Monoclonal Antibodies in Smoldering Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance: Current Status and Future Directions
Abstract
1. Introduction
2. Epidemiology and Clinical Presentation
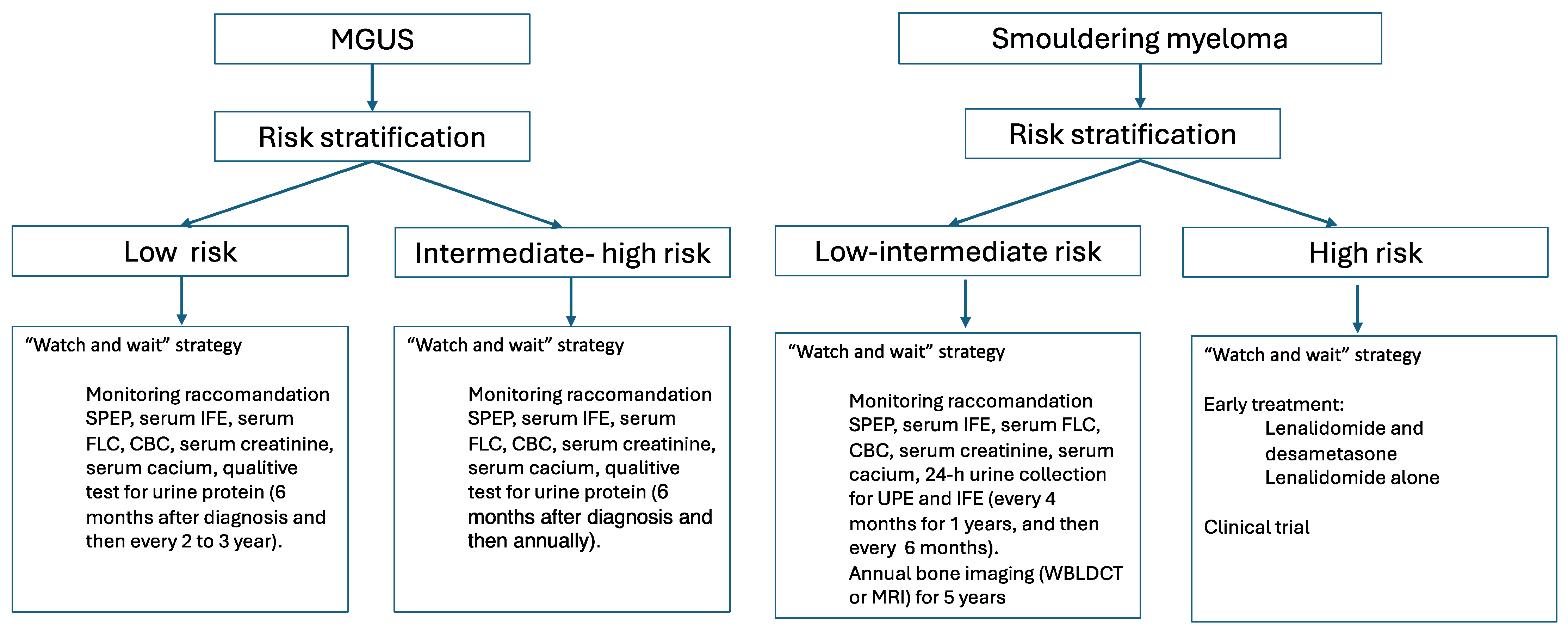
3. Prognosis and Risk Stratification
4. Pathogenesis and Immune Regulation
5. Initial Study on Early Treatment Approach
6. Monoclonal Antibodies and Future Directions
6.1. Monoclonal Antibodies in High-Risk SMM
6.1.1. Anti-CD-38 Monoclonal Antibodies
6.1.2. Anti-SLAM F7/CS1 Monoclonal Antibodies
6.1.3. Anti PD-1 Monoclonal Antibodies
6.1.4. Anti-Interleukin-6 Monoclonal Antibody
6.2. Monoclonal Antibodies in MGUS and SMM Low Risk
Anti-CD-38 Monoclonal Antibodies
6.3. Monoclonal Antibodies in MGRS
Anti-CD-38 Monoclonal Antibodies
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef]
- Varga, C.; Maglio, M.; Ghobrial, I.M.; Richardson, P.G. Current use of monoclonal antibodies in the treatment of multiple myeloma. Br. J. Haematol. 2018, 181, 447–459. [Google Scholar] [CrossRef]
- Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Terpos, E. Antibody therapies for multiple myeloma. Expert Opin. Biol. Ther. 2020, 20, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Landgren, O.; Mateos, M.-V. Smoldering multiple myeloma. Blood 2015, 125, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Hernandez, M.T.; Giraldo, P.; de la Rubia, J.; de Arriba, F.; Lopez Corral, L.; Rosinol, L.; Paiva, B.; Palomera, L.; Bargay, J.; et al. Lenalidomide plus Dexamethasone for High-Risk Smoldering Multiple Myeloma. N. Engl. J. Med. 2013, 369, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Jacobus, S.; Fonseca, R.; Weiss, M.; Kumar, S.; Orlowski, R.Z.; Kaufman, J.L.; Yacoub, A.M.; Buadi, F.K.; O’Brien, T.; et al. Randomized Trial of Lenalidomide Versus Observation in Smoldering Multiple Myeloma. J. Clin. Oncol. 2020, 38, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; González-Calle, V. Timing of treatment of smoldering myeloma: Early treatment. Blood Adv. 2018, 2, 3045–3049. [Google Scholar] [CrossRef]
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef]
- Weiss, B.M.; Abadie, J.; Verma, P.; Howard, R.S.; Kuehl, W.M. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009, 113, 5418–5422. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Larson, D.R.; Plevak, M.F.; Offord, J.R.; Dispenzieri, A.; Katzmann, J.A.; Melton, L.J.I. Prevalence of Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2006, 354, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; Katzmann, J.A.; Kyle, R.A.; Larson, D.R.; Melton, L.J.; Colby, C.L.; Therneau, T.M.; Clark, R.; Kumar, S.K.; Bradwell, A.; et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: A retrospective population-based cohort study. Lancet 2010, 375, 1721–1728. [Google Scholar] [CrossRef]
- Chee, C.E.; Kumar, S.; Larson, D.R.; Kyle, R.A.; Dispenzieri, A.; Gertz, M.A.; Colby, C.L.; Rajkumar, S.V. The importance of bone marrow examination in determining complete response to therapy in patients with multiple myeloma. Blood 2009, 114, 2617–2618. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; Plevak, M.F.; Melton, L.J., 3rd. A Long-Term Study of Prognosis in Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2002, 346, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Larson, D.R.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Cerhan, J.R.; Rajkumar, S.V. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2018, 378, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.R.; Kohlhagen, M.C.; Dasari, S.; Vanderboom, P.M.; Kyle, R.A.; Katzmann, J.A.; Willrich, M.A.; Barnidge, D.R.; Dispenzieri, A.; Murray, D.L. Comprehensive Assessment of M-Proteins Using Nanobody Enrichment Coupled to MALDI-TOF Mass Spectrometry. Clin. Chem. 2016, 62, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Barnidge, D.R.; Dasari, S.; Botz, C.M.; Murray, D.H.; Snyder, M.R.; Katzmann, J.A.; Dispenzieri, A.; Murray, D.L. Using Mass Spectrometry to Monitor Monoclonal Immunoglobulins in Patients with a Monoclonal Gammopathy. J. Proteome Res. 2014, 13, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.; Kumar, S.K.; Kyle, R.A.; Dispenzieri, A.; Dasari, S.; Larson, D.R.; Vachon, C.; Cerhan, J.R.; Rajkumar, S.V. Detection and prevalence of monoclonal gammopathy of undetermined significance: A study utilizing mass spectrometry-based monoclonal immunoglobulin rapid accurate mass measurement. Blood Cancer J. 2019, 9, 102. [Google Scholar] [CrossRef]
