Serum Metabolomics Uncovers the Mechanisms of Inulin in Preventing Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Results
2.1. Inulin Augmented Biochemical Indices in NAFLD
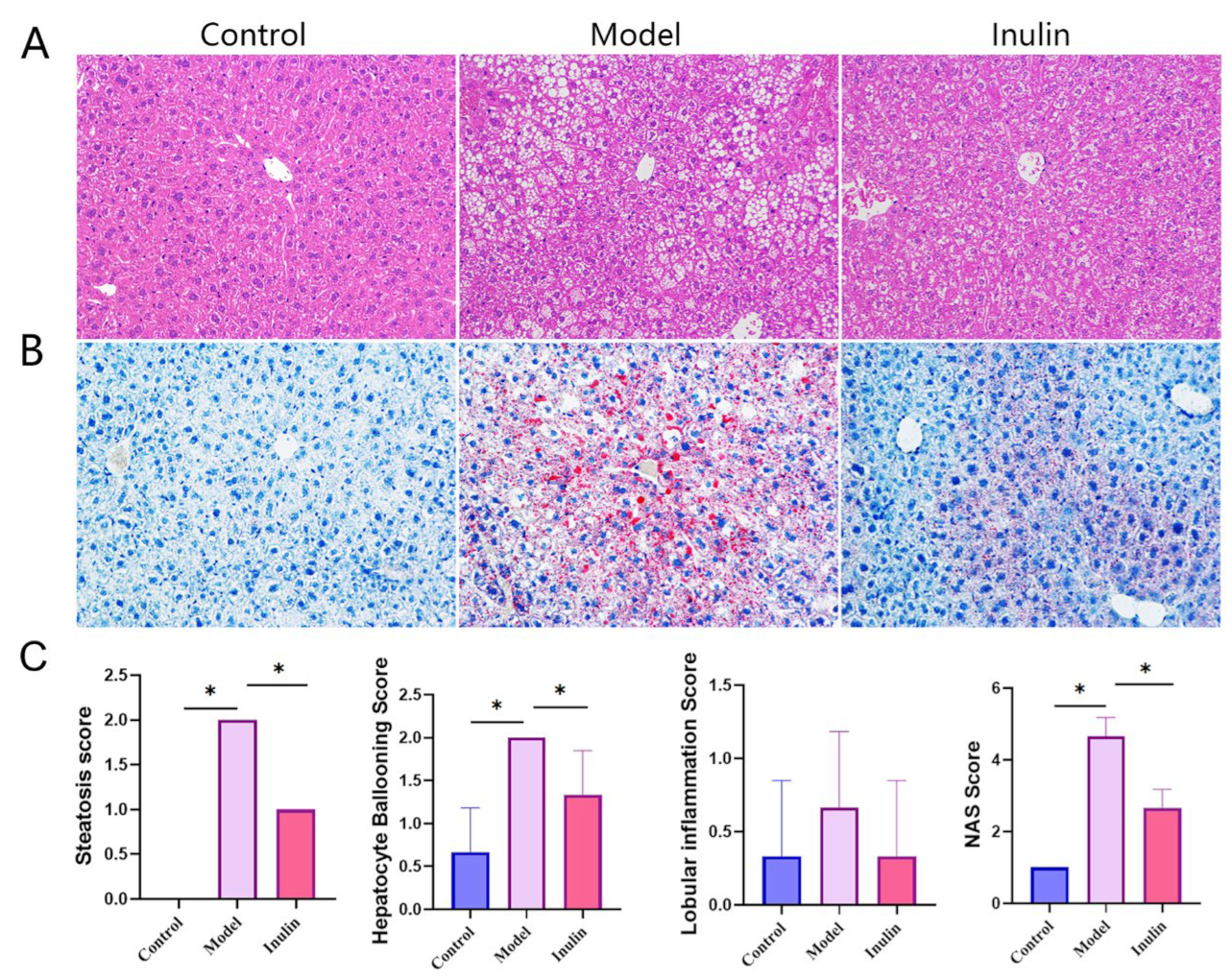
2.2. Inulin Restored Histopathological Changes Caused by NAFLD
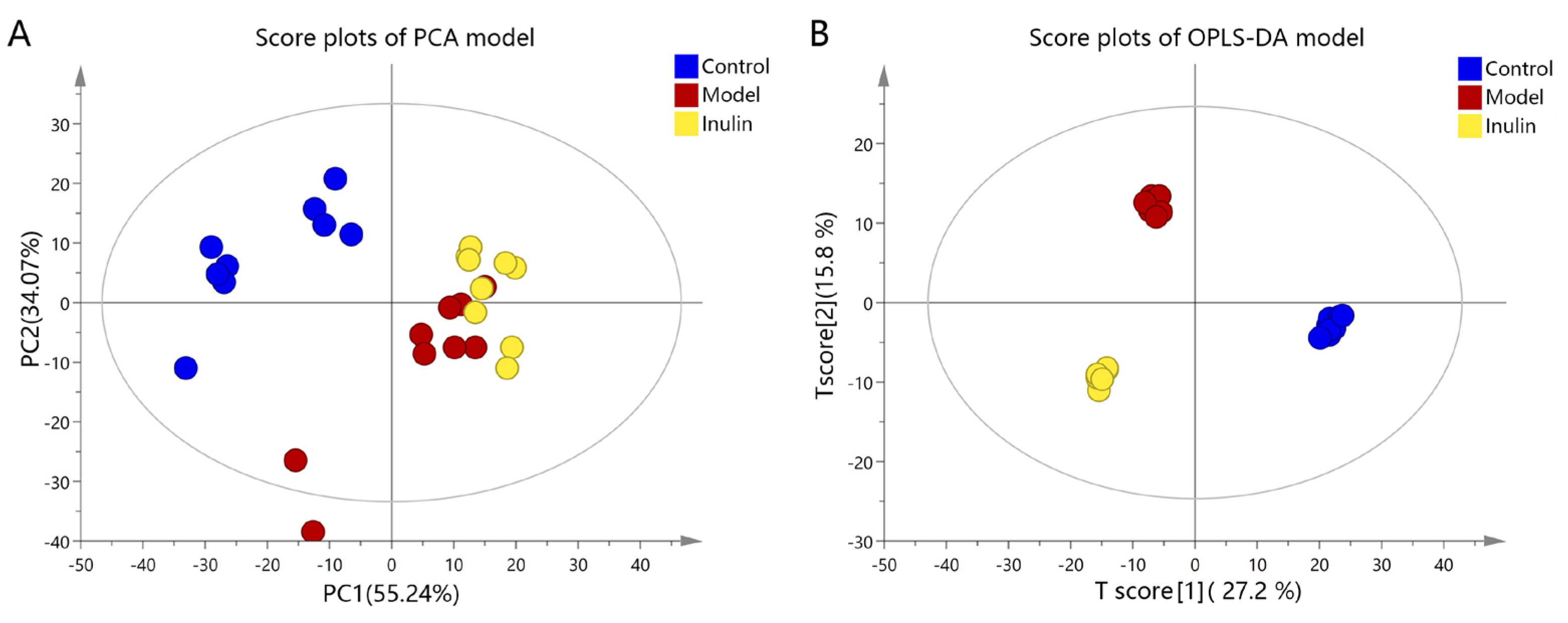
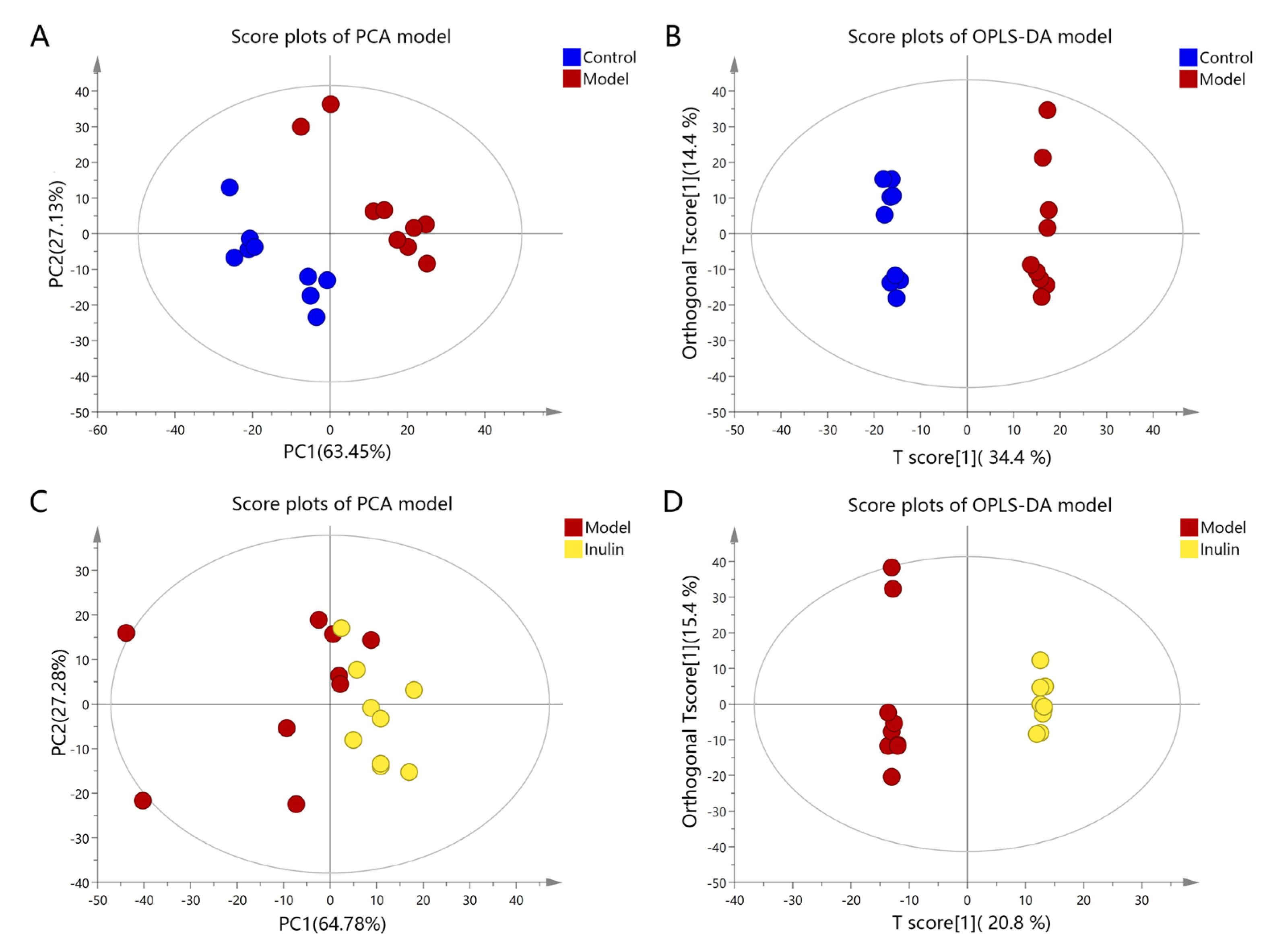
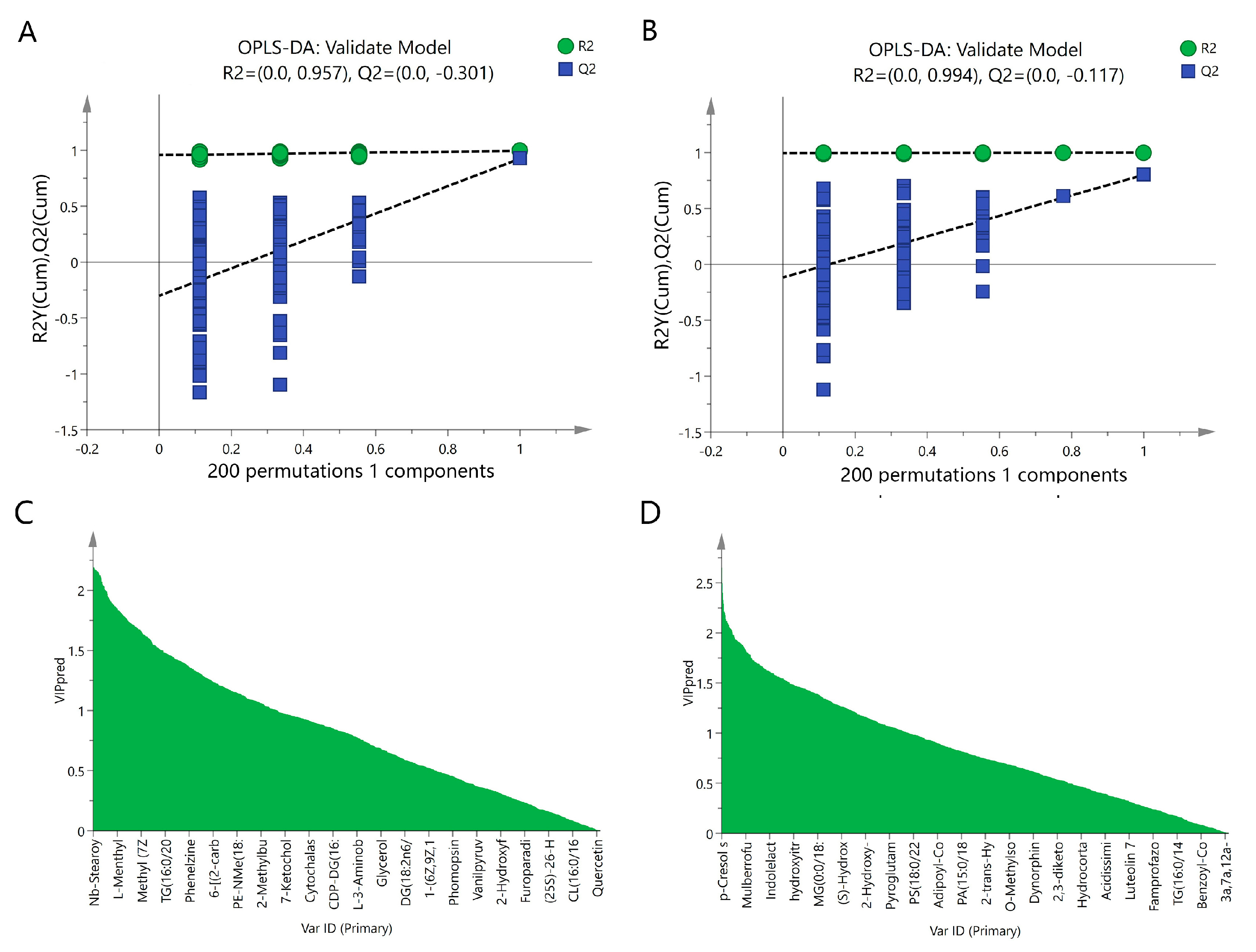
2.3. Multivariate Statistical Analysis of Serum Metabolites
2.4. Inulin Restored Changes in Metabolites in NAFLD
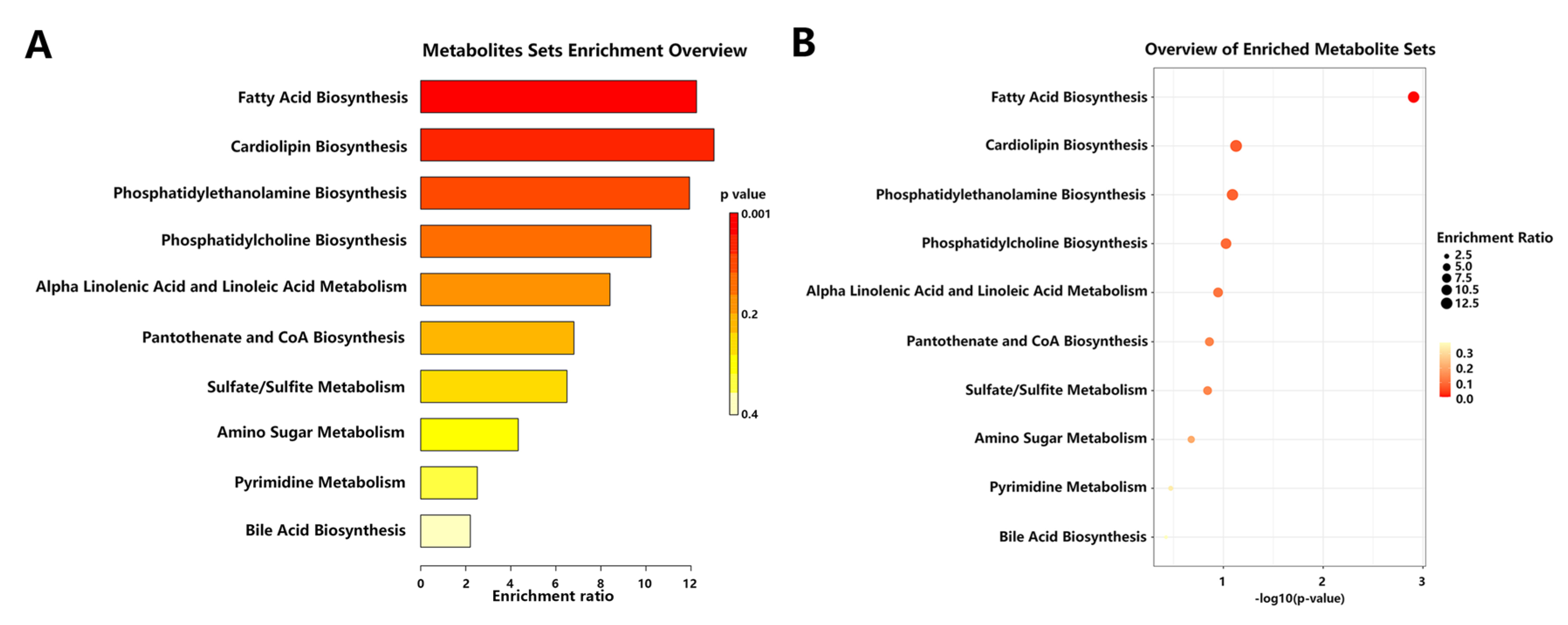
2.5. Inulin Altered Metabolic Pathways Affected by NAFLD
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Biochemical Index Analysis
4.3. Histopathological Analysis
4.4. Serum Metabolomics Data Acquisition
4.5. Reagents and Solvents
4.6. Metabolomics Data Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Rajak, S.; Upadhyay, A.; Tewari, A.; Anthony Sinha, R. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front. Biosci. 2021, 26, 206–237. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules 2022, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Zou, J.; Ran, W.; Qi, X.; Chen, Y.; Cui, H.; Guo, J. Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Front. Endocrinol. 2022, 13, 1087260. [Google Scholar] [CrossRef] [PubMed]
- Apolinario, A.C.; de Lima Damasceno, B.P.; de Macedo Beltrao, N.E.; Pessoa, A.; Converti, A.; da Silva, J.A. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydr. Polym. 2014, 101, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Q.; Wang, L.Y.; Yang, X.Y.; Xu, Y.J.; Fan, G.; Fan, Y.G.; Ren, J.N.; An, Q.; Li, X. Inulin: Properties and health benefits. Food Funct. 2023, 14, 2948–2968. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Du, Z.; Tian, Y.; Liu, M.; Zhu, K.; Zhao, Y.; Wang, H. Inulin accelerates weight loss in obese mice by regulating gut microbiota and serum metabolites. Front. Nutr. 2022, 9, 980382. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.Y.L.; Orr, D.; Plank, L.D.; Vatanen, T.; O’Sullivan, J.M.; Murphy, R. Randomised Double-Blind Placebo-Controlled Trial of Inulin with Metronidazole in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fang, T.; Shi, L.; Wang, Y.; Deng, X.; Wang, J.; Zhou, Y. The synbiotic combination of probiotics and inulin improves NAFLD though modulating gut microbiota. J. Nutr. Biochem. 2024, 125, 109546. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Su, H.; Lv, Y.; Tao, H.; Jiang, Y.; Ni, Z.; Peng, L.; Chen, X. Inulin intervention attenuates hepatic steatosis in rats via modulating gut microbiota and maintaining intestinal barrier function. Food Res. Int. 2023, 163, 112309. [Google Scholar] [CrossRef] [PubMed]
- Perez-Monter, C.; Alvarez-Arce, A.; Nuno-Lambarri, N.; Escalona-Nandez, I.; Juarez-Hernandez, E.; Chavez-Tapia, N.C.; Uribe, M.; Barbero-Becerra, V.J. Inulin Improves Diet-Induced Hepatic Steatosis and Increases Intestinal Akkermansia Genus Level. Int. J. Mol. Sci. 2022, 23, 991. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, Y.; Wang, W.; Shang, F.; Pei, B.; Zhao, Y.; Kong, D.; Fan, Z. Untargeted GC-MS-Based Metabolomics for Early Detection of Colorectal Cancer. Front. Oncol. 2021, 11, 729512. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Lu, H.; Lee, Y.H. Challenges and emergent solutions for LC-MS/MS based untargeted metabolomics in diseases. Mass Spectrom. Rev. 2018, 37, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Dang, X.; Feng, S.; He, Q.; Li, X.; Liu, T.; He, X. Integrated Analyses of Gut Microbiome and Host Metabolome in Children with Henoch-Schonlein Purpura. Front. Cell Infect. Microbiol. 