Fish Oil Containing Pro-Resolving Mediators Enhances the Antioxidant System and Ameliorates LPS-Induced Inflammation in Human Bronchial Epithelial Cells
Abstract
1. Introduction
2. Results
2.1. FO Exerts a Protective Role against LPS-Induced Cell Death
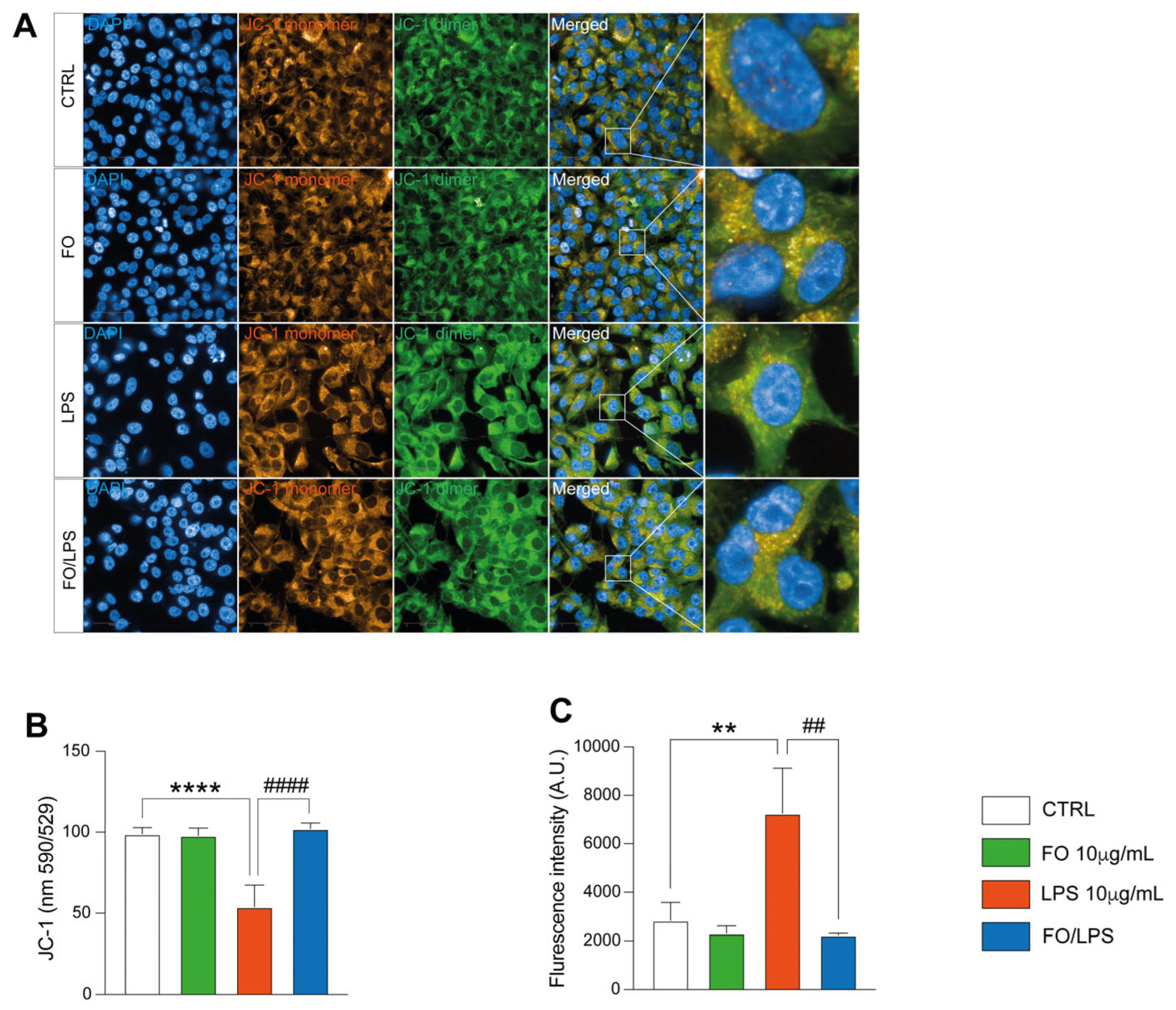
2.2. FO Supplementation Mitigate LPS-Induced Oxidative Stress
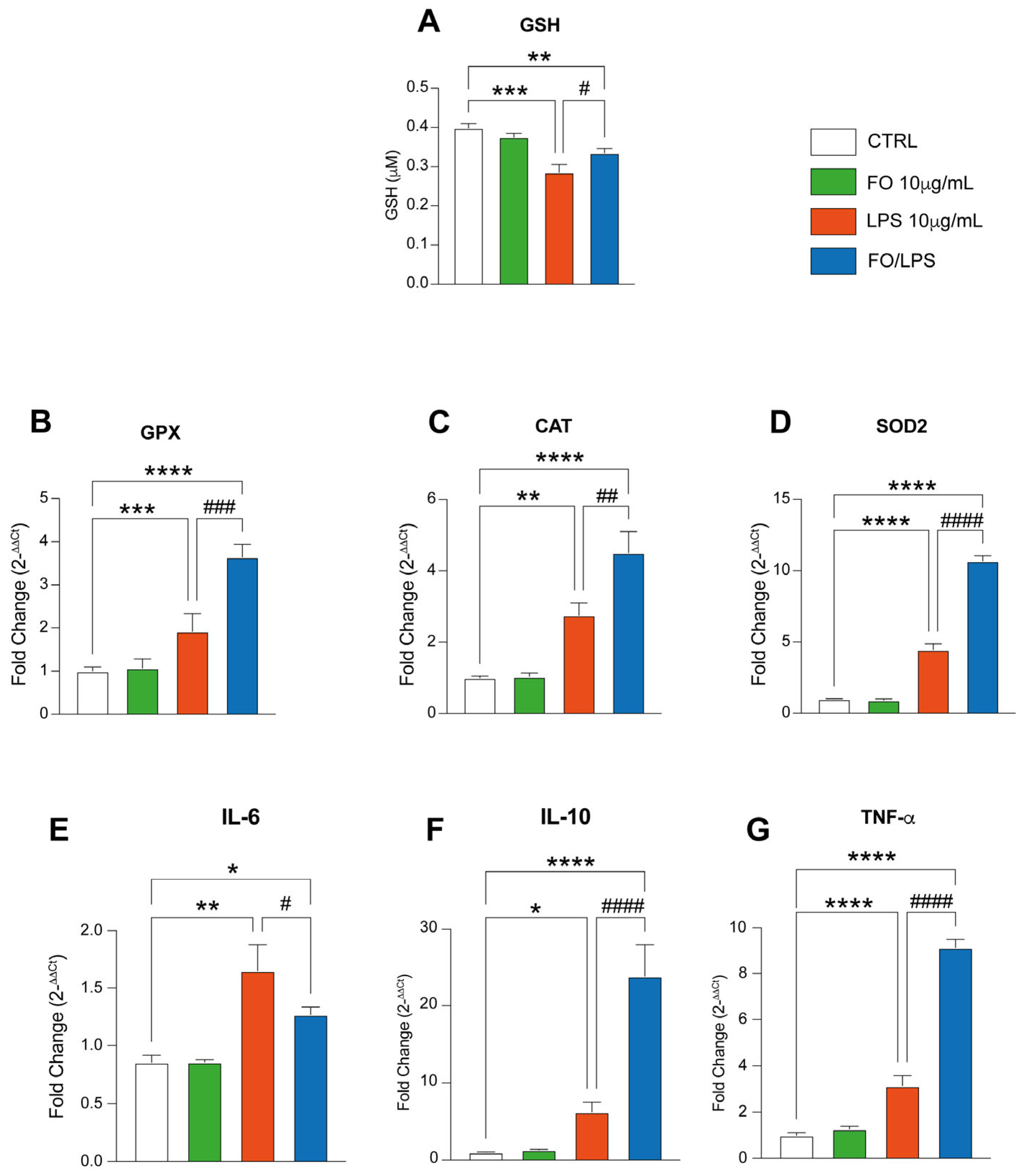
2.3. FO Triggers Antioxidant Defenses
2.4. FO Supplementation Recovers LPS-Mediated Inflammation by Boosting Pro-Resolving Mediators’ Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Fish Oil Characterization
4.3. Cell Treatment and Viability Assay
4.4. GSH Quantification
4.5. Mitochondrial Polarization Assessment
4.6. ROS Measurement
4.7. Real-Time PCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Johnson, J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004, 37, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Brustad, N.; Bonnelykke, K.; Chawes, B. Dietary prevention strategies for childhood asthma. Pediatr. Allergy Immunol. 2023, 34, e13984. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, S.; Zhao, Y.; Luo, Y.; Tong, H.; Su, L. Correlation analysis of omega-3 fatty acids and mortality of sepsis and sepsis-induced ARDS in adults: Data from previous randomized controlled trials. Nutr. J. 2018, 17, 57. [Google Scholar] [CrossRef]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Lehoczki, A.; Tarantini, S.; Varga, J.T. Effects of omega-3 supplementation on quality of life, nutritional status, inflammatory parameters, lipid profile, exercise tolerance and inhaled medications in chronic obstructive pulmonary disease. Ann. Palliat. Med. 2022, 11, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Mason, R.P.; Libby, P.; Steg, P.G.; Bhatt, D.L. Do patients benefit from omega-3 fatty acids? Cardiovasc. Res. 2024, 119, 2884–2901. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Carney, S.L.; Hicks, C.A.; Tree, B.; Broadmore, R.J. An in vivo investigation of the effect of anthraquinones on the turnover of aggrecans in spontaneous osteoarthritis in the guinea pig. Inflamm. Res. 1995, 44, 182–186. [Google Scholar] [CrossRef]
- Serhan, C.N. Novel lipid mediators and resolution mechanisms in acute inflammation: To resolve or not? Am. J. Pathol. 2010, 177, 1576–1591. [Google Scholar] [CrossRef] [PubMed]
