L-Carnitine Ameliorates Amiodarone-Mediated Alveolar Damage: Oxidative Stress Parameters, Inflammatory Markers, Histological and Ultrastructural Insights
Abstract
1. Introduction
Malondialdehyde
2. Results
2.1. Body Weight
2.2. Histopathological
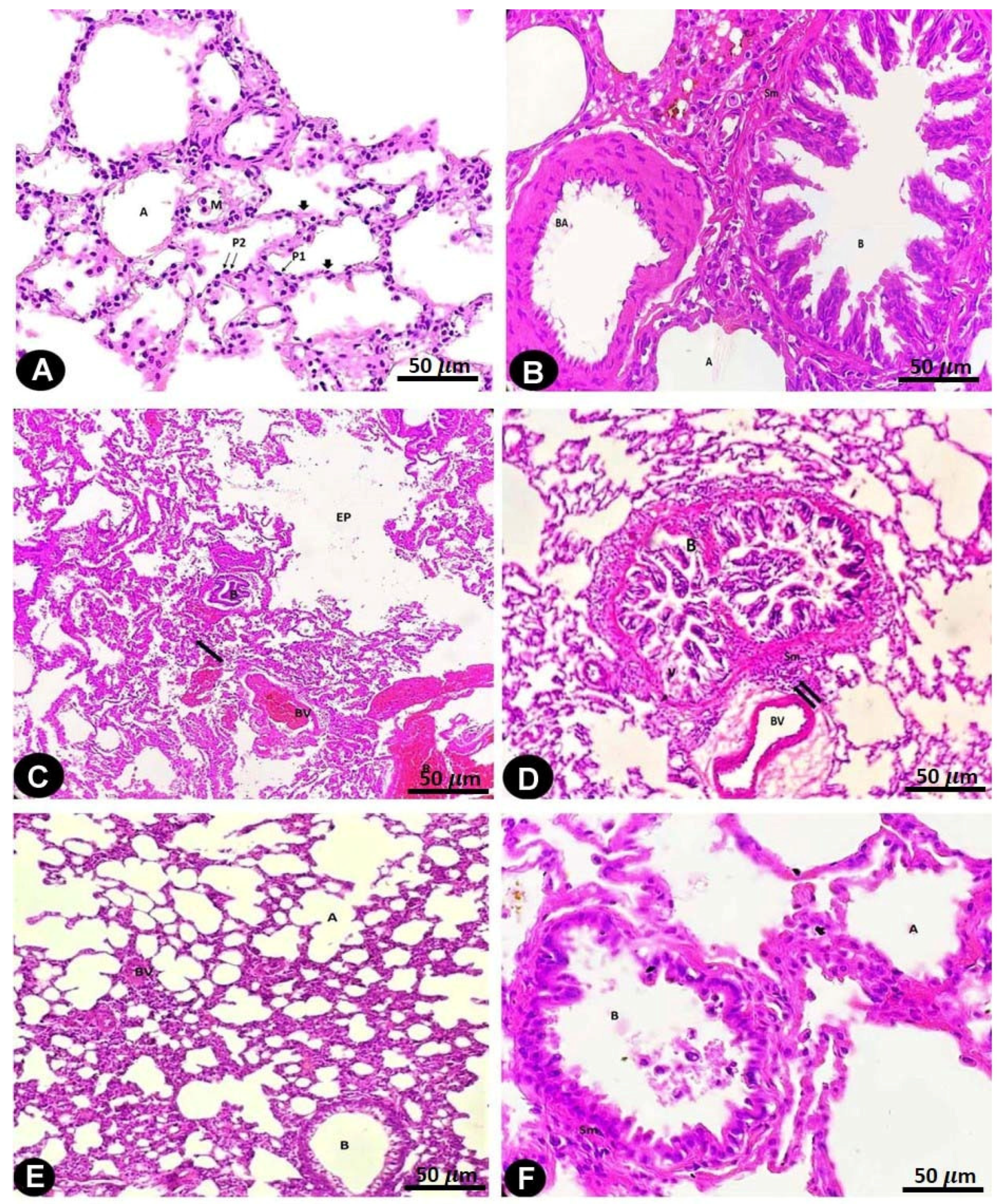
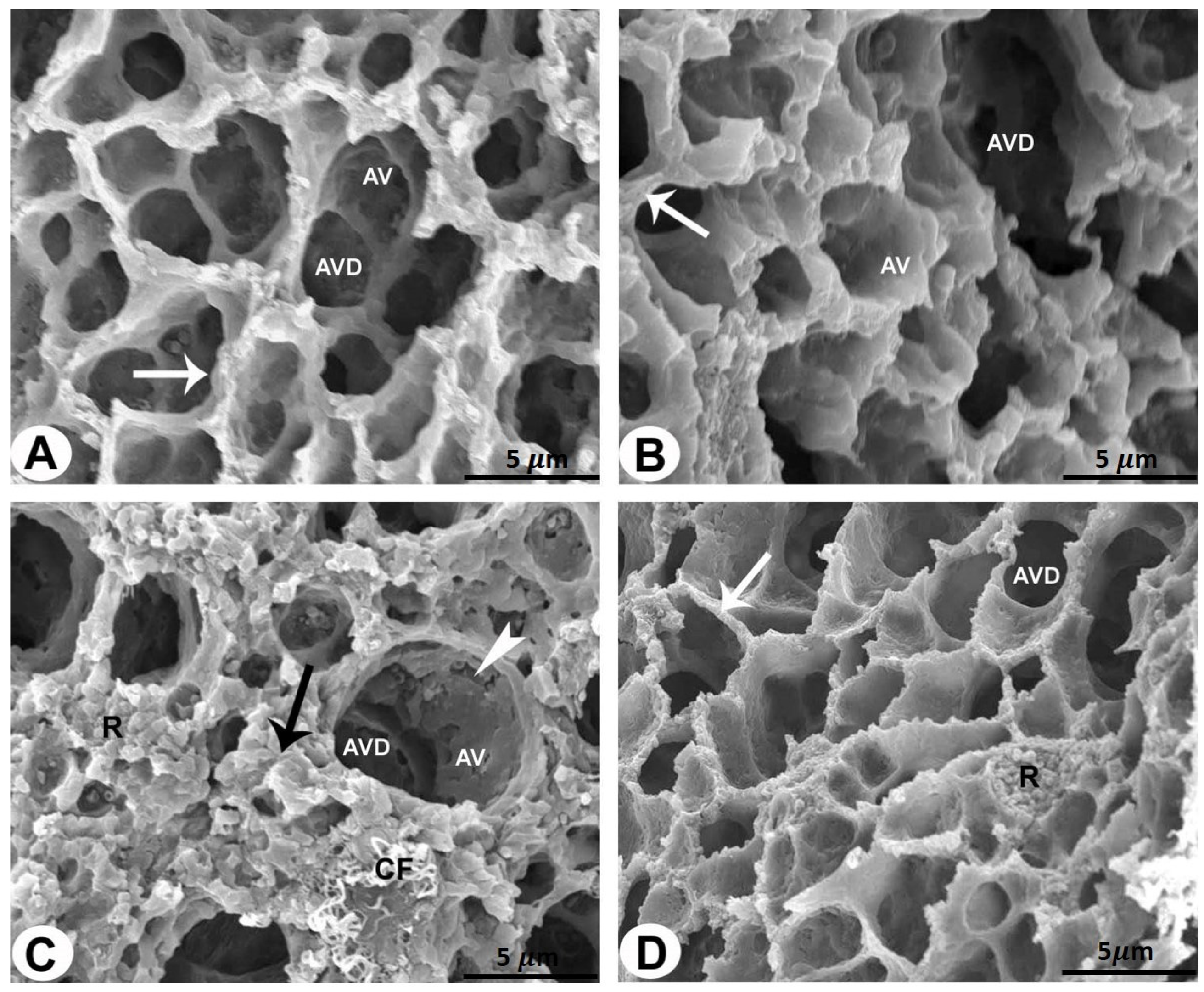
2.3. SEM
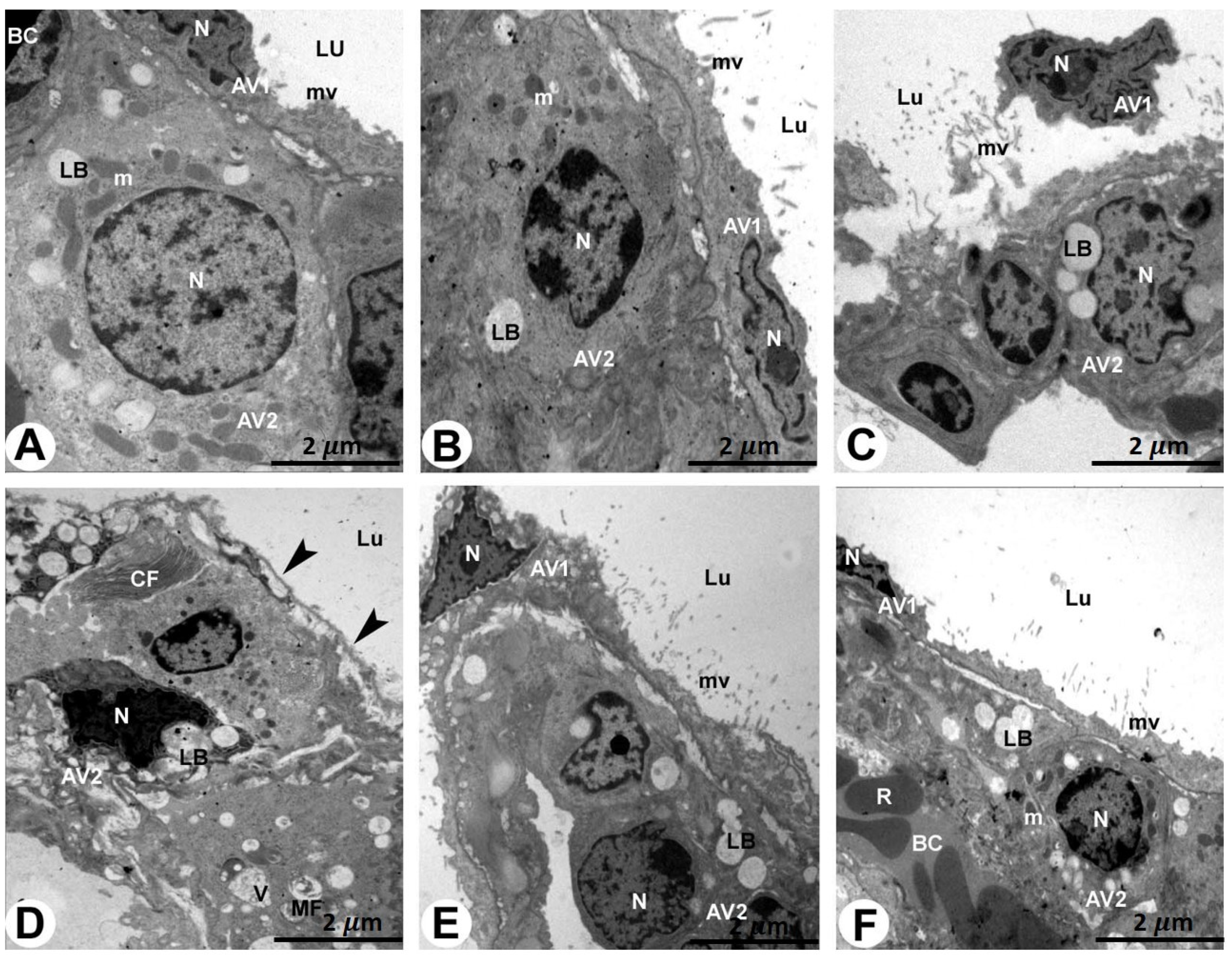
2.4. TEM
2.5. Biochemical Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals Yes Revised
4.3. Experimental Approach
4.4. Samples Collection
4.5. Light Microscopic Examination
4.6. Scanning Electron Microscopy (SEM) Examination
4.7. Transmission Electron Microscopy (TEM) Examination
4.8. Biochemical Analysis
4.8.1. Preparation of Lung Homogenate
4.8.2. Antioxidant Enzyme Markers and Malondialdehyde (MDA)
4.8.3. Estimation of TNF-α Marker
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryu, J.H.; Moua, T.; Daniels, C.E.; Hartman, T.E.; Eunhee, S.Y.; Utz, J.P.; Limper, A.H. Idiopathic Pulmonary Fibrosis: Evolving Concepts; Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands; pp. 1130–1142.
- El-Nahrawy, W.A.; Zaher, A.M.; Ali, S.M. Therapeutic effects of Laser and L-carnitine against amiodarone-induced pulmonary toxicity in adult male rats. Trop. J. Pharm. Res. 2017, 16, 1565–1571. [Google Scholar] [CrossRef][Green Version]
- Zaidel, E. Amiodarone: Updated review of its current usefulness. Arch. Clin. Exp. Cardiol. 2019, 1, 102. [Google Scholar]
- Tsai, I.L.; Huang, L.T.; Yu, Y.T.; Lee, C.T.; Huang, T.H. Variable radiographic and histologic presentations of amiodarone--related interstitial lung disease and the importance of avoiding re--exposure. Respirol. Case Rep. 2023, 11, e01165. [Google Scholar] [CrossRef]
- Gawad, F.A.E.-R.; Rizk, A.A.E.-E.; Jouakim, M.F.; Abd El Halem, M.Z. Amiodarone-induced lung toxicity and the protective role of Vitamin E in adult male albino rat. Eur. J. Anat. 2018, 22, 332–333. [Google Scholar]
- Galaly, S.R.; Abdul-Hamid, M.; Mahmoud, H.; Mostafa, F. Ameliorative effect of grape seed and ginkgo biloba against pulmonary damage induced by amiodarone in male albino rats. J. Adv. Pharm. Educ. Res. 2018, 8, 33–42. [Google Scholar]
