Novel Treatments in Refractory Recurrent Pericarditis
Abstract
1. Introduction
2. Clinical Features
3. Pathophysiology of Recurrent Pericarditis
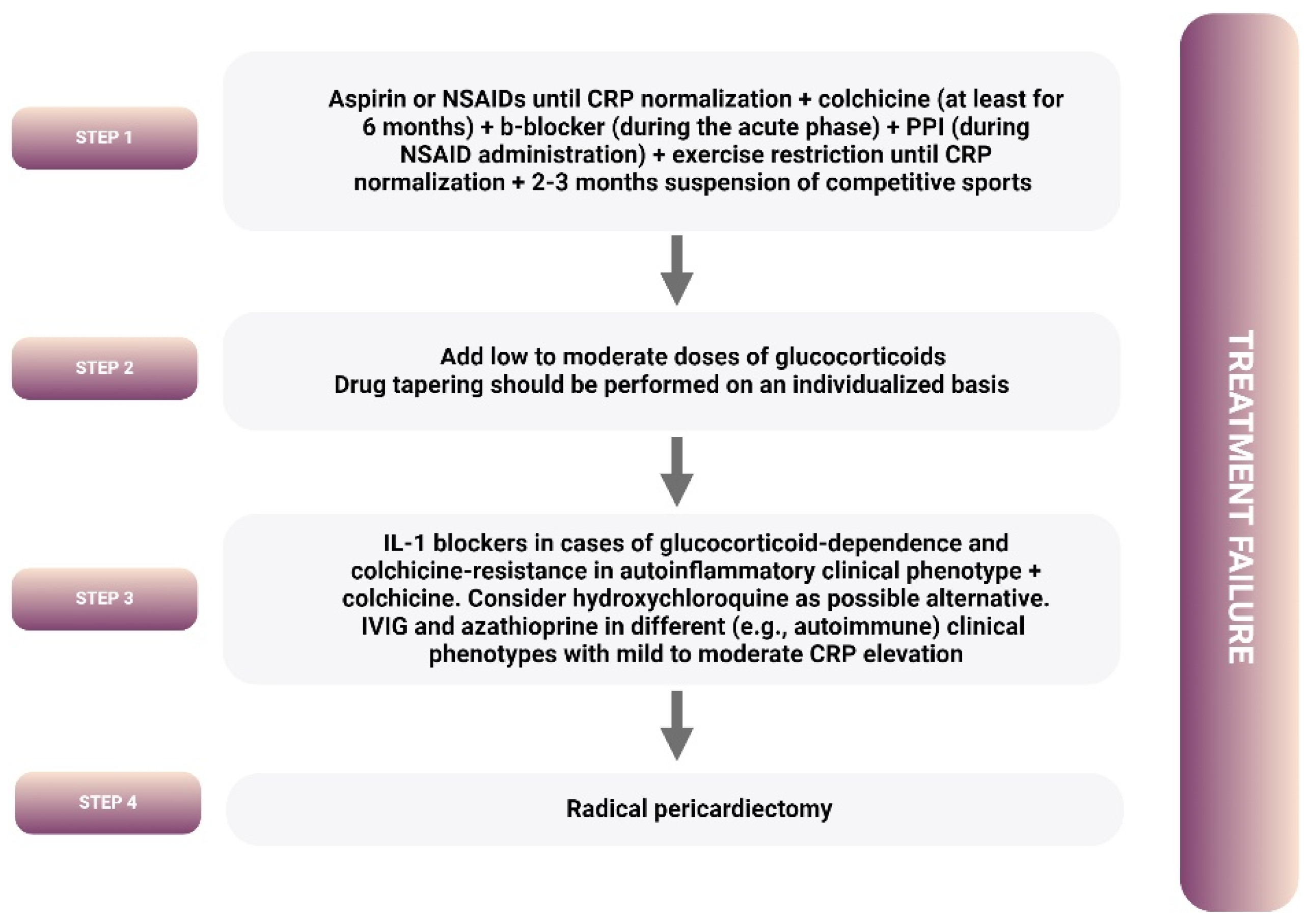
4. Treatment
4.1. Guideline-Recommended Treatment Algorithm
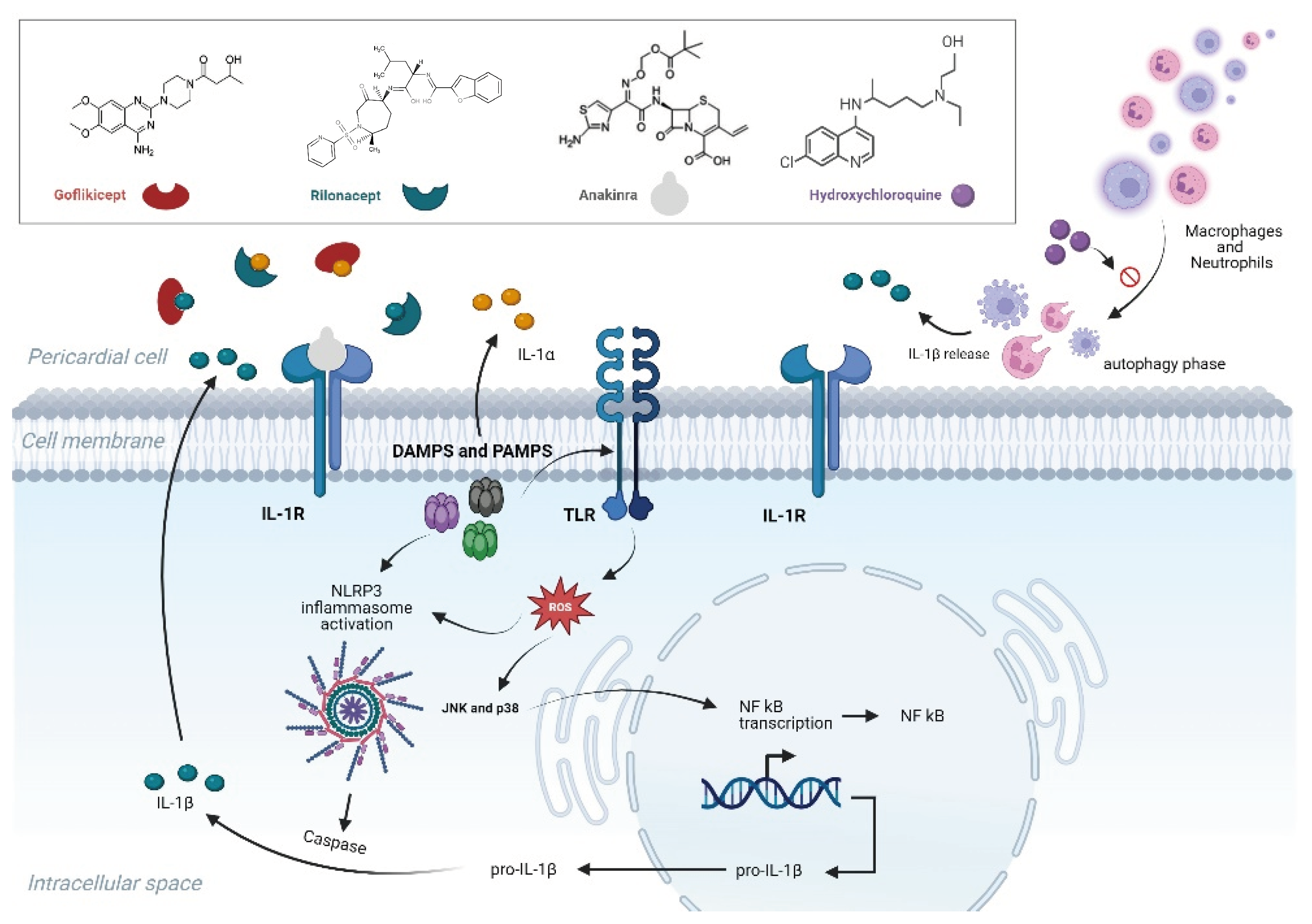
4.2. IL-1 Blockers
4.2.1. Anakinra
4.2.2. Rilonacept
4.2.3. Goflikicept
4.2.4. Canakinumab
4.2.5. Adverse Reactions of IL1 Blockers
4.2.6. IL1 Blockers in Specific Clinical Scenarios
4.3. New Treatment Proposals
4.3.1. Hydroxychloroquine
4.3.2. Beta Blockers
4.3.3. Cannabidiol
5. Gaps in Knowledge
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef] [PubMed]
- Vianello, F.; Cinetto, F.; Cavraro, M.; Battisti, A.; Castelli, M.; Imbergamo, S.; Marcolongo, R. Azathioprine in isolated recurrent pericarditis: A single centre experience. Int. J. Cardiol. 2011, 147, 477–478. [Google Scholar] [CrossRef]
- Imazio, M.; Lazaros, G.; Picardi, E.; Vasileiou, P.; Carraro, M.; Tousoulis, D.; Belli, R.; Gaita, F. Intravenous human immunoglobulins for refractory recurrent pericarditis: A systematic review of all published cases. J. Cardiovasc. Med. 2016, 17, 263–269. [Google Scholar] [CrossRef]
- Picco, P.; Brisca, G.; Traverso, F.; Loy, A.; Gattorno, M.; Martini, A. Successful treatment of idiopathic recurrent pericarditis in children with interleukin-1β receptor antagonist (anakinra): An unrecognized autoinflammatory disease? Arthritis Rheum. 2009, 60, 264–268. [Google Scholar] [CrossRef]
- Lopalco, G.; Rigante, D.; Cantarini, L.; Imazio, M.; Lopalco, A.; Emmi, G.; Venerito, V.; Fornaro, M.; Frediani, B.; Nivuori, M.; et al. The autoinflammatory side of recurrent pericarditis: Enlightening the pathogenesis for a more rational treatment. Trends Cardiovasc. Med. 2021, 31, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Peet, C.J.; Rowczenio, D.; Omoyinmi, E.; Papadopoulou, C.; Mapalo, B.R.R.; Wood, M.R.; Capon, F.; Lachmann, H.J. Pericarditis and Autoinflammation: A Clinical and Genetic Analysis of Patients with Idiopathic Recurrent Pericarditis and Monogenic Autoinflammatory Diseases at a National Referral Center. J. Am. Heart Assoc. 2022, 11, e024931. [Google Scholar] [CrossRef]
- Finetti, M.; Insalaco, A.; Cantarini, L.; Meini, A.; Breda, L.; Alessio, M.; D’Alessandro, M.; Picco, P.; Martini, A.; Gattorno, M. Long-Term Efficacy of Interleukin-1 Receptor Antagonist (Anakinra) in Corticosteroid-Dependent and Colchicine-Resistant Recurrent Pericarditis. J. Pediatr. 2014, 164, 1425–1431.e1. [Google Scholar] [CrossRef]
- Vassilopoulos, D.; Lazaros, G.; Tsioufis, C.; Vasileiou, P.; Stefanadis, C.; Pectasides, D. Successful treatment of adult patients with idiopathic recurrent pericarditis with an interleukin-1 receptor antagonist (anakinra). Int. J. Cardiol. 2012, 160, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Brucato, A.; Imazio, M.; Cremer, P.C.; Adler, Y.; Maisch, B.; Lazaros, G.; Gattorno, M.; Caforio, A.L.P.; Marcolongo, R.; Emmi, G.; et al. Recurrent pericarditis: Still idiopathic? The pros and cons of a well-honoured term. Intern. Emerg. Med. 2018, 13, 839–844. [Google Scholar] [CrossRef]
