Kalanchoe tomentosa: Phytochemical Profiling, and Evaluation of Its Biological Activities In Vitro, In Vivo, and In Silico
Abstract
1. Introduction
2. Results
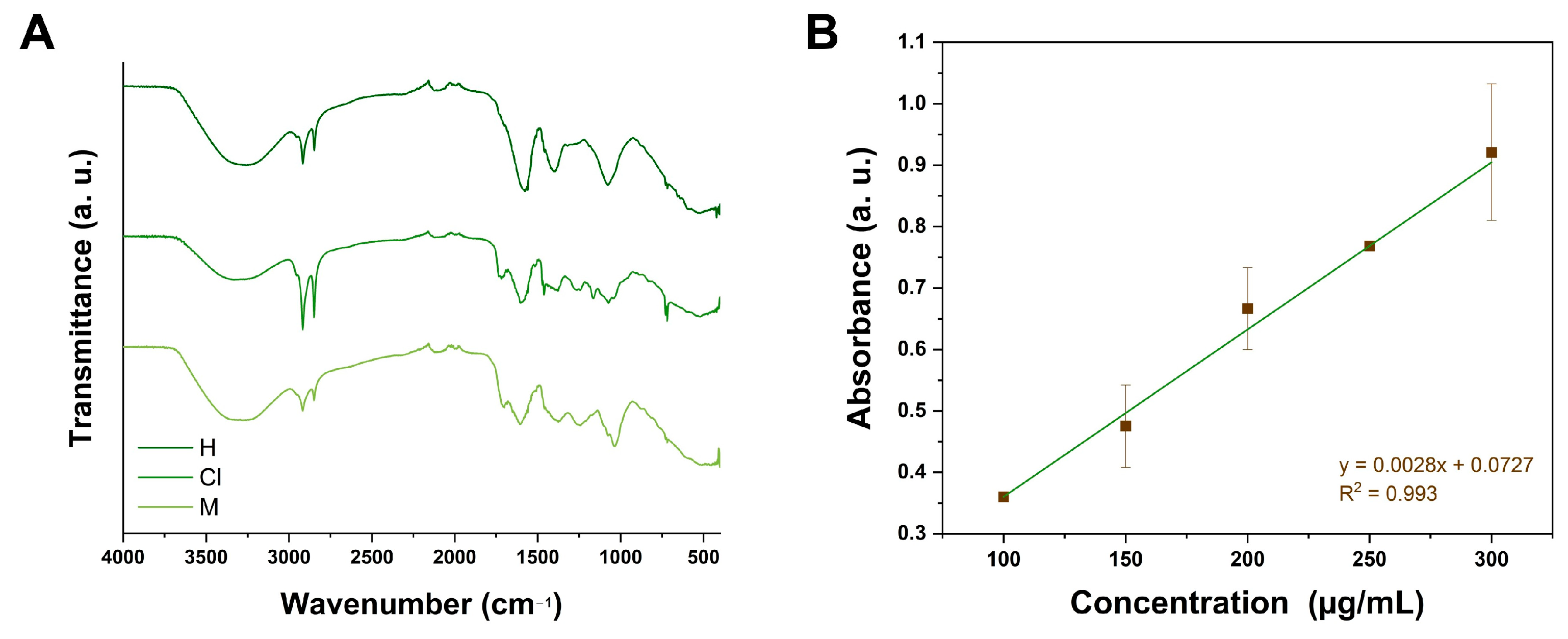
2.1. Total Flavonoid Content (TFC) and FTIR Analyses
2.2. GC/MS Analysis
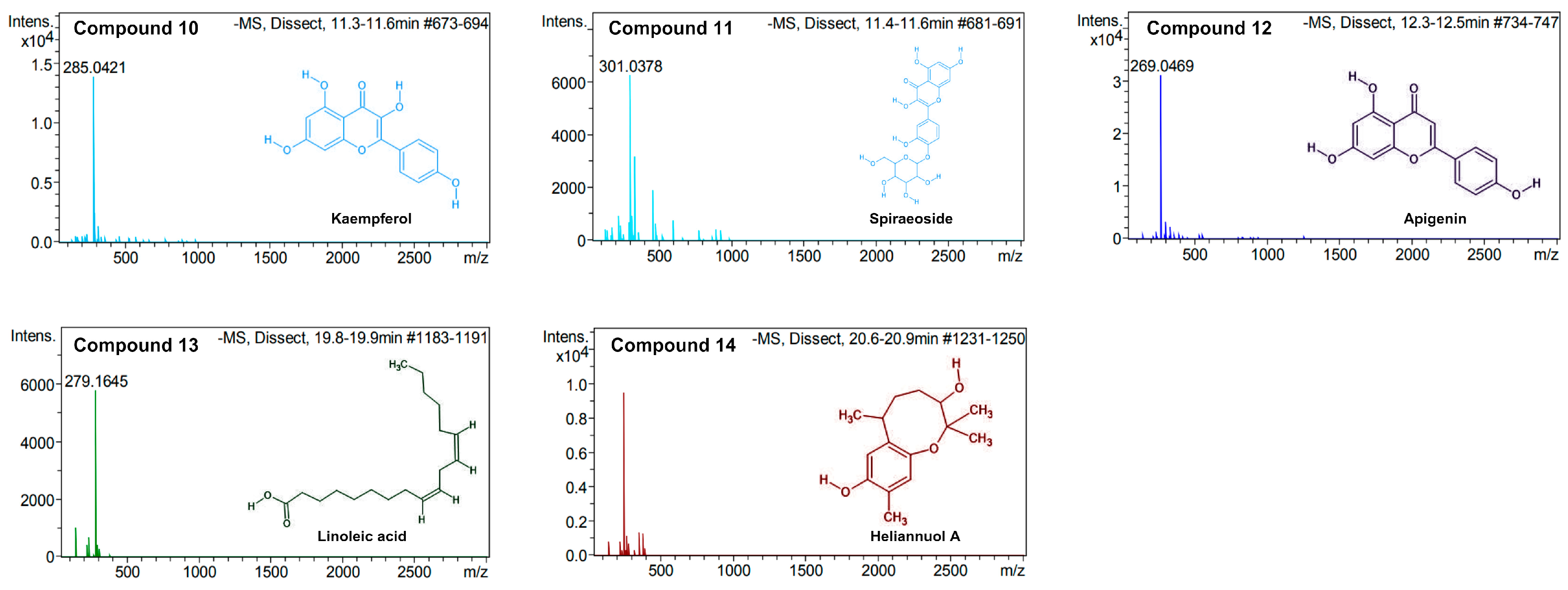
2.3. UPLC/MS/MS Analysis
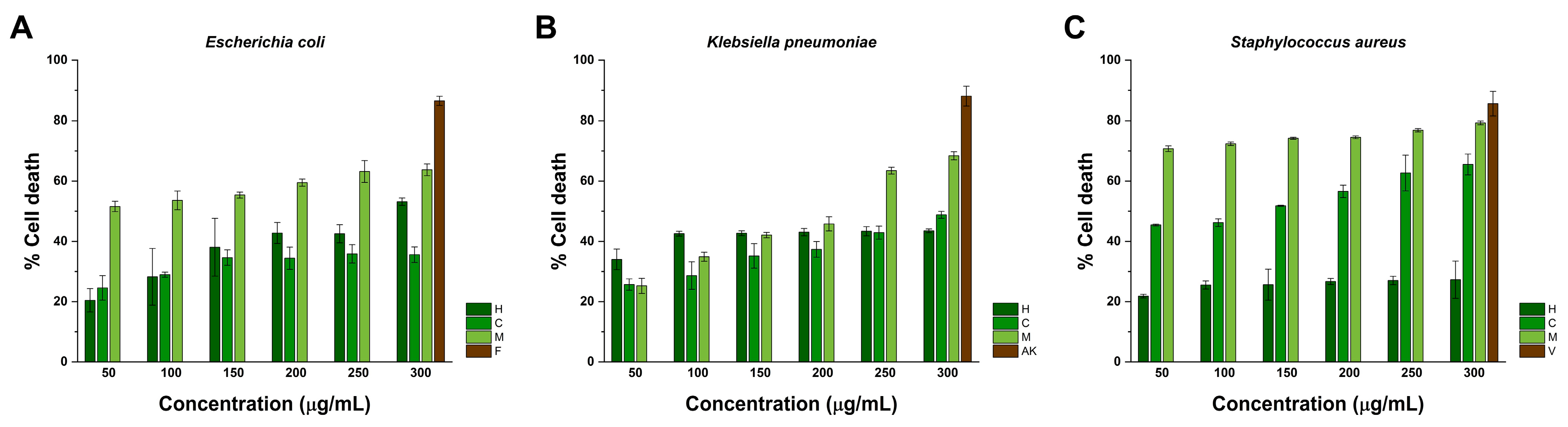
2.4. Antibacterial Activity
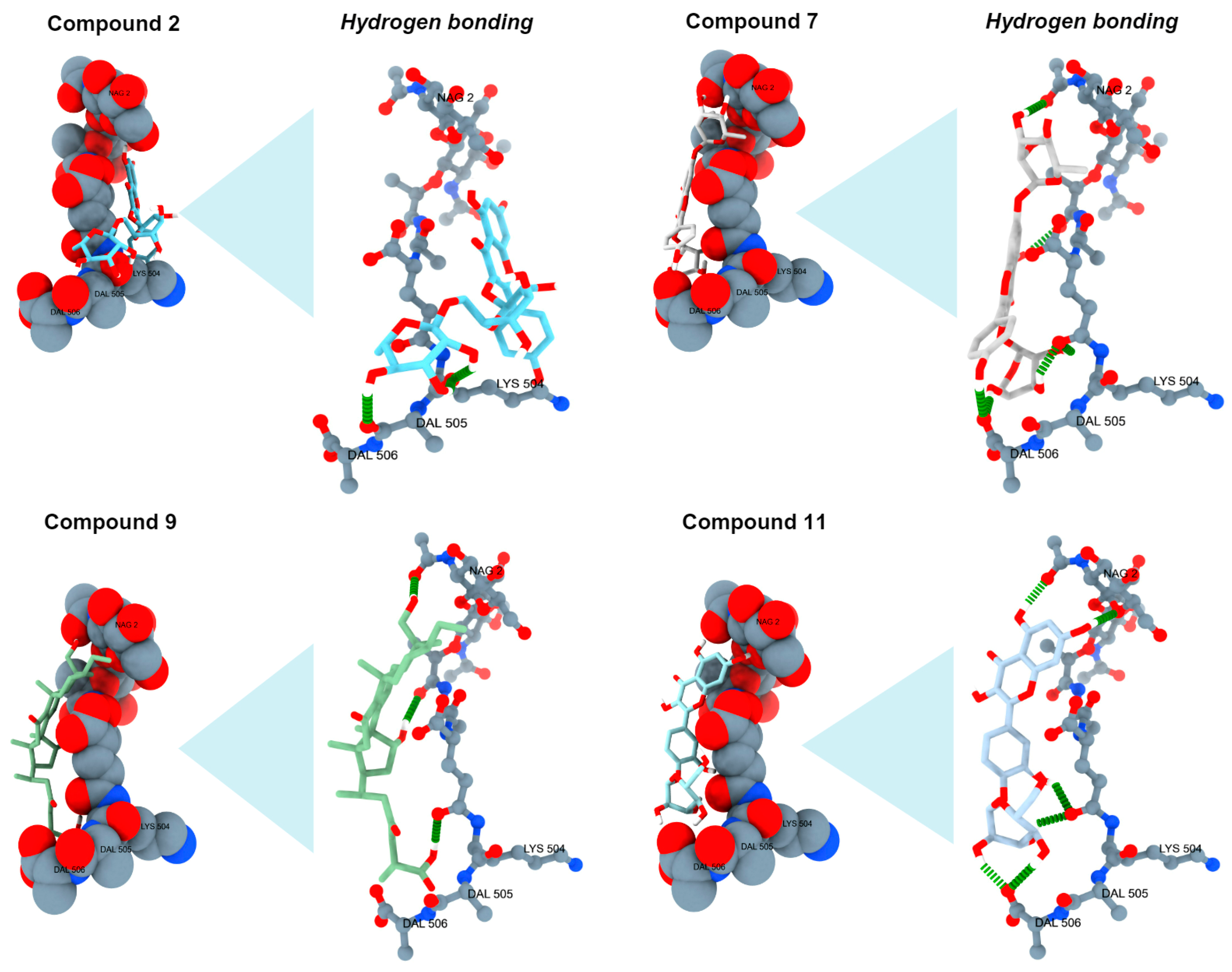
2.5. Analysis of Antibacterial Activity In Silico
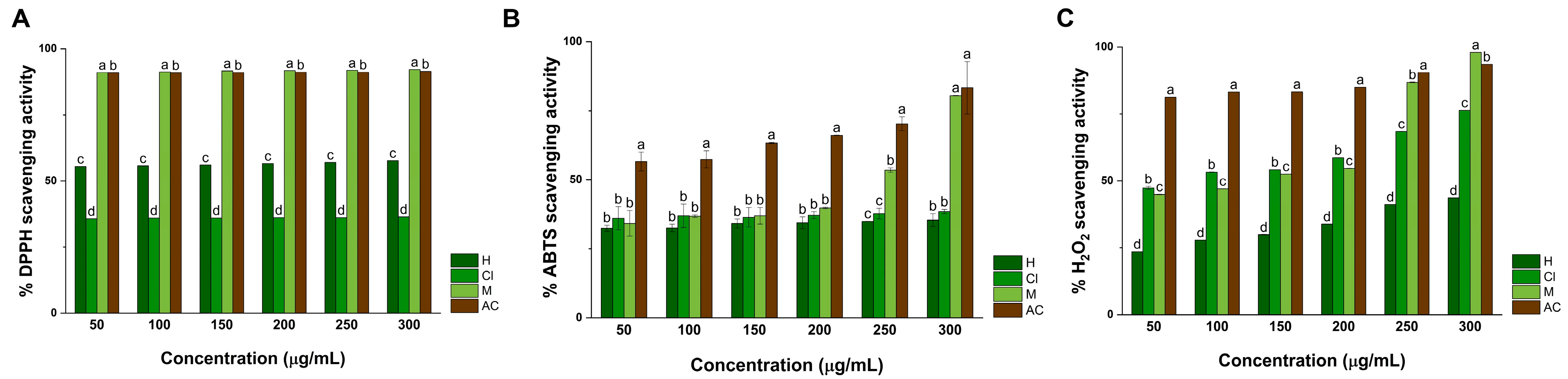
2.6. Antioxidant Activity
2.6.1. DPPH Assay
2.6.2. ABTS Assay
2.6.3. H2O2 Assay
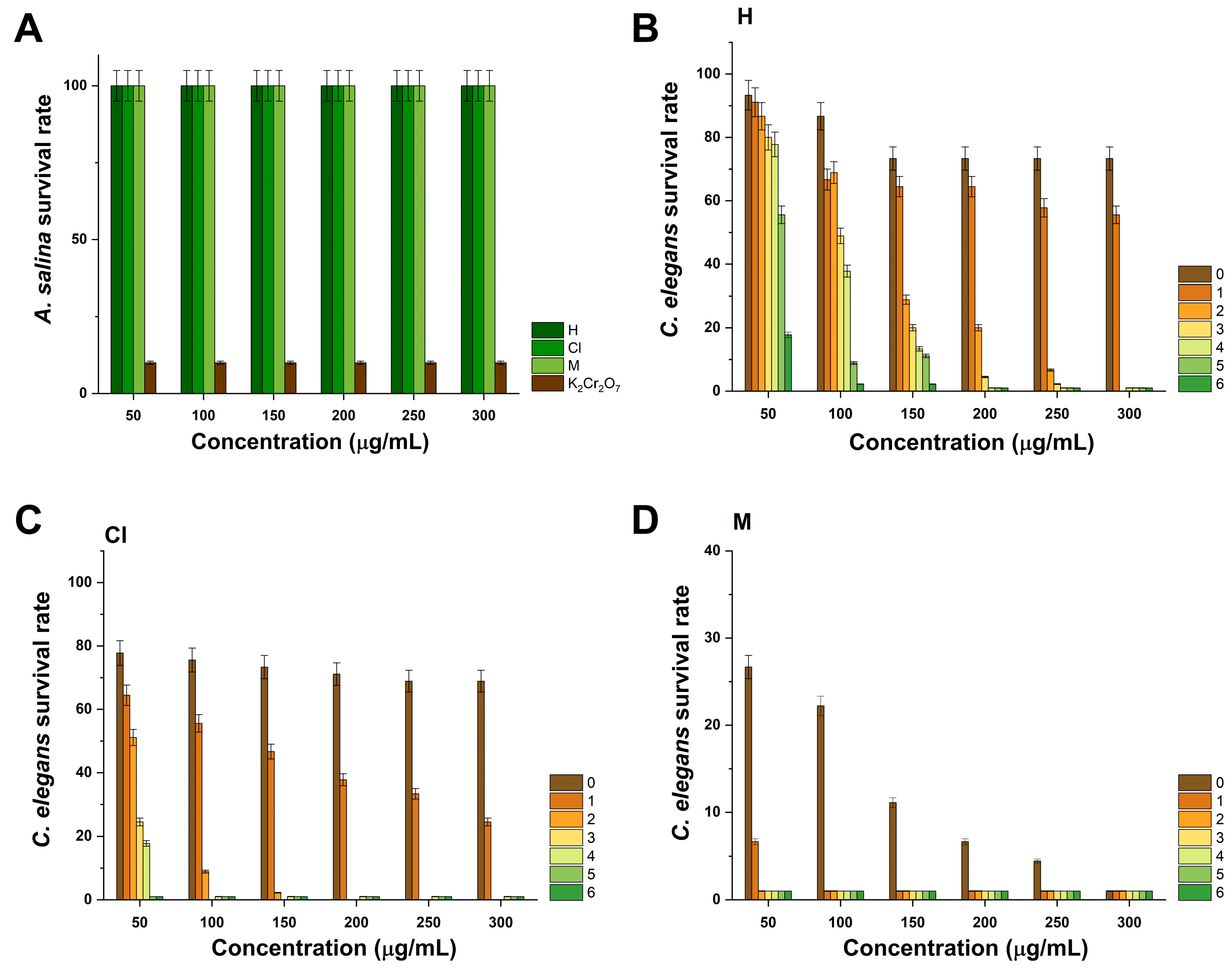
2.7. Evaluation of In Vivo Toxicity in A. salina and C. elegans
3. Discussion
4. Materials and Methods
4.1. Plant Collection and Identification
4.2. Extracts Preparation
4.3. TFC Analysis
4.4. FTIR Analysis
4.5. GC/MS Analysis
4.6. UPLC/MS/MS Analysis
4.7. Strains, Culture Media, and Antibacterial Assay
4.8. In Silico Analysis
4.9. Antioxidant Assays
4.10. Culture of In Vivo Models and Toxicity Assays
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Yan, X.; Zhao, M.; Zhang, S.; Jia, Z. Spatial and Temporal Distribution of Emerging Airborne Viral Infectious Diseases Outbreaks on a Global Scale. J. Public Health 2024, 32, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, V.D.; Yin, R.; Nercelles, P.; Rivera-Molina, S.E.; Jyoti, S.; Dongol, R.; Aguilar-De-Moros, D.; Tumu, N.; Alarcon-Rua, J.; Stagnaro, J.P.; et al. International Nosocomial Infection Control Consortium (INICC) Report of Health Care Associated Infections, Data Summary of 45 Countries for 2015 to 2020, Adult and Pediatric Units, Device-Associated Module. Am. J. Infect. Control 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.A.; Boeira, D.; Doeynart, R.; Longen, W.C.; Marqueze, L.F.; Silveira, P.C.L.; Thirupathi, A.; Gu, Y.; Pinho, R.A. Effects of Aerobic Exercise during Recovery from Eccentric Contraction on Muscular Performance, Oxidative Stress and Inflammation. Curr. Res. Physiol. 2024, 7, 100129. [Google Scholar] [CrossRef] [PubMed]
- Tiselko, A.V.; Misharina, E.V.; Yarmolinskaya, M.I.; Milyutina, Y.P.; Zalozniaia, I.V.; Korenevsky, A.V. Evaluation of Folliculogenesis and Oxidative Stress Parameters in Type 1 Diabetes Mellitus Women with Different Glycemic Profiles. Endocrine 2024. online ahead of print. [Google Scholar] [CrossRef]
- Jacques, C.; Marchand, F.; Chatelais, M.; Floris, I. Actives from the Micro-Immunotherapy Medicine 2LMIREG® Reduce the Expression of Cytokines and Immune-Related Markers Including Interleukin-2 and HLA-II While Modulating Oxidative Stress and Mitochondrial Function. J. Inflamm. Res. 2024, 17, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, Q.; Dai, Y.; Pan, X.; Luo, X.; Peng, R.; Guo, C.; Tan, L. Association of Co-Exposure to Polycyclic Aromatic Hydrocarbons and Phthalates with Oxidative Stress and Inflammation. Sci. Total Environ. 2024, 912, 169513. [Google Scholar] [CrossRef] [PubMed]
