Photodynamic Therapy in the Treatment of Cancer—The Selection of Synthetic Photosensitizers
Abstract
1. Introduction
2. Application of Photodynamic Therapy in Cancer Therapy
3. Photosensitizers in PDT
4. Second Generation of Photosensitizers
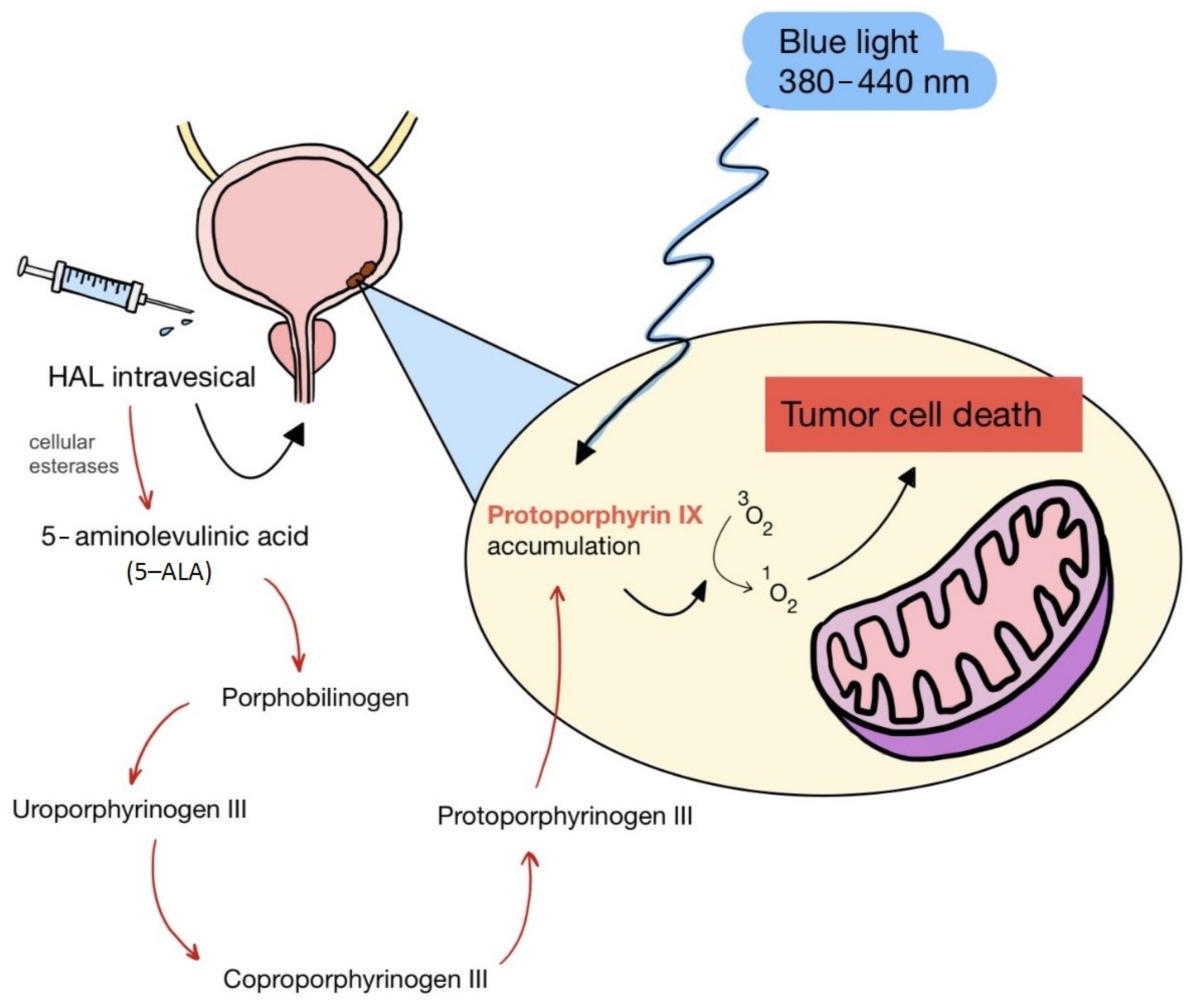
5. Second Generation of Photosensitizers—5-ALA and HAL
6. Second-Generation Photosensitizers—Porphyrin Derivatives: Examples from the Literature
7. Other Second-Generation Photosensitizers with Absorption Peaks in the Near-Infrared Range (NIR)
8. Third Generation of Photosensitizers
9. Evaluation of the Efficacy of Third-Generation Photosensitizers—Examples from the Literature
10. Third Generation of Photosensitizers—Antibody–Drug Conjugates (ADCs)
11. Heavy-Atom-Free Nonporphyrinoid Photosensitizers—Group Description
12. BODIPY
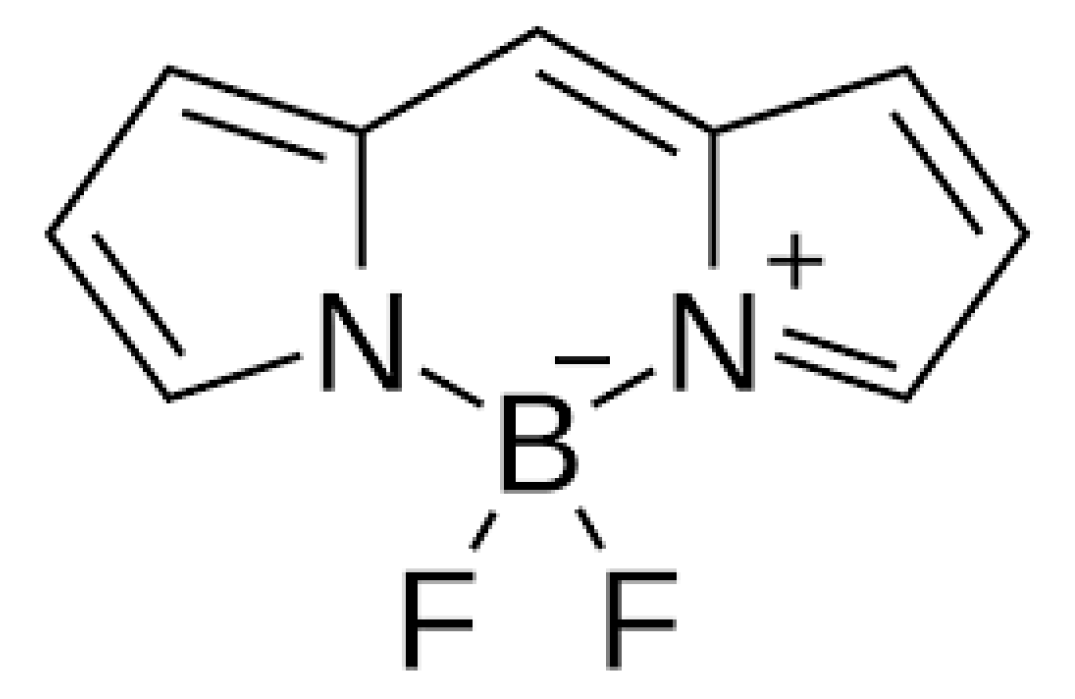
13. Heavy-Atom-Free Nonporphyrinoid Photosensitizers—Literature Examples
14. The Potential Problems of PDT
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, T.E.; Chang, J.E. Recent Studies in Photodynamic Therapy for Cancer Treatment: From Basic Research to Clinical Trials. Pharmaceutics 2023, 15, 2257. [Google Scholar] [CrossRef]
- Costa, L.D.; e Silva Jde, A.; Fonseca, S.M.; Arranja, C.T.; Urbano, A.M.; Sobral, A.J. Photophysical Characterization and in Vitro Phototoxicity Evaluation of 5,10,15,20-Tetra(quinolin-2-yl)porphyrin as a Potential Sensitizer for Photodynamic Therapy. Molecules 2016, 21, 439. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Phull, A.R.; Kim, S.J.; Hong, S.K.; Lee, K.H. Synthesis, Photophysical Properties and Application of New Porphyrin Derivatives for Use in Photodynamic Therapy and Cell Imaging. J. Fluoresc. 2018, 28, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Shi, Y.; Xie, L.; Zhang, K.; Wang, X.; Liu, Q.; Wang, P. Synthesis, Characterization, and Biological Evaluation of a Porphyrin-Based Photosensitizer and Its Isomer for Effective Photodynamic Therapy against Breast Cancer. J. Med. Chem. 2018, 61, 7189–7201. [Google Scholar] [CrossRef]
- Pushpan, S.K.; Venkatraman, S.; Anand, V.G.; Sankar, J.; Parmeswaran, D.; Ganesan, S.; Chandrashekar, T.K. Porphyrins in photodynamic therapy—A search for ideal photosensitizers. Current medicinal chemistry. Anti-Cancer Agents 2002, 2, 187–207. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer. Acc. Chem. Res. 2021, 54, 207–220. [Google Scholar] [CrossRef]
- Fingar, V.H.; Wieman, T.J.; Haydon, P.S. The effects of thrombocytopenia on vessel stasis and macromolecular leakage after photodynamic therapy using photofrin. Photochem. Photobiol. 1997, 66, 513–517. [Google Scholar] [CrossRef]
- Tromberg, B.J.; Orenstein, A.; Kimel, S.; Barker, S.J.; Hyatt, J.; Nelson, J.S.; Berns, M.W. In vivo tumor oxygen tension measurements for the evaluation of the efficiency of photodynamic therapy. Photochem. Photobiol. 1990, 52, 375–385. [Google Scholar] [CrossRef] [PubMed]
- de Vree, W.J.; Essers, M.C.; de Bruijn, H.S.; Star, W.M.; Koster, J.F.; Sluiter, W. Evidence for an important role of neutrophils in the efficacy of photodynamic therapy in vivo. Cancer Res. 1996, 56, 2908–2911. [Google Scholar]
- Dolmans, D.E.; Kadambi, A.; Hill, J.S.; Waters, C.A.; Robinson, B.C.; Walker, J.P.; Fukumura, D.; Jain, R.K. Vascular accumulation of a novel photosensitizer, MV6401, causes selective thrombosis in tumor vessels after photodynamic therapy. Cancer Res. 2002, 62, 2151–2156. [Google Scholar]
- Korbelik, M.; Krosl, G. Cellular levels of photosensitisers in tumours: The role of proximity to the blood supply. Br. J. Cancer 1994, 70, 604–610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Krysko, D.V. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Dis. 2022, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, N.; Samadani, A.A. Implications of photodynamic cancer therapy: An overview of PDT mechanisms basically and practically. J. Egypt. Natl. Cancer Inst. 2021, 33, 34. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cance. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Bartusik-Aebisher, D.; Żołyniak, A.; Barnaś, E.; Machorowska-Pieniążek, A.; Oleś, P.; Kawczyk-Krupka, A.; Aebisher, D. The Use of Photodynamic Therapy in the Treatment of Brain Tumors-A Review of the Literature. Molecules 2022, 27, 6847. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, T.; Karakuła, M.; Czop, M.; Kawczyk-Krupka, A.; Aebisher, D. Advances in Management of Bladder Cancer-The Role of Photodynamic Therapy. Molecules 2022, 27, 731. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Palasuberniam, P.; Myers, K.A.; Wang, C.; Chen, B. Effects of Silencing Heme Biosynthesis Enzymes on 5-Aminolevulinic Acid-mediated Protoporphyrin IX Fluorescence and Photodynamic Therapy. Photochem. Photobiol. 2015, 91, 923–930. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Garvey, K.L.; Bouras, A.; Cramer, G.; Stepp, H.; Jesu Raj, J.G.; Bozec, D.; Busch, T.M.; Hadjipanayis, C.G. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J. Neurooncol. 2019, 141, 595–607. [Google Scholar] [CrossRef]
- Eljamel, M.S.; Goodman, C.; Moseley, H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre Phase III randomised controlled trial. Lasers Med. Sci. 2008, 23, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Lamy, L.; Thomas, J.; Leroux, A.; Bisson, J.F.; Myren, K.; Godal, A.; Stensrud, G.; Bezdetnaya, L. Antitumor Effect and Induced Immune Response Following Exposure of Hexaminolevulinate and Blue Light in Combination with Checkpoint Inhibitor in an Orthotopic Model of Rat Bladder Cancer. Biomedicines 2022, 10, 548. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy. Photodiag. Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiag. Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef]
- Narumi, A.; Tsuji, T.; Shinohara, K.; Yamazaki, H.; Kikuchi, M.; Kawaguchi, S.; Mae, T.; Ikeda, A.; Sakai, Y.; Kataoka, H.; et al. Maltotriose-conjugation to a fluorinated chlorin derivative generating a PDT photosensitizer with improved water-solubility. Org. Biomol. Chem. 2016, 14, 3608–3613. [Google Scholar] [CrossRef]
- Waite, C.L.; Roth, C.M. Nanoscale drug delivery systems for enhanced drug penetration into solid tumors: Current progress and opportunities. Crit. Rev. Biomed. Eng. 2012, 40, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Krishnaswami, V.; Ponnusamy, C.; Madiyalakan, M.; Woo, T.; Palanisamy, R. Hypocrellin B and nano silver loaded polymeric nanoparticles: Enhanced generation of singlet oxygen for improved photodynamic therapy. Mater. Sci. Eng. C 2017, 77, 935–946. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Yu, X.T.; Sui, S.Y.; He, Y.X.; Yu, C.H.; Peng, Q. Nanomaterials-based photosensitizers and delivery systems for photodynamic cancer therapy. Biomater. Adv. 2022, 135, 212725. [Google Scholar] [CrossRef]
- Gierlich, P.; Mata, A.I.; Donohoe, C.; Brito, R.M.M.; Senge, M.O.; Gomes-da-Silva, L.C. Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment. Molecules 2020, 25, 5317. [Google Scholar] [CrossRef]
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, Z.; Li, W.; Wu, X.; Jiang, X.; Li, G.; Cao, L.; Zhang, D.; Wang, Q.; Xue, P.; et al. Photodynamic immunotherapy of cancers based on nanotechnology: Recent advances and future challenges. J. Nanobiotechnol. 2021, 19, 160. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S. Nanomaterials in tumor immunotherapy: New strategies and challenges. Mol. Cancer 2023, 22, 94. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Choi, W.; Jangili, P.; Ge, Y.; Xu, Y.; Kang, J.; Liu, L.; Zhang, B.; Xie, Z.; et al. Overcoming barriers in photodynamic therapy harnessing nano-formulation strategies. Chem. Soc. Rev. 2021, 50, 9152–9201. [Google Scholar] [CrossRef]
- Awuah, S.G.; You, Y. Boron Dipyrromethene (BODIPY)-based Photosensitizers for Photodynamic therapy. RSC Adv. 2012, 2, 11169–11183. [Google Scholar] [CrossRef]
- Malacarne, M.C.; Gariboldi, M.B.; Caruso, E. BODIPYs in PDT: A Journey through the Most Interesting Molecules Produced in the Last 10 Years. Int. J. Mol. Sci. 2022, 23, 10198. [Google Scholar] [CrossRef]
- Pollum, M.; Jockusch, S.; Crespo-Hernández, C.E. 2,4-Dithiothymine as a Potent UVAChemotherapeutic Agent. J. Am. Chem. Soc. 2014, 136, 17930–17933. [Google Scholar] [CrossRef]
- Mai, S.; Pollum, M.; Martínez-Fernández, L.; Dunn, N.; Marquetand, P.; Corral, I.; Crespo-Hernández, C.E.; González, L. The Origin of Efficient Triplet State Population in Sulfur-Substituted Nucleobases. Nat. Commun. 2016, 7, 13077. [Google Scholar] [CrossRef]
- Tang, J.; Wang, L.; Loredo, A.; Cole, C.; Xiao, H. Single-Atom Replacement as a General Approach Towards Visible-Light/Near-Infrared Heavy-Atom-Free Photosensitizers for Photodynamic Therapy. Chem. Sci. 2020, 11, 6701–6708. [Google Scholar] [CrossRef]