- Cantarini, L.; Lucherini, O.M.; Brucato, A.; Barone, L.; Cumetti, D.; Iacoponi, F.; Rigante, D.; Brambilla, G.; Penco, S.; Brizi, M.G.; et al. Clues to detect tumor necrosis factor receptor-associated periodic syndrome (TRAPS) among patients with idiopathic recurrent acute pericarditis: Results of a multicentre study. Clin. Res. Cardiol. 2012, 101, 525–531. [Google Scholar] [CrossRef]
- Cinteza, E.; Stefan, D.; Iancu, M.A.; Ioan, A.; Vasile, C.M.; Vatasescu, R.; Cochino, A. Autoinflammatory Recurrent Pericarditis Associated with a New NLRP12 Mutation in a Male Adolescent. Life 2023, 13, 2131. [Google Scholar] [CrossRef] [PubMed]
- Toldo, S.; Mezzaroma, E.; Buckley, L.F.; Potere, N.; Di Nisio, M.; Biondi-Zoccai, G.; Van Tassell, B.W.; Abbate, A. Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharmacol. Ther. 2022, 236, 108053. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Bonaventura, A.; Vecchié, A.; Moroni, F.; Golino, M.; Bressi, E.; De Ponti, R.; Dentali, F.; Montone, R.A.; Kron, J.; et al. Pathogenic pathways and therapeutic targets of inflammation in heart diseases: A focus on Interleukin-1. Eur. J. Clin. Investig. 2023, 54, e14110. [Google Scholar] [CrossRef]
- Toldo, S.; Abbate, A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases. Nat. Rev. Cardiol. 2023, 21, 219–237. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef]
- Imazio, M.; Mardigyan, V.; Andreis, A.; Franchin, L.; De Biasio, M.; Collini, V. New Developments in the Management of Recurrent Pericarditis. Can. J. Cardiol. 2023, 39, 1103–1110. [Google Scholar] [CrossRef]
- Dong, T.; Klein, A.L.; Wang, T.K.M. Paradigm Shift in Diagnosis and Targeted Therapy in Recurrent Pericarditis. Curr. Cardiol. Rep. 2023, 25, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.K.; Bonaventura, A.; Vecchié, A.; van Tassell, B.; Imazio, M.; Klein, A.; Luis, S.A.; Abbate, A. Interleukin-1 Blockers for the Treatment of Recurrent Pericarditis: Pathophysiology, Patient-Reported Outcomes, and Perspectives. J. Cardiovasc. Pharmacol. 2024, 83, 503–510. [Google Scholar] [CrossRef]
- Vecchié, A.; Del Buono, M.G.; Mauro, A.G.; Cremer, P.C.; Imazio, M.; Klein, A.L.; Abbate, A.; Dentali, F.; Bonaventura, A. Advances in pharmacotherapy for acute and recurrent pericarditis. Expert Opin. Pharmacother. 2022, 23, 681–691. [Google Scholar] [CrossRef]
- Bonaventura, A. The long journey of interleukin-1 in acute and recurrent pericarditis. Eur. Heart J. 2022, 43, 933–934. [Google Scholar] [CrossRef]