- Fejes, R.; Pilat, N.; Lutnik, M.; Weisshaar, S.; Weijler, A.M.; Krüger, K.; Draxler, A.; Bragagna, L.; Peake, J.M.; Woodman, R.J.; et al. Effects of Increased Nitrate Intake from Beetroot Juice on Blood Markers of Oxidative Stress and Inflammation in Older Adults with Hypertension. Free Radic. Biol. Med. 2024, 222, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.; Zhang, W.; Li, D.; Guo, P.; He, M.; Zhang, Y.; Li, J.; Guan, H.; Zhang, W.; Luo, D.; et al. Parkinson’s Disease with Anxiety: Clinical Characteristics and Their Correlation with Oxidative Stress, Inflammation, and Pathological Proteins. BMC Geriatr. 2024, 24, 433. [Google Scholar] [CrossRef] [PubMed]
- Abu Khadra, K.M.; Bataineh, M.I.; Khalil, A.; Saleh, J. Oxidative Stress and Type 2 Diabetes: The Development and the Pathogenesis, Jordanian Cross-Sectional Study. Eur. J. Med. Res. 2024, 29, 370. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of Oxidative Stress, Cellular Communication and Signaling Pathways in Cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Paudel, N.; Rai, M.; Adhikari, S.; Thapa, A.; Bharati, S.; Maharjan, B.; Shrestha, R.L.S.; Rav, K.; Singh, A.V. Green Extraction, Phytochemical Profiling, and Biological Evaluation of Dysphania ambrosioides: An In Silico and In Vitro Medicinal Investigation. J. Herbs Spices Med. Plants 2024, 30, 97–114. [Google Scholar] [CrossRef]
- Fauzi, A.; Kifli, N.; Noor, M.H.M.; Hamzah, H.; Azlan, A. Bioactivity, Phytochemistry Studies and Subacute In Vivo Toxicity of Ethanolic Leaf Extract of White Mulberry (Morus alba Linn.) in Female Mice. J. Ethnopharmacol. 2024, 325, 117914. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmed, A.; Shakeel, A.; Hussain, H.A.; Raza, S.A. The Efficacy of Linum Usitatissimum Seeds to Inhibit Estrogen Receptor as a Natural Therapy for PCOS: An In Silico and In Vitro Analysis. Cell Biochem. Funct. 2024, 42, e3897. [Google Scholar] [CrossRef]
- Qader, S.W.; Ozdemir, M.; Benjamin, I.; Chima, C.M.; Suvitha, A.; Rani, J.C.; Gber, T.E.; Kothandan, G. Toxicity, Pharmacokinetic Profile, and Compound-Protein Interaction Study of Polygonum minus Huds Extract. Appl. Biochem. Biotechnol. 2024, 196, 2425–2450. [Google Scholar] [CrossRef]
- Labhar, A.; El-Mernissi, Y.; Ahidar, N.; Zouhri, A.; Benamari, O.; Siddique, F.; Bashir, M.; Salhi, A.; Ahari, M.; Ibenmoussa, S.; et al. Seasonal Variations in the Essential Oil Composition and Biological Activities of Wild Lavandula dentata L. Nat. Prod. Commun. 2024, 19, 1934578X241230822. [Google Scholar] [CrossRef]
- Kan, J.; Zhang, S.; Wu, Z.; Bi, D. Exploring Plastomic Resources in Sempervivum (Crassulaceae): Implications for Phylogenetics. Genes 2024, 15, 441. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Zhang, D.-Q.; Zhang, J.-Q. Plastomic Data Shed New Light on the Phylogeny, Biogeography, and Character Evolution of the Family Crassulaceae. J. Syst. Evol. 2023, 61, 990–1003. [Google Scholar] [CrossRef]