- Arroyo Portilla, C.; Tomas, J.; Gorvel, J.P.; Lelouard, H. From Species to Regional and Local Specialization of Intestinal Macrophages. Front. Cell Dev. Biol. 2020, 8, 624213. [Google Scholar] [CrossRef]
- Zuniga-Hernandez, J.; Sambra, V.; Echeverria, F.; Videla, L.A.; Valenzuela, R. N-3 PUFAs and their specialized pro-resolving lipid mediators on airway inflammatory response: Beneficial effects in the prevention and treatment of respiratory diseases. Food Funct. 2022, 13, 4260–4272. [Google Scholar] [CrossRef]
- Guo, B.; Xue, M.; Zhang, T.; Gan, H.; Lin, R.; Liu, M.; Liao, Y.; Lyu, J.; Zheng, P.; Sun, B. Correlation between immune-related Tryptophan-Kynurenine pathway and severity of severe pneumonia and inflammation-related polyunsaturated fatty acids. Immun. Inflamm. Dis. 2023, 11, e1088. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin. Chest Med. 2014, 35, 71–86. [Google Scholar] [CrossRef]
- Rahman, I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: Cellular and molecular mechanisms. Cell Biochem. Biophys. 2005, 43, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, G.; Zhang, P.; Fan, J. Programmed cell death and its role in inflammation. Mil. Med. Res. 2015, 2, 12. [Google Scholar] [CrossRef]
- Longhitano, L.; Distefano, A.; Amorini, A.M.; Orlando, L.; Giallongo, S.; Tibullo, D.; Lazzarino, G.; Nicolosi, A.; Alanazi, A.M.; Saoca, C.; et al. (+)-Lipoic Acid Reduces Lipotoxicity and Regulates Mitochondrial Homeostasis and Energy Balance in an In Vitro Model of Liver Steatosis. Int. J. Mol. Sci. 2023, 24, 14491. [Google Scholar] [CrossRef] [PubMed]
- Giallongo, S.; Rehakova, D.; Biagini, T.; Lo Re, O.; Raina, P.; Lochmanova, G.; Zdrahal, Z.; Resnick, I.; Pata, P.; Pata, I.; et al. Histone Variant macroH2A1.1 Enhances Nonhomologous End Joining-dependent DNA Double-strand-break Repair and Reprogramming Efficiency of Human iPSCs. Stem Cells 2022, 40, 35–48. [Google Scholar] [CrossRef]
- Rasquel-Oliveira, F.S.; Silva, M.; Martelossi-Cebinelli, G.; Fattori, V.; Casagrande, R.; Verri, W.A., Jr. Specialized Pro-Resolving Lipid Mediators: Endogenous Roles and Pharmacological Activities in Infections. Molecules 2023, 28, 5032. [Google Scholar] [CrossRef]
- Ozerskaia, I.V.; Khachatryan, L.G.; Kolosova, N.G.; Polyanskaya, A.V.; Kasanave, E.V. The role of omega-3 polyunsaturated fatty acids in child development. Vopr. Pitan. 2024, 93, 6–18. [Google Scholar] [CrossRef]