- Kumar, S.; Khubber, S.; Reyaldeen, R.; Agrawal, A.; Cremer, P.C.; Imazio, M.; Kwon, D.H.; Klein, A.L. Advances in Imaging and Targeted Therapies for Recurrent Pericarditis: A Review. JAMA Cardiol. 2022, 7, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Lazaros, G.; Antonopoulos, A.S.; Antonatou, K.; Skendros, P.; Ritis, K.; Hadziyannis, E.; Lazarou, E.; Leontsinis, I.; Simantiris, S.; Vlachopoulos, C.; et al. Hydroxychloroquine for colchicine-resistant glucocorticoid-dependent idiopathic recurrent pericarditis: A pilot observational prospective study. Int. J. Cardiol. 2020, 311, 77–82. [Google Scholar] [CrossRef]
- Imazio, M.; Andreis, A.; Agosti, A.; Piroli, F.; Avondo, S.; Casula, M.; Paneva, E.; Squarotti, G.B.; Giustetto, C.; De Ferrari, G.M. Usefulness of Beta-Blockers to Control Symptoms in Patients with Pericarditis. Am. J. Cardiol. 2021, 146, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Lazaros, G.; Gattorno, M.; LeWinter, M.; Abbate, A.; Brucato, A.; Klein, A. Anti-interleukin-1 agents for pericarditis: A primer for cardiologists. Eur. Heart J. 2021, 43, 2946–2957. [Google Scholar] [CrossRef]
- Cremer, P.C.; Kumar, A.; Kontzias, A.; Tan, C.D.; Rodriguez, E.R.; Imazio, M.; Klein, A.L. Complicated Pericarditis: Understanding Risk Factors and Pathophysiology to Inform Imaging and Treatment. J. Am. Coll. Cardiol. 2016, 68, 2311–2328. [Google Scholar] [CrossRef] [PubMed]
- Andreis, A.; Imazio, M.; Giustetto, C.; Brucato, A.; Adler, Y.; De Ferrari, G.M. Anakinra for constrictive pericarditis associated with incessant or recurrent pericarditis. Heart 2020, 106, 1561–1565. [Google Scholar] [CrossRef]
- Brucato, A.; Brambilla, G.; Moreo, A.; Alberti, A.; Munforti, C.; Ghirardello, A.; Doria, A.; Shinar, Y.; Livneh, A.; Adler, Y.; et al. Long-Term Outcomes in Difficult-to-Treat Patients with Recurrent Pericarditis. Am. J. Cardiol. 2006, 98, 267–271. [Google Scholar] [CrossRef]
- Collini, V.; Vignut, L.S.; Angriman, F.; Braidotti, G.; De Biasio, M.; Imazio, M. Age-stratified patterns in clinical presentation, treatment and outcomes in acute pericarditis: A retrospective cohort study. Heart 2024. [Google Scholar] [CrossRef] [PubMed]
- Collini, V.; Andreis, A.; De Biasio, M.; De Martino, M.; Isola, M.; Croatto, N.; Lepre, V.; Cantarini, L.; Merlo, M.; Sinagra, G.; et al. Efficacy of colchicine in addition to anakinra in patients with recurrent pericarditis. Open Heart 2024, 11, e002599. [Google Scholar] [CrossRef]
- LeWinter, M.M. Clinical practice. Acute Pericarditis. N. Engl. J. Med. 2014, 371, 2410–2416. [Google Scholar] [CrossRef]
- Khandaker, M.H.; Espinosa, R.E.; Nishimura, R.A.; Sinak, L.J.; Hayes, S.N.; Melduni, R.M.; Oh, J.K. Pericardial Disease: Diagnosis and Management. Mayo Clin. Proc. 2010, 85, 572–593. [Google Scholar] [CrossRef]