2021, 11, 796410. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Liu, C.; Li, D.; Huang, Q.; Liu, D.; Zhang, Y.; Ye, C.; Zhou, D.; Wang, Y.; Tan, Y.; et al. Metabolomic analyses reveal new stage-specific features of COVID-19. Eur. Respir. J. 2022, 59, 2100284. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Duan, Y.; Yang, L.; Schnabl, B. Small metabolites, possible big changes: A microbiota-centered view of non-alcoholic fatty liver disease. Gut 2019, 68, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, R.S.; Diaz-Canestro, C.; Song, E.; Jia, X.; Liu, Y.; Wang, C.; Cheung, C.K.Y.; Panagiotou, G.; Xu, A. Distinct changes in serum metabolites and lipid species in the onset and progression of NAFLD in Obese Chinese. Comput. Struct. Biotechnol. J. 2024, 23, 791–800. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Su, Y.; Meng, D.; Wang, X.; Wang, J.; Ye, H. A pilot study optimizing metabolomic and lipidomic acquisition in serum for biomarker discovery in nonalcoholic fatty liver disease. J. Mass Spectrom. Adv. Clin. Lab 2021, 22, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, J.; Zhao, K.; Zhao, W.; Shi, Y.; Liu, J.; Zeng, L.; Wang, C.; Zeng, X.; Shi, J. Study on the mechanism of vitamin E alleviating non-alcoholic fatty liver function based on non-targeted metabolomics analysis in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 4299–4307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yuan, Y.; Dawa, Z.; Liu, F.; Yao, Y.; Wang, M.; Zhu, C.; Lin, C. Integrating metabolomics and network pharmacology to reveal the mechanisms of Delphinium brunonianum extract against nonalcoholic steatohepatitis. J. Ethnopharmacol. 2022, 293, 115268. [Google Scholar] [CrossRef] [PubMed]