- Trop-Steinberg, S.; Gal, M.; Azar, Y.; Kilav-Levin, R.; Heifetz, E.M. Effect of omega-3 supplements or diets on fertility in women: A meta-analysis. Heliyon 2024, 10, e29324. [Google Scholar] [CrossRef]
- Takic, M.; Rankovic, S.; Girek, Z.; Pavlovic, S.; Jovanovic, P.; Jovanovic, V.; Sarac, I. Current Insights into the Effects of Dietary alpha-Linolenic Acid Focusing on Alterations of Polyunsaturated Fatty Acid Profiles in Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 4909. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, C.; Mazzocchi, A.; Leone, L.; Agostoni, C.; Filocamo, G. Cardiovascular risk and inflammation in a population with autoimmune diseases: A narrative review. Front. Immunol. 2024, 15, 1380372. [Google Scholar] [CrossRef] [PubMed]
- Tutor, A.; O’Keefe, E.L.; Lavie, C.J.; Elagizi, A.; Milani, R.; O’Keefe, J. Omega-3 fatty acids in primary and secondary prevention of cardiovascular diseases. Prog. Cardiovasc. Dis. 2024, 84, 19–26. [Google Scholar] [CrossRef]
- Wang, C.; Han, D.; Feng, X.; Hu, L.; Wu, J. Docosahexaenoic acid alleviates LPS-induced cytotoxicity in HL-1 cardiac cells via improving stress-induced mitochondrial fragmentation. Heliyon 2023, 9, e22465. [Google Scholar] [CrossRef]
- Odetayo, A.F.; Adeyemi, W.J.; Olayaki, L.A. Omega-3 fatty acid ameliorates bisphenol F-induced testicular toxicity by modulating Nrf2/NFkB pathway and apoptotic signaling. Front. Endocrinol. 2023, 14, 1256154. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.J.; Sabaj, M.; Tolosa, G.; Herrera Vielma, F.; Zuniga, M.J.; Gonzalez, D.R.; Zuniga-Hernandez, J. Maresin-1 Prevents Liver Fibrosis by Targeting Nrf2 and NF-kappaB, Reducing Oxidative Stress and Inflammation. Cells 2021, 10, 3406. [Google Scholar] [CrossRef] [PubMed]
- Gortan Cappellari, G.; Aleksova, A.; Dal Ferro, M.; Cannata, A.; Semolic, A.; Guarnaccia, A.; Zanetti, M.; Giacca, M.; Sinagra, G.; Barazzoni, R. n-3 PUFA-Enriched Diet Preserves Skeletal Muscle Mitochondrial Function and Redox State and Prevents Muscle Mass Loss in Mice with Chronic Heart Failure. Nutrients 2023, 15, 3108. [Google Scholar] [CrossRef] [PubMed]