- Nascimento, L.B.d.S.; Casanova, L.M.; Costa, S.S. Bioactive Compounds from Kalanchoe Genus Potentially Useful for the Development of New Drugs. Life 2023, 13, 646. [Google Scholar] [CrossRef]
- Assis de Andrade, E.; Machinski, I.; Terso Ventura, A.C.; Barr, S.A.; Pereira, A.V.; Beltrame, F.L.; Strangman, W.K.; Williamson, R.T. A Review of the Popular Uses, Anatomical, Chemical, and Biological Aspects of Kalanchoe (Crassulaceae): A Genus of Plants Known as “Miracle Leaf”. Molecules 2023, 28, 5574. [Google Scholar] [CrossRef]
- Ramon, P.; Bergmann, D.; Abdulla, H.; Sparks, J.; Omoruyi, F. Bioactive Ingredients in K. Pinnata Extract and Synergistic Effects of Combined K. pinnata and Metformin Preparations on Antioxidant Activities in Diabetic and Non-Diabetic Skeletal Muscle Cells. Int. J. Mol. Sci. 2023, 24, 6211. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Hering, A.; Kowalczyk, M.; Hałasa, R.; Gucwa, M.; Ochocka, J.R. Kalanchoe sp. Extracts—Phytochemistry, Cytotoxic, and Antimicrobial Activities. Plants 2023, 12, 2268. [Google Scholar] [CrossRef]
- Nielsen, A.H.; Olsen, C.E.; Møller, B.L. Flavonoids in Flowers of 16 Kalanchoë blossfeldiana Varieties. Phytochemistry 2005, 66, 2829–2835. [Google Scholar] [CrossRef]
- Huang, H.-C.; Lin, M.-K.; Yang, H.-L.; Hseu, Y.-C.; Liaw, C.-C.; Tseng, Y.-H.; Tsuzuki, M.; Kuo, Y.-H. Cardenolides and Bufadienolide Glycosides from Kalanchoe tubiflora and Evaluation of Cytotoxicity. Planta Med. 2013, 79, 1362–1369. [Google Scholar] [CrossRef]
- Wu, P.-L.; Hsu, Y.-L.; Wu, T.-S.; Bastow, K.F.; Lee, K.-H. Kalanchosides A–C, New Cytotoxic Bufadienolides from the Aerial Parts of Kalanchoe Gracilis. Org. Lett. 2006, 8, 5207–5210. [Google Scholar] [CrossRef]
- Akulova-Barlow, Z. Kalanchoe. Cactus Succul. J. 2009, 81, 268–276. [Google Scholar] [CrossRef]
- Vargas, A.; Herrera, I.; Nualart, N.; Guézou, A.; Gómez-Bellver, C.; Freire, E.; Jaramillo Díaz, P.; López-Pujol, J. The Genus Kalanchoe (Crassulaceae) in Ecuador: From Gardens to the Wild. Plants 2022, 11, 1746. [Google Scholar] [CrossRef]
- Anwar, R.; Sukmasari, S.; Siti Aisyah, L.; Puspita Lestari, F.; Ilfani, D.; Febriani Yun, Y.; Diki Prestya, P. Antimicrobial Activity of β-Sitosterol Isolated from Kalanchoe Tomentosa Leaves against Staphylococcus aureus and Klebsiella pneumonia. Pak. J. Biol. Sci. 2022, 25, 602–607. [Google Scholar] [CrossRef]
- Saleh1, M.M.; Ghoneim1, M.M.; Kottb1, S.; El-Hela1, A.A. Biologically active secondary metabolites from kalanchoe tomentosa. J. Biomed. Pharm. Res. 2014, 3, 136–140. [Google Scholar]
- Aisyah, L.S.; Ilfani, D.; Lestari, F.P.; Yun, Y.F. α-Amylase Inhibition Activities by Flavonoid Compounds from Panda Plants (Kalanchoe tomentosa). J. Kim. Sains Apl. 2020, 23, 96–101. [Google Scholar] [CrossRef][Green Version]