- Schwaiblmair, M.; Berghaus, T.; Haeckel, T.; Wagner, T.; von Scheidt, W. Amiodarone-induced pulmonary toxicity: An under-recognized and severe adverse effect? Clin. Res. Cardiol. 2010, 99, 693–700. [Google Scholar] [CrossRef]
- Nacca, N.; Bhamidipati, C.M.; Yuhico, L.S.; Pinnamaneni, S.; Szombathy, T. Severe amiodarone induced pulmonary toxicity. J. Thorac. Dis. 2012, 4, 667. [Google Scholar]
- Kaya, S.B.; Deger, S.; Hacievliyagil, S.S.; Aytemur, Z.A. Acute amiodarone toxicity causing respiratory failure. Rev. Da Assoc. Médica Bras. 2017, 63, 210–212. [Google Scholar] [CrossRef]
- Papiris, S.A.; Triantafillidou, C.; Kolilekas, L.; Markoulaki, D.; Manali, E.D. Amiodarone: Review of pulmonary effects and toxicity. Drug Saf. 2010, 33, 539–558. [Google Scholar] [CrossRef]
- Estornut, C.; Milara, J.; Bayarri, M.A.; Belhadj, N.; Cortijo, J. Targeting oxidative stress as a therapeutic approach for idiopathic pulmonary fibrosis. Front. Pharmacol. 2022, 12, 794997. [Google Scholar] [CrossRef]
- Acar, Z.; Aga, M.; Kris, A.; Korkmaz, L.; Erkan, H.; Erkus, E. Mean platelet volumen on admission is associated with further left ventricular functions in primary PTCA patients. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1567–1569. [Google Scholar]
- Vereckei, A. The role of oxidative stress in amiodaron toxicity, in left ventricular dysfunction of hypertensive patients and in heart failure with preserved ejection fraction. Orvosi Hetil. 2015, 156, 1921–1925. [Google Scholar] [CrossRef][Green Version]
- Niewiem, M.; Grzybowska-Chlebowczyk, U. Intestinal barrier permeability in allergic diseases. Nutrients 2022, 14, 1893. [Google Scholar] [CrossRef]
- Abd-Allah, A.R.; Helal, G.K.; Al-Yahya, A.A.; Aleisa, A.M.; Al-Rejaie, S.S.; Al-Bakheet, S.A. Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. Oxidative Med. Cell. Longev. 2009, 2, 73–81. [Google Scholar] [CrossRef]
- De Simone, C.; Famularo, G.; Famularo, G.; Matricardi, F.; Nucera, E.; Santini, G.; De Simone, C. Carnitine deficiency: Primary and secondary syndromes. Carnitine Today 1997, 119–161. [Google Scholar] [CrossRef]
- Foster, D.W. The role of the carnitine system in human metabolism. Ann. N. Y. Acad. Sci. 2004, 1033, 1–16. [Google Scholar] [CrossRef]