- Lilly, L.S. Treatment of Acute and Recurrent Idiopathic Pericarditis. Circulation 2013, 127, 1723–1726. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Demichelis, B.; Parrini, I.; Giuggia, M.; Cecchi, E.; Gaschino, G.; Demarie, D.; Ghisio, A.; Trinchero, R. Day-hospital treatment of acute pericarditis: A management program for outpatient therapy. J. Am. Coll. Cardiol. 2004, 43, 1042–1046. [Google Scholar] [CrossRef]
- Imazio, M.; Andreis, A.; Lubian, M.; Lazaros, G.; Lazarou, E.; Brucato, A.; Adler, Y.; Giustetto, C.; Rinaldi, M.; De Ferrari, G.M. The Torino Pericarditis Score: A new-risk stratification tool to predict complicated pericarditis. Intern. Emerg. Med. 2021, 16, 1921–1926. [Google Scholar] [CrossRef]
- Lazarou, E.; Lazaros, G.; Antonopoulos, A.S.; Imazio, M.; Vasileiou, P.; Karavidas, A.; Toutouzas, K.; Vassilopoulos, D.; Tsioufis, C.; Tousoulis, D.; et al. A risk score for pericarditis recurrence. Eur. J. Clin. Investig. 2021, 51, e13602. [Google Scholar] [CrossRef]
- Lazarou, E.; Koutsianas, C.; Theofilis, P.; Lazaros, G.; Vassilopoulos, D.; Vlachopoulos, C.; Tsioufis, C.; Imazio, M.; Brucato, A.; Tousoulis, D. Interleukin-1 Blockers: A Paradigm Shift in the Treatment of Recurrent Pericarditis. Life 2024, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Spodick, D.H.; Brucato, A.; Trinchero, R.; Adler, Y. Controversial Issues in the Management of Pericardial Diseases. Circulation 2010, 121, 916–928. [Google Scholar] [CrossRef]
- Khandaker, M.H.; Schaff, H.V.; Greason, K.L.; Anavekar, N.S.; Espinosa, R.E.; Hayes, S.N.; Nishimura, R.A.; Oh, J.K. Pericardiectomy vs. Medical Management in Patients with Relapsing Pericarditis. Mayo Clin. Proc. 2012, 87, 1062–1070. [Google Scholar] [CrossRef]
- McGonagle, D.; McDermott, M.F. A Proposed Classification of the Immunological Diseases. PLoS Med. 2006, 3, e297. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Brucato, A.; Doria, A.; Brambilla, G.; Angelini, A.; Ghirardello, A.; Bottaro, S.; Tona, F.; Betterle, C.; Daliento, L.; et al. Anti-heart and anti-intercalated disk autoantibodies: Evidence for autoimmunity in idiopathic recurrent acute pericarditis. Heart 2010, 96, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Lazaros, G.; Antonatou, K.; Vassilopoulos, D. The Therapeutic Role of Interleukin-1 Inhibition in Idiopathic Recurrent Pericarditis: Current Evidence and Future Challenges. Front. Med. 2017, 4, 78. [Google Scholar] [CrossRef] [PubMed]
- Tombetti, E.; Giani, T.; Brucato, A.; Cimaz, R. Recurrent Pericarditis in Children and Adolescents. Front. Pediatr. 2019, 7, 419. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.S.; Raza, K.; Cooper, M.S. Therapeutic glucocorticoids: Mechanisms of actions in rheumatic diseases. Nat. Rev. Rheumatol. 2020, 16, 133–144. [Google Scholar] [CrossRef]