- Mayorga, O.A.S.; da Costa, Y.F.G.; da Silva, J.B.; Scio, E.; Ferreira, A.L.P.; de Sousa, O.V.; Alves, M.S. Kalanchoe brasiliensis Cambess., a Promising Natural Source of Antioxidant and Antibiotic Agents against Multidrug-Resistant Pathogens for the Treatment of Salmonella Gastroenteritis. Oxid. Med. Cell. Longev. 2019, 2019, e9245951. [Google Scholar] [CrossRef]
- Mejía-Méndez, J.L.; Bach, H.; Lorenzo-Leal, A.C.; Navarro-López, D.E.; López-Mena, E.R.; Hernández, L.R.; Sánchez-Arreola, E. Biological Activities and Chemical Profiles of Kalanchoe fedtschenkoi Extracts. Plants 2023, 12, 1943. [Google Scholar] [CrossRef]
- Farooqi, S.S.; Naveed, S.; Qamar, F.; Sana, A.; Farooqi, S.H.; Sabir, N.; Mansoor, A.; Sadia, H. Phytochemical Analysis, GC-MS Characterization and Antioxidant Activity of Hordeum vulgare Seed Extracts. Heliyon 2024, 10, e27297. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From Preclinical Evidence to Potential Clinical Applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef]
- Pal, S.; Chaudhuri, A.K.N. Studies on the Anti-Ulcer Activity of a Bryophyllum pinnatum Leaf Extract in Experimental Animals. J. Ethnopharmacol. 1991, 33, 97–102. [Google Scholar] [CrossRef]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as Potential Agents for the Chemoprevention and Therapy of Breast Cancer. Front. Biosci. (Landmark Ed.) 2011, 16, 980. [Google Scholar] [CrossRef]
- Yu, Y.; Chang, P.; Yu, H.; Ren, H.; Hong, D.; Li, Z.; Wang, Y.; Song, H.; Huo, Y.; Li, C. Productive Amyrin Synthases for Efficient α-Amyrin Synthesis in Engineered Saccharomyces cerevisiae. ACS Synth. Biol. 2018, 7, 2391–2402. [Google Scholar] [CrossRef]
- Nogueira, A.O.; Oliveira, Y.I.S.; Adjafre, B.L.; de Moraes, M.E.A.; Aragão, G.F. Pharmacological Effects of the Isomeric Mixture of Alpha and Beta Amyrin from Protium heptaphyllum: A Literature Review. Fundam. Clin. Pharmacol. 2019, 33, 4–12. [Google Scholar] [CrossRef]
- Siddiqui, S.; Faizi, S.; Siddiqui, B.S.; Sultana, N. Triterpenoids and Phenanthrenes from Leaves of Bryophyllum pinnatum. Phytochemistry 1989, 28, 2433–2438. [Google Scholar] [CrossRef]
- Siems, K.; Jas, G.; Arriaga-Giner, F.J.; Wollenweber, E.; Dörr, M. Notes: On the Chemical Nature of Epicuticular Waxes in Some Succulent Kalanchoe and Senecio Species. Z. Naturforschung C 1995, 50, 451–454. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Tsai, F.-S.; Lin, L.-W.; Wu, C.-R. Lupeol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 145–175. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, A Novel Anti-Inflammatory and Anti-Cancer Dietary Triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef]
- Xu, F.; Huang, X.; Wu, H.; Wang, X. Beneficial Health Effects of Lupenone Triterpene: A Review. Biomed. Pharmacother. 2018, 103, 198–203. [Google Scholar] [CrossRef]
- Navarrete-Carriola, D.V.; Paz-González, A.D.; Vázquez-Jiménez, L.K.; De Luna-Santillana, E.; Cruz-Hernández, M.A.; Bandyopadhyay, D.; Rivera, G. Comparative Analysis of a Secondary Metabolite Profile from Roots and Leaves of Iostephane heterophylla by UPLC-MS and GC-MS. ACS Omega 2024, 9, 5429–5439. [Google Scholar] [CrossRef]
