Transmission-Blocking Strategies for Malaria Eradication: Recent Advances in Small-Molecule Drug Development
Abstract
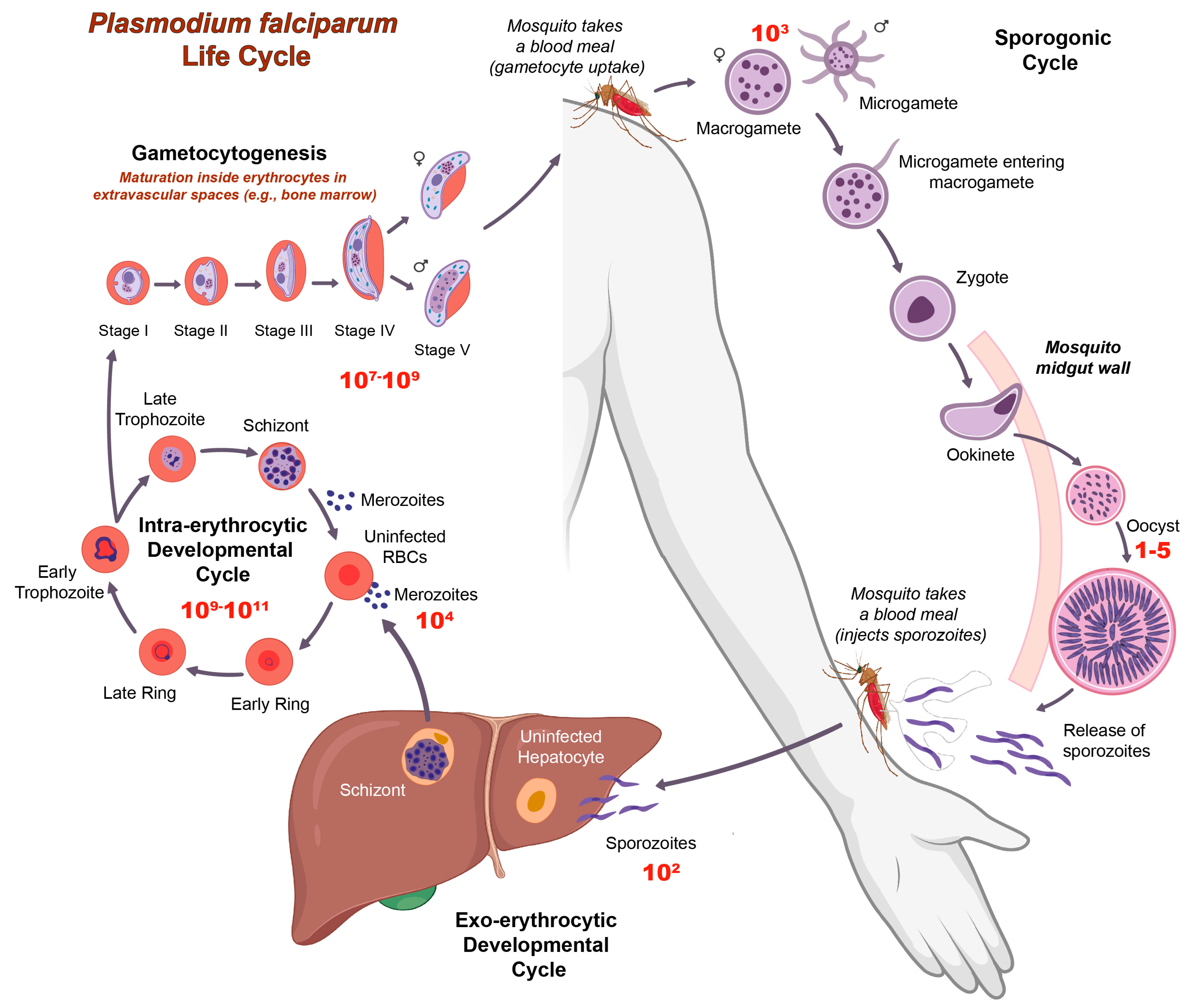
1. Introduction
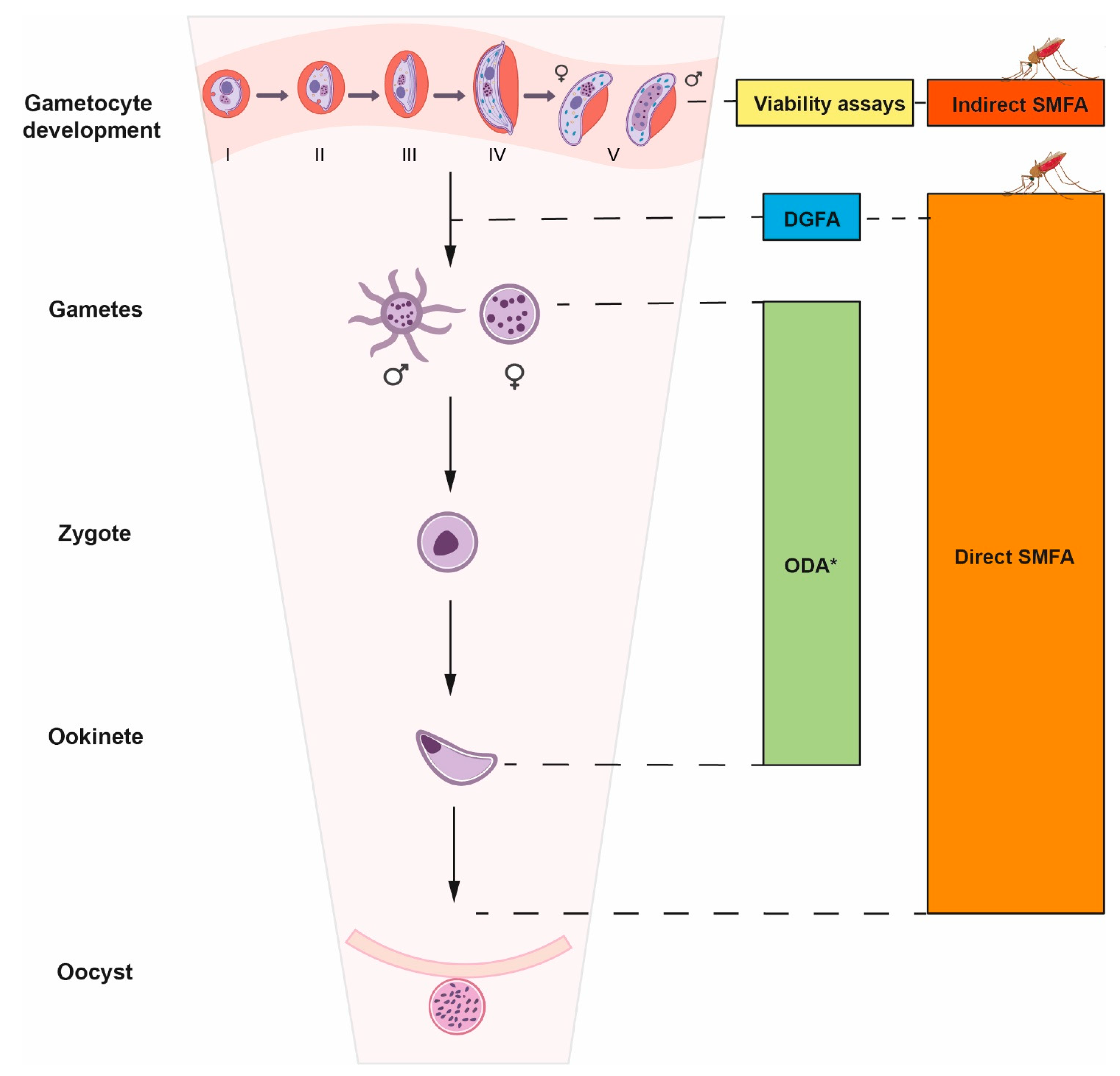
2. The Transmission-Blocking Screening Landscape
2.1. Gametocytocidal Assays
2.2. Dual Gamete Formation Assay (DGFA)
2.3. SMFA
2.4. Sporogonic Development Assays Using P. berghei
3. Transmission-Blocking Antimalarial Drugs
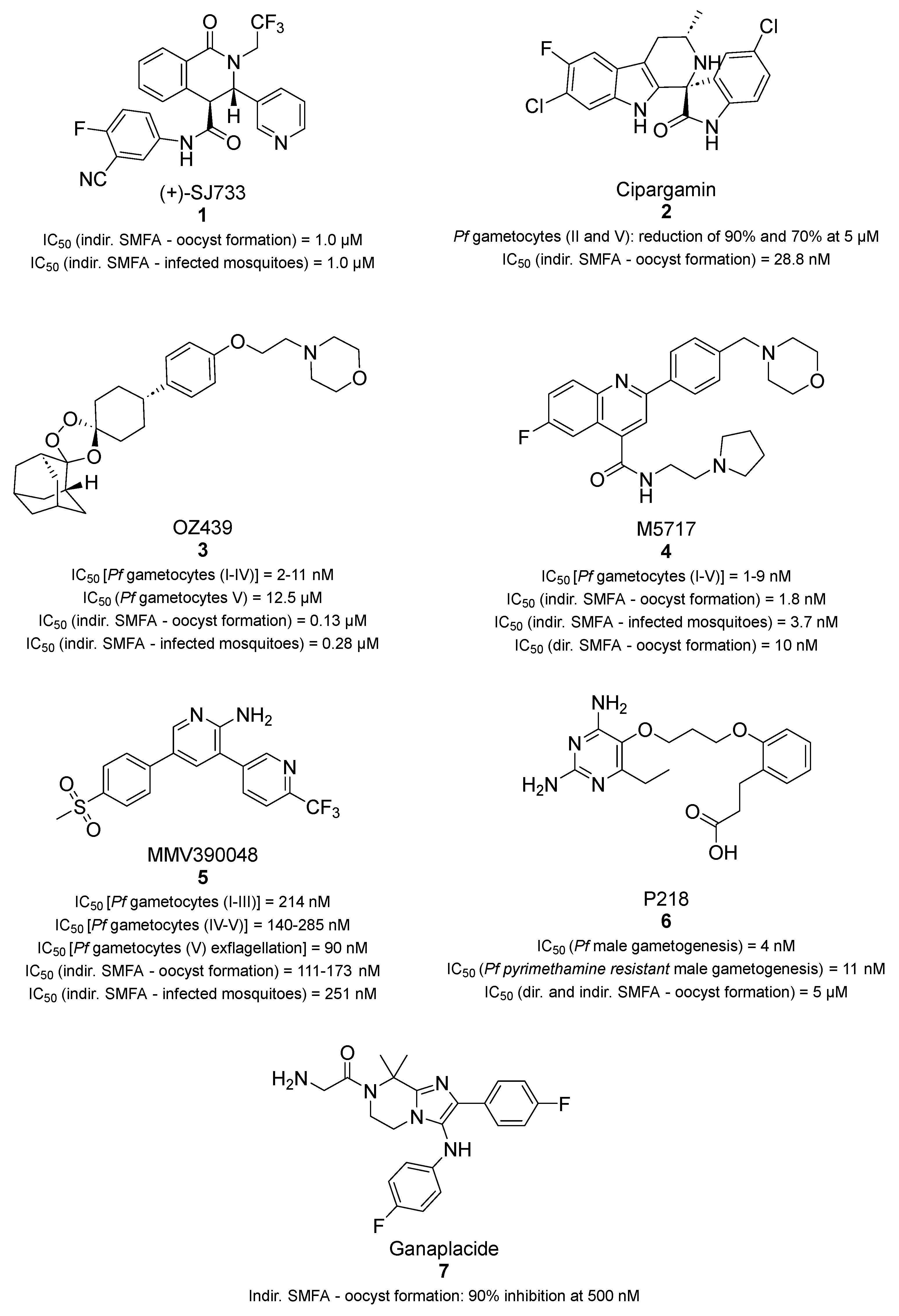
3.1. Transmission Blockers in Clinical Development
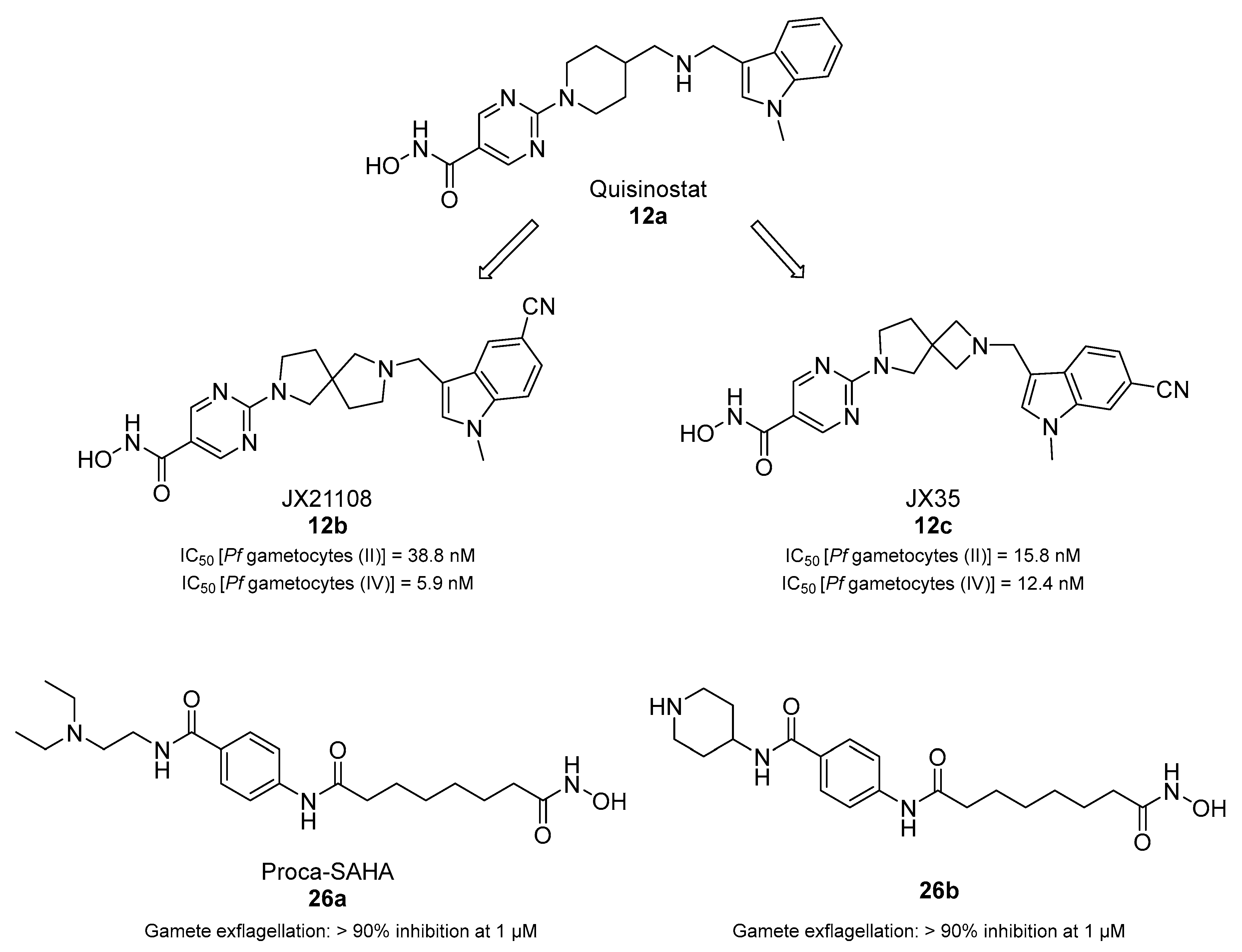
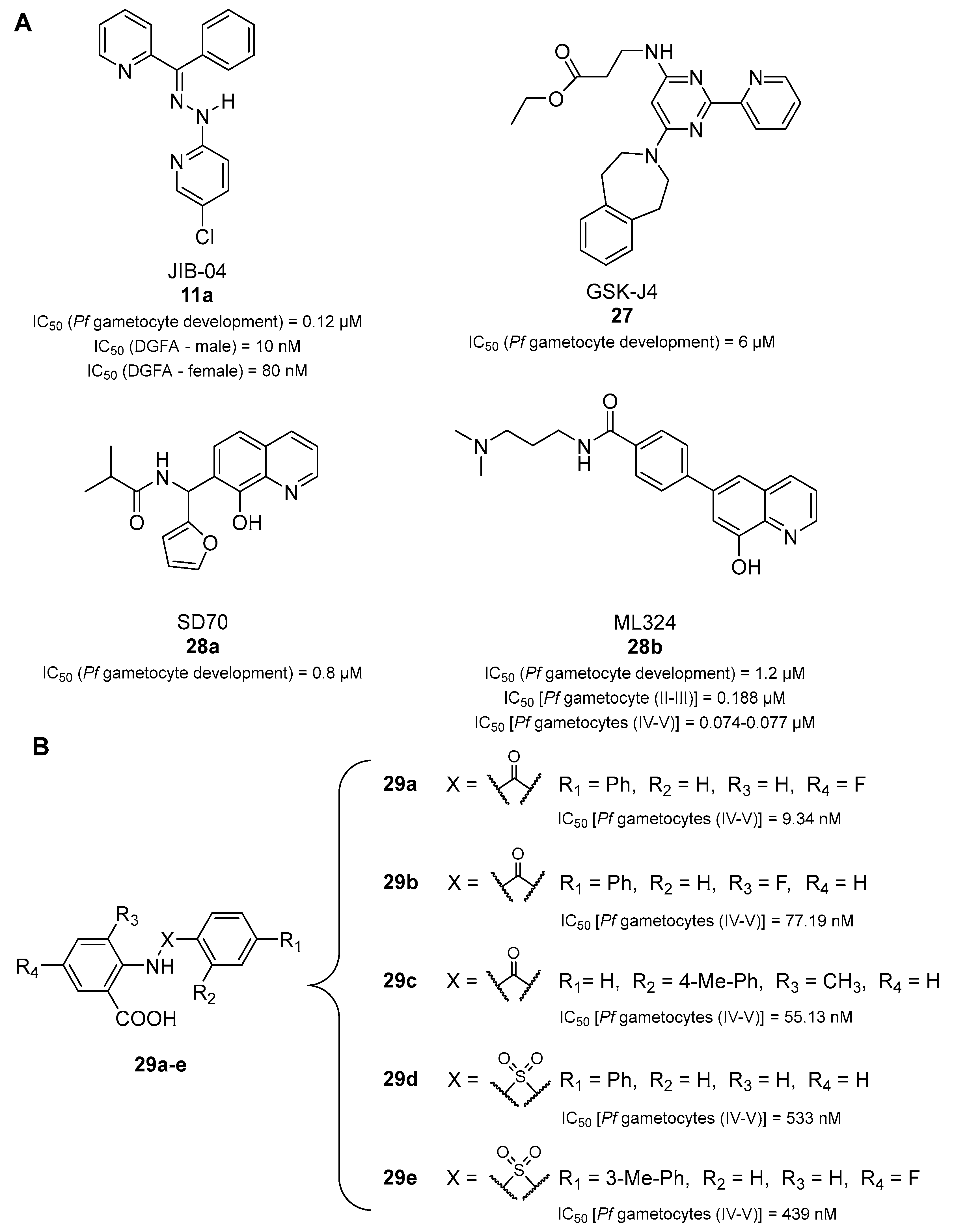
3.2. Epigenetic Transmission-Blocking Drugs
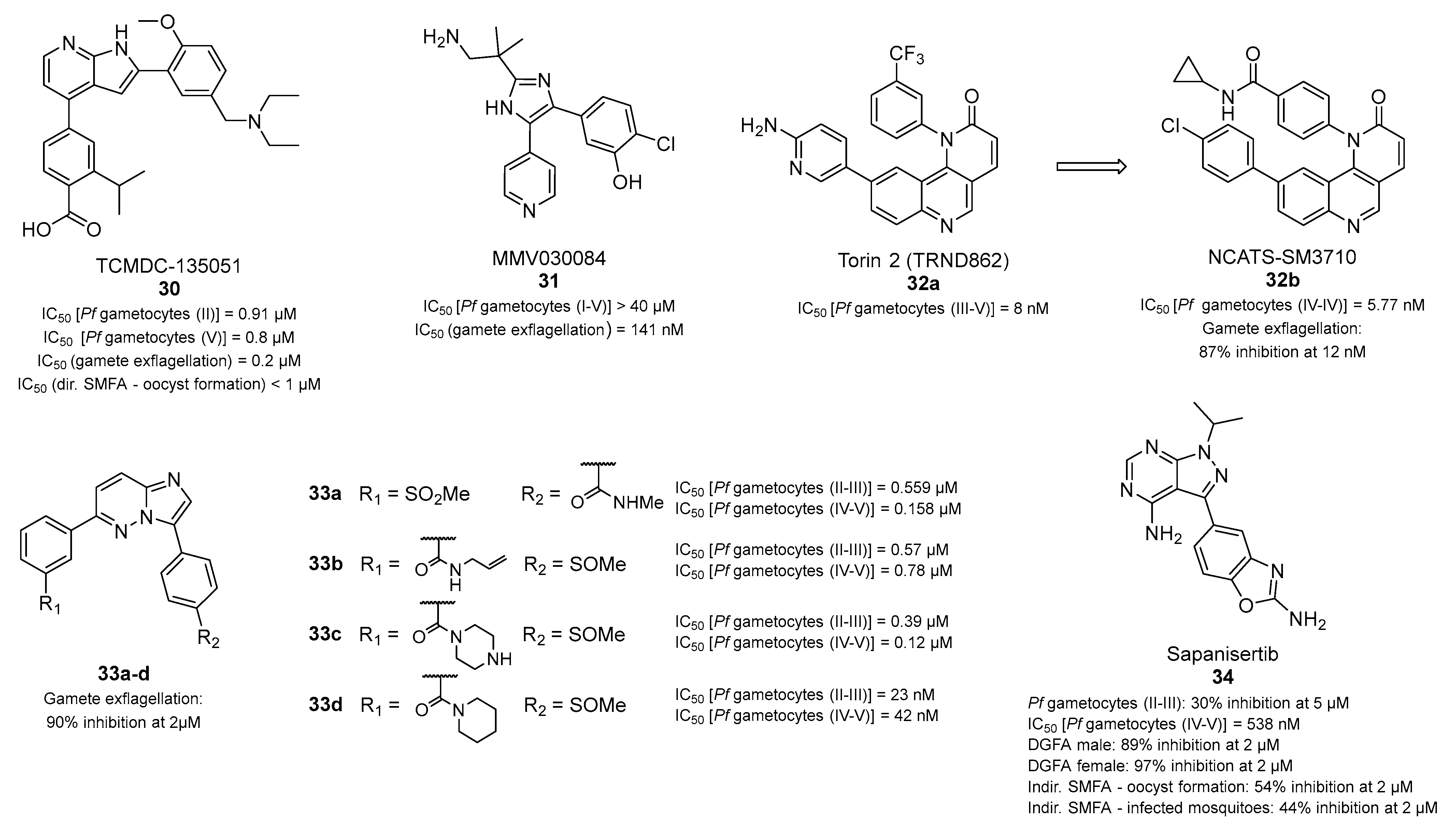
3.3. Antiplasmodial Transmission-Blocking Compounds Inhibiting Plasmodium Kinases
3.4. Aminoacyl-tRNA Synthetase Inhibitors
3.5. Pfs16 Inhibitors
3.6. Acetyl Coenzyme a Synthesis Inhibitors
3.7. Transmission-Blocking Compounds Altering Microtubule Assembly, Plasmepsins IX and X, and Pf20S Proteasome
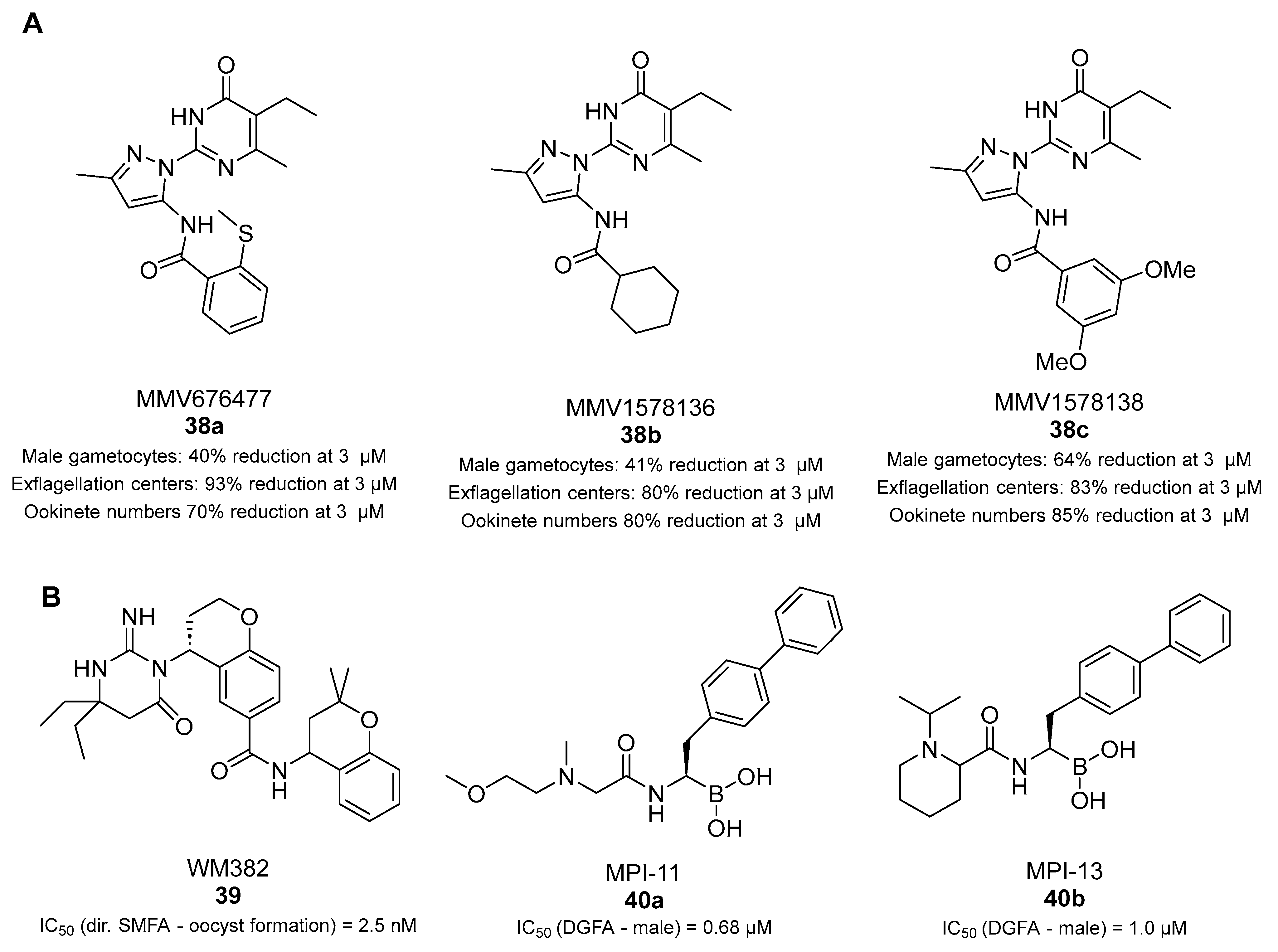
3.8. Drug Repurposing as an Approach to Developing Transmission-Blocking Compounds
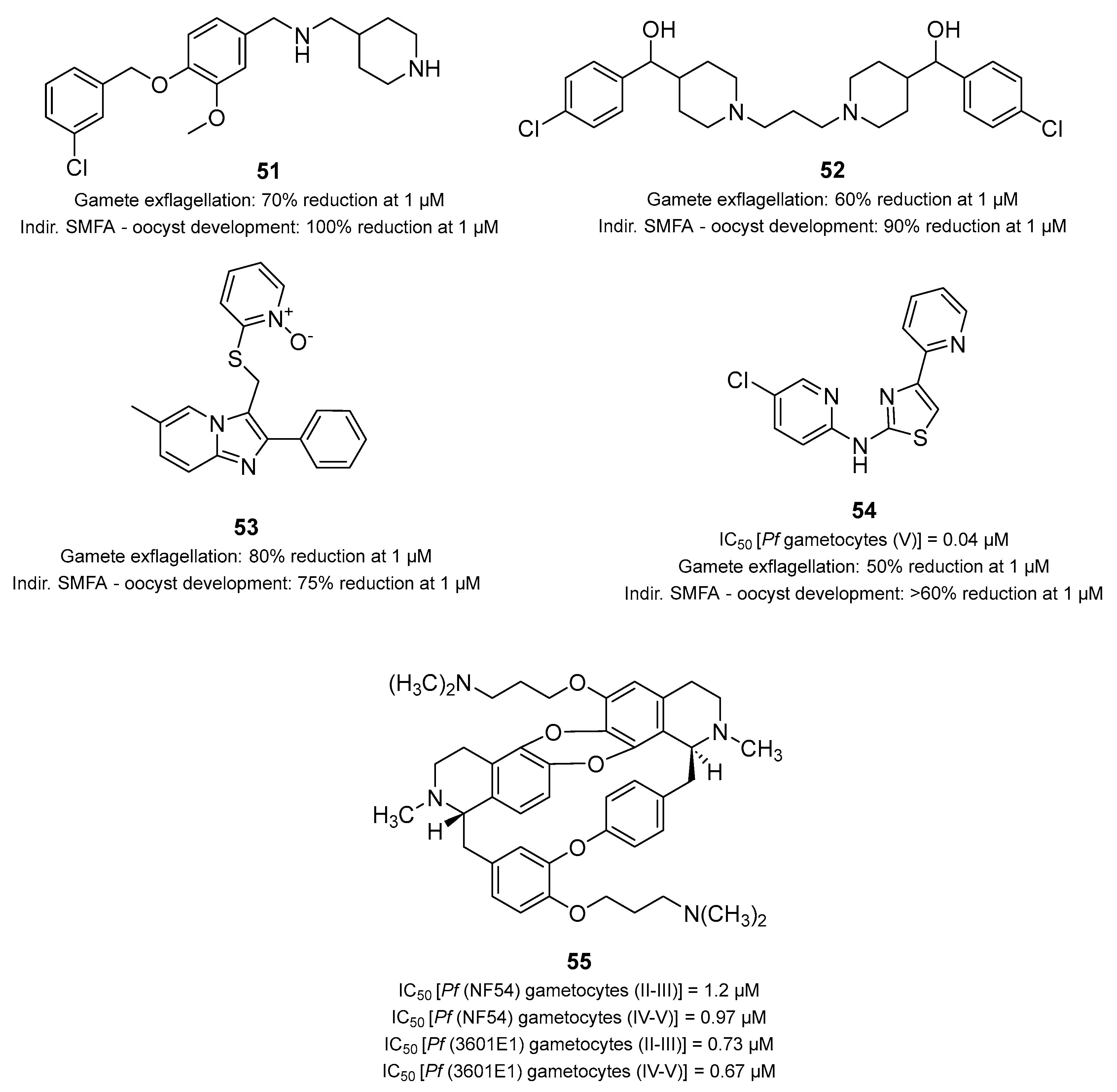
3.9. Multistage Active Drugs with Unknown Targets
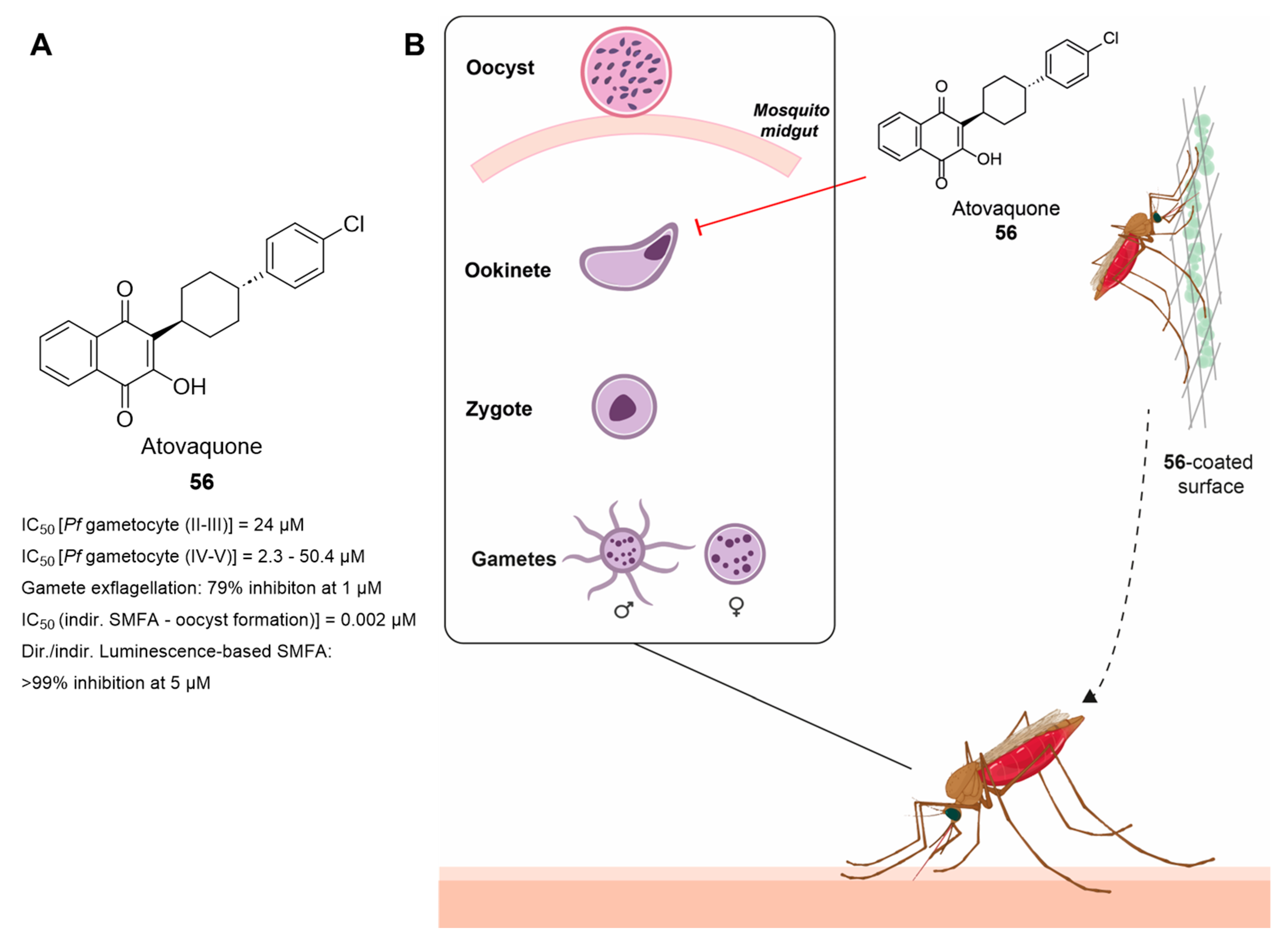