- Piao, Z.; Chai, B.; Wu, Y.; Diao, H.; He, Q.; Zheng, Q.; Yan, F.; Cui, W. The association between polyunsaturated fatty acids and chronic obstructive pulmonary disease: A meta-analysis. Food Funct. 2024, 15, 5929–5941. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.P.; Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Rogero, M.M.; Gualano, B.; Barroso, L.P.; Milne, G.L.; Pereira, R.M.R.; Castro, I.A. The severity of COVID-19 upon hospital admission is associated with plasma omega-3 fatty acids. Sci. Rep. 2024, 14, 10238. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.R.; Pereira, A.B.M.; de Souza, H.I.; Dos Santos, W.M.; de Assuncao, T.S.F.; de Vito, F.B.; de Souza, H.M.; da Silva, P.R.; da Silva, M.V.; Junior, V.R.; et al. Anti-inflammatory actions of aspirin-triggered resolvin D1 (AT-RvD1) in bronchial epithelial cells stimulated by cigarette smoke extract. Prostaglandins Other Lipid Mediat. 2024, 172, 106833. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Rahimi, S.; Karimi Torshizi, M.A.; Sharafi, M.; Masoudi, A.A.; Grimes, J.L. The effect of peroxisome proliferator-activated receptor gamma (PPARgamma) as a mediator of dietary fatty acids and thiazolidinedione in pulmonary arterial hypertension induced by cold stress of broilers. Res. Vet. Sci. 2024, 168, 105157. [Google Scholar] [CrossRef] [PubMed]
- Mickleborough, T.D. Omega-3 polyunsaturated fatty acids in physical performance optimization. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Hubbard, R.C.; Crystal, R.G. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1989, 139, 370–372. [Google Scholar] [CrossRef]
- Bunnell, E.; Pacht, E.R. Oxidized glutathione is increased in the alveolar fluid of patients with the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1993, 148, 1174–1178. [Google Scholar] [CrossRef]
- Roum, J.H.; Buhl, R.; McElvaney, N.G.; Borok, Z.; Crystal, R.G. Systemic deficiency of glutathione in cystic fibrosis. J. Appl. Physiol. 1993, 75, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.S.; Pabelick, C.M.; Sieck, G.C. Mitochondrial Dysfunction in Airway Disease. Chest 2017, 152, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, S.M.; Choi, A.M. Mitochondria in lung disease. J. Clin. Investig. 2016, 126, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Polito, A.J.; Proud, D. Epithelia cells as regulators of airway inflammation. J. Allergy Clin. Immunol. 