- El Abdellaoui, S.; Destandau, E.; Toribio, A.; Elfakir, C.; Lafosse, M.; Renimel, I.; André, P.; Cancellieri, P.; Landemarre, L. Bioactive Molecules in Kalanchoe pinnata Leaves: Extraction, Purification, and Identification. Anal. Bioanal. Chem. 2010, 398, 1329–1338. [Google Scholar] [CrossRef]
- Villarreal Romero, W.L.; Robles Camargo, J.E.; Costa, G.M. Phytochemical Standardization of an Extract Rich in Flavonoids from Flowers of Kalanchoe pinnata (Lam) Pers. Sci. Pharm. 2023, 91, 50. [Google Scholar] [CrossRef]
- de Barros-Santos, R.G.; Pimentel, T.C.; Amorim, T.A.; da Silva Nogueira, E.T.; de Oliveira Vilar, S.B.; de Souza, M.E.A.O.; de Brito Araújo Carvalho, A.J.; Magnani, M.; dos Santos Lima, M. Ultra-Fast Determination of Free Carotenoids in Fruit Juices by Rapid Resolution Liquid Chromatography (RRLC): Method Validation and Characterization of Brazilian Whole Fruit Juices. Food Anal. Methods 2023, 16, 808–818. [Google Scholar] [CrossRef]
- Osman, E.E.A.; Mohamed, A.S.; Elkhateeb, A.; Gobouri, A.; Abdel-Aziz, M.M.; Abdel-Hameed, E.-S.S. Phytochemical Investigations, Antioxidant, Cytotoxic, Antidiabetic and Antibiofilm Activities of Kalanchoe laxiflora Flowers. Eur. J. Integr. Med. 2022, 49, 102085. [Google Scholar] [CrossRef]
- Palumbo, A.; Casanova, L.M.; Corrêa, M.F.P.; Da Costa, N.M.; Nasciutti, L.E.; Costa, S.S. Potential Therapeutic Effects of Underground Parts of Kalanchoe gastonis-bonnieri on Benign Prostatic Hyperplasia. Evid.-Based Complement. Altern. Med. 2019, 2019, e6340757. [Google Scholar] [CrossRef]
- Ali, M.; Hassan, M.; Ansari, S.A.; Alkahtani, H.M.; Al-Rasheed, L.S.; Ansari, S.A. Quercetin and Kaempferol as Multi-Targeting Antidiabetic Agents against Mouse Model of Chemically Induced Type 2 Diabetes. Pharmaceuticals 2024, 17, 757. [Google Scholar] [CrossRef]
- Son, N.T.; Gianibbi, B.; Panti, A.; Spiga, O.; Bastos, J.K.; Fusi, F. 3,3′-O-Dimethylquercetin: A Bi-Functional Vasodilator Isolated from Green Propolis of the Caatinga Mimosa tenuiflora. Eur. J. Pharmacol. 2024, 967, 176400. [Google Scholar] [CrossRef]
- Singh, D.; Khan, M.A.; Mishra, D.; Goel, A.; Ansari, M.A.; Akhtar, K.; Siddique, H.R. Apigenin Enhances Sorafenib Anti-Tumour Efficacy in Hepatocellular carcinoma. Transl. Oncol. 2024, 43, 101920. [Google Scholar] [CrossRef]
- Aiesh, B.M.; Natsheh, M.; Amar, M.; AbuTaha, S.; Qadi, M.; AbuTaha, A.; Sabateen, A.; Zyoud, S.H. Epidemiology and Clinical Characteristics of Patients with Healthcare-Acquired Multidrug-Resistant Gram-Negative Bacilli: A Retrospective Study from a Tertiary Care Hospital. Sci. Rep. 2024, 14, 3022. [Google Scholar] [CrossRef]
- Oyardi, O.; Yilmaz, F.N.; Dosler, S. Efficacy of Zoliflodacin, a Spiropyrimidinetrione Antibiotic, against Gram-Negative Pathogens. Curr. Microbiol. 2024, 81, 241. [Google Scholar] [CrossRef]