- Kaur, J.; Valisekka, S.S.; Hameed, M.; Bandi, P.S.; Varma, S.; Onwughalu, C.J.; Ibrahim, H.; Mongia, H. Monoclonal Gammopathy of Undetermined Significance: A Comprehensive Review. Clin. Lymphoma Myeloma Leuk. 2023, 23, e195–e212. [Google Scholar] [CrossRef]
- Thorsteinsdóttir, S.; Gíslason, G.K.; Aspelund, T.; Rögnvaldsson, S.; Óskarsson, J.; Sigurðardóttir, G.; Þórðardóttir, R.; Viðarsson, B.; Önundarson, P.T.; Agnarsson, B.A.; et al. Prevalence of smoldering multiple myeloma based on nationwide screening. Nat. Med. 2023, 29, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moulopoulos, L.A.; Maniatis, A.; Alexanian, R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood 2000, 96, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moulopoulos, A.; Smith, T.; Delasalle, K.B.; Alexanian, R. Risk of disease progression in asymptomatic multiple myeloma. Am. J. Med. 1993, 94, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Miguel, J.-F.S. Treatment for High-Risk Smoldering Myeloma. N. Engl. J. Med. 2013, 369, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Preventive strategies in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Am. J. Hematol. 2012, 87, 453–454. [Google Scholar] [CrossRef]
- Kyle, R.A.; Remstein, E.D.; Therneau, T.M.; Dispenzieri, A.; Kurtin, P.J.; Hodnefield, J.M.; Larson, D.R.; Plevak, M.F.; Jelinek, D.F.; Fonseca, R.; et al. Clinical Course and Prognosis of Smoldering (Asymptomatic) Multiple Myeloma. N. Engl. J. Med. 2007, 356, 2582–2590. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kyle, R.A.; Therneau, T.M.; Melton, L.J.; Bradwell, A.R.; Clark, R.J.; Larson, D.R.; Plevak, M.F.; Dispenzieri, A.; Katzmann, J.A. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005, 106, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Turesson, I.; Kovalchik, S.A.; Pfeiffer, R.M.; Kristinsson, S.Y.; Goldin, L.R.; Drayson, M.T.; Landgren, O. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood 2014, 123, 338–345. [Google Scholar] [CrossRef]
- Perez-Persona, E.; Vidriales, M.B.; Mateo, G.; Garcia-Sanz, R.; Mateos, M.V.; de Coca, A.G.; Galende, J.; Martin-Nunez, G.; Alonso, J.M.; de Las Heras, N.; et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 2007, 110, 2586–2592. [Google Scholar] [CrossRef]
- Pérez-Persona, E.; Mateo, G.; García-Sanz, R.; Mateos, M.; Heras, N.D.L.; de Coca, A.G.; Hernández, J.M.; Galende, J.; Martín-Nuñez, G.; Bárez, A.; et al. Risk of progression in smouldering myeloma and monoclonal gammopathies of unknown significance: Comparative analysis of the evolution of monoclonal component and multiparameter flow cytometry of bone marrow plasma cells. Br. J. Haematol. 2010, 148, 110–114. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Kyle, R.A.; Katzmann, J.A.; Therneau, T.M.; Larson, D.; Benson, J.; Clark, R.J.; Melton, L.J., 3rd; Gertz, M.A.; Kumar, S.K.; et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood 2008, 111, 785–789. [Google Scholar] [CrossRef]
- Dhodapkar, M.V.; Sexton, R.; Waheed, S.; Usmani, S.; Papanikolaou, X.; Nair, B.; Petty, N.; Shaughnessy, J.D., Jr.; Hoering, A.; Crowley, J.; et al. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120). Blood 2014, 123, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, A.; Rajkumar, S.V.; Buadi, F.K.; Binder, M.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Dingli, D.; Fonder, A.L.; Hayman, S.R.; et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018, 8, 59. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Kumar, S.; Dimopoulos, M.A.; González-Calle, V.; Kastritis, E.; Hajek, R.; De Larrea, C.F.; Morgan, G.J.; Merlini, G.; Goldschmidt, H.; et al. International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J. 2020, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Kyle, R.A.; Buadi, F.K. Advances in the Diagnosis, Classification, Risk Stratification, and Management of Monoclonal Gammopathy of Undetermined Significance: Implications for Recategorizing Disease Entities in the Presence of Evolving Scientific Evidence. Mayo Clin. Proc. 2010, 85, 945–948. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Gupta, V.; Fonseca, R.; Dispenzieri, A.; Gonsalves, W.I.; Larson, D.; Ketterling, R.P.; Lust, J.A.; Kyle, R.A.; Kumar, S.K. Impact of primary molecular cytogenetic abnormalities and risk of progression in smoldering multiple myeloma. Leukemia 2013, 27, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Neben, K.; Jauch, A.; Hielscher, T.; Hillengass, J.; Lehners, N.; Seckinger, A.; Granzow, M.; Raab, M.S.; Ho, A.D.; Goldschmidt, H.; et al. Progression in Smoldering Myeloma Is Independently Determined by the Chromosomal Abnormalities del(17p), t(4;14), Gain 1q, Hyperdiploidy, and Tumor Load. J. Clin. Oncol. 2013, 31, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Rosiñol, L.; Bladé, J.; Esteve, J.; Aymerich, M.; Rozman, M.; Montoto, S.; Giné, E.; Nadal, E.; Filella, X.; Queralt, R.; et al. Smoldering multiple myeloma: Natural history and recognition of an evolving type. Br. J. Haematol. 2003, 123, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Kyle, R.A.; Larson, D.R.; Witzig, T.E.; Kumar, S.; Dispenzieri, A.; Morice, W.G.; Rajkumar, S.V. High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia 2013, 27, 680–685. [Google Scholar] [CrossRef]
- Hillengass, J.; Fechtner, K.; Weber, M.-A.; Bäuerle, T.; Ayyaz, S.; Heiss, C.; Hielscher, T.; Moehler, T.M.; Egerer, G.; Neben, K.; et al. Prognostic Significance of Focal Lesions in Whole-Body Magnetic Resonance Imaging in Patients with Asymptomatic Multiple Myeloma. J. Clin. Oncol. 2010, 28, 1606–1610. [Google Scholar] [CrossRef]
- Maura, F.; Bolli, N.; Angelopoulos, N.; Dawson, K.J.; Leongamornlert, D.; Martincorena, I.; Mitchell, T.J.; Fullam, A.; Gonzalez, S.; Szalat, R.; et al. Genomic landscape and chronological reconstruction of driver events in multiple myeloma. Nat. Commun. 2019, 10, 3835. [Google Scholar] [CrossRef] [PubMed]
- Bolli, N.; Maura, F.; Minvielle, S.; Gloznik, D.; Szalat, R.; Fullam, A.; Martincorena, I.; Dawson, K.J.; Samur, M.K.; Zamora, J.; et al. Genomic patterns of progression in smoldering multiple myeloma. Nat. Commun. 2018, 9, 3363. [Google Scholar] [CrossRef]
- Paiva, B.; Mateos, M.V.; Sanchez-Abarca, L.I.; Puig, N.; Vidriales, M.-B.; López-Corral, L.; Corchete, L.A.; Hernandez, M.T.; Bargay, J.; de Arriba, F.; et al. Immune status of high-risk smoldering multiple myeloma patients and its therapeutic modulation under LenDex: A longitudinal analysis. Blood 2016, 127, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.A.; Massagué, J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005, 8, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Viola, D.; Dona, A.; Caserta, E.; Troadec, E.; Besi, F.; McDonald, T.; Ghoda, L.; Gunes, E.G.; Sanchez, J.F.; Khalife, J.; et al. Daratumumab induces mechanisms of immune activation through CD38+ NK cell targeting. Leukemia 2021, 35, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Durie, B.G.; Rajkumar, S.V.; Landgren, O.; Blade, J.; Merlini, G.; Kroger, N.; Einsele, H.; Vesole, D.H.; Dimopoulos, M.; et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010, 24, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.; Palumbo, A.; Johnsen, H.E.; Engelhardt, M.; Gay, F.; Gregersen, H.; Hajek, R.; Kleber, M.; Ludwig, H.; Morgan, G.; et al. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: Recommendations from the European Myeloma Network. Haematologica 2014, 99, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Waxman, A.J. Multiple Myeloma Precursor Disease. JAMA 2010, 304, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; San-Miguel, J.F.; Mateos, M.-V.; Rajkumar, S.V. Monoclonal Gammopathy of Undetermined Significance and Smoldering Multiple Myeloma. Hematol. Oncol. Clin. N. Am. 2014, 28, 775–790. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Miguel, J.F.S. New Approaches to Smoldering Myeloma. Curr. Hematol. Malign Rep. 2013, 8, 270–276. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kumar, S.; Lonial, S.; Mateos, M.V. Smoldering multiple myeloma current treatment algorithms. Blood Cancer J. 2022, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.; Hellquist, L.; Holmberg, E.; Magnusson, B.; Rödjer, S.; Westin, J. Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I—A randomized study. Eur. J. Haematol. 1993, 50, 95–102. [Google Scholar] [CrossRef]
