Rudrapal M, Dintakurthi Sree Naga Bala Krishna P, et al. In Silico and In Vivo Studies of β-Sitosterol Nanoparticles as a Potential Therapy for Isoprenaline-Induced Cognitive Impairment in Myocardial Infarction, Targeting Myeloperoxidase. Pharmaceuticals. 2024; 17(8):1093. https://doi.org/10.3390/ph17081093
Chicago/Turabian StyleTallapalli, Partha Saradhi, Yennam Dastagiri Reddy, Deepak A. Yaraguppi, Surya Prabha Matangi, Ranadheer Reddy Challa, Bhaskar Vallamkonda, Sheikh F. Ahmad, Haneen A. Al-Mazroua, Mithun Rudrapal, Prasanth Dintakurthi Sree Naga Bala Krishna, and et al. 2024. "In Silico and In Vivo Studies of β-Sitosterol Nanoparticles as a Potential Therapy for Isoprenaline-Induced Cognitive Impairment in Myocardial Infarction, Targeting Myeloperoxidase" Pharmaceuticals 17, no. 8: 1093. https://doi.org/10.3390/ph17081093
APA StyleTallapalli, P. S., Reddy, Y. D., Yaraguppi, D. A., Matangi, S. P., Challa, R. R., Vallamkonda, B., Ahmad, S. F., Al-Mazroua, H. A., Rudrapal, M., Dintakurthi Sree Naga Bala Krishna, P., & Pasala, P. K. (2024). In Silico and In Vivo Studies of β-Sitosterol Nanoparticles as a Potential Therapy for Isoprenaline-Induced Cognitive Impairment in Myocardial Infarction, Targeting Myeloperoxidase. Pharmaceuticals, 17(8), 1093. https://doi.org/10.3390/ph17081093