- Spronk, H.M.; ten Cate, H.; van der Meijden, P.E. Differential roles of tissue factor and phosphatidylserine in activation of coagulation. Thromb. Res. 2014, 133 (Suppl. S1), S54–S56. [Google Scholar] [CrossRef]
- Copic, A.; Dieudonne, T.; Lenoir, G. Phosphatidylserine transport in cell life and death. Curr. Opin. Cell Biol. 2023, 83, 102192. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Chang, W. Phosphatidylserine in Diabetes Research. Mol. Pharm. 2023, 20, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alvarez, M.I.; Sebastian, D.; Vives, S.; Ivanova, S.; Bartoccioni, P.; Kakimoto, P.; Plana, N.; Veiga, S.R.; Hernandez, V.; Vasconcelos, N.; et al. Deficient Endoplasmic Reticulum-Mitochondrial Phosphatidylserine Transfer Causes Liver Disease. Cell 2019, 177, 881–895.e17. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Xu, Y.; Wang, Z.; Wang, T.; Du, X.; Song, X.; Guo, X.; Peng, J.; Zhang, J.; Liang, Y.; et al. Tim-3 Hampers Tumor Surveillance of Liver-Resident and Conventional NK Cells by Disrupting PI3K Signaling. Cancer Res. 2020, 80, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, H.; Gu, Y. Multiple roles of dihomo-gamma-linolenic acid against proliferation diseases. Lipids Health Dis. 2012, 11, 25. [Google Scholar] [CrossRef]
- Mustonen, A.M.; Nieminen, P. Dihomo-gamma-Linolenic Acid (20:3n-6)-Metabolism, Derivatives, and Potential Significance in Chronic Inflammation. Int. J. Mol. Sci. 2023, 24, 2116. [Google Scholar] [CrossRef] [PubMed]