- Pham, T.C.; Hoang, T.T.H.; Choi, Y.; Lee, S.; Joo, S.W.; Kim, G.; Kim, D.; Jung, O.S.; Lee, S. Dual Molecular Design toward a Lysosome-Tagged AIEgen and Heavy-Atom-Free Photosensitizers for Hypoxic Cancer Photodynamic Therapy. Biosensors 2022, 12, 420. [Google Scholar] [CrossRef]
- Alberto, M.E.; De Simone, B.C.; Marino, T.; Toscano, M.; Russo, N. Chalcogen Effects in the Photophysical Properties of Dimethylamino-1,8-naphthalimide Dyes Revealed by DFT Investigation. J. Phys. Chem. A 2022, 126, 5167–5172. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, R.; Zhang, X.F.; Liu, J.; Luo, L. Halogenated BODIPY photosensitizers: Photophysical processes for generation of excited triplet state, excited singlet state and singlet oxygen. Spectrochimica acta. Part A Mol. Biomol. Spectrosc. 2022, 272, 120965. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, T.; Mao, C. Enhancement of Photodynamic Cancer Therapy by Physical and Chemical Factors. Angew. Chem. 2019, 58, 14066–14080. (In English) [Google Scholar] [CrossRef]
- Gutman, R.L. Targeted drug delivery for brain cancer treatment. J. Control. Release 2000, 65, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Yang, X.X.; Liu, R.Q.; Zhao, D.; Guo, C.X.; Zhu, A.C.; Wen, M.N.; Liu, Z.; Qu, G.F.; Meng, H.X. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef] [PubMed]
- Quirk, B.J.; Brandal, G.; Donlon, S.; Vera, J.C.; Mang, T.S.; Foy, A.B.; Lew, S.M.; Girotti, A.W.; Jogal, S.; LaViolette, P.S.; et al. Photodynamic therapy (PDT) for malignant brain tumors—Where do we stand? Photodiagnosis Photodyn Ther. 2015, 12, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Gomer, C.J.; Razum, N.J. Acute skin response in albino mice following porphyrin photosensitization under oxic and anoxic conditions. Photochem. Photobiol. 1984, 40, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Pass, H.I. Photodynamic therapy in oncology: Mechanisms and clinical use. J. Natl. Cancer Inst. 1993, 85, 443–456. [Google Scholar] [CrossRef]
- Allison, R.R.; Bagnato, V.S.; Cuenca, R.; Downie, G.H.; Sibata, C.H. The future of photodynamic therapy in oncology. Future Oncol. 2006, 2, 53–71. [Google Scholar] [CrossRef]
- Vrouenraets, M.B.; Visser, G.W.; Snow, G.B.; van Dongen, G.A. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003, 23, 505–522. [Google Scholar] [PubMed]
- Luksiene, Z. Photodynamic therapy: Mechanism of action and ways to improve the efficiency of treatment. Medicina 2003, 39, 1137–1150. [Google Scholar] [PubMed]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy: Novel third-generation photosensitizers one step close? Br. J. Pharmacol. 2008, 154, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.M.F.; Bernegossi, J.; de Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wang, L.; Tang, H.; Cao, D. Recent advances in type I organic photosensitizers for efficient photodynamic therapy for overcoming tumor hypoxia. J. Mater. Chem. B 2023, 11, 4600–4618. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3086257, Photofrin II. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Photofrin-II (accessed on 21 March 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 123608, Aminolevulinic Acid Hydrochloride. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Aminolevulinic-Acid-Hydrochloride (accessed on 21 March 2024).
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 137, Aminolevulinic Acid. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/137 (accessed on 21 March 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6433083, Hexaminolevulinate. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hexaminolevulinate (accessed on 21 March 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 157922, Methyl Aminolevulinate. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/157922 (accessed on 21 March 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 60751, Temoporfin. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/60751 (accessed on 21 March 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5488036, Talaporfin sodium. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Talaporfin-sodium (accessed on 21 March 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 86287614, Redaporfin. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Redaporfin (accessed on 21 March 2024).