1998, 102, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Duru, E.A.; Ameredes, B.T. Role of IL-10 in the resolution of airway inflammation. Curr. Mol. Med. 2008, 8, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Hodge-Dufour, J.; Marino, M.W.; Horton, M.R.; Jungbluth, A.; Burdick, M.D.; Strieter, R.M.; Noble, P.W.; Hunter, C.A.; Pure, E. Inhibition of interferon gamma induced interleukin 12 production: A potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc. Natl. Acad. Sci. USA 1998, 95, 13806–13811. [Google Scholar] [CrossRef] [PubMed]
- Eickmeier, O.; Seki, H.; Haworth, O.; Hilberath, J.N.; Gao, F.; Uddin, M.; Croze, R.H.; Carlo, T.; Pfeffer, M.A.; Levy, B.D. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2013, 6, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Xia, L.; Shannahan, J.H. Enhanced silver nanoparticle-induced pulmonary inflammation in a metabolic syndrome mouse model and resolvin D1 treatment. Part. Fibre Toxicol. 2022, 19, 54. [Google Scholar] [CrossRef]
- Hernandez, J.; Schaffer, J.; Herden, C.; Pflieger, F.J.; Reiche, S.; Korber, S.; Kitagawa, H.; Welter, J.; Michels, S.; Culmsee, C.; et al. n-3 Polyunsaturated Fatty Acids Modulate LPS-Induced ARDS and the Lung-Brain Axis of Communication in Wild-Type versus Fat-1 Mice Genetically Modified for Leukotriene B4 Receptor 1 or Chemerin Receptor 23 Knockout. Int. J. Mol. Sci. 2023, 24, 13524. [Google Scholar] [CrossRef]
- Nienaber, A.; Ozturk, M.; Dolman, R.; Blaauw, R.; Zandberg, L.L.; van Rensburg, S.; Britz, M.; Hayford, F.E.A.; Brombacher, F.; Loots, D.T.; et al. n-3 long-chain PUFA promote antibacterial and inflammation-resolving effects in Mycobacterium tuberculosis-infected C3HeB/FeJ mice, dependent on fatty acid status. Br. J. Nutr. 2022, 127, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Serhan, C.N. Specialized pro-resolving mediator network: An update on production and actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar] [CrossRef]
- Leroy, V.; Cai, J.; Tu, Z.; McQuiston, A.; Sharma, S.; Emtiazjoo, A.; Atkinson, C.; Upchurch, G.R., Jr.; Sharma, A.K. Resolution of post-lung transplant ischemia-reperfusion injury is modulated via Resolvin D1-FPR2 and Maresin 1-LGR6 signaling. J. Heart Lung Transplant. 2023, 42, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Wang, Q.; Mei, H.X.; Zheng, S.X.; Ali, A.M.; Wu, Q.X.; Ye, Y.; Xu, H.R.; Xiang, S.Y.; Jin, S.W. RvD1 ameliorates LPS-induced acute lung injury via the suppression of neutrophil infiltration by reducing CXCL2 expression and release from resident alveolar macrophages. Int. Immunopharmacol. 