3.10. Innovative Approaches: Atovaquone-Coated Surfaces to Block Parasite Transmission
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABSs | Asexual blood stages |
| Ac-COA | Acetyl coenzyme A |
| ACS | Acyl-CoA synthetase |
| AMS | Adenosine-5′-sulfamate |
| aaRSs | Aminoacyl-tRNA synthetases |
| ATP | Adenosine triphosphate |
| BCKDH | Branched-chain keto-dehydrogenase |
| CETSA | Cellular thermal shift assay |
| CYP | Cytochrome P450 |
| DGFA | Dual gamete formation assay |
| DMSO | Dimethyl sulfoxide |
| DNMT | DNA methyltransferase |
| EC50 | 50% effective concentration |
| ED90 | 90% effective dose |
| EIA | Exflagellation inhibition assay |
| EM | Electron microscopy |
| ETC | Electron transport chain |
| FGAA | Female gamete activation assay |
| G6PD | Glucose-6-phosphate dehydrogenase |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GFP | Green fluorescent protein |
| GST | Glutathione S-transferase |
| HDAC | Histone deacetylase |
| HEA | Hydroxyethylamine |
| HMT | Histone methyltransferase |
| IAP | Inhibitor of apoptosis |
| IC50 | 50% inhibitory concentration |
| IF | Immunofluorescence |
| iPanAms | Inverted pantothenamides |
| JmjC | Jumonji C |
| KDM | Lysine demethylase |
| LDH | Lactate dehydrogenase |
| MMV | Medicines for Malaria Venture |