- Fekete, K.; Gyorei, E.; Lohner, S.; Verduci, E.; Agostoni, C.; Decsi, T. Long-chain polyunsaturated fatty acid status in obesity: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Tsurutani, Y.; Inoue, K.; Sugisawa, C.; Saito, J.; Omura, M.; Nishikawa, T. Increased Serum Dihomo-gamma-linolenic Acid Levels Are Associated with Obesity, Body Fat Accumulation, and Insulin Resistance in Japanese Patients with Type 2 Diabetes. Intern. Med. 2018, 57, 2929–2935. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hayashi, T.; Kashima, K.; Kurotani, K.; Shirouchi, B.; Mizoue, T.; Sato, M. Alteration of Serum Phospholipid n-6 Polyunsaturated Fatty Acid Compositions in Nonalcoholic Fatty Liver Disease in the Japanese Population: A Cross-Sectional Study. Lipids 2020, 55, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Kawamoto, T.; Tamura, R. Predictive value of serum dihomo-gamma-linolenic acid level and estimated Delta-5 desaturase activity in patients with hepatic steatosis. Obes. Res. Clin. Pract. 2017, 11, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.C.; McKenna, M.C. L-Carnitine and Acetyl-L-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Savic, D.; Hodson, L.; Neubauer, S.; Pavlides, M. The Importance of the Fatty Acid Transporter L-Carnitine in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Abolfathi, M.; Mohd-Yusof, B.N.; Hanipah, Z.N.; Mohd Redzwan, S.; Yusof, L.M.; Khosroshahi, M.Z. The effects of carnitine supplementation on clinical characteristics of patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 48, 102273. [Google Scholar] [CrossRef] [PubMed]