2019, 76, 105877. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Norling, L.V.; Dalli, J.; Flower, R.J.; Serhan, C.N.; Perretti, M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: Receptor-dependent actions. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1970–1978. [Google Scholar] [CrossRef]
- Monteiro Bastos da Silva, J.; Chaker, J.; Martail, A.; Costa Moreira, J.; David, A.; Le Bot, B. Improving Exposure Assessment Using Non-Targeted and Suspect Screening: The ISO/IEC 17025: 2017 Quality Standard as a Guideline. J. Xenobiot. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- GOED. GOED Voluntary Monograph. Available online: https://goedomega3.com/goed-monograph (accessed on 7 July 2024).
- Spampinato, M.; Carota, G.; Sferrazzo, G.; Fuochi, V.; Distefano, A.; Ronsisvalle, S.; Sipala, F.; Giuffrida, R.; Furneri, P.M.; Di Rosa, M.; et al. Effects of Mangiferin on LPS-Induced Inflammation and SARS-CoV-2 Viral Adsorption in Human Lung Cells. Pharmaceutics 2022, 14, 2845. [Google Scholar] [CrossRef]



| Determinations | Specification | Result |
|---|---|---|
| Fatty Acid Profile | ||
| EPA mg/g (as FFA) | 50–150 | 101.0 |
| DHA mg/g (as FFA) | 100–225 | 175.8 |
| Total Omega-3 mg/g (as FFA) * | 250–425 | 325.1 |
| * (Sum of 18:3 w3, 18:4 w3, 20:4 w3, 20:5 w3, 21:5 w3, 22:5 w3, 22:6 w3) | ||
| SPM Content | ||
| 17-HDHA as FFA (mg/kg) | 40–200 | 137.7 |
| 18-HEPE as FFA (mg/kg) | 25–200 | 97.5 |
| 14-HDHA as FFA (mg/kg) | 20–100 | 22.7 |
| Analytical Data | ||
| Acid Value (mg KOH/g) | Max. 3 | 0.47 |
| Peroxide Index (meq O2/kg) | Max. 5 | 2.38 (at time of release) |
| Anisidine value | Max. 20 | 11.0 (at time of release) |
| Weight Variation (Upon Fill) | Avg. 10% | Complies |
| Disintegration time (min) | Max. 30 | 7 |
| Contaminant Data | ||
| Arsenic (mg/kg) | Max. 1 | <0.05 |
| Cadmium(mg/kg) | Max. 1 | <0.01 |
| Lead (mg/kg) | Max. 0.33 | <0.05 |
| Mercury (mg/kg) | Max. 0.1 | <0.01 |
| Microbiological parameters | ||
| Aerobic Plate Count (CFU/g) | Max. 1000 | <10 |
| Yeast and Mould (CFU/g) | Max. 100 | <10 |
| S.aureus | Negative/10 g | Negative |
| Salmonella | Negative/10 g | Negative |
| E. coli | Negative/10 g | Negative |
| Primer | Forward | Reverse |