- Sohrabi, M.; Pirbonyeh, N.; Alizade Naini, M.; Rasekhi, A.; Ayoub, A.; Hashemizadeh, Z.; Shahcheraghi, F. A Challenging Case of Carbapenem Resistant Klebsiella pneumoniae-Related Pyogenic Liver Abscess with Capsular Polysaccharide Hyperproduction: A Case Report. BMC Infect. Dis. 2024, 24, 433. [Google Scholar] [CrossRef]
- González, M.I.; González-Arjona, M.; Cussó, L.; Morcillo, M.Á.; Aguilera-Correa, J.J.; Esteban, J.; Kestler, M.; Calle, D.; Cerón, C.; Cortes-Canteli, M.; et al. In Vivo Detection of Staphylococcus aureus Infections Using Radiolabeled Antibodies Specific for Bacterial Toxins. Int. J. Biomed. Imaging 2024, 2024, 3655327. [Google Scholar] [CrossRef]
- Periyasami, G.; Karuppiah, P.; Karthikeyan, P.; Palaniappan, S. Anti-Infective Efficacy of Duloxetine against Catheter-Associated Urinary Tract Infections Caused by Gram-Positive Bacteria. ACS Omega 2023, 8, 48317–48325. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Pasiński, B.; Ponczek, M.B.; Moniuszko-Szajwaj, B.; Kowalczyk, M.; Pecio, Ł.; Nowak, P.; Stochmal, A. Bufadienolides from Kalanchoe daigremontiana Modulate the Enzymatic Activity of Plasmin—In Vitro and in Silico Analyses. Int. J. Biol. Macromol. 2018, 120, 1591–1600. [Google Scholar] [CrossRef]
- Agarwal, H.; Shanmugam, V.K. Anti-Inflammatory Activity Screening of Kalanchoe pinnata Methanol Extract and Its Validation Using a Computational Simulation Approach. Inform. Med. Unlocked 2019, 14, 6–14. [Google Scholar] [CrossRef]
- Souza, L.; da Silva Oliveira, J.P.; da Silva Fernandes, A.; Macedo, A.F.; Araujo-Lima, C.F.; Felzenszwalb, I. UHPLC-MS Metabolomic Profile and in Silico Pharmacokinetic Approach of Kalanchoe daigremontiana Raym.-Hamet & H. Perrier Aqueous Extracts. J. Pharm. Biomed. Anal. 2024, 238, 115827. [Google Scholar] [CrossRef]
- Pratap Singh, R.; Pattnaik, A.K. Elucidating the Anti-Obesity Potential of Bioactive Fractions of Kalanchoe pinnata (Lam.) Leaves Extract Using a Combination of In Vitro, In Vivo and in Silico Methods along with Characterisation of Lead Compounds through an HPTLC MS-MSn Analytical Study. Nat. Prod. Res. 2024, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, S.; Krupa, J.; Raj, C.D.; Gurav, S.S.; Gandhi, G.R.; Ayyanar, M. Antidiabetic and Antioxidant Activities of the Hydroalcoholic Extracts of Canthium coromandelicum (Burm.f.) Alston Leaf in Streptozotocin-Induced Diabetic Rats. Process Biochem. 2024, 137, 85–98. [Google Scholar] [CrossRef]
- Ismaila, M.S.; Sanusi, K.O.; Iliyasu, U.; Imam, M.U.; Georges, K.; Sundaram, V.; Jones, K.R. Antioxidant and Anti-Inflammatory Properties of Quail Yolk Oil via Upregulation of Superoxide Dismutase 1 and Catalase Genes and Downregulation of EIGER and Unpaired 2 Genes in a D. Melanogaster Model. Antioxidants 2024, 13, 75. [Google Scholar] [CrossRef]