- Raszeja-Wyszomirska, J.; Safranow, K.; Milkiewicz, M.; Milkiewicz, P.; Szynkowska, A.; Stachowska, E. Lipidic last breath of life in patients with alcoholic liver disease. Prostaglandins Other Lipid Mediat. 2012, 99, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, D.; Drozd, A.; Skonieczna-Zydecka, K.; Skorka-Majewicz, M.; Dec, K.; Jakubczyk, K.; Pilutin, A.; Stachowska, E. Eicosanoids in Nonalcoholic Fatty Liver Disease (NAFLD) Progression. Do Serum Eicosanoids Profile Correspond with Liver Eicosanoids Content during NAFLD Development and Progression? Molecules 2020, 25, 2026. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, J.S.; Roh, Y.S. Molecular insights into the role of mitochondria in non-alcoholic fatty liver disease. Arch. Pharm. Res. 2019, 42, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Abulikemu, A.; Zhao, X.; Xu, H.; Li, Y.; Ma, R.; Yao, Q.; Wang, J.; Sun, Z.; Li, Y.; Guo, C. Silica nanoparticles aggravated the metabolic associated fatty liver disease through disturbed amino acid and lipid metabolisms-mediated oxidative stress. Redox Biol. 2023, 59, 102569. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 14205–14218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, X.; Wang, K.; Zhao, Z.; Dang, Y.; Ji, G.; Li, F.; Zhou, W. Lingguizhugan decoction improves non-alcoholic steatohepatitis partially by modulating gut microbiota and correlated metabolites. Front. Cell Infect. Microbiol. 2023, 13, 1066053. [Google Scholar] [CrossRef]

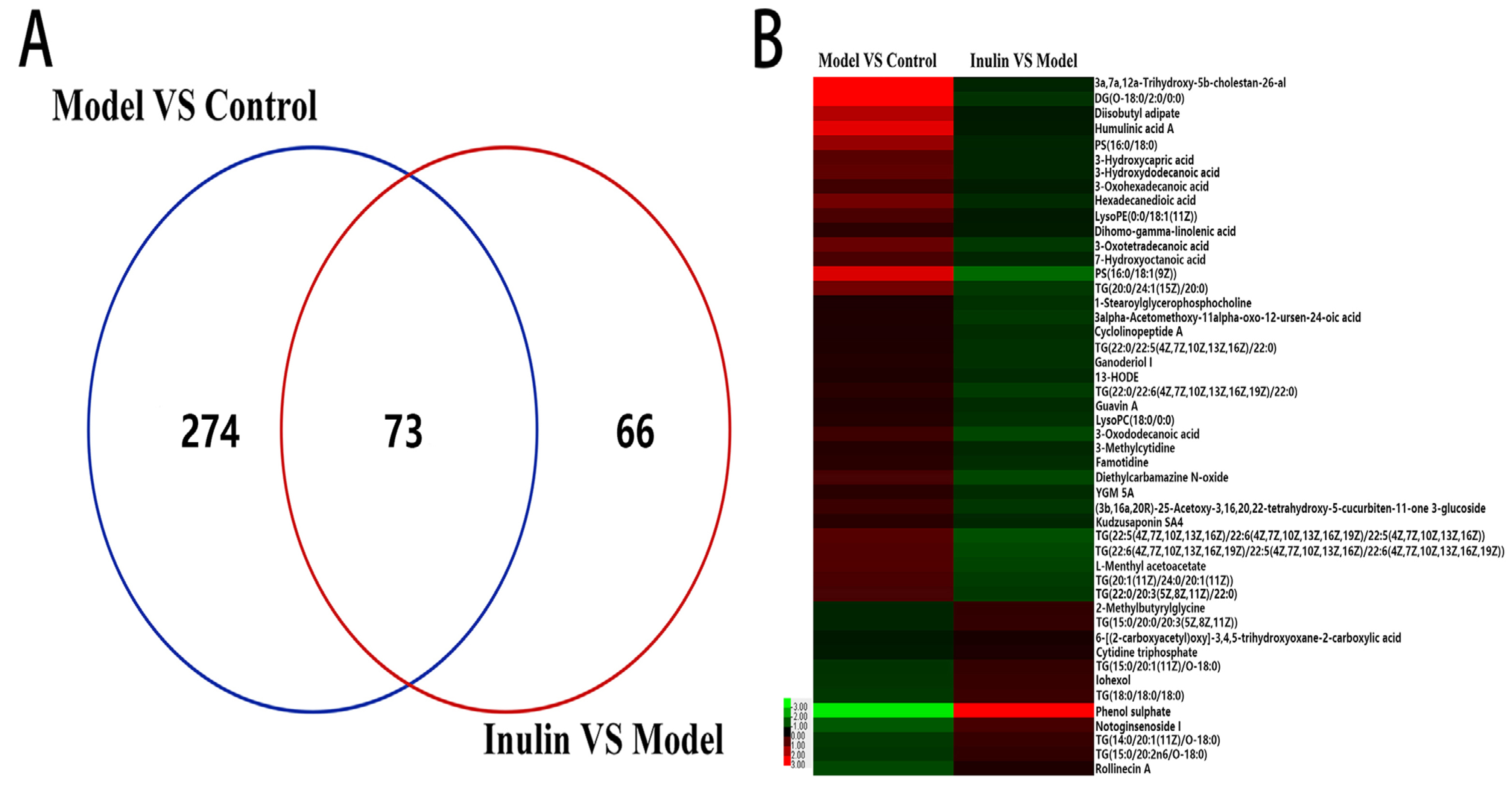
| Metabolites | Model vs. Control Fold Change | Model vs. Control p Value | Inulin vs. Model Fold Change | Inulin vs. Model p Value |
|---|---|---|---|---|
| (3b,16a,20R)-25-Acetoxy-3,16,20,22-tetrahydroxy-5-cucurbiten-11-one 3-glucoside | 1.6 | 0.01 | 0.65 | 0.01 |
| 13-HODE | 1.27 | 0.01 | 0.71 | 0 |