- Chen, Z.; Liu, L.; Liang, R.; Luo, Z.; He, H.; Wu, Z.; Tian, H.; Zheng, M.; Ma, Y.; Cai, L. Bioinspired Hybrid Protein Oxygen Nanocarrier Amplified Photodynamic Therapy for Eliciting Anti-tumor Immunity and Abscopal Effect. ACS Nano 2018, 12, 8633–8645. [Google Scholar] [CrossRef]
- Wang, D.; Wang, T.; Liu, J.; Yu, H.; Jiao, S.; Feng, B.; Zhou, F.; Fu, Y.; Yin, Q.; Zhang, P.; et al. Acid-Activatable Versatile Micelleplexes for PD-L1 Blockade-Enhanced Cancer Photodynamic Immunotherapy. Nano Lett. 2016, 16, 5503–5513. [Google Scholar] [CrossRef]
- Ai, X.; Hu, M.; Wang, Z.; Lyu, L.; Zhang, W.; Li, J.; Yang, H.; Lin, J.; Xing, B. Enhanced Cellular Ablation by Attenuating Hypoxia Status and Reprogramming Tumor-Associated Macrophages via NIR Light-Responsive Upconversion Nanocrystals. Bioconjugate Chem. 2018, 29, 928–938. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Lin, W. Nanoscale metal-organic framework for highly effective photodynamic therapy of resistant head and neck cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715. [Google Scholar] [CrossRef] [PubMed]
- Pucelik, B.; Arnaut, L.G.; Stochel, G.; Dąbrowski, J.M. Design of Pluronic-Based Formulation for Enhanced Redaporfin-Photodynamic Therapy against Pigmented Melanoma. ACS Appl. Mater. Interfaces 2016, 8, 22039–22055. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, L.E.; Beaugé, L.; Arias-Ramos, N.; Rivarola, V.A.; Chesta, C.A.; López-Larrubia, P.; Palacios, R.E. Trojan horse monocyte-mediated delivery of conjugated polymer nanoparticles for improved photodynamic therapy of glioblastoma. Nanomedicine 2020, 15, 1687–1707. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.I.; Pereira, N.A.M.; Cardoso, A.L.; Nascimento, B.F.O.; Pineiro, M.; Schaberle, F.A.; Gomes-da-Silva, L.C.; Brito, R.M.M.; Pinho, E.; Melo, T.M.V.D. Novel Foscan®-derived ring-fused chlorins for photodynamic therapy of cancer. Bioorganic Med. Chem. 2023, 93, 117443. [Google Scholar] [CrossRef] [PubMed]
- Fatima, D.; Leger, D.Y.; Diab-Assaf, M.; Sol, V.; Liagre, B. Porphyrin/Chlorin Derivatives as Promising Molecules for Therapy of Colorectal Cancer. Molecules 2021, 26, 7268. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin e6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Linares, I.A.P.; Martinelli, L.P.; Moritz, M.; Selistre-de-Araujo, H.; de Oliveira, K.T.; Perussi, J.R. Cytotoxicity of structurally-modified chlorins aimed for photodynamic therapy applications. J. Photochem. Photobiol. A Chem. 2022, 425, 113647. [Google Scholar] [CrossRef]
- Chandra, B.; Soman, R.; Sathish Kumar, B.; Jose, K.V.J.; Panda, P.K. Meso-Free Boron(III)subchlorin and Its μ-Oxo Dimer with Interacting Chromophores. Org Lett. 2020, 22, 9735–9739. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5489033, Chlorin p6. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chlorin-p6 (accessed on 24 March 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6683, Purpurin. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Purpurin (accessed on 24 March 2024).
- National Center for Biotechnology Information. PubChem Substance Record for SID 482115541, Photochlor, Source: Probes & Drugs portal. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hpph (accessed on 24 March 2024).
- Rahman, K.M.M.; Giram, P.; Foster, B.A.; You, Y. Photodynamic Therapy for Bladder Cancers, A Focused Review. Photochem. Photobiol. 2023, 99, 420–436. [Google Scholar] [CrossRef]
- Sternberg, E.D.; Dolphin, D. Second generation photodynamic agents: A review. J. Clin. Laser Med. Surg. 1993, 11, 233–241. [Google Scholar] [CrossRef]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [PubMed]
- Drăgoescu, O.; Tomescu, P.; Pănuş, A.; Enache, M.; Maria, C.; Stoica, L.; Pleşea, I.E. Photodynamic diagnosis of non-muscle invasive bladder cancer using hexaminolevulinic acid. Rom. J. Morphol. Embryol. 2011, 52, 123–127. [Google Scholar] [PubMed]
- Simões, J.C.S.; Sarpaki, S.; Papadimitroulas, P.; Therrien, B.; Loudos, G. Conjugated Photosensitizers for Imaging and PDT in Cancer Research. J. Med. Chem. 2020, 63, 14119–14150. [Google Scholar] [CrossRef] [PubMed]
- Kogan, E.A.; Meerovich, G.A.; Karshieva, S.S.; Makarova, E.A.; Romanishkin, I.D.; Akhlyustina, E.V.; Meerovich, I.G.; Zharkov, N.V.; Koudan, E.V.; Demura, T.A.; et al. Photodynamic therapy of lung cancer with photosensitizers based on polycationic derivatives of synthetic bacteriochlorin (experimental study). Photodiag. Photodyn. Ther. 2023, 42, 103647. [Google Scholar] [CrossRef] [PubMed]