- Abd-Allah, A.R.; Al-Majed, A.A.; Al-Yahya, A.A.; Fouda, S.I.; Al-Shabana, O.A. L-Carnitine halts apoptosis and myelosuppression induced by carboplatin in rat bone marrow cell cultures (BMC). Arch. Toxicol. 2005, 79, 406–413. [Google Scholar] [CrossRef]
- Liu, J.; Head, E.; Kuratsune, H.; Cotman, C.W.; Ames, B.N. Comparison of the effects of L--carnitine and acetyl--L--carnitine on carnitine levels, ambulatory activity, and oxidative stress biomarkers in the brain of old rats. Ann. N. Y. Acad. Sci. 2004, 1033, 117–131. [Google Scholar] [CrossRef]
- Buyse, J.; Swennen, Q.; Niewold, T.A.; Klasing, K.C.; Janssens, G.P.; Baumgartner, M.; Goddeeris, B.M. Dietary L-carnitine supplementation enhances the lipopolysaccharide-induced acute phase protein response in broiler chickens. Vet. Immunol. Immunopathol. 2007, 118, 154–159. [Google Scholar] [CrossRef]
- Manoli, I.; De Martino, M.U.; Kino, T.; Alesci, S. Modulatory effects of L--carnitine on glucocorticoid receptor activity. Ann. N. Y. Acad. Sci. 2004, 1033, 147–157. [Google Scholar] [CrossRef]
- Lango, R.; Smoleński, R.T.; Rogowski, J.; Siebert, J.; Wujtewicz, M.; Słomińska, E.M.; Łysiak-Szydłowska, W.; Yacoub, M.H. Propionyl-L-carnitine improves hemodynamics and metabolic markers of cardiac perfusion during coronary surgery in diabetic patients. Cardiovasc. Drugs Ther. 2005, 19, 267–275. [Google Scholar] [CrossRef]
- Virmani, M.A.; Cirulli, M. The role of l-carnitine in mitochondria, prevention of metabolic inflexibility and disease initiation. Int. J. Mol. Sci. 2022, 23, 2717. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, J.; Zheng, J.; Zhang, X.; Shao, J.; Zhao, L.; Hao, J. Anti-inflammatory and antioxidant effects of acetyl-L-carnitine on atherosclerotic rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e920250-1. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, B.; Khalifa, M.; Bakheet, S.A.; Yasser, M. A Mechanistic Study on the Amiodarone--Induced Pulmonary Toxicity. Oxidative Med. Cell. Longev. 2016, 2016, 6265853. [Google Scholar] [CrossRef]
- Mahdy, A.A. The possible ameliorative effect of selenium and vitamins combination against amiodarone-induced alveolar damage in albino rat: Histological and immunohistochemical study. J. Am. Sci. 2014, 10, 61–71. [Google Scholar]