| ODA | Ookinete development assay |
| PABP1 | Polyadenylate-binding protein 1 complex |
| PCNA1 | Proliferating cell nuclear antigen |
| PfCLK3 | P. falciparum cyclin-dependent-like kinase |
| PfDHFR | P. falciparum dihydrofolate reductase |
| PfATP4 | P. falciparum Na+-efflux ATPase ATP4 |
| PfPANK1 | P. falciparum pantothenate kinase 1 |
| PfPI4Kβ | P. falciparum phosphatidylinositol 4-kinase type III β |
| PfeEF2 | P. falciparum translation elongation factor 2 |
| PfYRS | P. falciparum tyrosine-tRNA synthetase |
| PfvapA | P. falciparum V-type H + -ATPase |
| Pf20S | P. falciparum 20S proteasome |
| PheRS | Phenylalanyl-tRNA synthetase |
| PK | Pharmacokinetics |
| PM | Plasmepsins |
| PMIX | Plasmepsin IX |
| PMX | Plasmepsin X |
| PVM | Parasitophorous vacuole membrane |
| RBCs | Red blood cells |
| SAHA | Suberoylanilide hydroxamic acid |
| SaLSSA | Saponin-lysis sexual stage assay |
| SAR | Structure–activity relationship |
| SMFA | Standard membrane feeding assay |
| SPR | Surface plasmon resonance |
| TAP | Triaminopyrimidine |
| YRS | Tyrosine-tRNA synthetase |
| WHO | World Health Organization |
References
- WHO. World Malaria Report 2022. 2022. Available online: https://www.who.int/publications/i/item/9789240064898 (accessed on 10 June 2024).