- Imazio, M.; Brucato, A.; Cemin, R.; Ferrua, S.; Maggiolini, S.; Beqaraj, F.; Demarie, D.; Forno, D.; Ferro, S.; Maestroni, S.; et al. A Randomized Trial of Colchicine for Acute Pericarditis. N. Engl. J. Med. 2013, 369, 1522–1528. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Imazio, M. Noninfectious pericarditis: Management challenges for cardiologists. Kardiologia Polska 2020, 78, 396–403. [Google Scholar] [CrossRef]
- De La Serna, A.; Soldevila, J.; Claramunt, V.; De Luna, A. Colchicine for recurrent pericarditis. Lancet 1987, 2, 1517. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Adler, Y.; de Luna, A.B.; Imazio, M. Colchicine in Pericarditis. Eur. Heart J. 2017, 38, 1706–1709. [Google Scholar] [CrossRef]
- Imazio, M.; Bobbio, M.; Cecchi, E.; Demarie, D.; Pomari, F.; Moratti, M.; Ghisio, A.; Belli, R.; Trinchero, R. Colchicine as first-choice therapy for recurrent pericarditis: Results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch. Intern. Med. 2005, 165, 1987–1991. [Google Scholar] [CrossRef]
- Imazio, M.; Brucato, A.; Cemin, R.; Ferrua, S.; Belli, R.; Maestroni, S.; Trinchero, R.; Spodick, D.H.; Adler, Y. Colchicine for recurrent pericarditis (CORP): A randomized trial. Ann. Intern. Med. 2011, 155, 409–414. [Google Scholar] [CrossRef]
- Imazio, M.; Belli, R.; Brucato, A.; Cemin, R.; Ferrua, S.; Beqaraj, F.; Demarie, D.; Ferro, S.; Forno, D.; Maestroni, S.; et al. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): A multicentre, double-blind, placebo-controlled, randomised trial. Lancet 2014, 383, 2232–2237. [Google Scholar] [CrossRef]
- Cohen, S.B. The use of anakinra, an interleukin-1 receptor antagonist, in the treatment of rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2004, 30, 365–380. [Google Scholar] [CrossRef]
- Brucato, A.; Imazio, M.; Gattorno, M.; Lazaros, G.; Maestroni, S.; Carraro, M.; Finetti, M.; Cumetti, D.; Carobbio, A.; Ruperto, N.; et al. Effect of Anakinra on Recurrent Pericarditis among Patients with Colchicine Resistance and Corticosteroid Dependence: The AIRTRIP Randomized Clinical Trial. JAMA 2016, 316, 1906–1912. [Google Scholar] [CrossRef]
- Imazio, M.; Andreis, A.; De Ferrari, G.M.; Cremer, P.C.; Mardigyan, V.; Maestroni, S.; Luis, S.A.; Lopalco, G.; Emmi, G.; Lotan, D.; et al. Anakinra for corticosteroid-dependent and colchicine-resistant pericarditis: The IRAP (International Registry of Anakinra for Pericarditis) study. Eur. J. Prev. Cardiol. 2020, 27, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.L.; Lin, D.; Cremer, P.C.; Nasir, S.; Luis, S.A.; Abbate, A.; Ertel, A.; LeWinter, M.; Beutler, A.; Fang, F.; et al. Efficacy and safety of rilonacept for recurrent pericarditis: Results from a phase II clinical trial. Heart 2020, 107, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.L.; Imazio, M.; Cremer, P.; Brucato, A.; Abbate, A.; Fang, F.; Insalaco, A.; LeWinter, M.; Lewis, B.S.; Lin, D.; et al. Phase 3 Trial of Interleukin-1 Trap Rilonacept in Recurrent Pericarditis. N. Engl. J. Med. 2021, 384, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Brucato, A.; Lim-Watson, M.Z.; Klein, A.; Imazio, M.; Cella, D.; Cremer, P.; LeWinter, M.M.; Luis, S.A.; Lin, D.; Lotan, D.; et al. Interleukin-1 Trap Rilonacept Improved Health-Related Quality of Life and Sleep in Patients with Recurrent Pericarditis: Results from the Phase 3 Clinical Trial RHAPSODY. J. Am. Heart Assoc. 