- Mahavadi, P.; Henneke, I.; Ruppert, C.; Knudsen, L.; Venkatesan, S.; Liebisch, G.; Chambers, R.C.; Ochs, M.; Schmitz, G.; Vancheri, C. Altered surfactant homeostasis and alveolar epithelial cell stress in amiodarone-induced lung fibrosis. Toxicol. Sci. 2014, 142, 285–297. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Schwarz, M.I.; Brown, K.; Tooze, J.A.; Colby, T.V.; Waldron, J.A., Jr.; Flint, A.; Thurlbeck, W.; Cherniack, R.M. Idiopathic pulmonary fibrosis: Relationship between histopathologic features and mortality. Am. J. Respir. Crit. Care Med. 2001, 164, 1025–1032. [Google Scholar] [CrossRef]
- Mitrofan, C.E.; Cretu, A.; Mitrofan, C.; Bar, C.; Ghiciuc, C.M. Amiodarone induced lung disease. Arch. Clin. Cases 2022, 9, 126. [Google Scholar] [CrossRef]
- Ashrafian, H.; Davey, P. Is amiodarone an underrecognized cause of acute respiratory failure in the ICU? Chest 2001, 120, 275–282. [Google Scholar] [CrossRef]
- Shimizu, S.; Gabazza, E.C.; Taguchi, O.; Yasui, H.; Taguchi, Y.; Hayashi, T.; Ido, M.; Shimizu, T.; Nakagaki, T.; Kobayashi, H. Activated protein C inhibits the expression of platelet-derived growth factor in the lung. Am. J. Respir. Crit. Care Med. 2003, 167, 1416–1426. [Google Scholar] [CrossRef]
- Nicolescu, A.C.; Comeau, J.L.; Hill, B.C.; Bedard, L.L.; Takahashi, T.; Brien, J.F.; Racz, W.J.; Massey, T.E. Aryl radical involvement in amiodarone-induced pulmonary toxicity: Investigation of protection by spin-trapping nitrones. Toxicol. Appl. Pharmacol. 2007, 220, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Wiegman, C.H.; Michaeloudes, C.; Haji, G.; Narang, P.; Clarke, C.J.; Russell, K.E.; Bao, W.; Pavlidis, S.; Barnes, P.J.; Kanerva, J. Oxidative stress–induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015, 136, 769–780. [Google Scholar] [CrossRef]
- Srichai, M.B. Computed Tomography and Magnetic Resonance of the Thorax; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Rebrova, T.Y.; Afanasyev, S. Free radical lipid peroxidation during amiodarone therapy for postinfarction cardiosclerosis. Bull. Exp. Biol. Med. 2008, 146, 283. [Google Scholar] [CrossRef]
- Kinnula, V.L.; Fattman, C.L.; Tan, R.J.; Oury, T.D. Oxidative stress in pulmonary fibrosis: A possible role for redox modulatory therapy. Am. J. Respir. Crit. Care Med. 2005, 172, 417–422. [Google Scholar] [CrossRef]