|---|---|---|
| GPX | ACAAGAACGGCTGCGTGGTGAA | GCCACACACTTGTGGAGCTAGA |
| CAT | GTGCGGAGATTCAACACTGCCA | CGGCAATGTTCTCACACAGACG |
| SOD2 | CTGGACAAACCTCAGCCCTAAC | AACCTGAGCCTTGGACACCAAC |
| IL-6 | AGACAGCCACTCACCTCTTCAG | TTCTGCCAGTGCCTCTTTGCTG |
| IL-10 | TCTCCGACAAGGCTTGGCAACCCA | TCAGACAAGGCTTGGCAACCCA |
| TNF-a | CTCTTCTGCCTGCTGCACTTTG | ATGGGCTACAGGCTTGTCACTC |
| PTGS1 | GATGAGCAGCTTTTCCAGACGAC | CCAGACTGGATGCAGGACACAA |
| ALOX15 | ACCTTCCTGCTCGCCTAGTGTT | GGCTACAGAATGACGTTGGC |
| FPR2 | GCCTTTTGGCTGGTTCCTGTGT | CCAGACTGGATGCAGGACACAA |
| GPR32 | GTGATCGCTCTTGTTCCAGGAAG | TGCGTGCCATACGGAAGACAGT |
| b-Actin | CACCATTGGCAATGAGCGGTTC | AGGTCTTTGCGGATGTCCACGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Distefano, A.; Orlando, L.; Giallongo, S.; Tropea, E.; Spampinato, M.; Santisi, A.; Longhitano, L.; Parisi, G.; Leonardi, S.; Russo, A.; et al. Fish Oil Containing Pro-Resolving Mediators Enhances the Antioxidant System and Ameliorates LPS-Induced Inflammation in Human Bronchial Epithelial Cells. Pharmaceuticals 2024, 17, 1066. https://doi.org/10.3390/ph17081066
Distefano A, Orlando L, Giallongo S, Tropea E, Spampinato M, Santisi A, Longhitano L, Parisi G, Leonardi S, Russo A, et al. Fish Oil Containing Pro-Resolving Mediators Enhances the Antioxidant System and Ameliorates LPS-Induced Inflammation in Human Bronchial Epithelial Cells. Pharmaceuticals. 2024; 17(8):1066. https://doi.org/10.3390/ph17081066
Chicago/Turabian StyleDistefano, Alfio, Laura Orlando, Sebastiano Giallongo, Emanuela Tropea, Mariarita Spampinato, Annalisa Santisi, Lucia Longhitano, Giuseppe Parisi, Salvatore Leonardi, Arcangelo Russo, and et al. 2024. "Fish Oil Containing Pro-Resolving Mediators Enhances the Antioxidant System and Ameliorates LPS-Induced Inflammation in Human Bronchial Epithelial Cells" Pharmaceuticals 17, no. 8: 1066. https://doi.org/10.3390/ph17081066
APA StyleDistefano, A., Orlando, L., Giallongo, S., Tropea, E., Spampinato, M., Santisi, A., Longhitano, L., Parisi, G., Leonardi, S., Russo, A., Caruso, M., Di Rosa, M., Tibullo, D., Salamone, M., Li Volti, G., & Barbagallo, I. A. (2024). Fish Oil Containing Pro-Resolving Mediators Enhances the Antioxidant System and Ameliorates LPS-Induced Inflammation in Human Bronchial Epithelial Cells. Pharmaceuticals, 17(8), 1066. https://doi.org/10.3390/ph17081066