- Boscencu, R.; Manda, G.; Radulea, N.; Socoteanu, R.P.; Ceafalan, L.C.; Neagoe, I.V.; Ferreira Machado, I.; Basaga, S.H.; Vieira Ferreira, L.F. Studies on the Synthesis, Photophysical and Biological Evaluation of Some Unsymmetrical Meso-Tetrasubstituted Phenyl Porphyrins. Molecules 2017, 22, 1815. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, H.; Sun, Y.; Zu, G.; Zhang, X.; Song, Y.; Zhao, F.; Wang, J. New porphyrin photosensitizers-Synthesis, singlet oxygen yield, photophysical properties and application in PDT. Spectrochimica acta. Part A Mol. Biomol. Spectrosc. 2022, 279, 121447. [Google Scholar] [CrossRef] [PubMed]
- Laville, I.; Pigaglio, S.; Blais, J.C.; Doz, F.; Loock, B.; Maillard, P.; Grierson, D.S.; Blais, J. Photodynamic efficiency of diethylene glycol-linked glycoconjugated porphyrins in human retinoblastoma cells. J. Med. Chem. 2006, 49, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Dereje, D.M.; Pontremoli, C.; Moran Plata, M.J.; Visentin, S.; Barbero, N. Polymethine dyes for PDT: Recent advances and perspectives to drive future applications. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2022, 21, 397–419. [Google Scholar] [CrossRef]
- Nicola, Z.; Capozzi, M.A.M.; Porcheddu, A.; Farinola, G.M.; Punzi, A. Solvent-free Reactions for the Synthesis of Indolenine-based Squaraines and Croconaines: Comparison of Thermal Heating, Mechanochemical Milling, and IR Irradiation. ChemSusChem 2021, 14, 1363–1369. [Google Scholar]
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjugate Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef]
- Lange, N.; Szlasa, W.; Saczko, J.; Chwiłkowska, A. Potential of Cyanine Derived Dyes in Photodynamic Therapy. Pharmaceutics 2021, 13, 818. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, Y.; Blum, N.T.; Huang, P.; Lin, J. Recent Advances in Croconaine Dyes for Bioimaging and Theranostics. Bioconjugate Chem. 2020, 31, 2072–2084. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Woźnicki, P.; Dynarowicz, K.; Aebisher, D. Photosensitizers for Photodynamic Therapy of Brain Cancers—A Review. Brain Sci. 2023, 13, 1299. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, F.; Fan, W.; Li, L.; Yang, Z. Editorial: Synthesis of novel photosensitizers for cancer theranostics. Front. Chem. 2023, 11, 1188243. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Dong, X.; Li, B. Nano-photosensitizers for enhanced photodynamic therapy. Photodiag. Photodyn. Therapy. 2021, 36, 102597. [Google Scholar] [CrossRef]
- Kim, J.; Jo, Y.U.; Na, K. Photodynamic therapy with smart nanomedicine. Arch. Pharmacal Res. 2020, 43, 22–31. [Google Scholar] [CrossRef]
- Cui, S.; Yin, D.; Chen, Y.; Di, Y.; Chen, H.; Ma, Y.; Achilefu, S.; Gu, Y. In vivo targeted deep-tissue photodynamic therapy based on near-infrared light triggered upconversion nanoconstruct. ACS Nano 2013, 7, 676–688. [Google Scholar] [CrossRef]
- Sandland, J.; Boyle, R.W. Photosensitizer Antibody-Drug Conjugates: Past, Present, and Future. Bioconjugate Chem. 2019, 30, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cheng, J.; Xu, J.; Ruf, W.; Lockwood, C.J. Tissue factor is an angiogenic-specific receptor for factor VII-targeted immunotherapy and photodynamic therapy. Angiogenesis 2017, 20, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, J.; Khirehgesh, M.R.; Safari, F.; Akbari, B. EGFR and anti-EGFR nanobodies: Review and update. J. Drug Target. 2021, 29, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Jin, C.S.; Lee, D.; Ujiie, H.; Fujino, K.; Hu, H.P.; Wada, H.; Wu, L.; Chen, J.; Weersink, R.A.; et al. Preclinical investigation of folate receptor-targeted nanoparticles for photodynamic therapy of malignant pleural mesothelioma. Int. J. Oncol. 2018, 53, 2034–2046. [Google Scholar]
- Jin, G.; He, R.; Liu, Q.; Dong, Y.; Lin, M.; Li, W.; Xu, F. Theranostics of Triple-Negative Breast Cancer Based on Conjugated Polymer Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 10634–10646. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, N.; Mohammadnejad, F.; Doustvandi, M.A.; Shadbad, M.A.; Amini, M.; Tajalli, H.; Mokhtarzadeh, A.; Baghbani, E.; Silvestris, N.; Baradaran, B. Photodynamic therapy with zinc phthalocyanine enhances the anti-cancer effect of tamoxifen in breast cancer cell line: Promising combination treatment against triple-negative breast cancer? Photodiag. Photodyn. Ther. 2023, 41, 103212. [Google Scholar] [CrossRef] [PubMed]