- Grignani, G.; Gobbi, P.; Formisano, R.; Pieresca, C.; Ucci, G.; Brugnatelli, S.; Riccardi, A.; Ascari, E. A prognostic index for multiple myeloma. Br. J. Cancer 1996, 73, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, A.; Mora, O.; Tinelli, C.; Valentini, D.; Brugnatelli, S.; Spanedda, R.; De Paoli, A.; Barbarano, L.; Di Stasi, M.; Giordano, M.; et al. Long-term survival of stage I multiple myeloma given chemotherapy just after diagnosis or at progression of the disease: A multicentre randomized study. Br. J. Cancer 2000, 82, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Witzig, T.E.; Laumann, K.M.; Lacy, M.Q.; Hayman, S.R.; Dispenzieri, A.; Kumar, S.; Reeder, C.B.; Roy, V.; Lust, J.A.; Gertz, M.A.; et al. A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia 2013, 27, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Geyer, S.M.; Iturria, N.; Kumar, S.; Lust, J.A.; Kyle, R.A.; et al. Thalidomide as initial therapy for early-stage myeloma. Leukemia 2003, 17, 775–779. [Google Scholar] [CrossRef]
- Weber, D.; Rankin, K.; Gavino, M.; Delasalle, K.; Alexanian, R. Thalidomide Alone or With Dexamethasone for Previously Untreated Multiple Myeloma. J. Clin. Oncol. 2003, 21, 16–19. [Google Scholar] [CrossRef]
- D’Arena, G.; Gobbi, P.G.; Broglia, C.; Sacchi, S.; Quarta, G.; Baldini, L.; Iannitto, E.; Falcone, A.; Guariglia, R.; Pietrantuono, G.; et al. Pamidronate versus observation in asymptomatic myeloma: Final results with long-term follow-up of a randomized study. Leuk. Lymphoma 2011, 52, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Musto, P.; Petrucci, M.T.; Bringhen, S.; Guglielmelli, T.; Caravita, T.; Bongarzoni, V.; Andriani, A.; D’Arena, G.; Balleari, E.; Pietrantuono, G.; et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer 2008, 113, 1588–1595. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Hernández, M.-T.; Giraldo, P.; de la Rubia, J.; de Arriba, F.; Corral, L.L.; Rosiñol, L.; Paiva, B.; Palomera, L.; Bargay, J.; et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): Long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016, 17, 1127–1136. [Google Scholar] [CrossRef]
- Mateos, M.V.; Hernandez, M.T.; Salvador, C. Over ten years of follow-up for phase II trial in smoldering myeloma at high risk of progression to myeloma: Sustained TTP and OS benefit with RD versus no treatment. In Proceedings of the 25th EHA Congress, European Hematology Association, The Hague, The Netherlands, 11–21 June 2020. [Google Scholar]
- Lonial, S.; Susanna, J.; Jacobus, M.W.; Kumar, S.; Orlowski, R.Z.; Kaufman, J.L.; Yacoub, A.; Buadi, F.; O’Brien, T.E.; Matous, J.; et al. E3A06: Randomized phase Ill trial of lena- lidomide versus observation alone in patients with asymptomatic high-risk smol- dering multiple myeloma. J. Clin. Oncol. 2019, 37, 8001. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Bergsagel, P.L.; Kumar, S. Smoldering Multiple Myeloma: Observation Versus Control Versus Cure. Hematol. Clin. N. Am. 2024, 38, 293–303. [Google Scholar] [CrossRef]
- Cohen, C.; Royer, B.; Javaugue, V.; Szalat, R.; El Karoui, K.; Caulier, A.; Knebelmann, B.; Jaccard, A.; Chevret, S.; Touchard, G.; et al. Bortezomib produces high hematological response rates with prolonged renal survival in monoclonal immunoglobulin deposition disease. Kidney Int. 2015, 88, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, L.; Feng, J.; Mao, Y.-Y.; Cao, X.-X.; Zhou, D.-B.; Li, J. Bortezomib-based chemotherapy can improve renal and tubular functions in patients with light chain-associated Fanconi syndrome. Ann. Hematol. 2019, 98, 1095–1100. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Lopez, J.M.; Rodriguez-Otero, P.; Ocio, E.M.; Gonzalez, M.S.; Oriol, A.; Gutierrez, N.; Paiva, B.; Rios, R.; Rosinol, L.; et al. Curative Strategy for High-Risk Smoldering Myeloma (GEM-CESAR): Carlfizomib, Lenalidomide and Dexamethasone (KRd) As Induction Followed By HDT- ASCT, Consolidation with Krd and Maintenance with Rd. Blood 2017, 130, 402. [Google Scholar]
- Landgren, C.O.; Chari, A.; Cohen, Y.C.; Spencer, A.; Voorhees, P.; Estell, J.A.; Sandhu, I.; Jenner, M.W.; Williams, C.; Cavo, M.; et al. Daratumumab monotherapy for patients with intermediate-risk or high-risk smoldering multiple myeloma: A randomized, open-label, multicenter, phase 2 study (CENTAURUS). Leukemia 2020, 34, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, D.; Hill, E.; Dew, A.; Morrison, C.; Roswarski, J.; Korde, N.; Emanuel, M.; Petrosyan, A.; Bhutani, M.; Calvo, K.R.; et al. Carfilzomib, Lenalidomide, and Dexamethasone Followed by Lenalidomide Maintenance for Prevention of Symptomatic Multiple Myeloma in Patients with High-risk Smoldering Myeloma. JAMA Oncol. 2021, 7, 1678–1685. [Google Scholar] [CrossRef]