- Aborode, A.T.; David, K.B.; Uwishema, O.; Nathaniel, A.L.; Imisioluwa, J.O.; Onigbinde, S.B.; Farooq, F. Fighting COVID-19 at the Expense of Malaria in Africa: The Consequences and Policy Options. Am. J. Trop. Med. Hyg. 2021, 104, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Hooft van Huijsduijnen, R.; Wells, T.N. The antimalarial pipeline. Curr. Opin. Pharmacol. 2018, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Birkholtz, L.M.; Alano, P.; Leroy, D. Transmission-blocking drugs for malaria elimination. Trends Parasitol. 2022, 38, 390–403. [Google Scholar] [CrossRef] [PubMed]
- van der Watt, M.E.; Reader, J.; Birkholtz, L.M. Adapt or Die: Targeting Unique Transmission-Stage Biology for Malaria Elimination. Front. Cell. Infect. Microbiol. 2022, 12, 901971. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, D.; Hunziker, P. Transmission-blocking strategies: The roadmap from laboratory bench to the community. Malar. J. 2016, 15, 95. [Google Scholar] [CrossRef]
- Smith, R.C.; Vega-Rodriguez, J.; Jacobs-Lorena, M. The Plasmodium bottleneck: Malaria parasite losses in the mosquito vector. Mem. Inst. Oswaldo Cruz 2014, 109, 644–661. [Google Scholar] [CrossRef]
- Kehrer, J.; Formaglio, P.; Muthinja, J.M.; Weber, S.; Baltissen, D.; Lance, C.; Ripp, J.; Grech, J.; Meissner, M.; Funaya, C.; et al. Plasmodium sporozoite disintegration during skin passage limits malaria parasite transmission. EMBO Rep. 2022, 23, e54719. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, C.; Trindade, A.O.; Andrade, F.S.; Souza, M.F.; Rios-Velasquez, C.M.; Lacerda, M.V.G.; Monteiro, W.M.; Costa, F.T.M.; Amino, R.; Lopes, S.C.P. Transmission-blocking compound candidates against Plasmodium vivax using P. berghei as an initial screening. Mem. Inst. Oswaldo Cruz 2021, 116, e200513. [Google Scholar] [CrossRef] [PubMed]
- Duffy, P.E. Transmission-Blocking Vaccines: Harnessing Herd Immunity for Malaria Elimination. Expert. Rev. Vaccines 2021, 20, 185–198. [Google Scholar] [CrossRef]
- Caragata, E.P.; Dutra, H.L.C.; Sucupira, P.H.F.; Ferreira, A.G.A.; Moreira, L.A. Wolbachia as translational science: Controlling mosquito-borne pathogens. Trends Parasitol. 2021, 37, 1050–1067. [Google Scholar] [CrossRef]
- Yu, S.; Wang, J.; Luo, X.; Zheng, H.; Wang, L.; Yang, X.; Wang, Y. Transmission-Blocking Strategies Against Malaria Parasites During Their Mosquito Stages. Front. Cell. Infect. Microbiol. 2022, 12, 820650. [Google Scholar] [CrossRef]
- Consalvi, S.; Tammaro, C.; Appetecchia, F.; Biava, M.; Poce, G. Malaria transmission blocking compounds: A patent review. Expert. Opin. Ther. Pat. 2022, 32, 649–666. [Google Scholar] [CrossRef]
- Bancone, G.; Chu, C.S. G6PD Variants and Haemolytic Sensitivity to Primaquine and Other Drugs. Front. Pharmacol. 2021, 12, 638885. [Google Scholar] [CrossRef]
- Birkholtz, L.M.; Coetzer, T.L.; Mancama, D.; Leroy, D.; Alano, P. Discovering New Transmission-Blocking Antimalarial Compounds: Challenges and Opportunities. Trends Parasitol. 2016, 32, 669–681. [Google Scholar] [CrossRef][Green Version]
- Shaw, W.R.; Marcenac, P.; Catteruccia, F. Plasmodium development in Anopheles: A tale of shared resources. Trends Parasitol. 2022, 38, 124–135. [Google Scholar] [CrossRef]
- Munro, B.A.; McMorran, B.J. Antimalarial Drug Strategies to Target Plasmodium Gametocytes. Parasitologia 2022, 2, 101–124. [Google Scholar] [CrossRef]
- Azevedo, R.; Mendes, A.M.; Prudencio, M. Inhibition of Plasmodium sporogonic stages by ivermectin and other avermectins. Parasit. Vectors 2019, 12, 549. [Google Scholar] [CrossRef]
- Delves, M.J.; Ramakrishnan, C.; Blagborough, A.M.; Leroy, D.; Wells, T.N.; Sinden, R.E. A high-throughput assay for the identification of malarial transmission-blocking drugs and vaccines. Int. J. Parasitol. 2012, 42, 999–1006. [Google Scholar] [CrossRef]
- Paton, D.G.; Probst, A.S.; Ma, E.; Adams, K.L.; Shaw, W.R.; Singh, N.; Bopp, S.; Volkman, S.K.; Hien, D.F.S.; Pare, P.S.L.; et al. Using an antimalarial in mosquitoes overcomes Anopheles and Plasmodium resistance to malaria control strategies. PLoS Pathog. 2022, 18, e1010609. [Google Scholar] [CrossRef]
- Oke, C.E.; Ingham, V.A.; Walling, C.A.; Reece, S.E. Vector control: Agents of selection on malaria parasites? Trends Parasitol. 2022, 38, 890–903. [Google Scholar] [CrossRef]
- Wadi, I.; Nath, M.; Anvikar, A.R.; Singh, P.; Sinha, A. Recent advances in transmission-blocking drugs for malaria elimination. Future Med. Chem. 2019, 11, 3047–3088. [Google Scholar] [CrossRef] [PubMed]
- Wadi, I.; Singh, P.; Nath, M.; Anvikar, A.R.; Sinha, A. Malaria transmission-blocking drugs: Implications and future perspectives. Future Med. Chem. 2020, 12, 1071–1101. [Google Scholar] [CrossRef] [PubMed]
- Delves, M.J.; Angrisano, F.; Blagborough, A.M. Antimalarial Transmission-Blocking Interventions: Past, Present, and Future. Trends Parasitol. 2018, 34, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Yahiya, S.; Rueda-Zubiaurre, A.; Delves, M.J.; Fuchter, M.J.; Baum, J. The antimalarial screening landscape-looking beyond the asexual blood stage. Curr. Opin. Chem. Biol. 2019, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.L.; Dans, M.G.; Balbin, J.M.; de Koning-Ward, T.F.; Gilson, P.R.; Beeson, J.G.; Boyle, M.J.; Wilson, D.W. Targeting malaria parasite invasion of red blood cells as an antimalarial strategy. FEMS Microbiol. Rev. 2019, 43, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, K.; Burke, T.A.; Williamson, K.C.; Wiegand, R.C.; Wirth, D.F.; Marti, M. A high-throughput screen targeting malaria transmission stages opens new avenues for drug development. J. Infect. Dis. 2011, 203, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Dechering, K.J.; Timmerman, M.; Rensen, K.; Koolen, K.M.J.; Honarnejad, S.; Vos, M.W.; Huijs, T.; Henderson, R.W.M.; Chenu, E.; Laleu, B.; et al. Replenishing the malaria drug discovery pipeline: Screening and hit evaluation of the MMV Hit Generation Library 1 (HGL1) against asexual blood stage Plasmodium falciparum, using a nano luciferase reporter read-out. SLAS Discov. 2022, 27, 337–348. [Google Scholar] [CrossRef]
- Reader, J.; Botha, M.; Theron, A.; Lauterbach, S.B.; Rossouw, C.; Engelbrecht, D.; Wepener, M.; Smit, A.; Leroy, D.; Mancama, D.; et al. Nowhere to hide: Interrogating different metabolic parameters of Plasmodium falciparum gametocytes in a transmission blocking drug discovery pipeline towards malaria elimination. Malar. J. 2015, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Reader, J.; van der Watt, M.E.; Taylor, D.; Le Manach, C.; Mittal, N.; Ottilie, S.; Theron, A.; Moyo, P.; Erlank, E.; Nardini, L.; et al. Multistage and transmission-blocking targeted antimalarials discovered from the open-source MMV Pandemic Response Box. Nat. Commun. 2021, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Ponnudurai, T.; Leeuwenberg, A.D.; Meuwissen, J.H. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop. Geogr. Med. 1981, 33, 50–54. [Google Scholar]
- Duffy, S.; Loganathan, S.; Holleran, J.P.; Avery, V.M. Large-scale production of Plasmodium falciparum gametocytes for malaria drug discovery. Nat. Protoc. 2016, 11, 976–992. [Google Scholar] [CrossRef]
- Lu, G.; Nagbanshi, M.; Goldau, N.; Mendes Jorge, M.; Meissner, P.; Jahn, A.; Mockenhaupt, F.P.; Muller, O. Efficacy and safety of methylene blue in the treatment of malaria: A systematic review. BMC Med. 2018, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M.L.; Avery, V.M. Approaches to protozoan drug discovery: Phenotypic screening. J. Med. Chem. 2013, 56, 7727–7740. [Google Scholar] [CrossRef]
- Wadi, I.; Anvikar, A.R.; Nath, M.; Pillai, C.R.; Sinha, A.; Valecha, N. Critical examination of approaches exploited to assess the effectiveness of transmission-blocking drugs for malaria. Future Med. Chem. 2018, 10, 2619–2639. [Google Scholar] [CrossRef]
- Plouffe, D.M.; Wree, M.; Du, A.Y.; Meister, S.; Li, F.; Patra, K.; Lubar, A.; Okitsu, S.L.; Flannery, E.L.; Kato, N.; et al. High-Throughput Assay and Discovery of Small Molecules that Interrupt Malaria Transmission. Cell Host Microbe 2016, 19, 114–126. [Google Scholar] [CrossRef]
- Lucantoni, L.; Silvestrini, F.; Signore, M.; Siciliano, G.; Eldering, M.; Dechering, K.J.; Avery, V.M.; Alano, P. A simple and predictive phenotypic High Content Imaging assay for Plasmodium falciparum mature gametocytes to identify malaria transmission blocking compounds. Sci. Rep. 2015, 5, 16414. [Google Scholar] [CrossRef]
- Ruecker, A.; Mathias, D.K.; Straschil, U.; Churcher, T.S.; Dinglasan, R.R.; Leroy, D.; Sinden, R.E.; Delves, M.J. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob. Agents Chemother. 2014, 58, 7292–7302. [Google Scholar] [CrossRef] [PubMed]
- Delves, M.J.; Miguel-Blanco, C.; Matthews, H.; Molina, I.; Ruecker, A.; Yahiya, S.; Straschil, U.; Abraham, M.; Leon, M.L.; Fischer, O.J.; et al. A high throughput screen for next-generation leads targeting malaria parasite transmission. Nat. Commun. 2018, 9, 3805. [Google Scholar] [CrossRef]