- Abolarin, P.O.; Amin, A.; Nafiu, A.B.; Ogundele, O.M.; Owoyele, B.V. Optimization of Parkinson’s Disease Therapy with Plant Extracts and Nutrition’s Evolving Roles. IBRO Neurosci. Rep. 2024, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, A.A.; Ibraheem, F.; El-Hefny, M.; El-Senduny, F. Evaluation of Saudi Juniperus procera Extracts Cytotoxicity and Regulatory Mechanisms of Tumorigenesis against Two Breast Cancer Cell Lines. Nat. Prod. Commun. 2024, 19, 1934578X241232778. [Google Scholar] [CrossRef]
- Ogbu, P.N.; Famurewa, A.C.; Ugbor, C.K.; Ogbu, I.M.; Aloke, C.; Obasi, N.A.; Aliu, T.A.; Narayanankutty, A. HPLC Phytochemical Profiling, Antioxidant Activity and In Vitro Evaluation of Inhibitory Effects of Terminalia catappa Stem Bark Extract on Enzymes Linked to Diabetes, Hypertensive Vasoconstriction and Erectile Dysfunction. Pharmacol. Res. Nat. Prod. 2024, 4, 100064. [Google Scholar] [CrossRef]
- Ceballos-Sanchez, O.; Navarro-López, D.E.; Mejía-Méndez, J.L.; Sanchez-Ante, G.; Rodríguez-González, V.; Sánchez-López, A.L.; Sanchez-Martinez, A.; Duron-Torres, S.M.; Juarez-Moreno, K.; Tiwari, N.; et al. Enhancing Antioxidant Properties of CeO2 Nanoparticles with Nd3+ Doping: Structural, Biological, and Machine Learning Insights. Biomater. Sci. 2024, 12, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Bogucka-Kocka, A.; Zidorn, C.; Kasprzycka, M.; Szymczak, G.; Szewczyk, K. Phenolic Acid Content, Antioxidant and Cytotoxic Activities of Four Kalanchoë Species. Saudi J. Biol. Sci. 2018, 25, 622–630. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Hering, A.; Gucwa, M.; Sztormowska-Achranowicz, K.; Kowalczyk, M.; Soluch, A.; Ochocka, J.R. An In Vitro Anticancer, Antioxidant, and Phytochemical Study on Water Extract of Kalanchoe daigremontiana Raym.-Hamet and H. Perrier. Molecules 2022, 27, 2280. [Google Scholar] [CrossRef] [PubMed]
- Adeleke Ojo, O.; Busola Ojo, A.; Olaitan Ajiboye, B.; Olaiya, O.; Akawa, A.; Olaoye, O.; Anifowose, O.O.; Idowu, O.; Olasehinde, O.; Obafemi, T.; et al. Inhibitory Effect of Bryophyllum pinnatum (Lam.) Oken Leaf Extract and Their Fractions on α-Amylase, α-Glucosidase and Cholinesterase Enzyme. Pharmacogn. J. 2018, 10, 497–506. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Hoang Le, Q.; Salmen, S.H.; Karuppusamy, I.; Pugazhendhi, A. In Vitro Biofilm Inhibition Efficacy of Aerva lanata Flower Extract against Gram Negative and Gram-Positive Biofilm Forming Bacteria and Toxicity Analysis Using Artemia salina. Environ. Res. 2023, 238, 117118. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.D.; da Cruz Guedes, E.; Fernandes, I.C.O.; Pedroza, L.A.L.; da Silva Pereira, G.J.; Gubert, P. Exploring Caenorhabditis Elegans as Parkinson’s Disease Model: Neurotoxins and Genetic Implications. Neurotox. Res. 2024, 42, 11. [Google Scholar] [CrossRef] [PubMed]
- Höss, S.; Sanders, D.; van Egmond, R. Determining the Toxicity of Organic Compounds to the Nematode Caenorhabditis elegans Based on Aqueous Concentrations. Environ. Sci. Pollut. Res. 2023, 30, 96290–96300. [Google Scholar] [CrossRef] [PubMed]

| Extract | Compound | Rt (min) | Match | R Match |
|---|---|---|---|---|