- Landgren, O.; Chari, A.; Cohen, Y.C.; Spencer, A.; Voorhees, P.M.; Sandhu, I.; Jenner, M.W.; Smith, D.; Cavo, M.; van de Donk, N.W.; et al. Efficacy and Safety of Daratumumab (DARA) Monotherapy in Patients with Intermediate-Risk or High-Risk Smoldering Multiple Myeloma (SMM): Final Analysis of the Phase 2 Centaurus Study. Blood 2023, 142, 210. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Voorhees, P.M.; Goldschmidt, H.; Baker, R.I.; Shi, Y.; Rousseau, E.; Dennis, R.M.; Carson, R.L.; Rajkumar, S.V. Subcutaneous daratumumab (DARA SC) versus active monitoring in patients (pts) with high-risk smoldering multiple myeloma (SMM): Randomized, open-label, phase 3 AQUILA study. J. Clin. Oncol. 2022, 40, TPS8075. [Google Scholar] [CrossRef]
- Nadeem, O.; Redd, R.; Mo, C.C.; Laubach, J.; Prescott, J.; Metivier, A.; Davie, C.; Bertoni, M.; Murphy, E.; Sheehan, B.; et al. B-PRISM (Precision Intervention Smoldering Myeloma): A phase II trial of combination of daratumumab, bortezomib, lenalidomide, and dexamethasone in high-risk smoldering multiple myeloma. J. Clin. Oncol. 2022, 40, 8040. [Google Scholar] [CrossRef]
- Kumar, S.K.; Abdallah, A.-O.; Badros, A.Z.; Laplant, B.; Dhakal, B.; Alsina, M.; Abonour, R.; Rosenbaum, C.A.; Bensinger, W.I.; Bhutani, M.; et al. Aggressive Smoldering Curative Approach Evaluating Novel Therapies (ASCENT): A Phase 2 Trial of Induction, Consolidation and Maintenance in Subjects with High Risk Smoldering Multiple Myeloma (SMM): Initial Analysis of Safety Data. Blood 2020, 136, 35–36. [Google Scholar] [CrossRef]
- Kumar, S.K.; Alsina, M.; Laplant, B.; Badros, A.Z.; Abdallah, A.-O.; Abonour, R.; Asmus, E.J.; Dhakal, B.; Rosenbaum, C.A.; Egan, D.; et al. Fixed Duration Therapy with Daratumumab, Carfilzomib, Lenalidomide and Dexamethasone for High Risk Smoldering Multiple Myeloma-Results of the Ascent Trial. Blood 2022, 140, 1830–1832. [Google Scholar] [CrossRef]
- Manasanch, E.E.; Jagannath, S.; Lee, H.C.; Patel, K.K.; Graham, C.; Kaufman, G.P.; Thomas, S.K.; Iyer, S.; Mailankody, S.; Korde, N.; et al. A Multicenter Phase II Single Arm Trial of Isatuximab in Patients with High Risk Smoldering Multiple Myeloma (HRSMM). Blood 2019, 134, 3116. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Otero, P.R.; Koh, Y.; Martinez-Lopez, J.; Parmar, G.; Prince, H.M.; Quach, H.; Ribas, P.; Hermansen, E.; Hungria, V.T.; et al. Isatuximab in Combination with Lenalidomide and Dexamethasone in Patients with High-Risk Smoldering Multiple Myeloma: Updated Safety Run-in Results from the Randomized Phase 3 Ithaca Study. Blood 2022, 140, 7317–7319. [Google Scholar] [CrossRef]
- Jagannath, S.; Laubach, J.; Wong, E.; Stockerl-Goldstein, K.; Rosenbaum, C.; Dhodapkar, M.; Jou, Y.; Lynch, M.; Robbins, M.; Shelat, S.; et al. Elotuzumab monotherapy in patients with smouldering multiple myeloma: A phase 2 study. Br. J. Haematol. 2018, 182, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Ghobrial, I.M.; Bustoros, M.; Reyes, K.; Hornburg, K.; Badros, A.Z.; Vredenburgh, J.J.; Boruchov, A.; Matous, J.V.; Caola, A. Phase 2 trial of combination of elotuzumab, lenalidomide and dexamethasone for high-risk smoldering multiple myeloma. Blood 2018, 132, 134. [Google Scholar] [CrossRef]
- Manasanch, E.E.; Han, G.; Mathur, R.; Qing, Y.; Zhang, Z.; Lee, H.; Weber, D.M.; Amini, B.; Berkova, Z.; Eterovic, K.; et al. A pilot study of pembrolizumab in smoldering myeloma: Report of the clinical, immune, and genomic analysis. Blood Adv. 2019, 3, 2400–2408. [Google Scholar] [CrossRef]
- Brighton, T.A.; Khot, A.; Harrison, S.J.; Ghez, D.; Weiss, B.M.; Kirsch, A.; Magen, H.; Gironella, M.; Oriol, A.; Streetly, M.; et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Study of Siltuximab in High-Risk Smoldering Multiple Myeloma. Clin. Cancer Res. 2019, 25, 3772–3775. [Google Scholar] [CrossRef]