- Delves, M.; Lafuente-Monasterio, M.J.; Upton, L.; Ruecker, A.; Leroy, D.; Gamo, F.J.; Sinden, R. Fueling Open Innovation for Malaria Transmission-Blocking Drugs: Hundreds of Molecules Targeting Early Parasite Mosquito Stages. Front. Microbiol. 2019, 10, 2134. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Stone, W.J.; Koolen, K.M.; Deng, B.; Zhou, L.; van Gemert, G.J.; Locke, E.; Morin, M.; Bousema, T.; Sauerwein, R.W.; et al. An inter-laboratory comparison of standard membrane-feeding assays for evaluation of malaria transmission-blocking vaccines. Malar. J. 2016, 15, 463. [Google Scholar] [CrossRef]
- Miura, K.; Swihart, B.J.; Deng, B.; Zhou, L.; Pham, T.P.; Diouf, A.; Burton, T.; Fay, M.P.; Long, C.A. Transmission-blocking activity is determined by transmission-reducing activity and number of control oocysts in Plasmodium falciparum standard membrane-feeding assay. Vaccine 2016, 34, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Churcher, T.S.; Blagborough, A.M.; Delves, M.; Ramakrishnan, C.; Kapulu, M.C.; Williams, A.R.; Biswas, S.; Da, D.F.; Cohuet, A.; Sinden, R.E. Measuring the blockade of malaria transmission—An analysis of the Standard Membrane Feeding Assay. Int. J. Parasitol. 2012, 42, 1037–1044. [Google Scholar] [CrossRef]
- Azevedo, R.; Markovic, M.; Machado, M.; Franke-Fayard, B.; Mendes, A.M.; Prudencio, M. Bioluminescence Method for In Vitro Screening of Plasmodium Transmission-Blocking Compounds. Antimicrob. Agents Chemother. 2017, 61, e02699-16. [Google Scholar] [CrossRef]
- Jimenez-Diaz, M.B.; Ebert, D.; Salinas, Y.; Pradhan, A.; Lehane, A.M.; Myrand-Lapierre, M.E.; O’Loughlin, K.G.; Shackleford, D.M.; Justino de Almeida, M.; Carrillo, A.K.; et al. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc. Natl. Acad. Sci. USA 2014, 111, E5455–E5462. [Google Scholar] [CrossRef] [PubMed]
- Dechering, K.J.; Duerr, H.P.; Koolen, K.M.J.; Gemert, G.V.; Bousema, T.; Burrows, J.; Leroy, D.; Sauerwein, R.W. Modelling mosquito infection at natural parasite densities identifies drugs targeting EF2, PI4K or ATP4 as key candidates for interrupting malaria transmission. Sci. Rep. 2017, 7, 17680. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A.H.; McCarthy, J.S.; Panetta, J.C.; Dallas, R.H.; Woodford, J.; Tang, L.; Smith, A.M.; Stewart, T.B.; Branum, K.C.; Freeman, B.B., 3rd; et al. Safety, tolerability, pharmacokinetics, and antimalarial efficacy of a novel Plasmodium falciparum ATP4 inhibitor SJ733: A first-in-human and induced blood-stage malaria phase 1a/b trial. Lancet Infect. Dis. 2020, 20, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A.H.; Panetta, J.C.; Smith, A.M.; Dallas, R.H.; Freeman, B.B., 3rd; Stewart, T.B.; Tang, L.; John, E.; Branum, K.C.; Patel, N.D.; et al. Combining SJ733, an oral ATP4 inhibitor of Plasmodium falciparum, with the pharmacokinetic enhancer cobicistat: An innovative approach in antimalarial drug development. EBioMedicine 2022, 80, 104065. [Google Scholar] [CrossRef] [PubMed]
- Rottmann, M.; McNamara, C.; Yeung, B.K.; Lee, M.C.; Zou, B.; Russell, B.; Seitz, P.; Plouffe, D.M.; Dharia, N.V.; Tan, J.; et al. Spiroindolones, a potent compound class for the treatment of malaria. Science 2010, 329, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- van Pelt-Koops, J.C.; Pett, H.E.; Graumans, W.; van der Vegte-Bolmer, M.; van Gemert, G.J.; Rottmann, M.; Yeung, B.K.; Diagana, T.T.; Sauerwein, R.W. The spiroindolone drug candidate NITD609 potently inhibits gametocytogenesis and blocks Plasmodium falciparum transmission to anopheles mosquito vector. Antimicrob. Agents Chemother. 2012, 56, 3544–3548. [Google Scholar] [CrossRef]
- Schmitt, E.K.; Ndayisaba, G.; Yeka, A.; Asante, K.P.; Grobusch, M.P.; Karita, E.; Mugerwa, H.; Asiimwe, S.; Oduro, A.; Fofana, B.; et al. Efficacy of Cipargamin (KAE609) in a Randomized, Phase II Dose-Escalation Study in Adults in Sub-Saharan Africa With Uncomplicated Plasmodium falciparum Malaria. Clin. Infect. Dis. 2022, 74, 1831–1839. [Google Scholar] [CrossRef]
- Qiu, D.; Pei, J.V.; Rosling, J.E.O.; Thathy, V.; Li, D.; Xue, Y.; Tanner, J.D.; Penington, J.S.; Aw, Y.T.V.; Aw, J.Y.H.; et al. A G358S mutation in the Plasmodium falciparum Na(+) pump PfATP4 confers clinically-relevant resistance to cipargamin. Nat. Commun. 2022, 13, 5746. [Google Scholar] [CrossRef] [PubMed]
- Charman, S.A.; Arbe-Barnes, S.; Bathurst, I.C.; Brun, R.; Campbell, M.; Charman, W.N.; Chiu, F.C.K.; Chollet, J.; Craft, J.C.; Creek, D.J.; et al. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc. Natl. Acad. Sci. USA 2011, 108, 4400–4405. [Google Scholar] [CrossRef] [PubMed]
- Klonis, N.; Crespo-Ortiz, M.P.; Bottova, I.; Abu-Bakar, N.; Kenny, S.; Rosenthal, P.J.; Tilley, L. Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc. Natl. Acad. Sci. USA 2011, 108, 11405–11410. [Google Scholar] [CrossRef] [PubMed]
- Lang-Unnasch, N.; Murphy, A.D. Metabolic changes of the malaria parasite during the transition from the human to the mosquito host. Annu. Rev. Microbiol. 1998, 52, 561–590. [Google Scholar] [CrossRef] [PubMed]
- Hayward, R.E. Plasmodium falciparum phosphoenolpyruvate carboxykinase is developmentally regulated in gametocytes. Mol. Biochem. Parasitol. 2000, 107, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Baragana, B.; Hallyburton, I.; Lee, M.C.; Norcross, N.R.; Grimaldi, R.; Otto, T.D.; Proto, W.R.; Blagborough, A.M.; Meister, S.; Wirjanata, G.; et al. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 2015, 522, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Baragana, B.; Forte, B.; Choi, R.; Nakazawa Hewitt, S.; Bueren-Calabuig, J.A.; Pisco, J.P.; Peet, C.; Dranow, D.M.; Robinson, D.A.; Jansen, C.; et al. Lysyl-tRNA synthetase as a drug target in malaria and cryptosporidiosis. Proc. Natl. Acad. Sci. USA 2019, 116, 7015–7020. [Google Scholar] [CrossRef] [PubMed]
- Paquet, T.; Le Manach, C.; Cabrera, D.G.; Younis, Y.; Henrich, P.P.; Abraham, T.S.; Lee, M.C.S.; Basak, R.; Ghidelli-Disse, S.; Lafuente-Monasterio, M.J.; et al. Antimalarial efficacy of MMV390048, an inhibitor of Plasmodium phosphatidylinositol 4-kinase. Sci. Transl. Med. 2017, 9, eaad9735. [Google Scholar] [CrossRef] [PubMed]
- Yuthavong, Y.; Tarnchompoo, B.; Vilaivan, T.; Chitnumsub, P.; Kamchonwongpaisan, S.; Charman, S.A.; McLennan, D.N.; White, K.L.; Vivas, L.; Bongard, E.; et al. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc. Natl. Acad. Sci. USA 2012, 109, 16823–16828. [Google Scholar] [CrossRef]
- Posayapisit, N.; Pengon, J.; Prommana, P.; Shoram, M.; Yuthavong, Y.; Uthaipibull, C.; Kamchonwongpaisan, S.; Jupatanakul, N. Transgenic pyrimethamine-resistant Plasmodium falciparum reveals transmission-blocking potency of P218, a novel antifolate candidate drug. Int. J. Parasitol. 2021, 51, 635–642. [Google Scholar] [CrossRef]
- Vos, M.W.; Stone, W.J.R.; Koolen, K.M.; van Gemert, G.-J.; van Schaijk, B.; Leroy, D.; Sauerwein, R.W.; Bousema, T.; Dechering, K.J. A semi-automated luminescence based standard membrane feeding assay identifies novel small molecules that inhibit transmission of malaria parasites by mosquitoes. Sci. Rep. 2015, 5, 18704. [Google Scholar] [CrossRef] [PubMed]
- Chughlay, M.F.; Rossignol, E.; Donini, C.; El Gaaloul, M.; Lorch, U.; Coates, S.; Langdon, G.; Hammond, T.; Mohrle, J.; Chalon, S. First-in-human clinical trial to assess the safety, tolerability and pharmacokinetics of P218, a novel candidate for malaria chemoprotection. Br. J. Clin. Pharmacol. 2020, 86, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Chughlay, M.F.; El Gaaloul, M.; Donini, C.; Campo, B.; Berghmans, P.J.; Lucardie, A.; Marx, M.W.; Cherkaoui-Rbati, M.H.; Langdon, G.; Angulo-Barturen, I.; et al. Chemoprotective Antimalarial Activity of P218 against Plasmodium falciparum: A Randomized, Placebo-Controlled Volunteer Infection Study. Am. J. Trop. Med. Hyg. 2021, 104, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Kuhen, K.L.; Chatterjee, A.K.; Rottmann, M.; Gagaring, K.; Borboa, R.; Buenviaje, J.; Chen, Z.; Francek, C.; Wu, T.; Nagle, A.; et al. KAF156 is an antimalarial clinical candidate with potential for use in prophylaxis, treatment, and prevention of disease transmission. Antimicrob. Agents Chemother. 2014, 58, 5060–5067. [Google Scholar] [CrossRef] [PubMed]
- Duraisingh, M.T.; Skillman, K.M. Epigenetic Variation and Regulation in Malaria Parasites. Annu. Rev. Microbiol. 2018, 72, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Vanheer, L.N.; Zhang, H.; Lin, G.; Kafsack, B.F.C. Activity of Epigenetic Inhibitors against Plasmodium falciparum Asexual and Sexual Blood Stages. Antimicrob. Agents Chemother. 2020, 64, e02523-19. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, N.; von Gruning, H.; Opperman, D.; van der Watt, M.; Reader, J.; Birkholtz, L.M. Epigenetic inhibitors target multiple stages of Plasmodium falciparum parasites. Sci. Rep. 2020, 10, 2355. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, R.; Tang, T.; Ling, D.; Wang, M.; Xu, D.; Sun, M.; Zheng, L.; Zhu, F.; Min, H.; et al. A novel multistage antiplasmodial inhibitor targeting Plasmodium falciparum histone deacetylase 1. Cell Discov. 2020, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, T.; Li, R.; Huang, Z.; Ling, D.; Zheng, L.; Ding, Y.; Liu, T.; Xu, W.; Zhu, F.; et al. Drug Repurposing of Quisinostat to Discover Novel Plasmodium falciparum HDAC1 Inhibitors with Enhanced Triple-Stage Antimalarial Activity and Improved Safety. J. Med. Chem. 2022, 65, 4156–4181. [Google Scholar] [CrossRef]