- Hasan, H.; Thabet, N.M.; Abdel-Rafei, M.K. Methanolic extract of Moringa oleifera leaf and low doses of gamma radiation alleviated amiodarone-induced lung toxicity in albino rats. Arch. Biol. Sci. 2016, 68, 31–39. [Google Scholar] [CrossRef]
- Zickri, M.B.; Fadl, S.G.A.; Metwally, H.G. Comparative study between intravenous and intraperitoneal stem cell therapy in amiodarone induced lung injury in rat. Int. J. Stem Cells 2014, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cherian, D.A.; Peter, T.; Narayanan, A.; Madhavan, S.S.; Achammada, S.; Vynat, G.P. Malondialdehyde as a marker of oxidative stress in periodontitis patients. J. Pharm. Bioallied Sci. 2019, 11 (Suppl. 2), S297–S300. [Google Scholar] [CrossRef] [PubMed]
- Gado, A.; Aldahmash, B. Protective effect of L-carnitine against amiodarone-induced lung toxicity in rats. Internet J. Toxicol. 2013, 10, 1–9. [Google Scholar]
- Cantin, A.M.; Hubbard, R.C.; Crystal, R.G. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1989, 139, 370–372. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Et. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Ischiropoulos, H.; Zhu, L.; Chen, J.; Tsai, M.; Martin, J.C.; Smith, C.D.; Beckman, J.S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 1992, 298, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, R.; Laskin, J.D.; Laskin, D.L. Anti-TNFα therapy in inflammatory lung diseases. Pharmacol. Ther. 2017, 180, 90–98. [Google Scholar] [CrossRef]
- Mali, P.; Salzman, M.M.H.; Vidaillet, H.J.; Rezkalla, S.H. Amiodarone therapy for cardiac arrhythmias: Is it associated with the development of cancers? World J. Cardiovasc. Dis. 2014, 2014, 43923. [Google Scholar] [CrossRef][Green Version]
- Punithavathi, D.; Venkatesan, N.; Babu, M. Protective effects of curcumin against amiodarone--induced pulmonary fibrosis in rats. Br. J. Pharmacol. 2003, 139, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.G.; Chou, H.-C.; Chen, Y.-T.; Tung, S.-Y.; Ko, T.-L.; Buyandelger, B.; Wen, L.-L.; Juan, S.-H. L-Carnitine reduces reactive oxygen species/endoplasmic reticulum stress and maintains mitochondrial function during autophagy-mediated cell apoptosis in perfluorooctanesulfonate-treated renal tubular cells. Sci. Rep. 2022, 12, 4673. [Google Scholar] [CrossRef]