2022, 11, e023252. [Google Scholar] [CrossRef]
- Imazio, M.; Klein, A.L.; Brucato, A.; Abbate, A.; Arad, M.; Cremer, P.C.; Insalaco, A.; LeWinter, M.M.; Lewis, B.S.; Lin, D.; et al. Sustained Pericarditis Recurrence Risk Reduction with Long-Term Rilonacept. J. Am. Heart Assoc. 2024, 13, e032516. [Google Scholar] [CrossRef]
- Imazio, M.; Andreis, A.; Piroli, F.; Lazaros, G.; Gattorno, M.; Lewinter, M.; Klein, A.L.; Brucato, A. Anti-interleukin 1 agents for the treatment of recurrent pericarditis: A systematic review and meta-analysis. Heart 2021, 107, 1240–1245. [Google Scholar] [CrossRef]
- Myachikova, V.Y.; Maslyanskiy, A.L.; Moiseeva, O.M.; Vinogradova, O.V.; Gleykina, E.V.; Lavrovsky, Y.; Abbate, A.; Grishin, S.A.; Egorova, A.N.; Schedrova, M.L.; et al. Treatment of Idiopathic Recurrent Pericarditis with Goflikicept: Phase II/III Study Results. J. Am. Coll. Cardiol. 2023, 82, 30–40. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Kougkas, N.; Fanouriakis, A.; Papalopoulos, I.; Bertsias, G.; Avgoustidis, N.; Repa, A.; Sidiropoulos, P. Canakinumab for recurrent rheumatic disease associated-pericarditis: A case series with long-term follow-up. Rheumatology 2018, 57, 1494–1495. [Google Scholar] [CrossRef] [PubMed]
- Epçaçan, S.; Sahin, S.; Kasapcopur, O. Anaphylactic reaction to anakinra in a child with steroid-dependent idiopathic recurrent pericarditis and successful management with canakinumab. Cardiol. Young 2019, 29, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Signa, S.; D’alessandro, M.; Consolini, R.; Miniaci, A.; Bustaffa, M.; Longo, C.; Tosca, M.A.; Bizzi, M.; Caorsi, R.; Mendonça, L.O.; et al. Failure of anti Interleukin-1 β monoclonal antibody in the treatment of recurrent pericarditis in two children. Pediatr. Rheumatol. 2020, 18, 51. [Google Scholar] [CrossRef]
- Chang, Z.; Spong, C.Y.; Jesus, A.A.; Davis, M.A.; Plass, N.; Stone, D.L.; Chapelle, D.; Hoffmann, P.; Kastner, D.L.; Barron, K.; et al. Brief Report: Anakinra Use during Pregnancy in Patients with Cryopyrin-Associated Periodic Syndromes (CAPS). Arthritis Rheumatol. 2014, 66, 3227–3232. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.D.; Dey, M.; Flint, J.; Davie, P.; Allen, A.; Crossley, A.; Frishman, M.; Gayed, M.; Hodson, K.; Khamashta, M.; et al. British Society for Rheumatology guideline on prescribing drugs in pregnancy and breastfeeding: Immunomodulatory anti-rheumatic drugs and corticosteroids. Rheumatology 2023, 62, e48–e88. [Google Scholar] [CrossRef]