| H | β-sitosterol | 7.10 | 774 | 813 |
| α-amyrin | 8.43 | 801 | 811 | |
| β-amirone | 8.49 | 808 | 821 | |
| α-amyrin | 8.61 | 834 | 838 | |
| Lupeol | 9.14 | 826 | 832 | |
| β-amyrin | 11.63 | 825 | 856 | |
| Urs-12-ene | 16.77 | 780 | 833 | |
| Lupen-3-one | 21.43 | 815 | 836 | |
| α-amyrone | 21.79 | 842 | 851 | |
| Lupenone | 23.04 | 913 | 915 | |
| Cl | β-amyrin acetate | 10.67 | 807 | 834 |
| Urs-12-en-24-oic-3-oxo-methyl ester | 11.82 | 801 | 897 | |
| Octacosane | 22.82 | 898 | 932 | |
| Heptacosanol | 23.63 | 801 | 860 | |
| Lupen-3-one | 27.77 | 828 | 840 |
| Extract | E. coli | K. pneumoniae | S. aureus |
|---|---|---|---|
| H | 609.78 | 1857.98 | 3634.67 |
| Cl | 7997.28 | 758.74 | 574.28 |
| M | 776.65 | 423.59 | 648.30 |
| Ligand | Binding Energy (Kcal/mol) | SD | n |
|---|---|---|---|
| Compound 2 | −6.1 | 0.4 | 9 |
| Compound 7 | −5.9 | 0.3 | 20 |
| Compound 9 | −6.3 | 0.5 | 35 |
| Compound 11 | −5.7 | 0.4 | 29 |
| Vancomycin | −3.1 | 0.7 | 11 |
| Extract | DPPH | ABTS | H2O2 |
|---|---|---|---|
| H | 2.76 | 978.14 | 376.83 |
| Cl | 6209.56 | 1554.73 | 87.81 |
| M | 3.95 | 210.22 | 110.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía-Méndez, J.L.; Sánchez-Ante, G.; Minutti-Calva, Y.; Schürenkämper-Carrillo, K.; Navarro-López, D.E.; Buendía-Corona, R.E.; González-Chávez, M.d.C.Á.; Sánchez-López, A.L.; Lozada-Ramírez, J.D.; Sánchez-Arreola, E.; et al. Kalanchoe tomentosa: Phytochemical Profiling, and Evaluation of Its Biological Activities In Vitro, In Vivo, and In Silico. Pharmaceuticals 2024, 17, 1051. https://doi.org/10.3390/ph17081051
Mejía-Méndez JL, Sánchez-Ante G, Minutti-Calva Y, Schürenkämper-Carrillo K, Navarro-López DE, Buendía-Corona RE, González-Chávez MdCÁ, Sánchez-López AL, Lozada-Ramírez JD, Sánchez-Arreola E, et al. Kalanchoe tomentosa: Phytochemical Profiling, and Evaluation of Its Biological Activities In Vitro, In Vivo, and In Silico. Pharmaceuticals. 2024; 17(8):1051. https://doi.org/10.3390/ph17081051
Chicago/Turabian StyleMejía-Méndez, Jorge L., Gildardo Sánchez-Ante, Yulianna Minutti-Calva, Karen Schürenkämper-Carrillo, Diego E. Navarro-López, Ricardo E. Buendía-Corona, Ma. del Carmen Ángeles González-Chávez, Angélica Lizeth Sánchez-López, J. Daniel Lozada-Ramírez, Eugenio Sánchez-Arreola, and et al. 2024. "Kalanchoe tomentosa: Phytochemical Profiling, and Evaluation of Its Biological Activities In Vitro, In Vivo, and In Silico" Pharmaceuticals 17, no. 8: 1051. https://doi.org/10.3390/ph17081051
APA StyleMejía-Méndez, J. L., Sánchez-Ante, G., Minutti-Calva, Y., Schürenkämper-Carrillo, K., Navarro-López, D. E., Buendía-Corona, R. E., González-Chávez, M. d. C. Á., Sánchez-López, A. L., Lozada-Ramírez, J. D., Sánchez-Arreola, E., & López-Mena, E. R. (2024). Kalanchoe tomentosa: Phytochemical Profiling, and Evaluation of Its Biological Activities In Vitro, In Vivo, and In Silico. Pharmaceuticals, 17(8), 1051. https://doi.org/10.3390/ph17081051