- Guzel, N.A.; Orer, G.E.; Bircan, F.S.; Cevher, S.C. Effects of acute L-carnitine supplementation on nitric oxide production and oxidative stress after exhaustive exercise in young soccer players. J. Sports Med. Phys. Fit. 2015, 55, 9–15. [Google Scholar]
- Lee, B.-J.; Lin, J.-S.; Lin, Y.-C.; Lin, P.-T. Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: A randomized, placebo-controlled trial. Nutr. J. 2014, 13, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.-Y. Effects of exogenous carnitine on function of respiratory chain and antioxidant capacity in mitochondria of myocardium after exhaustive running in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi Chin. J. Appl. Physiol. 2012, 28, 405–409. [Google Scholar]
- Hassanein, E.H.; ABO-Youssef, A.M.; Messiha, B.A.; Hemeida, R.A. Protective effects of montelukast and L-carnitine on cyclophosphamide-induced lung injury. Pharm. Biosci. J. 2015, 3, 30–36. [Google Scholar] [CrossRef]
- Fathizadeh, H.; Milajerdi, A.; Reiner, Ž.; Amirani, E.; Asemi, Z.; Mansournia, M.A.; Hallajzadeh, J. The effects of L-carnitine supplementation on indicators of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2020, 19, 1879–1894. [Google Scholar] [CrossRef]
- Bancroft, J.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed.; Churchill Livingstone Pub: Edinburgh, UK, 2002; Volume 172, pp. 593–620. [Google Scholar]
- Bozzola, J.J.; Russell, L.D. Electron Microscopy: Principles and Techniques for Biologists; Jones & Bartlett Learning: Burlington, MA, USA, 1999. [Google Scholar]
- Akers, S.; Kautz, E.; Trevino-Gavito, A.; Olszta, M.; Matthews, B.E.; Wang, L.; Du, Y.; Spurgeon, S.R. Rapid and flexible segmentation of electron microscopy data using few-shot machine learning. Npj Comput. Mater. 2021, 7, 187. [Google Scholar] [CrossRef]
- Hayat, M.A. Principles and Techniques of Electron Microscopy: Biological Applications; Edward Arnold: London, UK, 1981. [Google Scholar]