- Chari, A.; Munder, M.; Weisel, K.; Jenner, M.; Bygrave, C.; Petrucci, M.T.; Boccadoro, M.; Cavo, M.; van de Donk, N.W.C.J.; Turgut, M.; et al. Evaluation of Cardiac Repolarization in the Randomized Phase 2 Study of Intermediate- or High-Risk Smoldering Multiple Myeloma Patients Treated with Daratumumab Monotherapy. Adv. Ther. 2021, 38, 1328–1341. [Google Scholar] [CrossRef]
- Collins, S.M.; Bakan, C.E.; Swartzel, G.D.; Hofmeister, C.C.; Efebera, Y.A.; Kwon, H.; Starling, G.C.; Ciarlariello, D.; Bhaskar, S.; Briercheck, E.L.; et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 2013, 62, 1841–1849. [Google Scholar] [CrossRef]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Bar, N.; Costa, F.; Das, R.; Duffy, A.; Samur, M.; McCachren, S.; Gettinger, S.N.; Neparidze, N.; Parker, T.L.; Bailur, J.K.; et al. Differential effects of PD-L1 versus PD-1 blockade on myeloid inflammation in human cancer. J. Clin. Investig. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Krauss, A.C.; Mulkey, F.; Shen, Y.-L.; Rosenberg, A.; Miller, B.; Carioti, T.; Scott, K.; Gormley, N.; Theoret, M.R.; Sridhara, R.; et al. FDA analysis of pembrolizumab trials in multiple myeloma: Immune related adverse events (irAEs) and response. J. Clin. Oncol. 2018, 36, 8008. [Google Scholar] [CrossRef]
- Gadó, K.; Domján, G.; Hegyesi, H.; Falus, A. Role of interleukin-6 in the pathogenesis of multiple myeloma. Cell Biol. Int. 2000, 24, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, O.; Redd, R.; Stampleman, L.V.; Matous, J.V.; Yee, A.J.; Zonder, J.A.; Kin, A.; Rosenblatt, J.; Bustoros, M.; Prescott, J.; et al. A Phase II Study of Daratumumab in Patients with High-Risk MGUS and Low-Risk Smoldering Multiple Myeloma: First Report of Efficacy and Safety. Blood 2019, 134, 1898. [Google Scholar] [CrossRef]
- Kastritis, E.; Theodorakakou, F.; Roussou, M.; Psimenou, E.; Gakiopoulou, C.; Marinaki, S.; Gatou, A.; Fotiou, D.; Migkou, M.; Kanellias, N.; et al. Daratumumab-based therapy for patients with monoclonal gammopathy of renal significance. Br. J. Haematol. 2021, 193, 113–118. [Google Scholar] [CrossRef]
- Zand, L.; Rajkumar, S.V.; Leung, N.; Sethi, S.; El Ters, M.; Fervenza, F.C. Safety and Efficacy of Daratumumab in Patients with Proliferative GN with Monoclonal Immunoglobulin Deposits. J. Am. Soc. Nephrol. 2021, 32, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Bolli, N.; Sgherza, N.; Curci, P.; Rizzi, R.; Strafella, V.; Delia, M.; Gagliardi, V.P.; Neri, A.; Baldini, L.; Albano, F.; et al. What Is New in the Treatment of Smoldering Multiple Myeloma? J. Clin. Med. 2021, 10, 421. [Google Scholar] [CrossRef]
- Visram, A.; Cook, J.; Warsame, R. Smoldering multiple myeloma: Evolving diagnostic criteria and treatment strategies. Hematology 2021, 2021, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Musto, P.; Engelhardt, M.; Caers, J.; Bolli, N.; Kaiser, M.; Van de Donk, N.; Terpos, E.; Broijl, A.; De Larrea, C.F.; Gay, F.; et al. 2021 European Myeloma Network review and consensus statement on smoldering multiple myeloma: How to distinguish (and manage) Dr. Jekyll and Mr. Hyde. Haematologica 2021, 106, 2799–2812. [Google Scholar] [CrossRef]
- Maclachlan, K.; Diamond, B.; Maura, F.; Hillengass, J.; Turesson, I.; Landgren, C.O.; Kazandjian, D. Second malignancies in multiple myeloma; emerging patterns and future directions. Best Pract. Res. Clin. Haematol. 2020, 33, 101144. [Google Scholar] [CrossRef] [PubMed]
- Musto, P.; Anderson, K.; Attal, M.; Richardson, P.; Badros, A.; Hou, J.; Comenzo, R.; Du, J.; Durie, B.; Miguel, J.S.; et al. Second primary malignancies in multiple myeloma: An overview and IMWG consensus. Ann. Oncol. 2018, 29, 1074. [Google Scholar] [CrossRef] [PubMed]
- Poh, C.; Keegan, T.; Rosenberg, A.S. Second primary malignancies in multiple myeloma: A review. Blood Rev. 2021, 46, 100757. [Google Scholar] [CrossRef] [PubMed]
- Corre, J.; Cleynen, A.; Du Pont, S.R.; Buisson, L.; Bolli, N.; Attal, M.; Munshi, N.; Avet-Loiseau, H. Multiple myeloma clonal evolution in homogeneously treated patients. Leukemia 2018, 32, 2636–2647. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kyle, R.A. Haematological cancer: Treatment of smoldering multiple myeloma. Nat. Rev. Clin. Oncol. 2013, 10, 554–555. [Google Scholar] [CrossRef]
- Mohyuddin, G.R.; Ouchveridze, E.; Goodman, A.; Prasad, V. The landscape of trials for smoldering multiple myeloma: Endpoints, trial design, and lessons learnt. Leuk. Lymphoma 2021, 62, 2793–2795. [Google Scholar] [CrossRef] [PubMed]