- Nardella, F.; Halby, L.; Dobrescu, I.; Viluma, J.; Bon, C.; Claes, A.; Cadet-Daniel, V.; Tafit, A.; Roesch, C.; Hammam, E.; et al. Procainamide-SAHA Fused Inhibitors of hHDAC6 Tackle Multidrug-Resistant Malaria Parasites. J. Med. Chem. 2021, 64, 10403–10417. [Google Scholar] [CrossRef]
- Matthews, K.A.; Senagbe, K.M.; Notzel, C.; Gonzales, C.A.; Tong, X.; Rijo-Ferreira, F.; Bhanu, N.V.; Miguel-Blanco, C.; Lafuente-Monasterio, M.J.; Garcia, B.A.; et al. Disruption of the Plasmodium falciparum Life Cycle through Transcriptional Reprogramming by Inhibitors of Jumonji Demethylases. ACS Infect. Dis. 2020, 6, 1058–1075. [Google Scholar] [CrossRef] [PubMed]
- Reader, J.; Opperman, D.F.L.; van der Watt, M.E.; Theron, A.; Leshabane, M.; da Rocha, S.; Turner, J.; Garrabrant, K.; Pina, I.; Mills, C.; et al. New Transmission-Selective Antimalarial Agents through Hit-to-Lead Optimization of 2-([1,1′-Biphenyl]-4-carboxamido)benzoic Acid Derivatives. ChemBioChem 2022, 23, e202200427. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Sanchez-Azqueta, A.; Janha, O.; Flannery, E.L.; Mahindra, A.; Mapesa, K.; Char, A.B.; Sriranganadane, D.; Brancucci, N.M.B.; Antonova-Koch, Y.; et al. Validation of the protein kinase PfCLK3 as a multistage cross-species malarial drug target. Science 2019, 365, eaau1682. [Google Scholar] [CrossRef] [PubMed]
- Mahindra, A.; Janha, O.; Mapesa, K.; Sanchez-Azqueta, A.; Alam, M.M.; Amambua-Ngwa, A.; Nwakanma, D.C.; Tobin, A.B.; Jamieson, A.G. Development of Potent PfCLK3 Inhibitors Based on TCMDC-135051 as a New Class of Antimalarials. J. Med. Chem. 2020, 63, 9300–9315. [Google Scholar] [CrossRef] [PubMed]
- Vanaerschot, M.; Murithi, J.M.; Pasaje, C.F.A.; Ghidelli-Disse, S.; Dwomoh, L.; Bird, M.; Spottiswoode, N.; Mittal, N.; Arendse, L.B.; Owen, E.S.; et al. Inhibition of Resistance-Refractory, P. falciparum Kinase PKG Delivers Prophylactic, Blood Stage, and Transmission-Blocking Antiplasmodial Activity. Cell Chem. Biol. 2020, 27, 806–816.e808. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Tanaka, T.Q.; Magle, C.T.; Huang, W.; Southall, N.; Huang, R.; Dehdashti, S.J.; McKew, J.C.; Williamson, K.C.; Zheng, W. Chemical signatures and new drug targets for gametocytocidal drug development. Sci. Rep. 2014, 4, 3743. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.K.; Ressurreição, A.S.; Buchholz, K.; Prudêncio, M.; Herman-Ornelas, J.D.; Rebelo, M.; Beatty, W.L.; Wirth, D.F.; Hänscheid, T.; Moreira, R.; et al. Torins are potent antimalarials that block replenishment of Plasmodium liver stage parasitophorous vacuole membrane proteins. Proc. Natl. Acad. Sci. USA 2013, 110, E2838–E2847. [Google Scholar] [CrossRef]
- Krishnan, K.; Ziniel, P.; Li, H.; Huang, X.; Hupalo, D.; Gombakomba, N.; Guerrero, S.M.; Dotrang, T.; Lu, X.; Caridha, D.; et al. Torin 2 Derivative, NCATS-SM3710, Has Potent Multistage Antimalarial Activity through Inhibition of P. falciparum Phosphatidylinositol 4-Kinase (Pf PI4KIIIbeta). ACS Pharmacol. Transl. Sci. 2020, 3, 948–964. [Google Scholar] [CrossRef]
- Cheuka, P.M.; Centani, L.; Arendse, L.B.; Fienberg, S.; Wambua, L.; Renga, S.S.; Dziwornu, G.A.; Kumar, M.; Lawrence, N.; Taylor, D.; et al. New Amidated 3,6-Diphenylated Imidazopyridazines with Potent Antiplasmodium Activity Are Dual Inhibitors of Plasmodium Phosphatidylinositol-4-kinase and cGMP-Dependent Protein Kinase. ACS Infect. Dis. 2021, 7, 34–46. [Google Scholar] [CrossRef]
- Arendse, L.B.; Murithi, J.M.; Qahash, T.; Pasaje, C.F.A.; Godoy, L.C.; Dey, S.; Gibhard, L.; Ghidelli-Disse, S.; Drewes, G.; Bantscheff, M.; et al. The anticancer human mTOR inhibitor sapanisertib potently inhibits multiple Plasmodium kinases and life cycle stages. Sci. Transl. Med. 2022, 14, eabo7219. [Google Scholar] [CrossRef]
- Khan, S. Recent advances in the biology and drug targeting of malaria parasite aminoacyl-tRNA synthetases. Malar. J. 2016, 15, 203. [Google Scholar] [CrossRef]
- Bhatt, T.K.; Kapil, C.; Khan, S.; Jairajpuri, M.A.; Sharma, V.; Santoni, D.; Silvestrini, F.; Pizzi, E.; Sharma, A. A genomic glimpse of aminoacyl-tRNA synthetases in malaria parasite Plasmodium falciparum. BMC Genomics 2009, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Nyamai, D.W.; Tastan Bishop, O. Aminoacyl tRNA synthetases as malarial drug targets: A comparative bioinformatics study. Malar. J. 2019, 18, 34. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, A. Plasmodium falciparum mitochondria import tRNAs along with an active phenylalanyl-tRNA synthetase. Biochem. J. 2015, 465, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.C.; Metcalfe, R.D.; Dunn, E.; Morton, C.J.; Huang, S.C.; Puhalovich, T.; Du, Y.; Wittlin, S.; Nie, S.; Luth, M.R.; et al. Reaction hijacking of tyrosine tRNA synthetase as a new whole-of-life-cycle antimalarial strategy. Science 2022, 376, 1074–1079. [Google Scholar] [CrossRef]
- Sundararaman, S.A.; Odom John, A.R. Adenosine sulfamates: Next generation of antimalarials. Cell Host Microbe 2022, 30, 1074–1076. [Google Scholar] [CrossRef] [PubMed]
- Manickam, Y.; Chaturvedi, R.; Babbar, P.; Malhotra, N.; Jain, V.; Sharma, A. Drug targeting of one or more aminoacyl-tRNA synthetase in the malaria parasite Plasmodium falciparum. Drug Discov. Today 2018, 23, 1233–1240. [Google Scholar] [CrossRef]
- Gill, J.; Sharma, A. Exploration of aminoacyl-tRNA synthetases from eukaryotic parasites for drug development. J. Biol. Chem. 2022, 299, 102860. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Comer, E.; Sakata-Kato, T.; Sharma, A.; Sharma, M.; Maetani, M.; Bastien, J.; Brancucci, N.M.; Bittker, J.A.; Corey, V.; et al. Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature 2016, 538, 344–349. [Google Scholar] [CrossRef]
- Dechering, K.J.; Thompson, J.; Dodemont, H.J.; Eling, W.; Konings, R.N. Developmentally regulated expression of pfs16, a marker for sexual differentiation of the human malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 1997, 89, 235–244. [Google Scholar] [CrossRef]
- Berry, A.; Deymier, C.; Sertorio, M.; Witkowski, B.; Benoit-Vical, F. Pfs 16 pivotal role in Plasmodium falciparum gametocytogenesis: A potential antiplasmodial drug target. Exp. Parasitol. 2009, 121, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Lasonder, E.; Rijpma, S.R.; van Schaijk, B.C.; Hoeijmakers, W.A.; Kensche, P.R.; Gresnigt, M.S.; Italiaander, A.; Vos, M.W.; Woestenenk, R.; Bousema, T.; et al. Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: Molecular insight into sex-specific processes and translational repression. Nucleic Acids Res. 2016, 44, 6087–6101. [Google Scholar] [CrossRef] [PubMed]
- Kongkasuriyachai, D.; Fujioka, H.; Kumar, N. Functional analysis of Plasmodium falciparum parasitophorous vacuole membrane protein (Pfs16) during gametocytogenesis and gametogenesis by targeted gene disruption. Mol. Biochem. Parasitol. 2004, 133, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Yahiya, S.; Saunders, C.N.; Hassan, S.; Straschil, U.; Fischer, O.J.; Rueda-Zubiaurre, A.; Haase, S.; Vizcay-Barrena, G.; Famodimu, M.T.; Jordan, S.; et al. A novel class of sulphonamides potently block malaria transmission by targeting a Plasmodium vacuole membrane protein. Dis. Model. Mech. 2023, 16, dmm049950. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Zubiaurre, A.; Yahiya, S.; Fischer, O.J.; Hu, X.; Saunders, C.N.; Sharma, S.; Straschil, U.; Shen, J.; Tate, E.W.; Delves, M.J.; et al. Structure-Activity Relationship Studies of a Novel Class of Transmission Blocking Antimalarials Targeting Male Gametes. J. Med. Chem. 2020, 63, 2240–2262. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cobbold, S.A.; Vaughan, A.M.; Lewis, I.A.; Painter, H.J.; Camargo, N.; Perlman, D.H.; Fishbaugher, M.; Healer, J.; Cowman, A.F.; Kappe, S.H.; et al. Kinetic flux profiling elucidates two independent acetyl-CoA biosynthetic pathways in Plasmodium falciparum. J. Biol. Chem. 2013, 288, 36338–36350. [Google Scholar] [CrossRef]
- Rajaram, K.; Tewari, S.G.; Wallqvist, A.; Prigge, S.T. Metabolic changes accompanying the loss of fumarate hydratase and malate-quinone oxidoreductase in the asexual blood stage of Plasmodium falciparum. J. Biol. Chem. 2022, 298, 101897. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- Prata, I.O.; Cubillos, E.F.G.; Kruger, A.; Barbosa, D.; Martins, J., Jr.; Setubal, J.C.; Wunderlich, G. Plasmodium falciparum Acetyl-CoA Synthetase Is Essential for Parasite Intraerythrocytic Development and Chromatin Modification. ACS Infect. Dis. 2021, 7, 3224–3240. [Google Scholar] [CrossRef]