- Youngstein, T.; Hoffmann, P.; Gül, A.; Lane, T.; Williams, R.; Rowczenio, D.M.; Ozdogan, H.; Ugurlu, S.; Ryan, J.; Harty, L.; et al. International multi-centre study of pregnancy outcomes with interleukin-1 inhibitors. Rheumatology 2017, 56, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.F.; Chambers, C.D. Five successful pregnancies with antenatal anakinra exposure. Rheumatology 2018, 57, 1271–1275. [Google Scholar] [CrossRef]
- Brien, M.-E.; Gaudreault, V.; Hughes, K.; Hayes, D.J.L.; Heazell, A.E.P.; Girard, S. A Systematic Review of the Safety of Blocking the IL-1 System in Human Pregnancy. J. Clin. Med. 2021, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Sammaritano, L.R.; Bermas, B.L.; Chakravarty, E.E.; Chambers, C.; Clowse, M.E.B.; Lockshin, M.D.; Marder, W.; Guyatt, G.; Branch, D.W.; Buyon, J.; et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Care Res. 2020, 72, 461–488. [Google Scholar] [CrossRef]
- Wu, T.-C.; Xu, K.; Martinek, J.; Young, R.R.; Banchereau, R.; George, J.; Turner, J.; Kim, K.I.; Zurawski, S.; Wang, X.; et al. IL1 Receptor Antagonist Controls Transcriptional Signature of Inflammation in Patients with Metastatic Breast Cancer. Cancer Res. 2018, 78, 5243–5258. [Google Scholar] [CrossRef]
- Xie, W.; Xiao, S.; Huang, Y.; Sun, X.; Gao, D.; Ji, L.; Li, G.; Zhang, Z. A meta-analysis of biologic therapies on risk of new or recurrent cancer in patients with rheumatoid arthritis and a prior malignancy. Rheumatology 2020, 59, 930–939. [Google Scholar] [CrossRef]
- Fleischmann, R.M.; Tesser, J.; Schiff, M.H.; Schechtman, J.; Burmester, G.-R.; Bennett, R.; Modafferi, D.; Zhou, L.; Bell, D.; Appleton, B. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 1006–1012. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Jiang, L. Role of Interleukin-1 in the pathogenesis of colorectal cancer: A brief look at anakinra therapy. Int. Immunopharmacol. 2022, 105, 108577. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Everett, B.M.; Libby, P.; Glynn, R.J.; Ridker, P.; Lorenzatti, A.; Krum, H.; Varigos, J.; et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef]
- Spera, A.M. Hepatitis B virus infection reactivation in patients under immunosuppressive therapies: Pathogenesis, screening, prevention and treatment. World J. Virol. 2022, 11, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kullenberg, T.; Löfqvist, M.; Leinonen, M.; Goldbach-Mansky, R.; Olivecrona, H. Long-term safety profile of anakinra in patients with severe cryopyrin-associated periodic syndromes. Rheumatology 2016, 55, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Anakinra. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MA, USA, 2012. Available online: http://www.ncbi.nlm.nih.gov/books/NBK548615/ (accessed on 29 January 2024).