| Model | Risk Factors | Outcomes |
|---|---|---|
| Mayo (2005) [27] | 1. MC > 15 g/L 2. Non Ig G subtype 3. Abnormal FLC ratio | PFS at 20 years: 0 risk factors: 5% 1 risk factor: 21% 2 risk factors: 37% 3 risk factors: 58% |
| Swedish study (2014) [28] | 1. MC > 15 g/L 2. Non Ig G subtype 3. Abnormal FLC ratio 4. Immunoparesis (reduction of ≥1 uninvolved heavy chain) | PFS at 10 years: 0 risk factors: 4% 1 risk factor: 6% 2 risk factors: 12% 3 risk factors: 23% 4 risk factors: 40% |
| PETHEMA (2007) [29] | 1. Aberrant phenotype in >95% of BMPCs 2. DNA aneuploidy | PFS at 5 years: 0 risk factors: 4% 1 risk factor: 46% 2 risk factors: 72% |
| PETHEMA (2010) [30] | 1. Aberrant phenotype in >95% of BMPCs 2. Evolving MGUS (>10% increase in M-protein by the third year as confirmed by two consecutive measurements separated by ≥1 month) | PFS at 7 years: 0 risk factors: 2% 1 risk factor: 15% 2 risk factors: 72% |
| Model | Risk Factors | Risk Groups | Outcomes |
|---|---|---|---|
| PETHEMA (2007) [29] | 1. ≥95% aberrant BMPCs by flow cytometry (defined as CD38+ cells with absence or underexpression of CD19 and/or CD45 or overexpression of CD56) 2. Immunoparesis (reduction of ≥1 uninvolved heavy chain) | 0, low risk 1, intermediate 2, high risk | PFS at 5 y: Low risk, 4% Intermediate risk, 46% High risk, 72% |
| Mayo (2008) [31] | 1. BMPCs ≥ 10% 2. MC ≥ 30 g/L 3. sFLC ratio ≤ 0.125 or ≥8 | 0/1, low risk 2, intermediate risk 3, high risk | PFS at 5 y: Low risk, 25% Intermediate risk, 51% High risk, 76% |
| SWOG (2014) [32] | 1. MC > 30 g/L, 2. Involved sFLC > 25 mg/dL, 3. GEP-70 > 0.26 | 0, Low 1, Intermediate (1 factor) ≥2 factors, High | PFS at 52 y: Low risk, 9.7% Intermediate risk, 26.3% High risk, 47.4% |
| Mayo 20/20/2 (2018) [33] | 1. BMPCs > 20% 2. MC > 20 g/L 3. sFLC ratio < 0.05 or >20 | 0, low risk 1, intermediate risk 2–3, high risk | Median TTP: Low risk, 110 mo Intermediate risk, 68 mo High risk, 29 mo |
| IMWG (2020) [34] | 1. sFLC ratio: 0–10, 0 points; 10–25, 2 points; 25–40, 3 points; >40, 5 points 2. MC (g/L): 0–1.5, 0 points; 1.5–3, 3 points; >3, 4 points 3. Percentage of BMPCs 0–15, 0 points; 15–20, 2 points; 20–30, 3 points; 30–40, 5 points; >40, 6 points 4. FISH abnormalities * No, 0 points; Yes, 2 points | 0–4 points, low risk 5–8 points, low/intermediate risk 9–12 points, intermediate risk >12 points, high risk | Risk of progression at 2 y: Low risk, 3.8% Low/intermediate risk, 51.1% Intermediate risk, 26.2% High risk, 72.5% |
| Study | Phase N pts | High-Risk Definition | Design | Control Arm | Primary Endpoint | Response Rates | Survival Outcomes |
|---|---|---|---|---|---|---|---|
| CENTAURUS/ NCT02316106 [67,69] | 2, Randomized N = 123 | BMPCs ≥ 10% and at least 1 of the following: MC ≥ 30 g/L (IgA ≥ 20 g/L), urine M protein > 500 mg per 24 h, abnormal sFLC ratio (<0.126 or >8), | Daratumumab 16 mg/kg IV in 8-wk cycles (C): Extended intense: C1 every 1 wk; C2–3 every other wk; C4–7 every 4 wks; C8–20 every 8 weeks. Intermediate intense: C1 every 1 wk and C2–20 every 8 weeks Short dosing: C1 every 1 wk | Observation | CR | CR at 15.8 months: 2.4% vs. 4.9% vs. 0% CR at 25.9 months: 4.9% vs. 9.8% vs. 0% CR at 84 months: 4.9% vs. 12.2% vs. 0% | PFS at 2 years: 89.9% intense vs. 82.0% intermediate vs. 75.3% short OS at 84 months: 81.3% intense vs. 89.5% vs. 88.1% short |
| AQUILA/ NCT03301220 [70] | 3, randomized N = 390 | BMPCs ≥ 10% and ≥1 of the following: MC ≥30 g/L, IgA SMM, immunoparesis, abnormal sFLC ratio ≥8 to < 100, or BMPCs > 50% to <60% | Daratumumab SC: C1–2, every 2 weeks in C3–6, and every 4 weeks thereafter until C39 (28 days/cycle), up to 36 months, or until disease progression | Observation | PFS | NA | NA |
| DETER-SMM/ NCT03937635 | 3, randomized N = 288 | The presence of 2 or more of the following factors: abnormal sFLC ratio (>20, or <100); MC ≥ 20 g/L; high-risk FISH; BMPCs > 20% | Daratumumab 16 mg/kg IV on days 1, 8, 15, and 22 of C1–2, days 1 and 15 of C3–6, and day 1 of C7–24. Lena daily on days 1–21. Dex on days 1, 8, 15, and 22 in C1–12. (28 days/cycle) up to 24 courses or until disease progression | Rd | OS | NA | NA |