- Salvador, G.; Tsung, A.; Hu, Z. Current Targets and Bioconjugation Strategies in Photodynamic Diagnosis and Therapy of Cancer. Molecules 2020, 25, 4964. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Mackman, N. Tissue Factor and Cancer: Regulation, Tumor Growth, and Metastasis. Semin. Thromb. Hemost. 2019, 45, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Rondon, A.M.R.; Kroone, C.; Kapteijn, M.Y.; Versteeg, H.H.; Buijs, J.T. Role of Tissue Factor in Tumor Progression and Cancer-Associated Thrombosis. Semin. Thromb. Hemost. 2019, 45, 396–412. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, 1900132. [Google Scholar] [CrossRef]
- Buglak, A.A.; Charisiadis, A.; Sheehan, A.; Kingsbury, C.J.; Senge, M.O.; Filatov, M.A. Quantitative Structure-Property Relationship Modelling for the Prediction of Singlet Oxygen Generation by Heavy-Atom-Free BODIPY Photosensitizers. Chemistry 2021, 27, 9934–9947. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef] [PubMed]
- Bröring, M.; Krüger, R.; Link, S.; Kleeberg, C.; Köhler, S.; Xie, X.; Ventura, B.; Flamigni, L. Bis(BF2)-2,2′-bidipyrrins (BisBODIPYs): Highly fluorescent BODIPY dimers with large stokes shifts. Chemistry 2008, 14, 2976–2983. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, Y.; Kolemen, S.; Duman, S.; Dede, Y.; Dolen, Y.; Kilic, B.; Kostereli, Z.; Yildirim, L.T.; Dogan, A.L.; Guc, D.; et al. Designing excited states: Theory-guided access to efficient photosensitizers for photodynamic action. Angew. Chem. 2011, 50, 11937–11941. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, L.; Yan, Y.; El-Zohry, A.M.; Toffoletti, A.; Zhao, J.; Barbon, A.; Dick, B.; Mohammed, O.F.; Han, G. Elucidation of the Intersystem Crossing Mechanism in a Helical BODIPY for Low-Dose Photodynamic Therapy. Angew. Chem. 2020, 59, 16114–16121. (In English) [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, D.; John, A.T.; Sunny, J.; Hariharan, M. Access to the triplet excited states of organic chromophores. Chem. Soc. Rev. 2020, 49, 6122–6140. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Zhou, Y.; Tohnai, N.; Nakatsuji, H.; Matsusaki, M.; Fujitsuka, M.; Miyata, M.; Majima, T. Aggregation-Induced Singlet Oxygen Generation: Functional Fluorophore and Anthrylphenylene Dyad Self-Assemblies. Chemistry 2018, 24, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Niu, L.Y.; Chen, P.Z.; Chen, Y.Z.; Yang, Q.Z.; Boulatov, R. Ratiometric O2 sensing based on selective self-sensitized photooxidation of donor-acceptor fluorophores. Chem. Commun. 2019, 55, 7017–7020. [Google Scholar] [CrossRef] [PubMed]
- Sartor, S.M.; McCarthy, B.G.; Pearson, R.M.; Miyake, G.M.; Damrauer, N.H. Exploiting Charge-Transfer States for Maximizing Intersystem Crossing Yields in Organic Photoredox Catalysts. J. Am. Chem. Soc. 2018, 140, 4778–4781. [Google Scholar] [CrossRef] [PubMed]
- Tsuga, Y.; Katou, M.; Kuwabara, S.; Kanamori, T.; Ogura, S.I.; Okazaki, S.; Ohtani, H.; Yuasa, H. A Twist-Assisted Biphenyl Photosensitizer Passable Through Glucose Channel. Chemistry 2019, 14, 2067–2071. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, R.; Zhao, J.; Li, C. Spin-orbit charge transfer intersystem crossing in n perylenemonoimide-phenothiazine compact electron donor-acceptor dyads. Chem. Commun. 2018, 54, 12329–12332. [Google Scholar] [CrossRef]
- Verhoeven, J.W. On the role of spin correlation in the formation, decay, and detection of long-lived, intramolecular charge-transfer states. J. Photochem. Photobiol. C Photochem. Rev. 2006, 7, 40–60. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Ha, J.; Jeong, H.; Cho, M.; Kim, G.; Yoon, J. Rational Molecular Design of Efficient Heavy-Atom-Free Photosensitizers for Cancer Photodynamic Therapy. ChemPlusChem 2022, 87, e2022000862022. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X. Heavy Atom-Free Triplet Photosensitizers: Molecular Structure Design, Photophysical Properties and Application in Photodynamic Therapy. Molecules 2023, 28, 2170. [Google Scholar] [CrossRef]
- Chen, K.; Dong, Y.; Zhao, X.; Imran, M.; Tang, G.; Zhao, J.; Liu, Q. Bodipy Derivatives as Triplet Photosensitizers and the Related Intersystem Crossing Mechanisms. Front. Chem. 2019, 7, 821. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, K.; Yang, W.; Wang, Z.; Zhong, F. The Triplet Excited State of Bodipy: Formation, Modulation and Application. Chem. Soc. Rev. 2015, 44, 8904–8939. [Google Scholar] [CrossRef] [PubMed]