- Winthrop, K.; Mariette, X.; Silva, J.; Benamu, E.; Calabrese, L.; Dumusc, A.; Smolen, J.; Aguado, J.; Fernández-Ruiz, M. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: An infectious diseases perspective (Soluble immune effector molecules [II]: Agents targeting interleukins, immunoglobulins and complement factors). Clin. Microbiol. Infect. 2018, 24 (Suppl. S2), S21–S40. [Google Scholar] [CrossRef]
- Naya, N.M.; Kelly, J.; Hogwood, A.; Abbate, A.; Toldo, S. Therapeutic potential of cannabidiol (CBD) in the treatment of cardiovascular diseases. Expert Opin. Investig. Drugs 2024, 33, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Lazaros, G.; Vasileiou, P.; Koutsianas, C.; Antonatou, K.; Stefanadis, C.; Pectasides, D.; Vassilopoulos, D. Anakinra for the management of resistant idiopathic recurrent pericarditis. Initial experience in 10 adult cases: Table 1. Ann. Rheum. Dis. 2014, 73, 2215–2217. [Google Scholar] [CrossRef]
- Aldajani, A.; Imazio, M.; Klein, A.; Mardigyan, V. How to Use Interleukin-1 Antagonists in Patients with Pericarditis. Can. J. Cardiol. 2023, 39, 1132–1135. [Google Scholar] [CrossRef]
- Caorsi, R.; Insalaco, A.; Bovis, F.; Martini, G.; Cattalini, M.; Chinali, M.; Rimini, A.; Longo, C.; Federici, S.; Celani, C.; et al. Pediatric Recurrent Pericarditis: Appropriateness of the Standard of Care and Response to IL-1 Blockade. J. Pediatr. 2023, 256, 18–26.e8. [Google Scholar] [CrossRef]
| Diagnostic Criteria for Pericarditis (Diagnosis Can Be Made with at Least 2 of the Following Criteria) | High-Risk Criteria Associated with Complicated Pericarditis (Such as Cardiac Tamponade, Arrhythmias, as Well as Recurrence and Constrictive Pericarditis during Follow-Up) and a Specific (Non-Idiopathic and Non-Viral) Etiology |
|---|---|
| Major criteria (emerged in multivariate analysis).
|
| Anakinra | Rilonacept | Goflikicept | |
|---|---|---|---|
| Mechanism of action: | Recombinant human IL-1 receptor antagonist | Dimeric fusion protein that combines two IL-1 receptors with an Fc immunoglobulin tail (IL-1α and 1β trap) | Heterodimeric fusion protein having high affinity for IL-1α and IL-1β |
| Route of administration: | SC or IV | SC | SC |
| Biological half-life: | 4–6 h | 7 days | 10 days |
| Dosing (full dose): | Every day 1–2 mg/kg/day up to 100 mg/day | Once a week Loading: 320 mg on the first day (or 4.4 mg/kg if <18 years of age) Maintenance: 160 mg (or 2.2 mg/kg if <18 years of age) | Every 2 weeks Loading: 160 mg Maintenance: 80 mg on weeks 1 and 2 and then 80 mg |
| Route of excretion: | Mostly kidney (no dose adjustment is generally required in CKD) | Reticuloendothelial system (no dose adjustment is generally required in CKD) | Not known |
| Serious side effects requiring drug discontinuation: | 3% | 3% | 4.5% |
| Main side effects: | |||
| Injection site reactions | 38% | 33% | 18% |
| Transaminasemia | 3% | 4% | 4.5% |
| Neutropenia | 1% | NR | 9.1% |
| Infections | 3% | 16% | ~23% |
| Arthralgias/myalgias | 6% | 12% | NR |
| Blood lipid elevation | NR | 8% | 18% |
| Treatment protocol | At least 3 months full-dose with at least 3 months tapering | At least 6–8 months | No data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarou, E.; Koutsianas, C.; Vlachakis, P.K.; Theofilis, P.; Vassilopoulos, D.; Tsioufis, C.; Lazaros, G.; Tousoulis, D. Novel Treatments in Refractory Recurrent Pericarditis. Pharmaceuticals 2024, 17, 1069. https://doi.org/10.3390/ph17081069
Lazarou E, Koutsianas C, Vlachakis PK, Theofilis P, Vassilopoulos D, Tsioufis C, Lazaros G, Tousoulis D. Novel Treatments in Refractory Recurrent Pericarditis. Pharmaceuticals. 2024; 17(8):1069. https://doi.org/10.3390/ph17081069
Chicago/Turabian StyleLazarou, Emilia, Christos Koutsianas, Panayotis K. Vlachakis, Panagiotis Theofilis, Dimitrios Vassilopoulos, Costas Tsioufis, George Lazaros, and Dimitris Tousoulis. 2024. "Novel Treatments in Refractory Recurrent Pericarditis" Pharmaceuticals 17, no. 8: 1069. https://doi.org/10.3390/ph17081069
APA StyleLazarou, E., Koutsianas, C., Vlachakis, P. K., Theofilis, P., Vassilopoulos, D., Tsioufis, C., Lazaros, G., & Tousoulis, D. (2024). Novel Treatments in Refractory Recurrent Pericarditis. Pharmaceuticals, 17(8), 1069. https://doi.org/10.3390/ph17081069