| B-PRISM, NCT04775550 [71] | 2, single arm N = 60 | BMPCs ≥ 10% and any one or more of the following: MC ≥ 30 g/L; immunoparesis; sFLC ratio (≥8<100); progressive increase in M protein level; BMPCs 50–60%; abnormal plasma cell immunophenotype; high-risk FISH; focal bone lesion or high risk according to IMWG/Mayo 2018 “20-2-20” Criteria (at least 2 of the following) | Daratumumab SC standard dose and schedule, bortezomib given weekly on days 1, 8, and 15 for C1–6 and then biweekly until completion of C24. Lena on days 1–21 and Dex weekly. Up to C24. | No | MRD negativity at 2 years | NA | NA |
| ASCENT, NCT03289299 [72,73] | 2, single arm N = 46 | The presence of any 2 of the following: MC > 20 g/L; sFLC ratio > 20; BMPC > 20%; IMWG score ≥ 9 using risk scoring system using sFLC ratio, MC, marrow plasma cell percentage, and presence of high-risk FISH | Daratumumab 16 mg/kg IV plus KRd standard dose and schedule. Induction: C1–6 of Dara-KRd Consolidation: C7–12 of consolidation with Dara-KRd Maintenance: C12–24 with Dara and Lena. | No | sCR |
ORR 97%, 37% sCR, 26% CR, 29% VGPR, 2% PR, 1% SD 84% patients MRDneg 61% also in CR | PFS at 3 years 89.9% |
| NCT02960555 [74] | 2, single arm N = 61 | NA | Isatuximab 20 mg/kg IV (28 days/cycle), C1 every 1 wk; C2–6 every other wk; C7–30 every 4 weeks | no | ORR | ORR 64%, CR 5%, with MRD negativity | NA |
| ITHACA/ NCT04270409 [75] | 3, randomized N = 337 | The Mayo ‘20-20-20′ and/or PETHEMA model criteria | Isatuximab 10 mg/kg IV on day 1, 8, 15, and 22 C1, day 1 and 15 C2–12, day 1 C13–36; plus Lena D1–21 (25 mg C1–9; 10 mg C10–24) and Dex weekly (40 mg, 20 mg for ≥75 yr-old pts C1–9; 20 mg C10–24). 28 days/cycle. | Rd | PFS |
ORR of 100% (median follow-up of 19.4 months): 13.0% (sCR), 30.4% CR 30.4% VGPR | NA |
| NT01441973 [76] | 2, single arm N = 41 | The presence of MC ≥ 30 g/L with BMPC ≥ 10%; or MC 10–30 g/L (alternatively urine M protein > 200 mg/24 h), BMPCs ≥ 10% and sFLC ratio < 0.125 or >8.0 | Elotuzumab monotherapy: 20 mg/kg (days 1 and 8 C1, monthly from C2) 10 mg/kg (weekly cycles 1 and 2, twice monthly from C3) | No | The association between NK cell status and M protein reduction | ORR: 10% | PFS at 2 years: 69% |
| NCT02279394 [77] | 2, single arm N = 51 | Mayo/or PETHEMA criteria | Induction: 28 day-cycle C1–2 Elotuzumab 10 mg/kg IV. days 1, 8, 15, 22 + Lena 25 mg days 1–21 + Dex 40 mg days 1, 8, 15, 22 C3–8: Stem cell collection; Elotuzumab 10 mg/kg IV days 1, 15 + Lena 25 mg days 1–21 + Dex 40 mg days 1, 8, 15 Maintenance: 28 day-cycle (C9–24) Elotuzumab 10 mg/kg IV. days 1 + Lena 25 mg days 1–21 | no | PFS at 2 years | ORR: 84% VGPR: 37% CR: 6% | None of the patients progressed to MM at 3 years |
| NCT02603887 [78] | Pilot study, single arm N = 13 | PETHEMA, Mayo 2008 or SWOG criteria | Pembrolizumab 200 mg IV every (21 days/cycle) × 8 cycles, with an option to continue up to 24 cycles if continued benefit. | no | ORR | ORR: 8%, CR: 8%, MRD negativity 8% | 15% of patients progressed to MM |
| NCT01484275 [79] | Pilot study, randomized N = 85 | BMPCs > 10% and either MC > 3 g/dL, or sFLC ratio < 0.126/>8 and MC > 10/<30 g/L | Siltuximab 15 mg/kg IV (28 days/cycle) until progressive disease. | Observation | PFS | NA | 1-year PFS: 84.5% vs. 74.4% (p < 0.06) Median PFS: NR vs. 23.5 months; OS NR in both arms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferla, V.; Farina, F.; Perini, T.; Marcatti, M.; Ciceri, F. Monoclonal Antibodies in Smoldering Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance: Current Status and Future Directions. Pharmaceuticals 2024, 17, 901. https://doi.org/10.3390/ph17070901
Ferla V, Farina F, Perini T, Marcatti M, Ciceri F. Monoclonal Antibodies in Smoldering Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance: Current Status and Future Directions. Pharmaceuticals. 2024; 17(7):901. https://doi.org/10.3390/ph17070901
Chicago/Turabian StyleFerla, Valeria, Francesca Farina, Tommaso Perini, Magda Marcatti, and Fabio Ciceri. 2024. "Monoclonal Antibodies in Smoldering Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance: Current Status and Future Directions" Pharmaceuticals 17, no. 7: 901. https://doi.org/10.3390/ph17070901
APA StyleFerla, V., Farina, F., Perini, T., Marcatti, M., & Ciceri, F. (2024). Monoclonal Antibodies in Smoldering Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance: Current Status and Future Directions. Pharmaceuticals, 17(7), 901. https://doi.org/10.3390/ph17070901