- Lam, J.; Katti, P.; Biete, M.; Mungai, M.; AshShareef, S.; Neikirk, K.; Garza Lopez, E.; Vue, Z.; Christensen, T.A.; Beasley, H.K. A universal approach to analyzing transmission electron microscopy with ImageJ. Cells 2021, 10, 2177. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.A. Handbook Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Misera, H.; Fridovich, I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for SOD. J. BiolChem 1972, 247, 3170–3175. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Draper, H.H.; Hadley, M. [43] Malondialdehyde determination as index of lipid Peroxidation. In Methods in Enzymozlogy; Elsevier: Amsterdam, The Netherlands, 1990; Volume 186, pp. 421–431. [Google Scholar]
- El Gamal, A.A.; AlSaid, M.S.; Raish, M.; Al-Sohaibani, M.; Al-Massarani, S.M.; Ahmad, A.; Hefnawy, M.; Al-Yahya, M.; Basoudan, O.A.; Rafatullah, S. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediat. Inflamm. 2014, 2014, 983952. [Google Scholar] [CrossRef]
- Tello, R.; Crewson, P.E. Hypothesis testing II: Means. Radiology 2003, 227, 1–4. [Google Scholar] [CrossRef]
 p < 0.05 in comparison to the AMD group.
p < 0.05 in comparison to the AMD group.
 p < 0.05 in comparison to the AMD group.
p < 0.05 in comparison to the AMD group.
 p < 0.05 in contrary to the AMD group. CAT: catalase; SOD: superoxide dismutase GPX: glutathione peroxidase.
p < 0.05 in contrary to the AMD group. CAT: catalase; SOD: superoxide dismutase GPX: glutathione peroxidase.
 p < 0.05 in contrary to the AMD group. CAT: catalase; SOD: superoxide dismutase GPX: glutathione peroxidase.
p < 0.05 in contrary to the AMD group. CAT: catalase; SOD: superoxide dismutase GPX: glutathione peroxidase.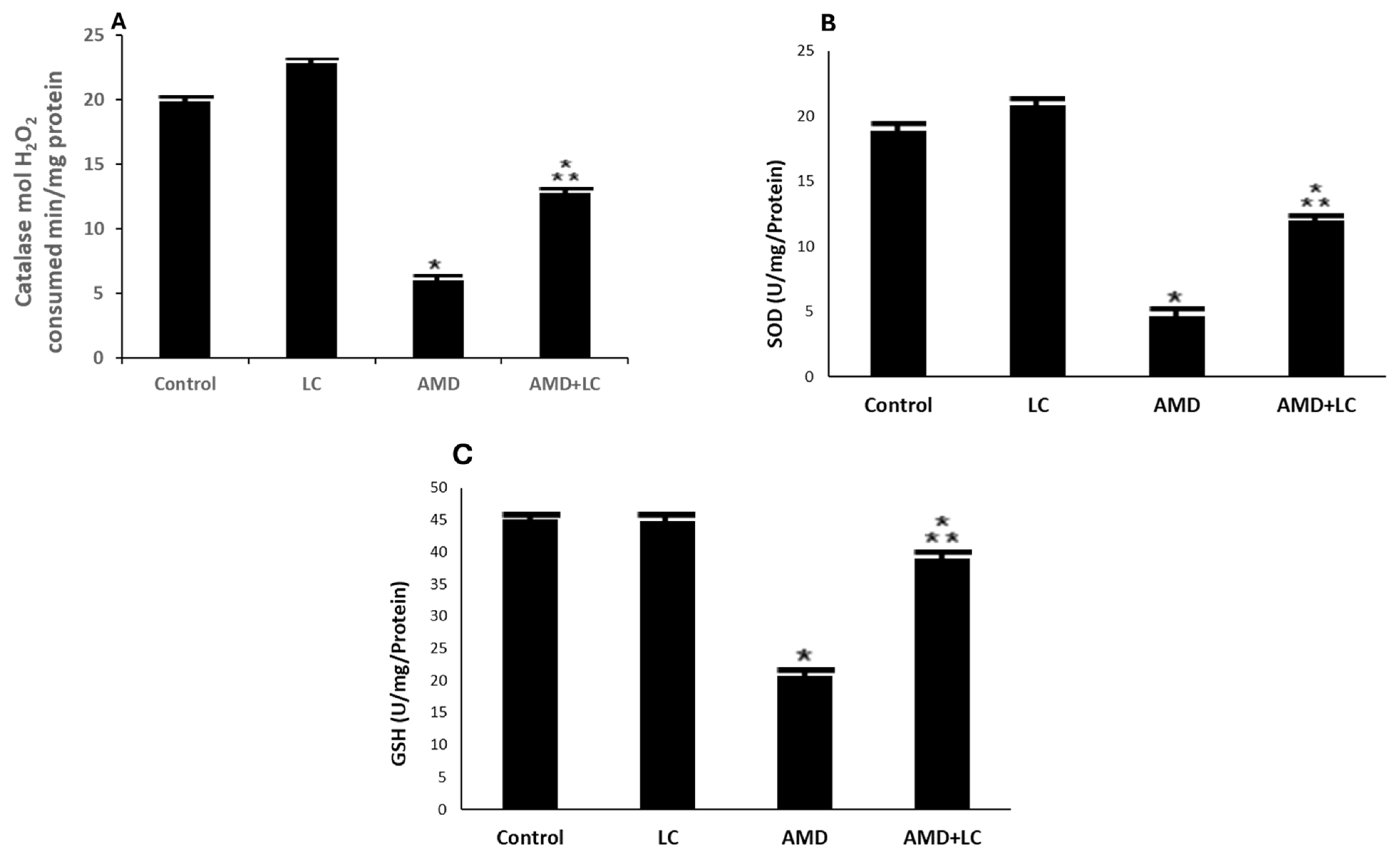
 p < 0.05 in comparison to the AMD group. TNF-α: tumor necrosis factor-α.
p < 0.05 in comparison to the AMD group. TNF-α: tumor necrosis factor-α.
 p < 0.05 in comparison to the AMD group. TNF-α: tumor necrosis factor-α.
p < 0.05 in comparison to the AMD group. TNF-α: tumor necrosis factor-α.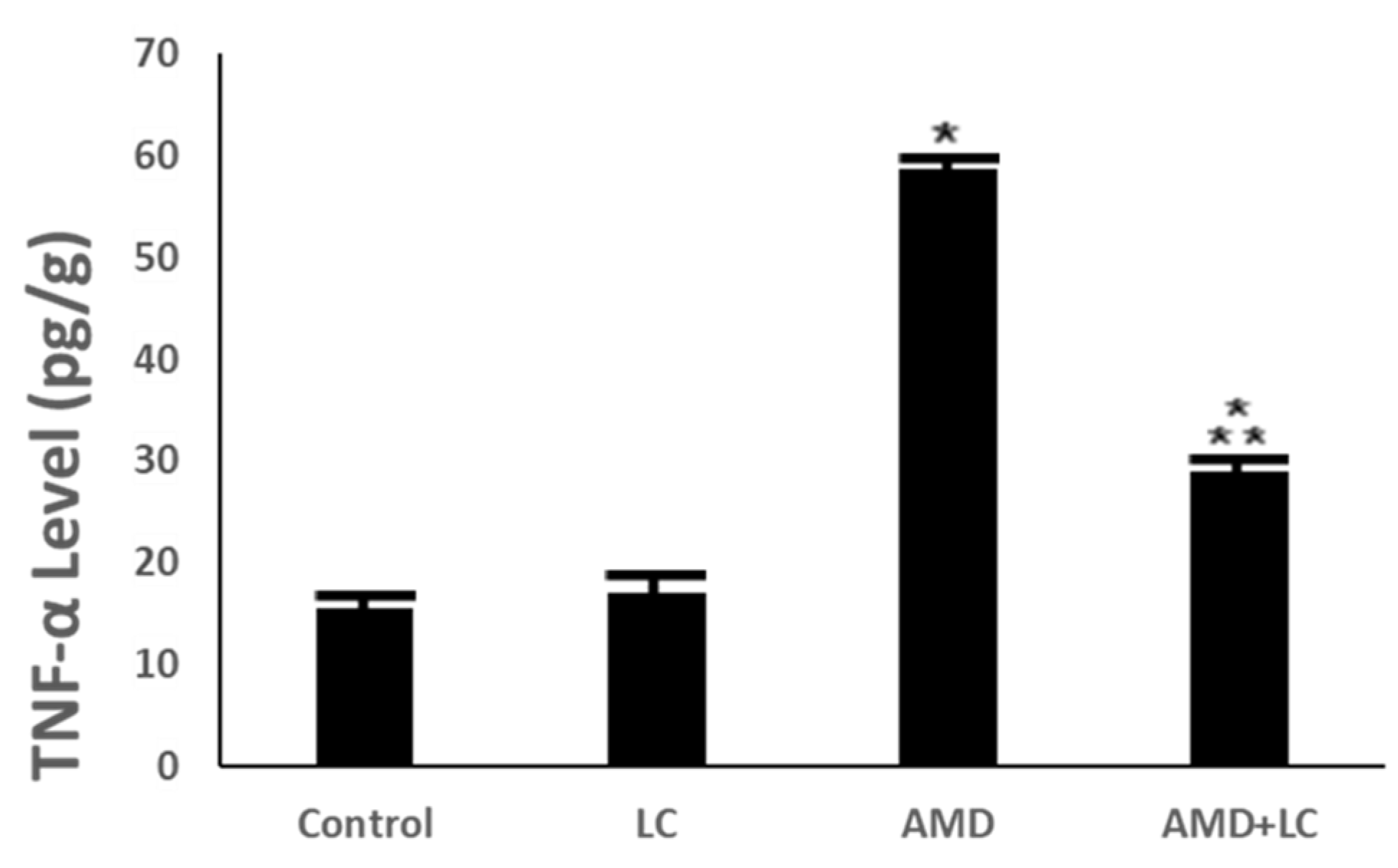
| Lung Structures | Percentages | ||||
|---|---|---|---|---|---|
| Control Group | LC Group | AMD Group | LC Plus AMD Group | ||
| Histopathological Analysis | Alveolar duct | 0–1% | 1–2% | 55–58% | 1–4% |
| Alveolar sacs | 0–1% | 2–3% | 35–40% | 2–3% | |
| Alveoli | 0–1% | 0–2% | 29–37% | 1–2% | |