- Filatov, M.A. Heavy-atom-free BODIPY photosensitizers with intersystem crossing mediated by intramolecular photoinduced electron transfer. Org. Biomol. Chem. 2019, 18, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cui, X.; Therrien, B.; Zhao, J. Energy-funneling-based broadband visible-light-absorbing bodipy-C60 triads and tetrads as dual functional heavy-atom-free organic triplet photosensitizers for photocatalytic organic reactions. Chemistry 2013, 19, 17472–17482. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hou, Y.; Wang, Z.; Li, Y.; Zhao, J. 3,5-Anthryl-Bodipy dyad/triad: Preparation, effect of F-B-F induced conformation restriction on the photophysical properties, and application in triplet-triplet-annihilation upconversion. J. Chem. Phys. 2020, 153, 224304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, J.; Di Donato, M.; Mazzone, G. Increasing the anti-Stokes shift in TTA upconversion with photosensitizers showing red-shifted spin-allowed charge transfer absorption but a non-compromised triplet state energy level. Chem. Commun. 2019, 55, 1510–1513. [Google Scholar] [CrossRef]
- Chen, K.; Yang, W.; Wang, Z.; Iagatti, A.; Bussotti, L.; Foggi, P.; Ji, W.; Zhao, J.; Di Donato, M. Triplet Excited State of BODIPY Accessed by Charge Recombination and Its Application in Triplet-Triplet Annihilation n Upconversion. J. Phys. Chem. A 2017, 121, 7550–7564. [Google Scholar] [CrossRef]
- Hou, Y.; Kurganskii, I.; Elmali, A.; Zhang, H.; Gao, Y.; Lv, L.; Zhao, J.; Karatay, A.; Luo, L.; Fedin, M. Electronic coupling and spin-orbit charge transfer intersystem crossing (SOCT-ISC) in compact BDP-carbazole dyads with different mutual orientations of the electron donor and acceptor. J. Chem. Phys. 2020, 152, 114701. [Google Scholar] [CrossRef]
- Dong, Y.; Sukhanov, A.A.; Zhao, J.; Elmali, A. Spin–orbit charge-transfer intersystem crossing (SOCT-ISC) in bodipy-phenoxazine dyads: Effect of chromophore orientation and conformation restriction on the photophysical properties. J. Phys. Chem. C 2019, 123, 22793–22811. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Yim, Y.; Kim, S.; Ryu, B.; Swamy, K.M.K.; Kim, G.; Kwon, N.; Kim, C.Y.; Park, S.; Yoon, J. Molecular Design of Highly Efficient Heavy-Atom-Free Triplet BODIPY Derivatives for Photodynamic Therapy and Bioimaging. Angew. Chem. 2020, 59, 8957–8962. (In English) [Google Scholar] [CrossRef]
- Nepomnyashchii, A.B.; Bard, A.J. Electrochemistry and electrogenerated chemiluminescence of BODIPY dyes. Acc. Chem. Res. 2012, 45, 1844–1853. [Google Scholar] [CrossRef]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 25058173, Bodipy. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bodipy (accessed on 21 March 2024).
- Huis In’t Veld, R.V.; Heuts, J.; Ma, S.; Cruz, L.J.; Ossendorp, F.A.; Jager, M.J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 330. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Rogóż, K.; Myśliwiec, A.; Dynarowicz, K.; Wiench, R.; Cieślar, G.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. The use of photodynamic therapy in medical practice. Front. Oncol. 2024, 14, 1373263. [Google Scholar] [CrossRef]
- Allison, R.R.; Huang, Z.; Dallimore, I.; Moghissi, K. Tools of clinical Photodynamic Therapy (PDT): A Mini Compendium. Photodiagn. Photodyn. Ther. 2024, 46, 104058. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Oncologic photodynamic therapy: Clinical strategies that modulate mechanisms of action. Photodiag. Photodyn. Ther. 2013, 10, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yuan, F.; Zheng, L.; Wen, L.; Gao, M.; Zhou, W.; Fan, X. Limitations of ALA-PDT as a reliable therapy for AK in clinical practice. Photodiag. Photodyn. Ther. 2023, 44, 103797. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yang, J.; Zhang, L.; Zhang, Y.; Yan, G.; Zhang, H.; Yang, J.; Wang, P.; Zhang, G.; Zhou, Z.; et al. Adverse reactions of ALA-PDT for the treatment of cutaneous diseases: A retrospective study. Photodiag. Photodyn. Ther. 2022, 38, 102783. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Giuffrida, R.; Caradonna, E.; Vaccaro, M.; Guarneri, F.; Cannavò, S.P. Early and Late Onset Side Effects of Photodynamic Therapy. Biomedicines 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.G.; Smith, K.M.; McCullough, J.L.; Berns, M.W. Skin photosensitivity and photodestruction of several potential photodynamic sensitizers. Photochem. Photobiol. 1989, 49, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.S.; Mouinga-Ondeme, A.G. Photodynamic Therapy: A Prospective Therapeutic Approach for Viral Infections and Induced Neoplasia. Pharmaceuticals 2022, 15, 1273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef] [PubMed]
- Mariño-Ocampo, N.; Dibona-Villanueva, L.; Escobar-Álvarez, E.; Guerra-Díaz, D.; Zúñiga-Núñez, D.; Fuentealba, D.; Robinson-Duggon, J. Recent Photosensitizer Developments, Delivery Strategies and Combination-based Approaches for Photodynamic Therapy. Photochem. Photobiol. 2023, 99, 469–497. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Carter, K.A.; Miranda, D.; Lovell, J.F. Chemophototherapy: An Emerging Treatment Option for Solid Tumors. Adv. Sci. 2017, 4, 1600106. [Google Scholar] [CrossRef] [PubMed]