- Summers, R.L.; Pasaje, C.F.A.; Pisco, J.P.; Striepen, J.; Luth, M.R.; Kumpornsin, K.; Carpenter, E.F.; Munro, J.T.; Lin, D.; Plater, A.; et al. Chemogenomics identifies acetyl-coenzyme A synthetase as a target for malaria treatment and prevention. Cell Chem. Biol. 2022, 29, 191–201.e198. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.E.; Lunghi, M.; Krishnan, A.; Kooij, T.W.A.; Soldati-Favre, D. Pantothenate and CoA biosynthesis in Apicomplexa and their promise as antiparasitic drug targets. PLoS Pathog. 2021, 17, e1010124. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.; Lucantoni, L.; Sykes, M.L.; Jones, A.J.; Holleran, J.P.; Saliba, K.J.; Avery, V.M. Biological characterization of chemically diverse compounds targeting the Plasmodium falciparum coenzyme A synthesis pathway. Parasit. Vectors 2016, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Spry, C.; Barnard, L.; Kok, M.; Powell, A.K.; Mahesh, D.; Tjhin, E.T.; Saliba, K.J.; Strauss, E.; de Villiers, M. Toward a Stable and Potent Coenzyme A-Targeting Antiplasmodial Agent: Structure-Activity Relationship Studies of N-Phenethyl-alpha-methyl-pantothenamide. ACS Infect. Dis. 2020, 6, 1844–1854. [Google Scholar] [CrossRef]
- Schalkwijk, J.; Allman, E.L.; Jansen, P.A.M.; de Vries, L.E.; Verhoef, J.M.J.; Jackowski, S.; Botman, P.N.M.; Beuckens-Schortinghuis, C.A.; Koolen, K.M.J.; Bolscher, J.M.; et al. Antimalarial pantothenamide metabolites target acetyl-coenzyme A biosynthesis in Plasmodium falciparum. Sci. Transl. Med. 2019, 11, eaas9917. [Google Scholar] [CrossRef] [PubMed]
- Kumari, G.; Jain, R.; Kumar Sah, R.; Kalia, I.; Vashistha, M.; Singh, P.; Prasad Singh, A.; Samby, K.; Burrows, J.; Singh, S. Multistage and transmission-blocking tubulin targeting potent antimalarial discovered from the open access MMV pathogen box. Biochem. Pharmacol. 2022, 203, 115154. [Google Scholar] [CrossRef]
- Pino, P.; Caldelari, R.; Mukherjee, B.; Vahokoski, J.; Klages, N.; Maco, B.; Collins, C.R.; Blackman, M.J.; Kursula, I.; Heussler, V.; et al. A multistage antimalarial targets the plasmepsins IX and X essential for invasion and egress. Science 2017, 358, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Nasamu, A.S.; Glushakova, S.; Russo, I.; Vaupel, B.; Oksman, A.; Kim, A.S.; Fremont, D.H.; Tolia, N.; Beck, J.R.; Meyers, M.J.; et al. Plasmepsins IX and X are essential and druggable mediators of malaria parasite egress and invasion. Science 2017, 358, 518–522. [Google Scholar] [CrossRef]
- Favuzza, P.; de Lera Ruiz, M.; Thompson, J.K.; Triglia, T.; Ngo, A.; Steel, R.W.J.; Vavrek, M.; Christensen, J.; Healer, J.; Boyce, C.; et al. Dual Plasmepsin-Targeting Antimalarial Agents Disrupt Multiple Stages of the Malaria Parasite Life Cycle. Cell Host Microbe 2020, 27, 642–658.e612. [Google Scholar] [CrossRef]
- Xie, S.C.; Metcalfe, R.D.; Mizutani, H.; Puhalovich, T.; Hanssen, E.; Morton, C.J.; Du, Y.; Dogovski, C.; Huang, S.C.; Ciavarri, J.; et al. Design of proteasome inhibitors with oral efficacy in vivo against Plasmodium falciparum and selectivity over the human proteasome. Proc. Natl. Acad. Sci. USA 2021, 118, e2107213118. [Google Scholar] [CrossRef]
- Ramesh, R.; Shingare, R.D.; Kumar, V.; Anand, A.B.S.; Veeraraghavan, S.; Viswanadha, S.; Ummanni, R.; Gokhale, R.; Srinivasa Reddy, D. Repurposing of a drug scaffold: Identification of novel sila analogues of rimonabant as potent antitubercular agents. Eur. J. Med. Chem. 2016, 122, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.G.; Barlaam, B.; Cadogan, E.; Campbell, A.; Chen, Y.; Colclough, N.; Davies, N.L.; de-Almeida, C.; Degorce, S.L.; Didelot, M.; et al. The Identification of Potent, Selective, and Orally Available Inhibitors of Ataxia Telangiectasia Mutated (ATM) Kinase: The Discovery of AZD0156 (8-{6-[3-(Dimethylamino)propoxy]pyridin-3-yl}-3-methyl-1-(tetrahydro-2 H-pyran-4-yl)-1,3-dihydro-2 H-imidazo[4,5- c]quinolin-2-one). J. Med. Chem. 2018, 61, 3823–3841. [Google Scholar] [CrossRef] [PubMed]
- Benetatos, C.A.; Mitsuuchi, Y.; Burns, J.M.; Neiman, E.M.; Condon, S.M.; Yu, G.; Seipel, M.E.; Kapoor, G.S.; LaPorte, M.G.; Rippin, S.R.; et al. Birinapant (TL32711), a Bivalent SMAC Mimetic, Targets TRAF2-Associated cIAPs, Abrogates TNF-Induced NF-κB Activation, and Is Active in Patient-Derived Xenograft Models. Mol. Cancer Ther. 2014, 13, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Camerini, S.; Bocedi, A.; Cecchetti, S.; Casella, M.; Carbo, M.; Morea, V.; Pozio, E.; Ricci, G.; Lalle, M. Proteomic and functional analyses reveal pleiotropic action of the anti-tumoral compound NBDHEX in Giardia duodenalis. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Dalzoppo, D.; Di Paolo, V.; Calderan, L.; Pasut, G.; Rosato, A.; Caccuri, A.M.; Quintieri, L. Thiol-Activated Anticancer Agents: The State of the Art. Anticancer. Agents Med. Chem. 2017, 17, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; De Maria, F.; Antonini, G.; Turella, P.; Bullo, A.; Stella, L.; Filomeni, G.; Federici, G.; Caccuri, A.M. 7-Nitro-2,1,3-benzoxadiazole derivatives, a new class of suicide inhibitors for glutathione S-transferases. Mechanism of action of potential anticancer drugs. J. Biol. Chem. 2005, 280, 26397–26405. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, G.; Di Paolo, V.; Rotili, D.; Migale, R.; Pedini, F.; Casella, M.; Camerini, S.; Dalzoppo, D.; Henderson, R.; Huijs, T.; et al. The Nitrobenzoxadiazole Derivative NBDHEX Behaves as Plasmodium falciparum Gametocyte Selective Inhibitor with Malaria Parasite Transmission Blocking Activity. Pharmaceuticals 2022, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Ciana, C.L.; Siegrist, R.; Aissaoui, H.; Marx, L.; Racine, S.; Meyer, S.; Binkert, C.; de Kanter, R.; Fischli, C.; Wittlin, S.; et al. Novel in vivo active anti-malarials based on a hydroxy-ethyl-amine scaffold. Bioorg. Med. Chem. Lett. 2013, 23, 658–662. [Google Scholar] [CrossRef]
- Jaudzems, K.; Tars, K.; Maurops, G.; Ivdra, N.; Otikovs, M.; Leitans, J.; Kanepe-Lapsa, I.; Domraceva, I.; Mutule, I.; Trapencieris, P.; et al. Plasmepsin inhibitory activity and structure-guided optimization of a potent hydroxyethylamine-based antimalarial hit. ACS Med. Chem. Lett. 2014, 5, 373–377. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Rajendran, V.; Singh, S.; Kumar, P.; Kumar, Y.; Singh, A.; Miller, W.; Potemkin, V.; Poonam; Grishina, M.; et al. Antiplasmodial activity of hydroxyethylamine analogs: Synthesis, biological activity and structure activity relationship of plasmepsin inhibitors. Bioorg. Med. Chem. 2018, 26, 3837–3844. [Google Scholar] [CrossRef]
- Cunico, W.; Gomes, C.R.; Moreth, M.; Manhanini, D.P.; Figueiredo, I.H.; Penido, C.; Henriques, M.G.; Varotti, F.P.; Krettli, A.U. Synthesis and antimalarial activity of hydroxyethylpiperazine derivatives. Eur. J. Med. Chem. 2009, 44, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Cheuka, P.M.; Dziwornu, G.; Okombo, J.; Chibale, K. Plasmepsin Inhibitors in Antimalarial Drug Discovery: Medicinal Chemistry and Target Validation (2000 to Present). J. Med. Chem. 2020, 63, 4445–4467. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kashif, M.; Singh, V.; Fontinha, D.; Mukherjee, B.; Kumar, D.; Singh, S.; Prudencio, M.; Singh, A.P.; Rathi, B. Novel Antiplasmodial Compounds Leveraged with Multistage Potency against the Parasite Plasmodium falciparum: In Vitro and In Vivo Evaluations and Pharmacokinetic Studies. J. Med. Chem. 2021, 64, 8666–8683. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.; Sharma, N.; Singh, S.; Romero, J.G.; Rajendran, V.; Mogire, R.M.; Kashif, M.; Beach, J.; Jeske, W.; Poonam; et al. The Multistage Antimalarial Compound Calxinin Perturbates, P. falciparum Ca(2+) Homeostasis by Targeting a Unique Ion Channel. Pharmaceutics 2022, 14, 1371. [Google Scholar] [CrossRef] [PubMed]
- Mambwe, D.; Korkor, C.M.; Mabhula, A.; Ngqumba, Z.; Cloete, C.; Kumar, M.; Barros, P.L.; Leshabane, M.; Coertzen, D.; Taylor, D.; et al. Novel 3-Trifluoromethyl-1,2,4-oxadiazole Analogues of Astemizole with Multi-stage Antiplasmodium Activity and In Vivo Efficacy in a Plasmodium berghei Mouse Malaria Infection Model. J. Med. Chem. 2022, 65, 16695–16715. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.M.; Lucantoni, L.; Chavchich, M.; Abraham, M.; De Paoli, A.; Luth, M.R.; Zeeman, A.M.; Delves, M.J.; Teran, F.S.; Straschil, U.; et al. The Novel bis-1,2,4-Triazine MIPS-0004373 Demonstrates Rapid and Potent Activity against All Blood Stages of the Malaria Parasite. Antimicrob. Agents Chemother. 2021, 65, e0031121. [Google Scholar] [CrossRef]
- Cohen, A.; Suzanne, P.; Lancelot, J.-C.; Verhaeghe, P.; Lesnard, A.; Basmaciyan, L.; Hutter, S.; Laget, M.; Dumètre, A.; Paloque, L.; et al. Discovery of new thienopyrimidinone derivatives displaying antimalarial properties toward both erythrocytic and hepatic stages of Plasmodium. Eur. J. Med. Chem. 2015, 95, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Bosson-Vanga, H.; Primas, N.; Franetich, J.F.; Lavazec, C.; Gomez, L.; Ashraf, K.; Tefit, M.; Soulard, V.; Dereuddre-Bosquet, N.; Le Grand, R.; et al. A New Thienopyrimidinone Chemotype Shows Multistage Activity against Plasmodium falciparum, Including Artemisinin-Resistant Parasites. Microbiol. Spectr. 2021, 9, e0027421. [Google Scholar] [CrossRef] [PubMed]