| 1-Stearoylglycerophosphocholine | 1.25 | 0 | 0.68 | 0.02 |
| 2-Methylbutyrylglycine | 0.74 | 0.01 | 1.49 | 0 |
| 3a,7a,12a-Trihydroxy-5b-cholestan-26-al | 8.42 | 0 | 0.75 | 0.02 |
| 3alpha-Acetomethoxy-11alpha-oxo-12-ursen-24-oic acid | 1.28 | 0 | 0.64 | 0 |
| 3-Hydroxycapric acid | 2.07 | 0 | 0.74 | 0.01 |
| 3-Hydroxydodecanoic acid | 2.19 | 0 | 0.74 | 0 |
| 3-Methylcytidine | 1.35 | 0 | 0.72 | 0.01 |
| 3-Oxododecanoic acid | 1.65 | 0.03 | 0.56 | 0 |
| 3-Oxohexadecanoic acid | 1.68 | 0 | 0.79 | 0.01 |
| 3-Oxotetradecanoic acid | 2.31 | 0 | 0.64 | 0 |
| 6-[(2-carboxyacetyl)oxy]-3,4,5-trihydroxyoxane-2-carboxylic acid | 0.82 | 0 | 1.22 | 0.04 |
| 7-Hydroxyoctanoic acid | 1.83 | 0 | 0.74 | 0 |
| Cyclolinopeptide A | 1.26 | 0 | 0.69 | 0.01 |
| Cytidine triphosphate | 0.8 | 0.01 | 1.25 | 0.01 |
| DG(O-18:0/2:0/0:0) | 16.85 | 0 | 0.67 | 0 |
| Diethylcarbamazine N-oxide | 1.76 | 0.01 | 0.56 | 0 |
| Dihomo-gamma-linolenic acid | 1.42 | 0 | 0.8 | 0.01 |
| Diisobutyl adipate | 4.37 | 0 | 0.81 | 0 |
| Famotidine | 1.39 | 0 | 0.69 | 0 |
| Ganoderiol I | 1.31 | 0 | 0.67 | 0.01 |
| Guavin A | 1.3 | 0 | 0.71 | 0.04 |
| Hexadecanedioic acid | 2.56 | 0 | 0.71 | 0.01 |
| Humulinic acid A | 6.49 | 0 | 0.8 | 0.01 |
| Iohexol | 0.66 | 0 | 1.57 | 0.02 |
| Kudzusaponin SA4 | 1.4 | 0.02 | 0.73 | 0.02 |
| L-Menthyl acetoacetate | 1.95 | 0 | 0.58 | 0 |
| LysoPC(18:0/0:0) | 1.34 | 0 | 0.68 | 0.01 |
| LysoPE(0:0/18:1(11Z)) | 1.83 | 0 | 0.81 | 0.02 |
| Notoginsenoside I | 0.48 | 0 | 1.79 | 0.02 |
| Phenol sulphate | 0.15 | 0 | 8.81 | 0 |
| PS(16:0/18:0) | 3.4 | 0 | 0.75 | 0.01 |
| PS(16:0/18:1(9Z)) | 5.99 | 0 | 0.42 | 0.01 |
| Rollinecin A | 0.56 | 0 | 1.27 | 0 |
| TG(14:0/20:1(11Z)/O-18:0) | 0.63 | 0 | 1.56 | 0.02 |