- Broekgaarden, M.; Rizvi, I.; Bulin, A.L.; Petrovic, L.; Goldschmidt, R.; Massodi, I.; Celli, J.P.; Hasan, T. Neoadjuvant photodynamic therapy augments immediate and prolonged oxali-platin efficacy in metastatic pancreatic cancer organoids. Oncotarget 2018, 9, 13009–13022. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Rollakanti, K.R.; Brankov, N.; Brash, D.E.; Hasan, T.; Maytin, E.V. Fluorouracil enhances photodynamic therapy of squamous cell carcinoma via a p53-independent mechanism that increases protoporphyrin IX levels and tumor cell death. Mol. Cancer Ther. 2017, 16, 1092–1101. [Google Scholar] [CrossRef]
- Carter, K.; Luo, D.; Razi, A.; Geng, J.; Shao, S.; Ortega, J.; Lovell, J.F. Sphingomyelin liposomes containing porphyrin-phospholipid for irinotecan chemophototherapy. Theranostics 2016, 6, 2329. [Google Scholar] [CrossRef]
- Li, M.; Long, S.; Kang, Y.; Guo, L.; Wang, J.; Fan, J.; Du, J.; Peng, X. De Novo Design of Phototheranostic Sensitizers Based on Structure-Inherent Targeting for Enhanced Cancer Ablation. J. Am. Chem. Soc. 2018, 140, 15820–15826. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Yu, L.; Sun, M. Progress and trends of photodynamic therapy: From traditional photosensitizers to AIE-based photosensitizers. Photodiag. Photodyn. Ther. 2021, 34, 102254. [Google Scholar] [CrossRef] [PubMed]
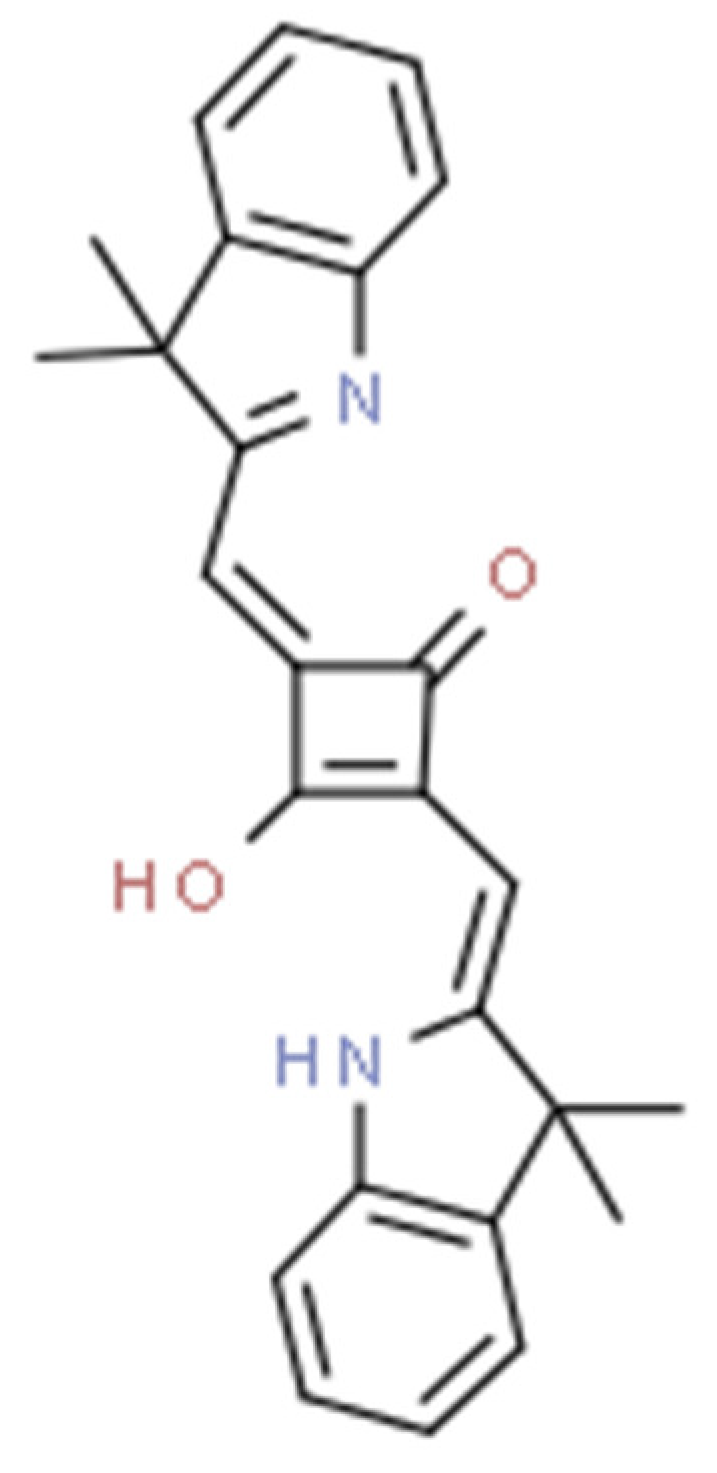
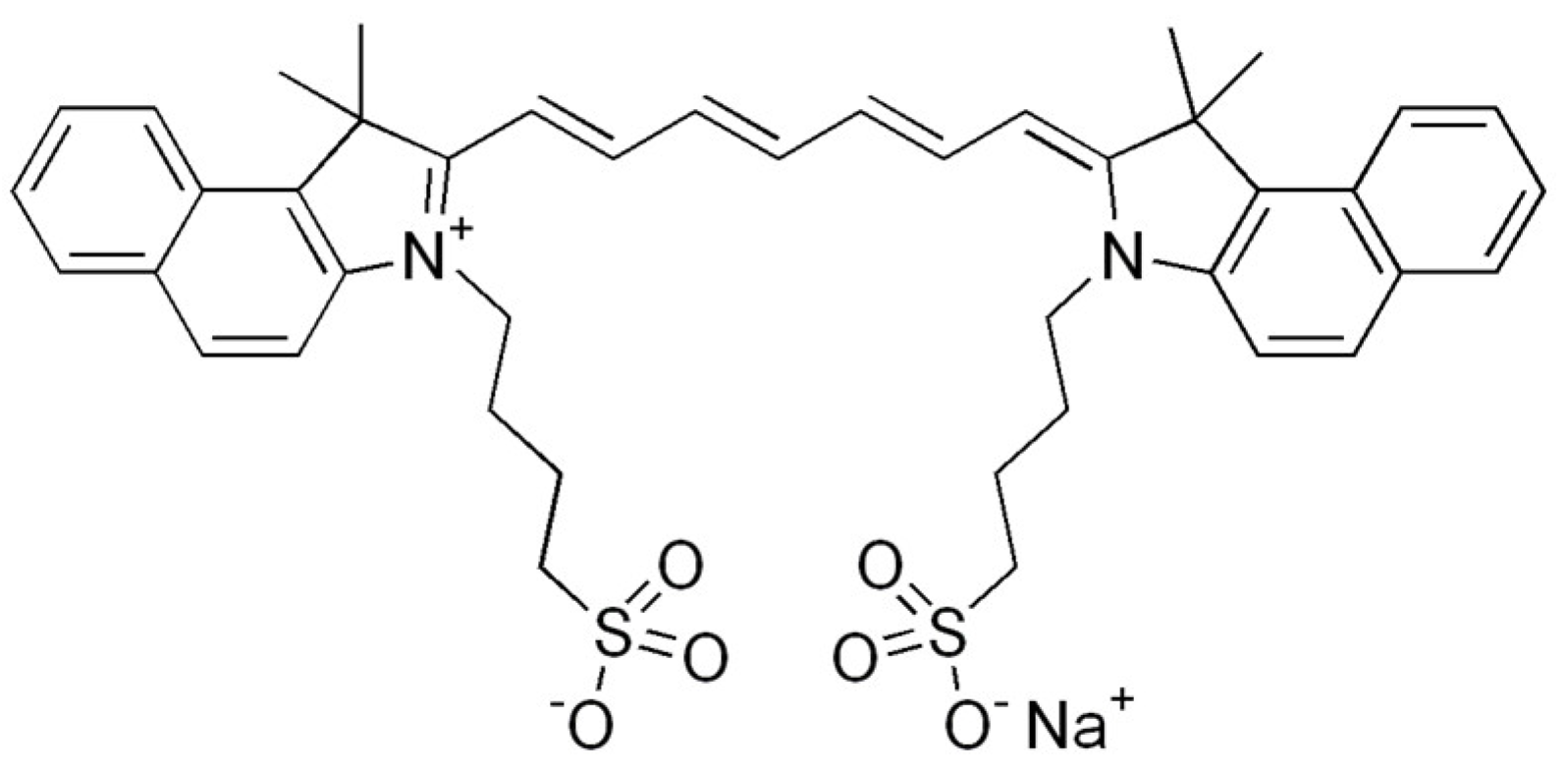

| PS Generation | Representative Compounds and the Activation Wavelength Range/Absorption Peak | Structure of the Molecule | Applications | References |
|---|---|---|---|---|
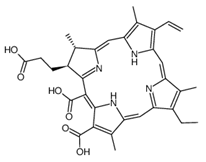
| I | Photofrin, 630 nm | 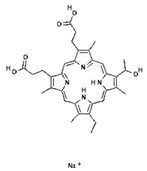 | Cancers of the esophagus, lungs and bronchi | [16,57] |
| II | Ameluz 635 nm | 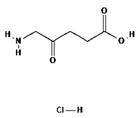 | Basal cell carcinoma of the skin and actinic keratosis (Ameluz) | [16,58,59] |
| II | 5-ALA, 630 nm | 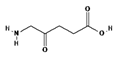 | Imaging of brain tumors | [16,17,60] |
| II | HAL/Hexvix 380–450 nm | 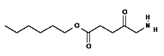 | Bladder cancer | [16,61] |
| II | Metvix, 570 to 670 nm |  | Basal cell carcinoma, Bowen’s disease and actinic keratosis | [16,59,62] |
| II | Foscan, 652 nm | 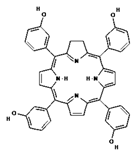 | Head and neck cancer | [16,17,59,63] |
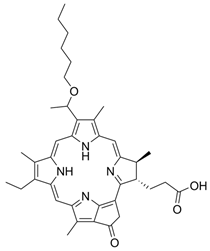
| II | Laserphyrin, 664 nm |  | Esophageal cancer, lung cancer and brain tumors | [16,17,59,64] |
| II | Redaporfin, 749 nm | 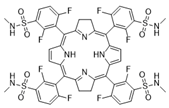 | Cancer of the bile ducts | [16,17,59,65] |
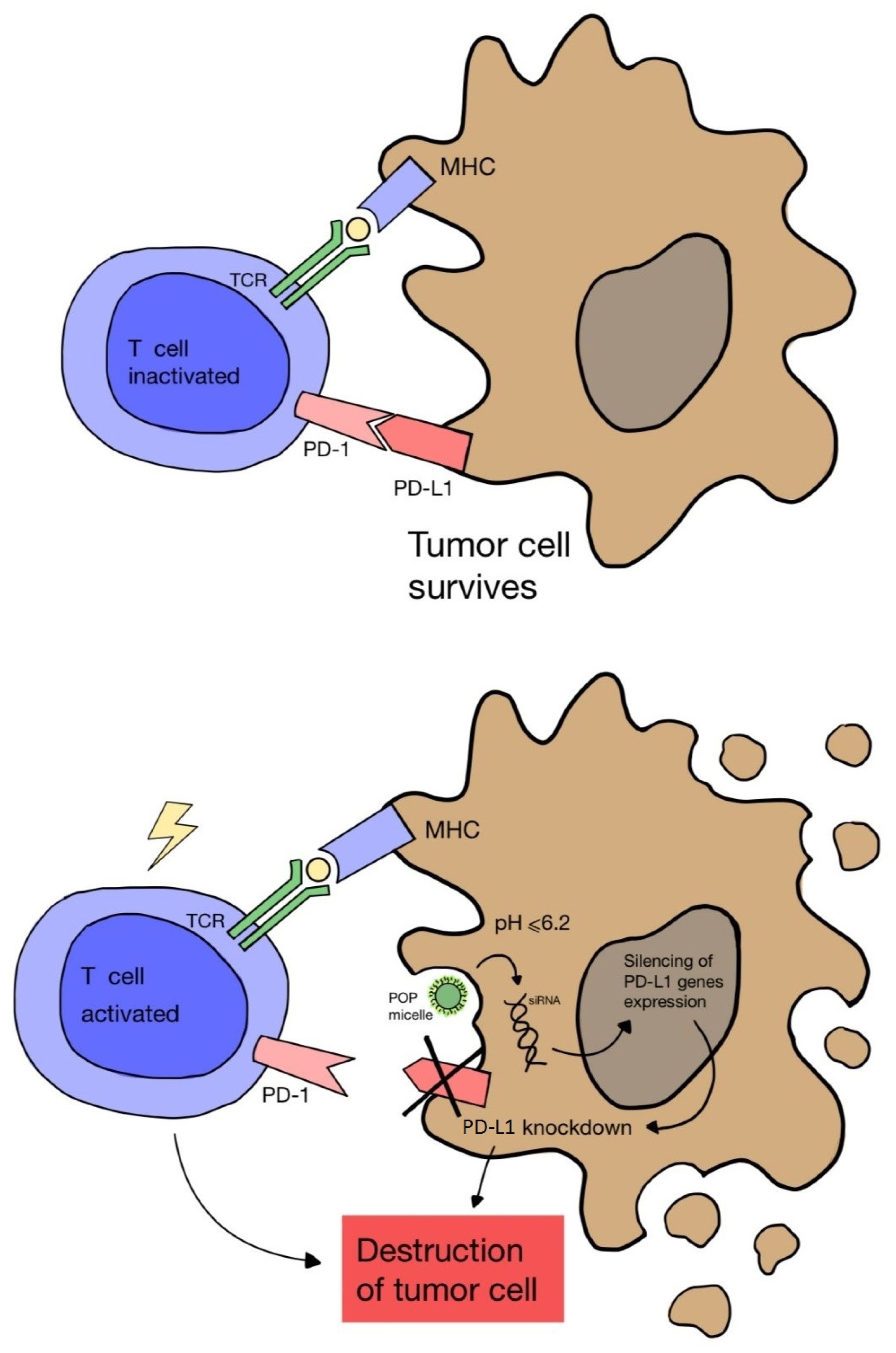
| III | Conjugates of a photosensitizer with a plasma protein (hemoglobin or albumin molecule) | - | Under investigation | [66] |
| III | Nanocomplexes of cationic micelle, photosensitizer and small interfering RNA (siRNA) | - | Under investigation | [67] |
| III | Nanoconjugates of UCNs and photosensitizer, made from a nanoparticle of manganese dioxide (MnO2) and a biopolymer of hyaluronic acid (HA) | - | Under investigation | [68] |
| III | Organometallic Hf-porphyrin: DBP-UiO | - | Under investigation | [69] |
| III | Pluronic redaporphyrin micelles | - | Under investigation | [70] |
| III | Polymer nanoparticles on a monocytic carrier | - | Under investigation | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aebisher, D.; Serafin, I.; Batóg-Szczęch, K.; Dynarowicz, K.; Chodurek, E.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Photodynamic Therapy in the Treatment of Cancer—The Selection of Synthetic Photosensitizers. Pharmaceuticals 2024, 17, 932. https://doi.org/10.3390/ph17070932
Aebisher D, Serafin I, Batóg-Szczęch K, Dynarowicz K, Chodurek E, Kawczyk-Krupka A, Bartusik-Aebisher D. Photodynamic Therapy in the Treatment of Cancer—The Selection of Synthetic Photosensitizers. Pharmaceuticals. 2024; 17(7):932. https://doi.org/10.3390/ph17070932
Chicago/Turabian StyleAebisher, David, Iga Serafin, Katarzyna Batóg-Szczęch, Klaudia Dynarowicz, Ewa Chodurek, Aleksandra Kawczyk-Krupka, and Dorota Bartusik-Aebisher. 2024. "Photodynamic Therapy in the Treatment of Cancer—The Selection of Synthetic Photosensitizers" Pharmaceuticals 17, no. 7: 932. https://doi.org/10.3390/ph17070932
APA StyleAebisher, D., Serafin, I., Batóg-Szczęch, K., Dynarowicz, K., Chodurek, E., Kawczyk-Krupka, A., & Bartusik-Aebisher, D. (2024). Photodynamic Therapy in the Treatment of Cancer—The Selection of Synthetic Photosensitizers. Pharmaceuticals, 17(7), 932. https://doi.org/10.3390/ph17070932