- Paonessa, G.; Siciliano, G.; Graziani, R.; Lalli, C.; Cecchetti, O.; Alli, C.; La Valle, R.; Petrocchi, A.; Sferrazza, A.; Bisbocci, M.; et al. Gametocyte-specific and all-blood-stage transmission-blocking chemotypes discovered from high throughput screening on Plasmodium falciparum gametocytes. Commun. Biol. 2022, 5, 547. [Google Scholar] [CrossRef]
- Spangenberg, T.; Burrows, J.N.; Kowalczyk, P.; McDonald, S.; Wells, T.N.C.; Willis, P. The Open Access Malaria Box: A Drug Discovery Catalyst for Neglected Diseases. PLoS ONE 2013, 8, e62906. [Google Scholar] [CrossRef]
- Almela, M.J.; Lozano, S.; Lelièvre, J.; Colmenarejo, G.; Coterón, J.M.; Rodrigues, J.; Gonzalez, C.; Herreros, E. A New Set of Chemical Starting Points with Plasmodium falciparum Transmission-Blocking Potential for Antimalarial Drug Discovery. PLoS ONE 2015, 10, e0135139. [Google Scholar] [CrossRef] [PubMed]
- Nardella, F.; Dobrescu, I.; Hassan, H.; Rodrigues, F.; Thiberge, S.; Mancio-Silva, L.; Tafit, A.; Jallet, C.; Cadet-Daniel, V.; Goussin, S.; et al. Hemisynthetic alkaloids derived from trilobine are antimalarials with sustained activity in multidrug-resistant Plasmodium falciparum. iScience 2023, 26, 105940. [Google Scholar] [CrossRef]
- MacRae, J.I.; Dixon, M.W.; Dearnley, M.K.; Chua, H.H.; Chambers, J.M.; Kenny, S.; Bottova, I.; Tilley, L.; McConville, M.J. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 2013, 11, 67. [Google Scholar] [CrossRef]
- Evers, F.; Cabrera-Orefice, A.; Elurbe, D.M.; Kea-Te Lindert, M.; Boltryk, S.D.; Voss, T.S.; Huynen, M.A.; Brandt, U.; Kooij, T.W.A. Composition and stage dynamics of mitochondrial complexes in Plasmodium falciparum. Nat. Commun. 2021, 12, 3820. [Google Scholar] [CrossRef]
- Hino, A.; Hirai, M.; Tanaka, T.Q.; Watanabe, Y.; Matsuoka, H.; Kita, K. Critical roles of the mitochondrial complex II in oocyst formation of rodent malaria parasite Plasmodium berghei. J. Biochem. 2012, 152, 259–268. [Google Scholar] [CrossRef]
- Ke, H.; Lewis, I.A.; Morrisey, J.M.; McLean, K.J.; Ganesan, S.M.; Painter, H.J.; Mather, M.W.; Jacobs-Lorena, M.; Llinas, M.; Vaidya, A.B. Genetic investigation of tricarboxylic acid metabolism during the Plasmodium falciparum life cycle. Cell Rep. 2015, 11, 164–174. [Google Scholar] [CrossRef]
- Sturm, A.; Mollard, V.; Cozijnsen, A.; Goodman, C.D.; McFadden, G.I. Mitochondrial ATP synthase is dispensable in blood-stage Plasmodium berghei rodent malaria but essential in the mosquito phase. Proc. Natl. Acad. Sci. USA 2015, 112, 10216–10223. [Google Scholar] [CrossRef] [PubMed]
- Boysen, K.E.; Matuschewski, K. Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase. J. Biol. Chem. 2011, 286, 32661–32671. [Google Scholar] [CrossRef] [PubMed]
- Fowler, R.E.; Billingsley, P.F.; Pudney, M.; Sinden, R.E. Inhibitory action of the anti-malarial compound atovaquone (566C80) against Plasmodium berghei ANKA in the mosquito, Anopheles stephensi. Parasitology 1994, 108 Pt 4, 383–388. [Google Scholar] [CrossRef]
- Mariebernard, M.; Mohanty, A.; Rajendran, V. A comprehensive review on classifying fast-acting and slow-acting antimalarial agents based on time of action and target organelle of Plasmodium sp. Pathog. Dis. 2022, 80, ftac015. [Google Scholar] [CrossRef]
- Benoit-Vical, F.; Lelièvre, J.; Berry, A.; Deymier, C.; Dechy-Cabaret, O.; Cazelles, J.; Loup, C.; Robert, A.; Magnaval, J.F.; Meunier, B. Trioxaquines are new antimalarial agents active on all erythrocytic forms, including gametocytes. Antimicrob. Agents Chemother. 2007, 51, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Blanco, C.; Lelièvre, J.; Delves, M.J.; Bardera, A.I.; Presa, J.L.; López-Barragán, M.J.; Ruecker, A.; Marques, S.; Sinden, R.E.; Herreros, E. Imaging-based high-throughput screening assay to identify new molecules with transmission-blocking potential against Plasmodium falciparum female gamete formation. Antimicrob. Agents Chemother. 2015, 59, 3298–3305. [Google Scholar] [CrossRef] [PubMed]
- Lelièvre, J.; Almela, M.J.; Lozano, S.; Miguel, C.; Franco, V.; Leroy, D.; Herreros, E. Activity of Clinically Relevant Antimalarial Drugs on Plasmodium falciparum Mature Gametocytes in an ATP Bioluminescence “Transmission Blocking” Assay. PLoS ONE 2012, 7, e35019. [Google Scholar] [CrossRef]
- Paton, D.G.; Childs, L.M.; Itoe, M.A.; Holmdahl, I.E.; Buckee, C.O.; Catteruccia, F. Exposing Anopheles mosquitoes to antimalarials blocks Plasmodium parasite transmission. Nature 2019, 567, 239–243. [Google Scholar] [CrossRef]
- Goodman, C.D.; Siregar, J.E.; Mollard, V.; Vega-Rodriguez, J.; Syafruddin, D.; Matsuoka, H.; Matsuzaki, M.; Toyama, T.; Sturm, A.; Cozijnsen, A.; et al. Parasites resistant to the antimalarial atovaquone fail to transmit by mosquitoes. Science 2016, 352, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Blake, L.D.; Johnson, M.E.; Siegel, S.V.; McQueen, A.; Iyamu, I.D.; Shaikh, A.K.; Shultis, M.W.; Manetsch, R.; Kyle, D.E. Menoctone Resistance in Malaria Parasites Is Conferred by M133I Mutations in Cytochrome b That Are Transmissible through Mosquitoes. Antimicrob. Agents Chemother. 2017, 61, e00689-17. [Google Scholar] [CrossRef]
- Calit, J.; Araujo, J.E.; Deng, B.; Miura, K.; Gaitan, X.A.; Araujo, M.D.S.; Medeiros, J.F.; Long, C.A.; Simeonov, A.; Eastman, R.T.; et al. Novel Transmission-Blocking Antimalarials Identified by High-Throughput Screening of Plasmodium berghei Ookluc. Antimicrob. Agents Chemother. 2023, 67, e0146522. [Google Scholar] [CrossRef]
- Kamiya, T.; Paton, D.G.; Catteruccia, F.; Reece, S.E. Targeting malaria parasites inside mosquitoes: Ecoevolutionary consequences. Trends Parasitol. 2022, 38, 1031–1040. [Google Scholar] [CrossRef]





| Compound | Structure | Target Class | Asexual IC50 ± SEM (nM) | Stage I–II Gametocyte IC50 ± SEM (nM) | Stage IV–V Gametocyte IC50 ± SEM (nM) |
|---|---|---|---|---|---|
| SGI-1027 8 | 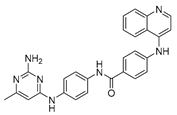 | DNMT | 50.8 ± 0.6 | 18.2 ± 2.1 | 322.4 ± 123.7 |
| Chaetocin 9 |  | HMT | 775.3 ± 366.0 | 292.3 ± 25.7 | 504.5 ± 92.3 |
| BIX01294 10a | 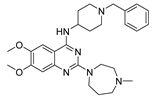 | HMT | 10.5 ± 3.6 | 12.3 ± 1.3 | 939.0 ± 84.7 |
| UNC0631 10b | 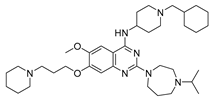 | HMT | 28.5 ± 5.9 | 14.8 ± 0.9 | 641.2 ± 83.0 |
| UNC0642 10c |  | HMT | 19.2 ± 10.4 | 14.6 ± 0.8 | 929.6 ± 199.0 |
| UNC0379 10d |  | HMT | 50.4 ± 2.3 | 21.3 ± 4.8 | >1000 |
| UNC0638 10e | 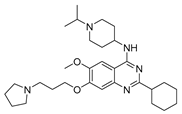 | HMT | 21.6 ± 2.0 | 16.4 ± 1.0 | >1000 |
| UNC0646 10f | 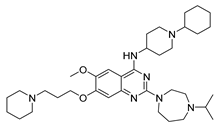 | HMT | 140.1 ± 3.8 | 66.8 ± 22.6 | >1000 |
| JIB-04 11a | 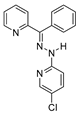 | KDM | 470.5 ± 28.3 | 133.1 ± 18.5 | 262.5 ± 113.0 |
| Quisinostat 12a |  | HDAC | <13 | <13 | 148.1 ± 145.8 |
| Panobinostat 13 | 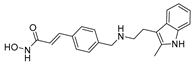 | HDAC | 8.7 ± 3.8 | 12.0 ± 4.8 | 515.3 ± 144.7 |
| Apicidin 14 | 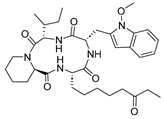 | HDAC | 23.1 ± 15.2 | 103.6 ± 2.9 | 590.2 ± 146.6 |
| HC Toxin 15 | 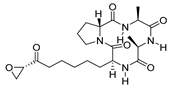 | HDAC | 15.1 ± 3.7 | 30.2 ± 0.1 | 351.4 ± 221.3 |
| CUDC-101 16 | 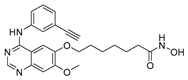 | HDAC | 35.6 ± 8.4 | 133.1 ± 6.3 | 2150.4 ± 744.3 |
| Trichostatin A 17 |  | HDAC | 62.3 ± 21.1 | 53.9 ± 5.4 | 3795.5 ± 1576.3 |
| Dacinostat 18 | 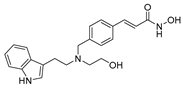 | HDAC | 40.8 ± 19.1 | 45.3 ± 0.9 | 2266.1 ± 843.2 |
| Fedratinib 19 | 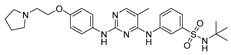 | Kinase | 66.9 ± 2.8 | 96.9 ± 19.7 | >1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appetecchia, F.; Fabbrizi, E.; Fiorentino, F.; Consalvi, S.; Biava, M.; Poce, G.; Rotili, D. Transmission-Blocking Strategies for Malaria Eradication: Recent Advances in Small-Molecule Drug Development. Pharmaceuticals 2024, 17, 962. https://doi.org/10.3390/ph17070962
Appetecchia F, Fabbrizi E, Fiorentino F, Consalvi S, Biava M, Poce G, Rotili D. Transmission-Blocking Strategies for Malaria Eradication: Recent Advances in Small-Molecule Drug Development. Pharmaceuticals. 2024; 17(7):962. https://doi.org/10.3390/ph17070962
Chicago/Turabian StyleAppetecchia, Federico, Emanuele Fabbrizi, Francesco Fiorentino, Sara Consalvi, Mariangela Biava, Giovanna Poce, and Dante Rotili. 2024. "Transmission-Blocking Strategies for Malaria Eradication: Recent Advances in Small-Molecule Drug Development" Pharmaceuticals 17, no. 7: 962. https://doi.org/10.3390/ph17070962
APA StyleAppetecchia, F., Fabbrizi, E., Fiorentino, F., Consalvi, S., Biava, M., Poce, G., & Rotili, D. (2024). Transmission-Blocking Strategies for Malaria Eradication: Recent Advances in Small-Molecule Drug Development. Pharmaceuticals, 17(7), 962. https://doi.org/10.3390/ph17070962