| Alveolar epithelium | 0–1% | 0–1% | 25–28% | 2–3% | |
| Type I pneumocytes | 0–1% | 1–2% | 35–41% | 2–3% | |
| Type II pneumocytes | 0–1% | 1–2% | 25–28% | 1–4% | |
| Interalveolar septum | 0–1% | 1–2% | 34–45% | 1–2% | |
| Bronchiole | 0–1% | 0–2% | 65–78% | 1–3% | |
| Ultrastructural Analysis | SEM Analysis | ||||
| ● Alveolar duct | 0–1% | 1–2% | 52–54% | 1–7% | |
| ● Alveoli | 0–1% | 1–2% | 24–30% | 1–5% | |
| ● Alveolar enhance ridge | 0–1% | 1–2% | 35–40% | 2–3% | |
| ● Collagen fibrils | 0–1% | 1–2% | 14–28% | 1–2% | |
| ● Erythrocytes | 0–1% | 1–2% | 12–21% | 1–3% | |
| TEM Analysis | |||||
| ● Type I pneumocytes | 0–1% | 1–2% | 37–43% | 1–2% | |
| ● Type II pneumocytes | 0–1% | 1–2% | 26–31% | 1–4% | |
| ● Blood capillary | 0–1% | 1–2% | 36–44% | 2–3% | |
| ● Collagen fibrils | 0–1% | 1–2% | 16–29% | 1–2% | |
| ● Myelin figures | 0–1% | 1–2% | 8–11% | 0–1% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawood, S.A.; Asseri, A.A.; Shati, A.A.; Eid, R.A.; El-Gamal, B.; Zaki, M.S.A. L-Carnitine Ameliorates Amiodarone-Mediated Alveolar Damage: Oxidative Stress Parameters, Inflammatory Markers, Histological and Ultrastructural Insights. Pharmaceuticals 2024, 17, 1004. https://doi.org/10.3390/ph17081004
Dawood SA, Asseri AA, Shati AA, Eid RA, El-Gamal B, Zaki MSA. L-Carnitine Ameliorates Amiodarone-Mediated Alveolar Damage: Oxidative Stress Parameters, Inflammatory Markers, Histological and Ultrastructural Insights. Pharmaceuticals. 2024; 17(8):1004. https://doi.org/10.3390/ph17081004
Chicago/Turabian StyleDawood, Samy A., Ali Alsuheel Asseri, Ayed A. Shati, Refaat A. Eid, Basiouny El-Gamal, and Mohamed Samir A. Zaki. 2024. "L-Carnitine Ameliorates Amiodarone-Mediated Alveolar Damage: Oxidative Stress Parameters, Inflammatory Markers, Histological and Ultrastructural Insights" Pharmaceuticals 17, no. 8: 1004. https://doi.org/10.3390/ph17081004
APA StyleDawood, S. A., Asseri, A. A., Shati, A. A., Eid, R. A., El-Gamal, B., & Zaki, M. S. A. (2024). L-Carnitine Ameliorates Amiodarone-Mediated Alveolar Damage: Oxidative Stress Parameters, Inflammatory Markers, Histological and Ultrastructural Insights. Pharmaceuticals, 17(8), 1004. https://doi.org/10.3390/ph17081004