| TG(15:0/20:0/20:3(5Z,8Z,11Z)) | 0.74 | 0.01 | 1.51 | 0.02 |
| TG(15:0/20:1(11Z)/O-18:0) | 0.66 | 0 | 1.53 | 0.03 |
| TG(15:0/20:2n6/O-18:0) | 0.65 | 0 | 1.47 | 0.03 |
| TG(18:0/18:0/18:0) | 0.64 | 0 | 1.6 | 0.02 |
| TG(20:0/24:1(15Z)/20:0) | 2.58 | 0 | 0.63 | 0 |
| TG(20:1(11Z)/24:0/20:1(11Z)) | 1.83 | 0 | 0.61 | 0 |
| TG(22:0/20:3(5Z,8Z,11Z)/22:0) | 1.76 | 0 | 0.64 | 0 |
| TG(22:0/22:5(4Z,7Z,10Z,13Z,16Z)/22:0) | 1.28 | 0 | 0.68 | 0 |
| TG(22:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/22:0) | 1.4 | 0 | 0.63 | 0 |
| TG(22:5(4Z,7Z,10Z,13Z,16Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/22:5(4Z,7Z,10Z,13Z,16Z)) | 2.01 | 0 | 0.53 | 0 |
| TG(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/22:5(4Z,7Z,10Z,13Z,16Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | 1.93 | 0 | 0.55 | 0 |
| YGM 5A | 1.41 | 0 | 0.71 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhou, W.; Zhu, M. Serum Metabolomics Uncovers the Mechanisms of Inulin in Preventing Non-Alcoholic Fatty Liver Disease. Pharmaceuticals 2024, 17, 895. https://doi.org/10.3390/ph17070895
Sun Y, Zhou W, Zhu M. Serum Metabolomics Uncovers the Mechanisms of Inulin in Preventing Non-Alcoholic Fatty Liver Disease. Pharmaceuticals. 2024; 17(7):895. https://doi.org/10.3390/ph17070895
Chicago/Turabian StyleSun, Yunhong, Wenjun Zhou, and Mingzhe Zhu. 2024. "Serum Metabolomics Uncovers the Mechanisms of Inulin in Preventing Non-Alcoholic Fatty Liver Disease" Pharmaceuticals 17, no. 7: 895. https://doi.org/10.3390/ph17070895
APA StyleSun, Y., Zhou, W., & Zhu, M. (2024). Serum Metabolomics Uncovers the Mechanisms of Inulin in Preventing Non-Alcoholic Fatty Liver Disease. Pharmaceuticals, 17(7), 895. https://doi.org/10.3390/ph17070895






